Abstract
Norovirus GII.4 is recognized as a worldwide cause of nonbacterial outbreaks. In particular, the GII.4 variant occurs every 2–3 years according to antigenic variation. The aim of our study was to identify GII.4 variants in outbreaks in Korea during 2004–2012. Partial VP1 sequence of norovirus GII.4-related outbreaks during 2004–2012 was analyzed. The partial VP1 sequence was detected with reverse transcription-polymerase chain reaction, seminested polymerase chain reaction, and nucleotide sequence of 312-314 base pairs for phylogenetic comparison. Nine variants emerged in outbreaks, with the Sydney variant showing predominance recently. This predominance may persist for at least 3 years, although new variants may appear in Korea.
Keywords: epidemics, GII.4 variants, norovirus
Noroviruses (NoVs) are recognized as a worldwide cause of epidemic acute gastroenteritis in all age groups as a result of their low infectious dose and highly aggressive characteristics [1]. NoVs are divided into five genogroups (G), and NoV GI (15 genotypes) and GII (21 genotypes) are known to contain pathogens that infect humans. In particular, NoV GII.4 is the prominent genotype detected in cases of human infection, and this genotype produces variants in epidemic years and shows genetic characteristics of quasispecies-associated population diversity 2, 3. Recently, the GII.4 variants have been divided into 13 subcluster types. The New-Orleans and Sydney variants have been reported as relatively new strains, described in 2010 and 2012 4, 5, 6. In Korea, NoV surveys have been performed continuously since 2004 under the laboratory surveillance system CaliciNet.
In this study, we analyzed the NoV GII.4 sequences deposited in the CaliciNet database, and described the predominance and emergence of the GII.4 variant strain in Korea from 2004 to 2012.
NoV-related outbreaks were reported in 298 cases between 2004 and 2012 in Korea (data not shown). Stool samples were provided from 17 regional institutes of health and environment research to analyze the GII.4 variants. The partial capsid sequence was used to determine the genotype associated with NoV-related outbreaks. Viral RNA was extracted using an automated prep kit (GM-AUTOPREP Kit; Greenmate Biotech Corp., Seoul, Korea), according to the manufacturer’s instructions. The NoV genome was detected by conducting a one-step reverse transcription-polymerase chain reaction (RT-PCR; AccuPower RT/PCR PreMix; Bioneer, Daejeon, Korea) with NoV-specific primers. Then, seminested PCR (AccuPower PCR PreMix; Bioneer) was performed based on the partial 312–314 base pair sequence of the capsid region. RT-PCR was performed for NV-GIF1, NV-GIR1, NV-GIIF1, and NV-GIIR1 according to the genogroup references. For seminested PCR, NV-GIF2 and NV-GIIF2 were added to the type-specific sense primers in the PCR mixture [7]. To improve the diagnostic efficiency, we included a web-based reporting system, K-CaliciNet, starting in 2007 and a one-step real-time PCR starting in 2008 in the laboratory surveillance system. Nucleotide sequencing was performed with an automated sequencing system (ABI3730xl; Applied Biosystems, Foster, CA, USA), and the results were reported to CaliciNet. The nucleotide sequences were aligned with the CLUSTAL W program in MegAlign package (Windows version 3.12e). In particular, the GII.4 variants were clustered with the neighbor-joining method. References for the GII.4 variants were provided by GenBank 4, 5, 6 and subdivided into 13 subcluster types.
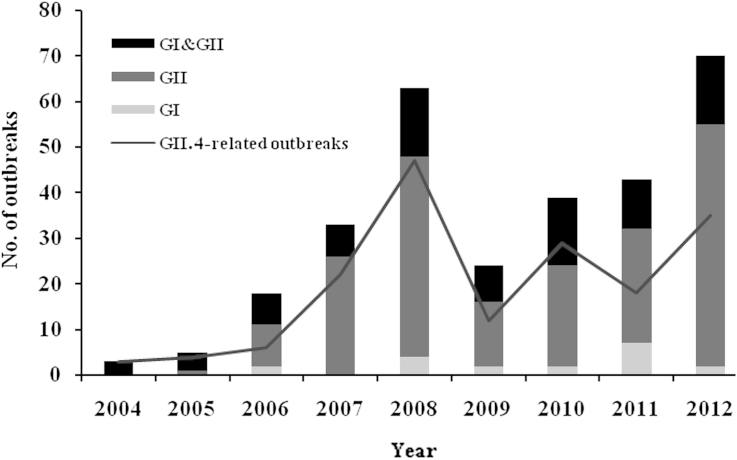
NoV-related outbreaks were continuously reported for 9 years. However, the incidence of NoV peaked in 2008 and 2012. Most of the NoV GI-associated outbreaks were caused by infections with mixed genotypes and/or mixed genogroups including GII, whereas the GII-associated outbreaks tended to be caused by a single genogroup or genotype. The distribution of GII.4-related outbreaks was compared with the total NoV-related outbreaks, based on the phylogenetic partial sequence. NoV GII.4 was the most prevalent genotype (60.3%) in NoV-associated outbreaks for each year examined (Figure 1).
Figure 1.
The number of NoV-related outbreaks from 2004 to 2012. Outbreaks associated with NoV were reported through the public health reporting system at KNIH. The genogroup was determined using a GI/GII-specific RT-PCR method. GI is shown as a gray bar, GII as a dark gray bar, and mixed infection (GI&GII) as a black bar. Notably, GII.4-related outbreaks are shown as a dark gray line. G = genogroup; KNIH = Korea National Institute of Health; Nov = norovirus; RT-PCR = reverse transcription-polymerase chain reaction.
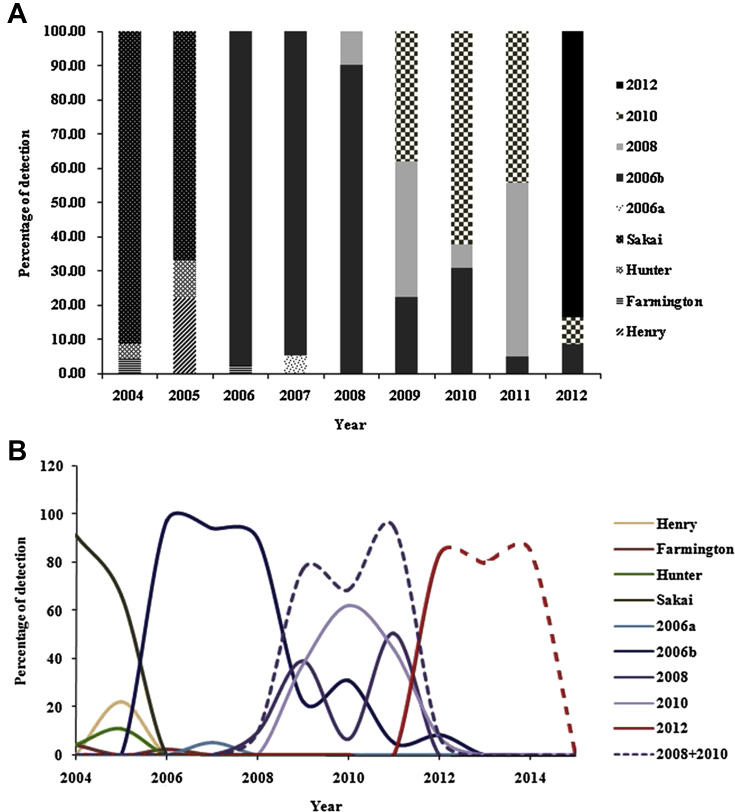
Nine variants were identified from the GII.4 strains detected in this period, including the Henry, Farmington, Hunter, Sakai, 2006a, 2006b, 2008, New Orleans (2010), and Sydney (2012) variants (Figure 2A). The 2006b variant was prominent during the years 2006–2008. The 2008 and New Orleans (2010) variants emerged between the years 2008 and 2011, but were not prominent, when compared to the 2006b variant. The Sydney (2012) variant was detected in 2011, and its prevalence sharply increased in 2012 (Figures 2A and 2B). The 2008 variant showed a biphasic and retrogressive prevalence pattern compared with the 2006b variant. The sequence identity of the 2006b strain showed high similarity to that of the New Orleans (2010) strain, which was attributed to one branch of the group (data not shown). The merged prevalence of these variants was similar to the prevalence pattern of the 2006b variant (Figure 2B).
Figure 2.
Distribution of NoV GII.4 variants in Korea from 2004 to 2012. (A) NoV GII.4 variants are shown in a bar graph, divided according to the total NoV GII.4 variants in Korea. (B) NoV GII.4 variants are drawn as a full line and predicted variants as a dotted line. The sum of the 2008 and 2010 variants showed a similar epidemic pattern to that of the 2006b variant. The epidemic pattern of the Sydney variant was assumed based on the 2006b variant and the sum of the 2008 and 2010 variants. G = genogroup; Nov = norovirus.
NoVs are recognized as important pathogens for gastroenteritis, although their mechanisms of infection and pathogenesis are poorly understood because of the absence of a cell culture system or animal model. For this reason, most research has been performed through molecular epidemiological studies and has concentrated on the NoV GII.4 strain, which is the most prevalent genotype worldwide. NoV GII.4 is also a prominent strain in Korea, causing both outbreak and sporadic cases, as determined by the laboratory surveillance system (data not shown). The NoV detection rate increased after the detection method was changed from multiplex real-time PCR to conventional RT-PCR, to assess the outbreak response and laboratory surveillance system starting in 2008. Conventional RT-PCR is able to genotype and sequence directly, but carries a risk of contamination and takes a long time to perform. Despite this weakness, conventional RT-PCR is used as an additional analysis method for sequencing and genotyping.
The partial VP1 sequence of GII.4 was genetically analyzed for its variation in the nucleotide sequence. Emergence of the GII.4 variant was reported as a biennial event. The 2008 and New Orleans (2010) variants were reported as different variants, but they were remarkably identical compared with the other variants. The New Orleans (2010) variant closely clustered with the 2008 variant, but not the Sydney (2012) variant, on a phylogenetic tree (data not shown). The merged prevalence of the 2008 and New Orleans (2010) variants showed a similar epidemic trend to that of the 2006b variant in Korea (Figure 2B). Thus, it is reasonable to assume that the emergence of scientific variants represents a biennial event, whereas the emergence of an epidemic variant represents a quadrennial event, on the premise that the 2008 and New Orleans (2010) variants originated from one index strain. In this respect, there may have been three epidemic events of the NoV GII.4 variants in Korea: the 2006b, 2008 and New Orleans (2010), and Sydney (2012) variants (Figure 2A and 2B). The first epidemic event occurred due to the 2006b variant from 2006 to 2009, but was completely terminated in 2013. The second epidemic event was caused by the 2008 and New Orleans (2010) variants from 2009 to 2011. The third epidemic event was caused by the Sydney variant in 2012, at the same time as a global outbreak, and this event may persist for 3 years.
NoV GII.4 variants have caused several pandemics 8, 9. One variant 95/96 led to a pandemic outbreak between 1995 and 2001 9, 10, along with two other variants in 2002 11, 12 and 2004 [13]. These outbreaks suggest that reinfection with other types arises from genetic and antigenic diversity and that immunity is not permanent, as infection due to repeated exposure was statistically significant 14, 15. Therefore, a continuous study of human NoVs is essential in many fields, including diagnostic methods, epidemiology, and development of vaccines among others. The sequence database used in this study is the major tool for epidemiological research in Korea. However, no conclusions could be drawn about the partial VP1 sequence in this study because a complete VP1 sequence or three-dimensional-prediction model synthesis is not yet available. However, these data provided significant insights into the distribution of GII.4 variants and the predicted emerging strains in Korea since 2004. Efforts are in progress to obtain additional information on various strains, and we hope to study the complete viral genome for NoV soon.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This study was supported by National Norovirus Surveillance System (K-CaliciNet, 4851-304-210) and the Regional Institute of Health and Environment Research.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Rockx B., De Wit M., Vennema H. Natural history of human calicivirus infection: a prospective cohort study. Clin Infect Dis. 2002 Aug;35(3):246–253. doi: 10.1086/341408. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson E.F., Lindesmith L.C., Lobue A.D. Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations. Immunol Rev. 2008 Oct;225:190–211. doi: 10.1111/j.1600-065X.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 3.Escarmis C., Lazaro E., Manrubia S.C. Population bottlenecks in quasispecies dynamics. Curr Top Microbiol Immunol. 2006;299:141–170. doi: 10.1007/3-540-26397-7_5. [DOI] [PubMed] [Google Scholar]
- 4.Belliot G., Kamel A.H., Estienney M. Evidence of emergence of new GGII.4 norovirus variants from gastroenteritis outbreak survey in France during the 2007-to-2008 and 2008-to-2009 winter seasons. J Clin Microbiol. 2010 Mar;48(3):994–998. doi: 10.1128/JCM.01888-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Beek J., Ambert-Balay K., Botteldoorn N. Indications for worldwide increased norovirus activity associated with emergence of a new variant of genotype II.4, late 2012. Euro Surveil. 2013 Jan;18(1):8–9. [PubMed] [Google Scholar]
- 6.Vega E., Barclay L., Gregoricus N. Novel surveillance network for norovirus gastroenteritis outbreaks, United States. Emerg Infect Dis. 2011 Aug;17(8):1389–1395. doi: 10.3201/eid1708.101837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S.H., Cheon D.S., Kim J.H. Outbreaks of gastroenteritis that occurred during school excursions in Korea were associated with several waterborne strains of norovirus. J Clin Microbiol. 2005 Sep;43(9):4836–4839. doi: 10.1128/JCM.43.9.4836-4839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koopmans M., Vinjé J., Duizer E. Molecular epidemiology of human enteric caliciviruses in the Netherlands. Novartis Found Symp. 2001;238:197–214. doi: 10.1002/0470846534.ch12. discussion 214–8. [DOI] [PubMed] [Google Scholar]
- 9.Noel J.S., Fankhauser R.L., Ando T. Identification of a distinct common strain of “Norwalk-like viruses” having a global distribution. J Infect Dis. 1999 Jun;179(6):1334–1344. doi: 10.1086/314783. [DOI] [PubMed] [Google Scholar]
- 10.Lau C.S., Wong D.A., Tong L.K. High rate and changing molecular epidemiology pattern of norovirus infections in sporadic cases and outbreaks of gastroenteritis in Hong Kong. J Med Virol. 2004 May;73(1):113–117. doi: 10.1002/jmv.20066. [DOI] [PubMed] [Google Scholar]
- 11.Ike A.C., Brockmann S.O., Hartelt K. Molecular epidemiology of norovirus in outbreaks of gastroenteritis in southwest Germany from 2001 to 2004. J Clin Microbiol. 2006 Apr;44(4):1262–1267. doi: 10.1128/JCM.44.4.1262-1267.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopman B., Vennema H., Kohli E. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet. 2004 Feb;363(9410):682–688. doi: 10.1016/S0140-6736(04)15641-9. [DOI] [PubMed] [Google Scholar]
- 13.Bull R.A., Hansman G.S., Clancy L.E. Norovirus recombination in ORF1/ORF2 overlap. Emerg Infect Dis. 2005 Jul;11(7):1079–1085. doi: 10.3201/eid1107.041273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallimore C.I., Cubitt D., du Plessis N. Asymptomatic and symptomatic excretion of noroviruses during a hospital outbreak of gastroenteritis. J Clin Microbiol. 2004 May;42(5):2271–2274. doi: 10.1128/JCM.42.5.2271-2274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson P.C., Mathewson J.J., DuPont H.L. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis. 1990 Jan;161(1):18–21. doi: 10.1093/infdis/161.1.18. [DOI] [PubMed] [Google Scholar]