Abstract
Alpha-defensins, including human neutrophil peptides 1-3 (HNP1-3) and human defensin 5 (HD5), are elevated at the genital mucosa in individuals with sexually transmitted infections (STIs). The presence of STIs is associated with an increased risk of human immunodeficiency virus (HIV) transmission, suggesting there may be a role for defensins in early events of HIV transmission. HD5 has been demonstrated to contribute to STI-mediated increased HIV infectivity in vitro. HNPs exhibit anti-HIV activity in vitro. However, increased levels of HNPs have been associated with enhanced HIV acquisition and higher viral load in breast milk. This study found that HNP1, but not HD5, significantly disrupted epithelial integrity and promoted HIV traversal of epithelial barriers. Linear HNP1 with the same charges did not affect epithelial permeability, indicating that the observed effect of HNP1 on the epithelial barrier was structure dependent. These results suggest a role for HNP1 in STI-mediated enhancement of HIV transmission.
Introduction
Mucosal epithelial cells and their secreted products provide the first line of defense against human immunodeficiency virus (HIV) during genital and rectal transmission (13,14). However, HIV has been demonstrated to cross the epithelial barrier in a cell-free or cell-associated manner in vitro (1,3,4,15,23), and cell-free infection plays a major role in mucosal transmission of SIV in vivo (24,31). Sexually transmitted infections (STIs) increase the risk of HIV mucosal transmission in part by altering epithelial barriers (10,16,24). Individuals with STIs exhibit elevated levels of antimicrobial peptides, including human defensin 5 (HD5) produced by genital epithelial cells and human neutrophil peptides (HNPs) produced by infiltrating neutrophils (9,27,30,35). These defensins are believed to play a role in controlling bacterial infection. However, increasing evidence indicates that defensins could exhibit pleotropic effects on viral infection (8,34). HD5 contributes to Neisseria gonorrhoeae-mediated enhancement of HIV infectivity in vitro (18,29), whereas HNPs block HIV infection in vitro through multiple mechanisms (7,22,33). In contrast to their anti-HIV activity in vitro, elevated levels of HNPs in cervicovaginal fluid have been associated with an increase in HIV acquisition (21). The levels of HNPs in breast milk are also correlated with HIV RNA viral load (2,5,19). However, their association with mother-to-child HIV transmission has not been consistent (5,19).
This study examined the effect of HNP1 and HD5 on epithelial integrity and the subsequent impact on HIV traversal of the epithelial barrier. We found that HNP1, but not HD5, significantly increased epithelial permeability, which was accompanied by an increase in HIV traversal of the barrier in a polarized system, suggesting a role for HNP1 in promoting HIV transmission.
Materials and Methods
Reagents
HNP1 and HD5 and their linear analogs were chemically synthesized as previously described (36). Cysteine residues were replaced by alanine for synthesis of linear HNP1; whereas, isosteric α-aminobutyric acid (Abu) replaced cysteine residues for synthesis of linear HD5. Rabbit anti-ZO-1 (Mid, catalog #40-220) polyclonal antibody, mouse anti-occludin (OC-3F10, #33-1500) monoclonal antibody, and Prolong Gold Antifade Mountant with DAPI (4′,6-Diamidino-2-phenylindole) were purchased from Invitrogen, Life Technologies. Fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 fragment of anti-rabbit immunoglobulin G (IgG) and Cy3-conjugated sheep F(ab′)2 fragment of anti-mouse IgG were obtained from Sigma-Aldrich. CCR5-tropic HIV-1BaL virus was purchased from Advanced Biotechnologies, Inc.
Measurement of transepithelial electrical resistance
Colorectal epithelial (Caco-2) cells (3 × 104 cells/well) were grown and polarized on 24-well transwell inserts with a 0.4 μm pore size (Corning) in 10% fetal bovine serum Dulbecco's modified Eagle's medium (DMEM) for 5 days. Polarized cells were used when transepithelial electrical resistance (TER) reached ≥500 Ω by the EVOM voltmeter (World Precision Instruments). Polarized cells were then placed in serum-free DMEM, and transwell inserts were transferred to a CellZscope (nanoAnalytics) instrument. The CellZscope was used to measure TER in multiple wells simultaneously for 0–24 h. Once the instrument was initialized, defensins at various concentrations were added to the apical chamber. Data were analyzed using the nanoAnalytics CellZscope software.
FITC-dextran diffusion assay
FITC-dextran (Sigma, FD4) at 1 mg/mL in Hank's balanced salt solution (HBSS) was added to the apical chamber of polarized cells treated with ethylenediaminetetraacetic acid (EDTA) or defensin for 4 h. After 1 h incubation at 37°C, the florescence intensity of FITC-dextran in the basolateral compartment was measured using EnSpire Multimode Plate Reader (Perkin Elmer) at 490 nm for excitation and 520 nm for emission.
Immunofluorescence microscopy
Caco-2 monolayers (3 × 104 cells/dish) were cultured on 35 mm poly-D-lysine coated MatTek dishes (P35GC-0-10-C; MatTek), and polarized cells were cultured on transwell inserts as described above. Cells in plates and transwells were treated with defensins for 4 h at 37°C, followed by fixation with 4% paraformaldehyde for 10 min at room temperature, and permeabilization with 0.1% Triton-X for 10 min. Samples were incubated in blocking solution (50 mM ETDA, 1% fish gelatin, 1% bovine serum albumin IgG free, 1% horse serum) for 1 h at room temperature, and incubated with diluted (1:200) anti-ZO-1 or anti-occludin antibody in blocking solution at 4°C. After overnight incubation, samples were washed five times with room temperature phosphate-buffered saline (PBS) followed by incubation with (1:500) diluted FITC-conjugated antirabbit IgG or Cy3-conjugated sheep antimouse IgG for 2 h at room temperature. Samples were washed five times with PBS, and mounted using Prolong Gold Antifade Mountant with DAPI. Images for polarized cells were captured on a Zeiss Axio Observer Z1 microscope, and data were analyzed using ZEN imaging software (Zeiss) to enhance brightness and contrast of all images uniformly and to insert the scale bar for the images. No background was subtracted. Cells grown in a monolayer on MatTek dishes were visualized using a NikonA1Rsi confocal microscope. Images were quantified by using NIS Element Advanced Research software (v4.13) to determine the values of blue (DAPI), green (ZO-1), or red (occludin) pixels in images. The fluorescence intensity of tight junctions markers in each image was first normalized to DAPI expression by dividing the values of pixels for ZO-1 or occludin by those for DAPI. The ratios of pixels for tight junction markers expressed in treated cells were then divided by those in untreated control.
Assay for HIV traversal of the polarized epithelium
Polarized Caco-2 cells were treated with or without EDTA or defensins for 4 h. HIV-1BAL in 100 μL of serum-free medium at a concentration of 500 ng/mL HIV p24 was placed in the apical chamber. The basolateral chamber contained 500 μL of the medium in a 24-well plate. At indicated time points after HIV exposure, HIV p24 levels in the bottom chamber were determined by HIV-1 alphaLISA HIV p24 kit (Perkin-Elmer). HIV p24 levels in the bottom chamber of the untreated control samples were approximately 7 pg/mL and 14 pg/mL after HIV exposure for 24 and 48 h, respectively.
Statistical analysis
Statistical analysis was performed using a two-tailed Student's t-test (Fig. 3B, EDTA) or one-way analysis of variance with Bonferroni correction; p < 0.05 was considered significant.
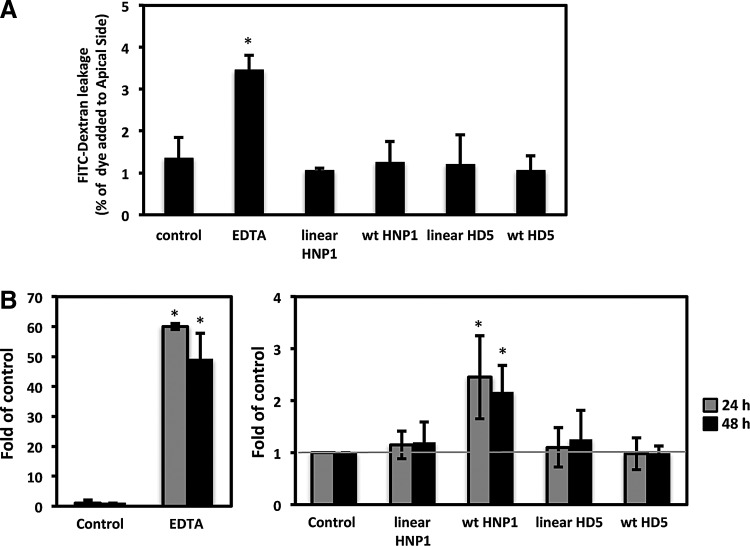
FIG. 3.
HNP1, but not HD5, promotes HIV traversal of polarized intestinal epithelial cells. (A) The effect of defensins on epithelial permeability was determined by FITC-dextran diffusion assays. EDTA or linear defensins were included as a comparison. To calculate percentage of FITC-dextran leakage, the florescence intensity in the basal chamber was divided by the fluorescent intensity in the apical chamber, then multiplied by 100. Data represent an average of two independent experiments. (B) Polarized Caco-2 cells were treated with EDTA at 2.5 mM, or defensins and their linear analogs at 6 μM for 4 h, before addition of HIV-1BAL to the apical chamber. The HIV levels in the bottom chamber at the indicated times were determined by HIV p24 alphaLISA. The fold changes of HIV traversal compared to untreated control samples were determined. Data are mean ± SD of three to five independent experiments. The gray line indicates no change in HIV traversal compared to the untreated control samples. EDTA, ethylenediaminetetraacetic acid; FITC, fluorescein isothiocyanate.
Results
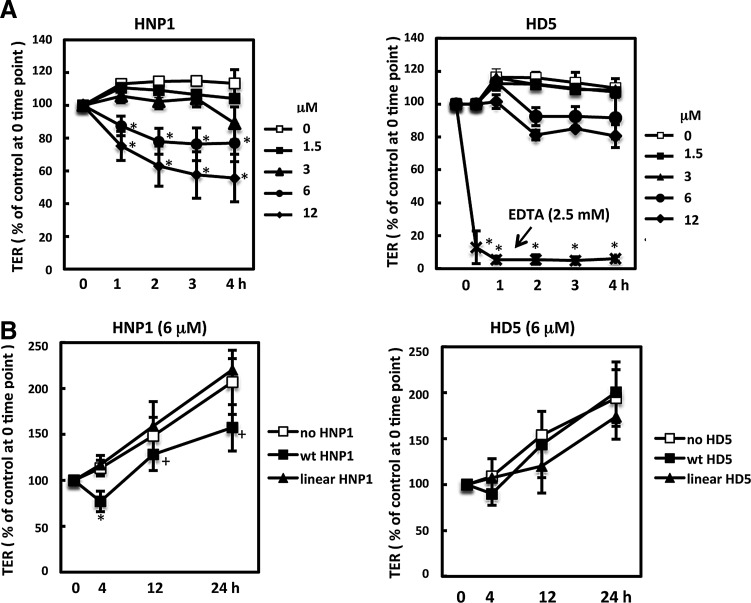
To determine the effect of alpha-defensins on epithelial integrity, HNP1 and HD5 were added to the apical side of polarized Caco-2 cells, a colorectal carcinoma epithelial cell line. The concentrations of HNP1 and HD5 used in this study did not cause cytotoxic effects as determined by a CytoTox-Glo cytotoxicity kit (Promega; data not shown). Kinetics of TER as a measure of epithelial permeability was examined by CellZscope (nanoAnalytics) in multiple wells under different conditions simultaneously at 37°C. Analysis of the impact of defensins on short-term tight junction dynamics (within 4 h) revealed that HNP1 significantly increased epithelial permeability in a time- and dose-dependent manner (Fig. 1A). Reduction of TER by HNP1 was observed as early as 1 h post-treatment, suggesting a direct effect of the HNP1 on epithelium. EDTA (2.5 mM), which is known to increase paracellular transport, was also included as a comparison. As expected, EDTA reduced TERs significantly within 30 min and continued to decline within 4 h (Fig. 1A, right panel). TER increased over time in the presence of HNP1, suggesting that HNP1 did not prevent recovery of the tight junction network (Fig. 1B). However, TER values in HNP1-treated cells were lower than untreated cells after 24 h, although the difference was not significant after Bonferroni correction. HD5 did not significantly affect epithelial permeability within 4 or 24 h of treatment (Fig. 1A and B, right panel). Linear analogs of HNP1 and HD5 did not affect epithelial permeability, indicating that the impact of the alpha-defensin on epithelial integrity was structure dependent (Fig. 1B).
FIG. 1.
Human neutrophil peptide 1 (HNP1), but not human defensin 5 (HD5) or linear analogs, increases epithelial permeability. (A) The effect of HNP1 and HD5 on short-term dynamics of epithelial integrity in polarized Caco-2 cells were determined by measuring transepithelial electrical resistance (TER) using a CellZscope. (B) Effect of defensins and their linear analogs (6 μM) on epithelial integrity. TER values were normalized by the value at time 0 for each treatment. Data are mean ± standard deviation (SD) of averages of three to six experiments; *p < 0.05, defensin-treated versus untreated control at the same time point. After Bonferroni correction, the difference between untreated control and HNP1 at 12 or 24 h was not significant (+, p > 0.05).
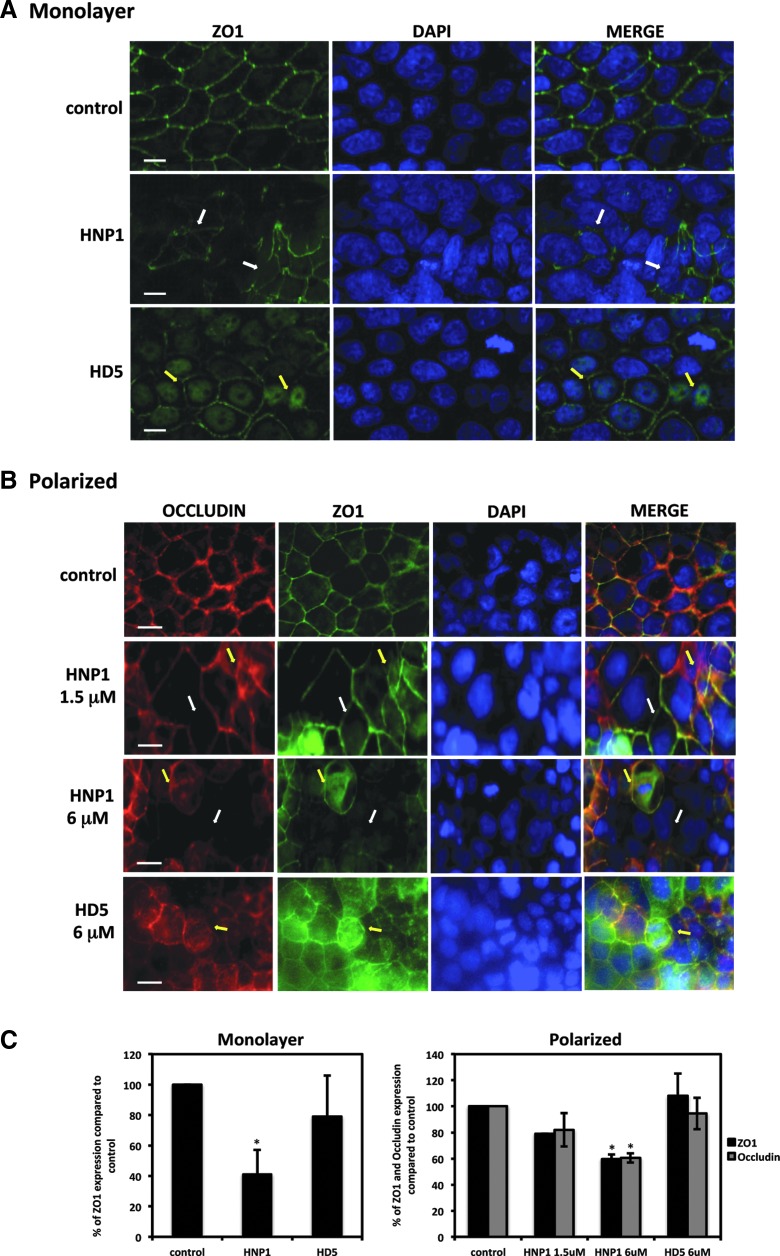
To examine whether changes in epithelial permeability induced by defensins were associated with altered distribution of tight junction markers, Caco-2 cells grown in a monolayer or a polarized system were treated with defensins for 4 h, and the distribution of tight junction markers, zonula occludens-1 (ZO-1) and occludin were examined by immunofluorescence microscopy. HNP1-treated intestinal monolayers lost the typical honeycomb distribution found in untreated cells (Fig. 2A), whereas HD5-treated cells maintained their overall tight junctions. HD5 treatment caused internalization of ZO-1, indicative of a decrease in cell–cell contact (12). Similar results were found in defensin-treated polarized cells (Fig. 2B). At a concentration of 1.5 μM, HNP1 caused significant discontinuation and translocation of occludin and ZO-1. The extent of tight junction disruption was even more apparent when cells were treated with HNP1 at 6 μM. Although disruption of intercellular borders was not apparent, internalization of occludin and ZO-1 was detected in HD5-treated cells. Quantitative analysis indicated 6 μM of HNP1, but not HD5, significantly reduced tight junction expression compared with untreated cells (Fig. 2C).
FIG. 2.
Defensins alter the distribution of tight junction markers. Monolayers of Caco-2 cells grown on MatTek plates (A) or polarized Caco-2 cells grown in transwell inserts (B) were treated with defensins (6 μM) for 4 h followed by immunostaining with antibodies against tight junction markers ZO-1 and occludin. White arrows indicate loss of intercellular tight junction borders; yellow arrows indicate translocation of markers. Images in (A) were acquired on a Nikon A1Rsi confocal microscope; images in (B) were acquired on a Zeiss Axio Observer Z1 microscope. Scale bar = 10 μm (A) or 20 μm (B). Immunofluorescence intensity of tight junction markers was quantified as described in Materials and Methods (C). The results represent means of a minimum of three representative fields for each experiment and averages of two independent experiments as described by Galvin and Cohen (11).
The effects of defensins on epithelial integrity could facilitate subsequent HIV traversal of the barrier. FITC-dextran permeability assay showed there was an increase in FITC-dextran diffusion after EDTA treatment for 4 h (Fig. 3A). However, the treatment with defensins or their linear analogs did not have an apparent effect on FITC-dextran diffusion from the apical to the basolateral chamber. The effect of EDTA, defensins, or their linear analogs on HIV translocation were then determined. Defensins at 6 μM that reduced TER by 20% were used to avoid the potential cytotoxicity of defensins at high concentrations after long-term incubation. HIV was added to the apical side of polarized epithelial cells at 4 h after various treatments. The levels of HIV particles that crossed the polarized epithelium were determined by alphaLISA HIV p24 kit. As expected, EDTA treatment for 4 h significantly increased HIV translocation by nearly 60-fold after HIV exposure for 24 h (Fig. 3B). In agreement with the results of TER (Fig. 1) and tight junction disruption (Fig. 2), there was a two-fold increase in HIV p24 at the bottom chamber in HNP1-treated cells when compared with untreated or linear HNP1-treated cells at 24 h or 48 h after HIV exposure (Fig. 3B). Although HD5 led to nuclear translocation of tight junction markers, there was no difference in HIV traversal in HD5-treated cells compared to control and linear HD5-treated cells (Fig. 3B), which was consistent with the TER analysis (Fig. 1).
Discussion
Antimicrobial peptides, such as alpha-defensins, exhibit diverse biological functions (20). Although the anti-HIV activity of HNP1 in vitro is well established (8), its association with an increased risk of HIV acquisition is not clear. This study found that HNP1 increased epithelial permeability and disrupted tight junctions, facilitating HIV traversal of polarized colorectal epithelial cells. Although HIV has been demonstrated to cross the epithelium via transcytosis using some cell models (3,4), increased HIV traversal of HNP1-treated epithelium is most likely through a paracellular transport mechanism based on HNP1-mediated disruption of the tight junction structure. The ability of defensins to disrupt epithelial barriers is probably not a consequence of their being positively charged, since linear peptides with the same charge did not alter epithelial permeability (Fig. 1B). In contrast to HNP1, HD5, which is known to promote HIV infectivity (18), did not have a significant impact on epithelial integrity. Although treatment with a high concentration of HD5 led to internalization of tight junction markers, this did not promote HIV traversal of the barrier. These results demonstrate differential effects of alpha-defensins HD5 and HNP-1 on epithelial integrity, which affect HIV traversal of the epithelial barrier. The findings suggest that HNPs may play a role in promoting HIV transmission, especially in the setting of STIs that can cause inflammation.
At high concentrations (≥9 μM) and prolonged treatment (≥8 h), HNP1-3 has been shown to reduce epithelial integrity in monolayers of canine kidney epithelial cells (26). Nygaard et al. showed no increase in the permeability of mannitol after HNP1-3 (12 μM) treatment for 4 h but a significant reduction in TER within 2 h treatment (26). Similarly, this study found that HNP1 treatment increased epithelial permeability within 1 h, indicating a direct effect on epithelial cells. However, HNP did not have a significant impact on FITC-dextran diffusion after 4 h treatment, suggesting that the TER measurement is more sensitive than the diffusion assay for monitoring polarized epithelial integrity. The discrepancy between FITC-dextran diffusion and HIV traversal could be due to the different incubation time in these two assays (1 h vs. 24–48 h, respectively). Additionally, because HIV is known to reduce TERs and disrupt tight junctions (25,32), HNP1 treatment may facilitate HIV-mediated tight junction disruption, leading to an increase in HIV traversal. The intestinal epithelial cells recovered over time, as indicated by an increase in TER values. HNP1 is known to interfere with PKC signaling (7,26). The PKC signaling pathway is associated with redistribution of tight junction markers, leading to an increase in TER values (6). It remains to be determined whether increased permeability induced by HNP1 is a consequence of interference with the PKC signaling pathway.
The present results suggest that HNP1 and HD5 mediate distinct functions in facilitating transmission of HIV. Although intestinal and genital epithelia are constantly being regenerated, defensin-mediated disruption of mucosal barriers offers a window of opportunity for HIV to invade the host. Interestingly, the impact of HD5 and HNP1 on epithelial integrity and the subsequent effect on HIV transmission contrast with their effect on HIV infection in vitro. Similarly, beta-defensins HBD1 and HBD3 promote tight junction barriers in keratinocytes (11,17); HBD3, but not HBD1, exhibited anti-HIV activity (28). Defensins are known to exhibit immune-modulatory activities in addition to their antimicrobial functions. The current results on epithelial integrity provide further evidence indicating a complex role of defensins in modulation of HIV transmission. In particular, the possibility that HIV traversal may be increased by defensins should be considered prior to development of defensins as microbicides for HIV prevention.
Acknowledgments
We thank Alison J. Quayle and Lyndsey Buckner for helpful discussions and critical reading of the manuscript. This work was supported by NIH AI081559 (to T.L.C.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alfsen A, Yu H, Magerus-Chatinet A, et al. . HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol Biol Cell 2005;16:4267–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baroncelli S, Andreotti M, Guidotti G, et al. . Tumor necrosis factor-alpha, Interleukin-10, and alpha-defensins in plasma and breast milk of HIV-infected highly active antiretroviral therapy-treated and untreated pregnant women in Mozambique. J Acquir Immune Defic Syndr 2008;47:647–649 [DOI] [PubMed] [Google Scholar]
- 3.Bobardt MD, Chatterji U, Selvarajah S, et al. . Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol 2007;81:395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med 1997;3:42–47 [DOI] [PubMed] [Google Scholar]
- 5.Bosire R, John-Stewart GC, Mabuka JM, et al. . Breast milk alpha-defensins are associated with HIV type 1 RNA and CC chemokines in breast milk but not vertical HIV type 1 transmission. AIDS Res Hum Retroviruses 2007;23:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cario E, Gerken G, and Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 2004;127:224–238 [DOI] [PubMed] [Google Scholar]
- 7.Chang TL, Vargas J, Jr, DelPortillo A, et al. . Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. J Clin Invest 2005;115:765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding J, Chou YY, and Chang TL. Defensins in viral infections. J Innate Immun 2009;1:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan SR, Liu XP, and Liao QP. Human defensins and cytokines in vaginal lavage fluid of women with bacterial vaginosis. Int J Gynaecol Obstet 2008;103:50–54 [DOI] [PubMed] [Google Scholar]
- 10.Galvin SR, and Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2004;2:33–42 [DOI] [PubMed] [Google Scholar]
- 11.Goto H, Hongo M, Ohshima H, et al. . Human beta defensin-1 regulates the development of tight junctions in cultured human epidermal keratinocytes. J Dermatol Sci 2013;71:145–148 [DOI] [PubMed] [Google Scholar]
- 12.Gottardi CJ, Arpin M, Fanning AS, et al. . The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci U S A 1996;93:10779–10784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010;464:217–223 [DOI] [PubMed] [Google Scholar]
- 14.Hladik F, and McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol 2008;8:447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hocini H, Becquart P, Bouhlal H, et al. . Active and selective transcytosis of cell-free human immunodeficiency virus through a tight polarized monolayer of human endometrial cells. J Virol 2001;75:5370–5374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horbul JE, Schmechel SC, Miller BR, et al. . Herpes simplex virus-induced epithelial damage and susceptibility to human immunodeficiency virus type 1 infection in human cervical organ culture. PloS One 2011;6:e22638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiatsurayanon C, Niyonsaba F, Smithrithee R, et al. . Host defense (Antimicrobial) peptide, human beta-defensin-3, improves the function of the epithelial tight-junction barrier in human keratinocytes. J Investig Dermatol 2014;134:2163–2173 [DOI] [PubMed] [Google Scholar]
- 18.Klotman ME, Rapista A, Teleshova N, et al. . Neisseria gonorrhoeae-induced human defensins 5 and 6 increase HIV infectivity: role in enhanced transmission. J Immunol 2008;180:6176–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn L, Trabattoni D, Kankasa C, et al. . Alpha-defensins in the prevention of HIV transmission among breastfed infants. J Acquir Immune Defic Syndr 2005;39:138–142 [PMC free article] [PubMed] [Google Scholar]
- 20.Lehrer RI, and Lu W. alpha-Defensins in human innate immunity. Immunol Rev 2012;245:84–112 [DOI] [PubMed] [Google Scholar]
- 21.Levinson P, Kaul R, Kimani J, et al. . Levels of innate immune factors in genital fluids: association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS 2009;23:309–317 [DOI] [PubMed] [Google Scholar]
- 22.Mackewicz CE, Yuan J, Tran P, et al. . alpha-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. AIDS 2003;17:F23–32 [DOI] [PubMed] [Google Scholar]
- 23.Meng G, Wei X, Wu X, et al. . Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med 2002;8:150–156 [DOI] [PubMed] [Google Scholar]
- 24.Miller CJ, and Shattock RJ. Target cells in vaginal HIV transmission. Microbes Infect 2003;5:59–67 [DOI] [PubMed] [Google Scholar]
- 25.Nazli A, Chan O, Dobson-Belaire WN, et al. . Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathogens 2010;6:e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nygaard SD, Ganz T, and Peterson MW. Defensins reduce the barrier integrity of a cultured epithelial monolayer without cytotoxicity. Am J Respir Cell Mol Biol 1993;8:193–200 [DOI] [PubMed] [Google Scholar]
- 27.Porter E, Yang H, Yavagal S, et al. . Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell–neutrophil interactions. Infect Immun 2005;73:4823–4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinones-Mateu ME, Lederman MM, Feng Z, et al. . Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 2003;17:F39–48 [DOI] [PubMed] [Google Scholar]
- 29.Rapista A, Ding J, Benito B, et al. . Human defensins 5 and 6 enhance HIV-1 infectivity through promoting HIV attachment. Retrovirology 2011;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simhan HN, Anderson BL, Krohn MA, et al. . Host immune consequences of asymptomatic Trichomonas vaginalis infection in pregnancy. Am J Obstet Gynecol 2007;196:59..e51–55. [DOI] [PubMed] [Google Scholar]
- 31.Sodora DL, Gettie A, Miller CJ, et al. . Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses 1998;14:S119–123 [PubMed] [Google Scholar]
- 32.Sufiawati I, and Tugizov SM. HIV-associated disruption of tight and adherens junctions of oral epithelial cells facilitates HSV-1 infection and spread. PloS One 2014;9:e88803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Owen SM, Rudolph DL, et al. . Activity of alpha- and theta-defensins against primary isolates of HIV-1. J Immunol 2004;173:515–520 [DOI] [PubMed] [Google Scholar]
- 34.Wiens ME, Wilson SS, Lucero CM, et al. . Defensins and viral infection: dispelling common misconceptions. PLoS Pathogens 2014;10:e1004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiesenfeld HC, Heine RP, Krohn MA, et al. . Association between elevated neutrophil defensin levels and endometritis. J Infect Dis 2002;186:792–797 [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, Ericksen B, Tucker K, et al. . Synthesis and characterization of human alpha-defensins 4-6. J Peptide Res 2004;64:118–125 [DOI] [PubMed] [Google Scholar]