Abstract
Aims: To study the frequency of methylation abnormalities among Estonian patients selected according to published clinical diagnostic scoring systems for Silver–Russell syndrome (SRS) and Beckwith–Wiedemann syndrome (BWS). Materials and Methods: Forty-eight patients with clinical suspicion of SRS (n = 20) or BWS (n = 28) were included in the study group, to whom methylation-specific multiplex ligation-dependant probe amplification analysis of 11p15 region was made. In addition, to patients with minimal diagnostic score for either SRS or BWS, multilocus methylation-specific single nucleotide primer extension assay was performed. Results: Five (38%) SRS patients with positive clinical scoring had abnormal methylation pattern at chromosome 11p15, whereas in the BWS group, only one patient was diagnosed with imprinting control region 2 (ICR2) hypomethylation (8%). An unexpected hypomethylation of the PLAGL1 (6q24) and IGF2R (6q25) genes in the patient with the highest BWS scoring was found. Conclusions: Compared to BWS, diagnostic criteria used for selecting SRS patients gave us a similar detection rate of 11p15 imprinting disorders as seen in other studies. A more careful selection of patients with possible BWS should be considered to improve the detection of molecularly confirmed cases. Genome-wide multilocus methylation tests could be used in routine clinical practice as it increases the detection rates of imprinting disorders.
Introduction
Silver–Russell syndrome (SRS) and Beckwith–Wiedemann syndrome (BWS) are two genetically and clinically opposite growth-affecting disorders that are, among other factors, caused by imprinting disturbances in chromosome 11p15 (Schneid et al., 1993; Weksberg et al., 1993; Gicquel et al., 2005). Chromosome 11p15 contains imprinting control regions (ICR) 1 and 2 that control fetal and postnatal growth. ICR1 contains the maternally expressed H19 and the paternally expressed IGF2 gene. ICR2 contains the maternally expressed KCNQ1 and CDKN1C genes and the paternally expressed KCNQ1OT1 gene. Genes expressed in one allele are imprinted and inactive in the other allele, dependent on the parent of origin. The epigenetic alterations in 11p15 are the most common molecular causes of SRS and BWS. In SRS, hypomethylation at ICR1 is found among 37–63% of patients. In case of SRS, up to 10% of patients have maternal uniparental disomy of chromosome 7 (UPD7) or different chromosomal aberrations; however, in many cases, the cause remains unknown (Netchine et al., 2007; Bartholdi et al., 2009; Peñaherrera et al., 2010). In BWS, epigenetic alterations in chromosome 11p15 (mainly hypomethylation of ICR2) are diagnosed in up to 77% of the patients, but CDKN1C mutations, paternal UPD of chromosome 11p15, and structural rearrangements in chromosome 11p have also been associated with the syndrome (for review, see Weksberg et al., 2010). Moreover, during the recent years, many patients with disturbed methylation at multiple imprinted loci have been reported in the literature. This group of conditions has been named as multilocus methylation defects (MLMD). Different MLMD are found in 25% of BWS patients with ICR2 hypomethylation and in 10% of SRS patients with ICR1 hypomethylation (Mackay et al., 2015).
Our aim was to study the frequency of methylation abnormalities among Estonian patients selected by the previously published clinical diagnostic scoring systems for SRS (Bartholdi et al., 2009) and BWS (Weksberg et al., 2010).
Methods
Study group
Altogether, 48 patients with clinical suspicion of SRS or BWS were included in the study group. The patients were selected among the patients investigated in the Children's Clinic and the Department of Genetics of Tartu University Hospital and Tallinn Children's Hospital by clinical geneticists or pediatricians.
For selecting patients with the suspicion of SRS (N = 20), a slightly modified Bartholdi et al. (2009) scoring system (birth weight and length ≤10th centile, relative macrocephaly, postnatal height ≤3rd centile, normal head circumference, normal cognitive development, asymmetry of face, body, and/or limbs, distinctive facial features, and other features) was used. Patients were clinically classified as SRS when the total score was ≥8 points. The difference between our scoring and the Bartholdi et al. scoring was that we gave the patients with facial, body, and/or limb asymmetry “0–3” points, depending on how many body parts were affected, instead of “0” or “3” points.
For choosing patients with the suspicion of BWS (N = 28), a referral form with Weksberg's major (abdominal wall defect, macroglossia, macrosomia, ear creases or pits, visceromegaly, embryonal tumor, hemihyperplasia, cytomegaly of adrenal fetal cortex, renal abnormalities, positive family history of BWS, and cleft palate) and minor criteria (pregnancy-related findings, neonatal hypoglycemia, nevus flammeus, cardiac anomalies, characteristic facies, diastasis recti, and advanced bone age) (Weksberg et al., 2010) was used. Patients were clinically classified as BWS when at least three major findings or two major findings, and one minor finding were present. Some patients were referred as incomplete BWS with one or two positive criteria.
In addition to clinical data obtained from referral forms, hospital electronic database was used to get additional clinical information about the patients to correct and/or add the data. Fenton (2003) Intrauterine Growth Curves and Estonian age- and gender-specific growth curves were used to evaluate and correct growth parameters at birth and later (Uibo et al., 2010). The age and growth parameters were corrected until 2 years of age in all children born <32 gestational weeks.
In all 48 patients with clinical suspicion of SRS or BWS, the methylation analysis of 11p15 region was performed at first. To the majority of patients with typical phenotype for SRS and BWS, who met the diagnostic criteria according to the above-mentioned scoring systems for SRS and BWS (12 and 11, respectively), a multilocus methylation-specific single nucleotide primer extension (MS-SNuPE) assay and/or testing for imprinted locuses in chromosome 6, 7, and 14 were performed (SALSA MLPA ME032 UPD7-UPD14). The aim of these analyses was to exclude MLMD or single alterations in other imprinted regions, as well as UPD7 and other chromosomes among these patients.
In addition, the parents of patients with abnormal methylation status were also studied for methylation defects in the 11p15 region. Ten healthy and normal-stature individuals as references were tested for the 11p15-imprinted region by methylation-specific multiplex ligation-dependant probe amplification (MS-MLPA).
This study was approved by the Research Ethics Committee of the University of Tartu.
Molecular analyses
Methylation analysis in 11p15 region and UPD(6, 7, 14) MS-MLPA testing
DNA was extracted from peripheral blood samples of patients and control individuals by a standard salting-out procedure. MS-MLPA was performed using SALSA® MS-MLPA® probemix ME030-B2 BWS/SRS and probemix ME032 UPD7-UPD14 (MRC-Holland, Amsterdam, the Netherlands) according to the manufacturer's instructions. Polymerase chain reaction (PCR) products were analyzed on a fluorescent capillary sequencer using the Genescan software (ABI 3130XL Genetic Analyzer; Applied Biosystems, Darmstadt, Germany). All patients were tested twice.
The BWS/SRS probemix contains 26 probes, 14 of which are methylation specific for the BWS/SRS region in 11p15,
MLPA data analysis was performed with the Coffalyser software (MRC-Holland), an Excel-based program able to perform all data normalization steps, corrections for signal sloping and standard deviation (SD) calculations for each probe automatically. Expected normalized values for copy number analysis were 1.0 (range 0.85–1.15) in absence of any change and 0.5 or 1.5 in case of a heterozygous deletion or duplication, respectively. For methylation status analysis, the expected methylation index for normal individuals is 0.5.
Testing for imprinted regions in chromosomes 6, 7, and 14 was performed by using SALSA MS-MLPA probe mixture ME032 UPD7-UPD14 (MRC-Holland). This probemix contains probes that target following imprinted regions: PLAGL1, 6q24; GRB10, 7p12; MEST, 7q32; DLK1, MEG3, RTL1, and MIR380 in 14q32 (MRC-Holland, product description version 05; December 10, 2014).
Multilocus MS-SNuPE assay
The MS-SNuPE assay is based on the ABI PRISM® SNaPshot® Multiplex Kit (Applied Biosystems). PCR products were analyzed on a fluorescent capillary sequencer using the GeneMapper software (AB 3130 Genetic Analyzer; Applied Biosystems). The MS-SNuPE assay allowed the simultaneous characterization of 10 imprinted loci in five chromosomes (PLAGL1, 6q24; IGF2R, 6q25; GRB10, 7p12; MEST, 7q32; ICR1, ICR2, and IGF2P0 in 11p15.5; MEG3 and IG-DMR in 14q32; SNRPN, 15q11.2). A detailed description of the method was published elsewhere (Gonzalgo and Liang, 2007; Begemann et al., 2012).
Results
SRS group
Of the referred 20 patients (15 girls, 5 boys) with clinical suspicion of SRS, 13 had at least eight positive features according to the Bartholdi et al. (2009) scoring system and therefore met the criteria required for the clinical diagnosis of SRS (Table 1). The most frequent findings were poor postnatal growth (18/20, 90%), low birth weight (17/20, 85%) and height (16/20, 80%), clinodactyly, and distinctive facial features (65–80%). Four patients (including two sisters, patients 1 and 2 in Table 1, published previously; Ounap et al., 2004), had hypomethylation in ICR1 in chromosome 11p15, and one patient had a 1.3 Mb duplication in 11p15.5 involving both ICR1 and ICR2. The clinical score of molecularly confirmed cases ranged from 9 to 14 points. None of the patients with a score <8 points had an imprinting disorder in 11p15.
Table 1.
Corrected Data of Referred Patients with Clinical Diagnosis (Score ≥8) of Silver–Russell Syndrome (SRS)
| Patients | 1a | 2a | 3a | 4 | 5 | 6 | 7b | 8a | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth weight ≤10th ct | + | + | + | + | + | − | + | + | + | + | + | + | + |
| Birth length ≤10th ct | + | + | + | + | + | − | + | + | + | + | + | + | + |
| Relative macrocephaly >50th ct | + | + | + | − | − | − | − | − | − | − | − | − | − |
| Postnatal height ≤3rd ct | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Normal postnatal head circumference | + | + | + | + | − | + | − | − | − | − | + | + | − |
| Normal cognitive development | + | + | + | + | − | − | + | + | − | − | + | + | − |
| Facial asymmetry | + | + | − | + | − | + | − | − | − | − | − | − | − |
| Body asymmetry | + | − | − | − | + | + | + | − | − | − | − | − | − |
| Limb asymmetry | + | + | + | − | − | + | − | + | − | − | − | − | + |
| Triangular face | + | + | + | − | + | + | + | + | + | + | − | + | − |
| Prominent forehead | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Other facial features | + | + | + | + | + | − | + | − | + | + | − | + | + |
| Clinodactyly of fifth finger | + | + | + | + | + | − | + | + | + | + | + | − | + |
| Genital abnormality | − | − | − | − | + | + | − | − | − | + | − | − | + |
| Otherc | + | + | − | + | + | + | − | + | + | − | + | − | − |
| Total score | 14 | 13 | 11 | 10 | 10 | 9 | 9 | 9 | 8 | 8 | 8 | 8 | 8 |
Patients with imprinting control region 1 (ICR1) hypomethylation in 11p15.
Patient with duplication in region 11p15.5.
For example, brachymesophalangy, syndactyly of toes, inguinal hernia, and pigmentary changes.
Molecular analyses
All referred 20 patients and 10 controls were blind-tested by MS-MLPA at least twice. Molecular diagnostics confirmed the SRS diagnosis in 5 of 13 patients clinically scored as SRS patients (38%). All 10 reference samples were tested repeatedly (n = 45) and gave all reproducible results with all the probes both for copy number and methylation analysis, except the probe 08745-L08765, which was noninformative (mentioned also by the manufacturer). The mean methylation indices for the normal reference samples were 0.56 (SD = 0.03; range 0.48–0.60) for four MS-MLPA probes at ICR1 and 0.61 (SD = 0.04; range 0.57–0.68) for four MS-MLPA probes at ICR2.
Compared to the reference group, all four SRS patients with ICR1 hypomethylation showed lower methylation between 0.11 and 0.40 in all four analyzed MS-MLPA probes, whereas the methylation ratios for ICR2 were in the normal range, similar to controls (Table 2). Copy number variant (CNV) analyses of these patients were normal.
Table 2.
Summary of the Methylation-Specific Multiplex Ligation-Dependant Probe Amplification (MS-MLPA) Methylation Ratios (MR) in SRS and Beckwith–Wiedemann Syndrome (BWS) Patients and Controls
| ICR1 MR rangea | Mean MR ± SD | ICR2 MR rangea | Mean MR ± SD | |
|---|---|---|---|---|
| Controlsb | 0.48–0.6 | 0.56 ± 0.03 | 0.57–0.68 | 0.61 ± 0.04 |
| BWS patient | 0.51–0.6 | 0.56 ± 0.02 | 0.13–0.25 | 0.2 ± 0.07 |
| SRS patient No. 1 | 0.11–0.24 | 0.17 ± 0.08 | 0.48–0.6 | 0.6 ± 0.09 |
| SRS patient No. 2 | 0.27–0.33 | 0.33 ± 0.06 | 0.48–0.58 | 0.54 ± 0.06 |
| SRS patient No. 3 | 0.28–0.4 | 0.36 ± 0.06 | 0.54–0.67 | 0.6 ± 0.04 |
| SRS patient No. 8 | 0.29–0.4 | 0.36 ± 0.1 | 0.52–0.63 | 0.55 ± 0.07 |
| SRS patient with duplication (No. 7) | 0.29–0.5 | 0.4 ± 0.06 | 0.64–0.82 | 0.71 ± 0.05 |
Anomalous MR are outlined in bold.
Methylation ratio range includes the methylation results of all four MS-MLPA probes in ICR1 or ICR2, respectively.
Control group included 10 individuals, who were tested repeatedly. Results from 45 tests were obtained and taken into account.
SD, standard deviation.
The investigation of the SRS patient with a 1.3 Mb duplication revealed an increase in CNV ratios in altogether 26 MLPA probes in the whole analyzed 11p15.5 region. This familial case report is published in detail elsewhere (Vals et al., 2015).
None of the 12 patients (including 4 patients with 11p15 methylation disorder), to whom MS-SNuPE or UPD(6, 7, 14) MS-MLPA analyses were done, showed a reliable alteration of methylation in any of the analyzed positions.
BWS group
Twenty-eight patients (16 girls, 12 boys) were referred with clinical suspicion of BWS. After the correction of data, only 12 of them met the Weksberg's criteria required for clinical diagnosis of BWS (Table 3). The most frequent major findings were macrosomia (13/28, 46%), hemihyperplasia, and distinctive ear features (both 9/28, 30%). The most frequent minor finding was characteristic facies (13/28, 46%). Only one patient had ICR2 hypomethylation in chromosome 11p15 (1/12, 8%). BWS was clinically diagnosed in the male patient at the age of 1.5 and molecularly confirmed at the age of 7. He had two major findings (macroglossia and macrosomia) and three minor findings (neonatal hypoglycemia, congenital heart anomaly, and characteristic facies). Birth parameters were within normal limits.
Table 3.
Corrected Data of Referred Patients with Clinical Diagnosis of BWS
| Patients | 1a | 2 | 3 | 4 | 5 | 6 | 7 | 8b | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdominal wall defect | + | − | − | − | + | − | − | − | + | − | − | − |
| Macroglossia | + | − | + | − | + | + | + | + | − | + | − | − |
| Macrosomyc | − | + | + | + | + | + | + | + | − | − | + | + |
| Pits and creases | + | + | − | + | − | + | − | − | − | − | + | − |
| Visceromegaly | + | + | − | + | − | − | − | − | − | + | − | + |
| Childhood cancer | − | − | − | − | − | − | − | − | − | − | − | − |
| Hemihyperplasia | + | − | + | − | − | − | + | − | + | − | − | − |
| Cytomegaly of adrenal fetal cortex | − | − | − | − | − | − | − | − | − | − | − | − |
| Renal abnormalities | − | − | − | − | − | − | − | − | − | − | − | − |
| Positive family history | − | − | − | − | − | − | − | − | − | − | − | − |
| Cleft palate | − | − | − | − | − | − | − | − | − | − | − | − |
| Pregnancy-related pathology | + | − | − | − | + | − | − | − | − | − | − | − |
| Neonatal hypoglycemia | + | + | + | − | − | − | − | + | − | − | − | − |
| Naevus flammeus | + | + | + | − | − | − | − | − | − | − | − | − |
| Cardiomegaly/structural heart defect | − | + | − | + | − | − | − | + | − | − | − | − |
| Characteristic facies | + | − | + | − | − | + | − | + | + | + | + | − |
| Diastasis recti | + | − | − | − | − | − | − | − | + | − | − | − |
| Advanced bone age | − | − | − | + | − | − | − | − | − | − | − | + |
| Major + minor criteria | 5 + 5 | 3 + 3 | 3 + 3 | 3 + 2 | 3 + 1 | 3 + 1 | 3 + 0 | 2 + 3 | 2 + 2 | 2 + 1 | 2 + 1 | 2 + 1 |
Patient with hypomethylation of PLAGL1 (6q24) and IGF2R (6q25) genes.
Patient with ICR2 hypomethylation.
Height and weight >97 ct.
Molecular analyses
The only molecularly confirmed BWS patient showed hypomethylation in the ICR2 region with methylation indices between 0.13 and 0.25 (Table 2).
MS-SNuPE analysis revealed an unexpected hypomethylation of PLAGL1 (6q24) and IGF2R (6q25) genes in the patient with the highest BWS scoring (see case report below). MS-SNuPE and UPD7-UPD14 MS-MLPA analyses did not show any methylation alteration in other patients from the BWS group.
We also studied the parents of four SRS patients and one BWS patient. All the parents, except one, had normal CNV and methylation analyses. The mother of a patient with 11p15 duplication and SRS syndrome (Table 2, patient 7) had also the same duplication, but with opposite methylation and clinical diagnosis of BWS (Vals et al., 2015).
Case report
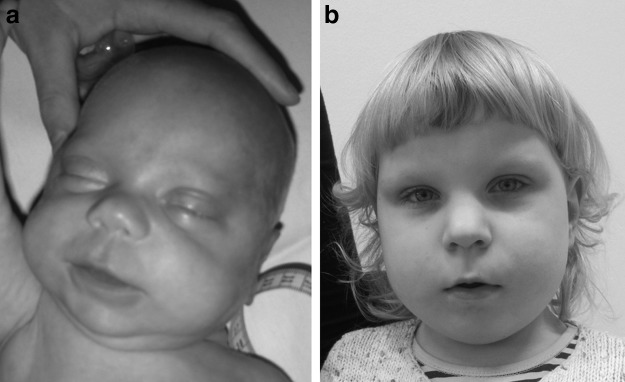
The proband (Table 3, patient 1), age 3, is the only child in the family. Because of complicated pregnancy anamnesis, elevated maternal serum alpha-fetoprotein, and human chorionic gonadotropin (2.3 MoM and 3.1 MoM, respectively), an amniocentesis was performed at the 17th gestational week. Fetal karyotype was 46,XX. At the 20th week of pregnancy, an ultrasound scan revealed slight polyhydramnios and pericardial effusion. She was born prematurely at the 30th gestational week. Birth weight was 1696 g (>75th percentile) and Apgar scores 7 and 8 at 1 and 5 min, respectively. During the first week of life, the child had feeding difficulties and several episodes of hypoglycemia treated with glucose infusion. Later, coarse facial features, small omphalocele, macroglossia, hemihypertrophy, asymmetrical tongue, ear fistulas, inverted nipples, and exomphtalmos were noted (Fig. 1a). At the age of 2 months, the child was referred to the geneticist with a suspicion of BWS. The 11p15.5 region MS-MLPA analysis of DNA derived from patient's blood and skin cells and the CDKN1C gene sequencing and deletion/duplication analysis did not show any genetic or epigenetic alteration in the BWS region. Also, her chromosomal microarray analysis was normal. Both the MS-SnuPE and UPD7-UPD14 MS-MLPA analyses of this patient revealed loss of methylation of PLAGL1 (6q24) and IGF2R (6q25) genes. The same result was obtained using DNA from patient's fibroblasts and buccal swab. It was assumed that the patient has paternal heterodisomy of chromosome 6, but comparative analysis of the SNPs using her mother's chromosomal microarray results, excluded any uniparental disomy.
FIG. 1.
(a) Patient 1 in the Beckwith–Wiedemann syndrome (BWS) group as a newborn and (b) at the age of 3 years. Note the characteristic BWS facies.
At the age of 3, she had typical facial features (Fig. 1b), slight organomegaly, and her weight, height, and head circumference are at the 97th, 75th, and 75th percentiles, respectively.
Discussion
In our study, we evaluated the frequency of methylation abnormalities among Estonian patients who were selected according to published clinical diagnostic scoring systems for SRS (Bartholdi et al., 2009) and BWS (Weksberg et al., 2010). We did not have any preselection and we studied all the referred patients, as there exists a recommendation for both syndromes to look for imprinting disorders in every patient with a suspicion of SRS or BWS, even if the clinical picture is incomplete. Although over the years, many clinical criteria and scoring systems for diagnosis of SRS and BWS have been revised and suggested (see for review Tables 4 and 5) (Elliott and Maher, 1994; Lai et al., 1994; DeBaun and Tucker, 1998; Price et al., 1999; Netchine et al., 2007; Bartholdi et al., 2009; Weksberg et al., 2010; Dias et al., 2013; Ibrahim et al., 2014), neither syndrome has common consensus diagnostic criteria yet. This is one of the main aims of the European Network of Congenital Imprinting Disorders (www.imprinting-disorders.eu) in the nearest future to help clinicians recognize and diagnose both syndromes and select the candidates for further molecular studies in everyday practice. Recently, more simple and objective scoring systems were published (Dias et al., 2013; Ibrahim et al., 2014). These scoring systems do not focus on subjective features.
Table 4.
Clinical Diagnostic Criteria for SRS
| Lai et al. (1994) | Price et al. (1999) | Netchine et al. (2007) | Bartholdi et al. (2009) | Dias et al. (2013) | |
|---|---|---|---|---|---|
| Minimal score for clinical diagnosis of SRS | 3 of 5 | 4 of 5 | Prenatal growth retardation plus 3 of 5 | 8 of 15 | 3 of 4 |
| Prenatal growth parameters | Birth weight ≤ −2 SD | Birth weight ≤ −2 SD | Birth weight and/or length ≤ −2 SD | Birth weight and length ≤10th ct | Birth weight < −2 SD |
| Postnatal growth parameters | Height ≤ −2 SD | Height ≤ −2 SD | Height ≤ −2 SD at 2 years of age | Height ≤3rd ct, normal head circumference (3rd–97th ct) | Height < −2 SD after 2 years of age |
| Relative macrocephaly | + | + (at birth) | + (at birth) | + | |
| Body and/or limb asymmetry | + | + | + | + (3 points) | + |
| Facial phenotype | Classical | Classical | Prominent forehead | Prominent forehead, triangular face and other features | |
| Clinodactyly | + | + | |||
| Feeding difficulties | + | ||||
| Normal cognitive development | + | ||||
| Genital abnormalities | + | ||||
| Other features | + |
Table 5.
Criteria for BWS
| Elliott and Maher (1994) | DeBaun and Tucker (1998) | Weksberg et al. (2010) | Ibrahim et al. (2014) | |
|---|---|---|---|---|
| Minimal score for clinical diagnosis of BWS | Three major features or two major and three minor features | Diagnosis made by physician plus two of five most common features | Three major features or two major and one minor features | Minimum score 3.5 points |
| Major criteria | ||||
| Abdominal wall defect | + | + (Incl. diastasis recti) | + | + (1.5 points) |
| Macroglossia | + | + | + | + (2.5 points) |
| Macrosomia | Pre- and postnatal height >90th ct | Birth weight >90th ct | Height and weight >97th ct | + (1 point) |
| Visceromegaly | + (Intra-abdominal organ(s)) | + (1 point) | ||
| Embryonal tumor in childhood | + | |||
| Hemihyperplasia | + | |||
| Cytomegaly of adrenal fetal cortex | + | |||
| Renal abnormalities | + | |||
| Positive family history of BWS | + | |||
| Cleft palate | + | |||
| Minor criteria | ||||
| Ear creases and/or pits | + | + | + | |
| Facial naevus flammeus | + | + | + (1 point) | |
| Neonatal hypoglycemia | + | + | + | + (0.5 points) |
| Hemihypertrophy | + | + (0.5 points) | ||
| Visceromegaly | + (nephromegaly) | |||
| Pregnancy-related findings | + | |||
| Cardiac anomalies | + | |||
| Characteristic facies | + | |||
| Diastasis recti | + | |||
| Advanced bone age | + | |||
After the correction of clinical data in our study group, some initially positive criteria were changed to negative (e.g., growth-related data), especially among the BWS group patients. Most of our BWS group patients were referred because of macrosomia, but some of them did not have previously defined overgrowth and their actual clinical score did not meet the required minimum. Also, macrosomia can be caused by other factors like overlapping syndromes or simple overweight/obesity (some patients had weight, but not height >97th centile), as we later diagnosed Coffin–Siris syndrome caused by ARID1B gene mutation in one patient of this investigation group (Vals et al., 2014). However, not all the BWS patients are macrosomic (Elliott and Maher, 1994) and therefore it is not mandatory to present with overgrowth in case of BWS.
Unfortunately, we could not control and correct subjective criteria (e.g., distinctive facial phenotype).
Compared to other studies (Gaston et al., 2001; Bliek et al., 2009; Calvello et al., 2013; Mussa et al., 2013; Ibrahim et al., 2014), our detection rate of epigenetic alterations in chromosome 11p15 was significantly lower in the BWS group, but comparable in the SRS group (Bartholdi et al., 2009; Peñaherrera et al., 2010). In our group, 5 of 13 (38%) SRS patients with positive clinical scoring had abnormal methylation in chromosome 11p15. In the BWS group, of all the patients who met the clinical criteria, only one patient was diagnosed with ICR2 hypomethylation (8%), whereas in other studies, the detection rate for imprinting disorders in 11p15 has been 28–72% (Gaston et al., 2001; Calvello et al., 2013; Mussa et al., 2013; Baskin et al., 2014; Eggermann et al., 2014). When we compared the symptoms of our BWS group patients with recent criteria by Ibrahim et al. (2014), eight patients met the minimum score for BWS, but nevertheless the detection rate remained low (12.5%). Interestingly, it seems that for our specialists, it is easier to suspect and detect SRS because the phenotype is more familiar, most of the criteria can be more easily assessed at once, and additional investigations are not required. Our results also support that the Bartholdi diagnostic criteria (Bartholdi et al., 2009) for SRS are working well.
In addition, we studied UPD7, MLMD, and/or single alterations in other imprinted regions among the patients who met at least the minimal diagnostic score for SRS and BWS. None of the patients with 11p15 imprinting disorders had MLMD. For our surprise, we detected one patient in the BWS group who had hypomethylation of PLAGL1 (6q24) and IGF2R (6q25) genes without 11p15 imprinting disorder. Hypomethylation of PLAGL1 gene should theoretically result in 6q24-related transient neonatal diabetes mellitus (TNDM) (Docherty et al., 2013; Temple et al., 2015). This is a rare imprinting disorder characterized by intrauterine growth retardation, transient neonatal diabetes, and in some cases, macroglossia, abdominal wall defects, hypotonia, congenital visceral anomalies, and developmental delay. Some symptoms of TNDM such as macroglossia and omphalocele overlap with symptoms of BWS and this circumstance can explain several phenotypic features of this patient. However, the patient did not have major diagnostic symptoms of transient neonatal diabetes and therefore it is not possible to clinically confirm this diagnosis. As it is known, the clinical and laboratory features of TNDM can sometimes be very mild and nonspecific. The meaning of hypomethylation of PLAGL1 and IGF2R in this patient is not clear at the moment. In a literature, cases have been described where individuals with 6q24 TNDM mutations did not present in the neonatal period, but later in life that is, disorders such as insulin resistance or gestational diabetes (Boonen et al., 2013). Therefore, blood glucose levels should be followed regularly in our patient. Also, as MLMD have been described in TNDM as well as in BWS patients in rather high levels and mutations in ZFP57 gene have been associated with TNDM symptoms, sequencing of ZFP57 gene should be performed in these patients sharing common features/symptoms of TNDM and BWS (Mackay et al., 2008, 2015; Bliek et al., 2009).
In conclusion, it seems that among our cohort, diagnostic criteria used for selecting SRS patients worked as expected and gave us a similar detection rate of 11p15 imprinting disorders as seen in other studies. In contrast, to confirm patients with BWS caused by imprinting disorders, a more careful selection of patients and perhaps an overlook of BWS clinical criteria should be considered in our center, to improve the detection of molecularly confirmed cases. Genome-wide DNA methylation analysis should be considered to patients with growth disturbances, in whom an imprinting disorder is suspected, as imprinting disorders frequently share common symptoms.
Acknowledgments
We thank the patients and their families for participating in this study. This work was supported by grant 8175 and 355P from the Estonian Science Foundation. Four authors (T.K., K.Õ., M.Y., and T.E.) are members of the European Network of Congenital Imprinting Disorders (EUCID.net), which is supported by COST (BM1208).
Author Disclosure Statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bartholdi D, et al. (2009) Epigenetic mutations of the imprinted IGF2-H19 domain in Silver-Russell syndrome (SRS): results from a large cohort of patients with SRS and SRS-like phenotypes. J Med Genet 46:192–197 [DOI] [PubMed] [Google Scholar]
- Baskin B, et al. (2014) High frequency of copy number variations (CNVs) in the chromosome 11p15 region in patients with Beckwith-Wiedemann syndrome. Hum Genet 133:321–330 [DOI] [PubMed] [Google Scholar]
- Begemann M, et al. (2012) Use of multilocus methylation-specific single nucleotide primer extension (MS-SNuPE) technology in diagnostic testing for human imprinted loci. Epigenetics 7:473–481 [DOI] [PubMed] [Google Scholar]
- Bliek J, et al. (2009) Hypomethylation at multiple maternally methylated imprinted regions including PLAGL1 and GNAS loci in Beckwith-Wiedemann syndrome. Eur J Human Genet 17:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonen SE, et al. (2013) Transient neonatal diabetes, ZFP57, and hypomethylation of multiple imprinted loci: a detailed follow-up. Diabetes Care 36:505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvello M, et al. (2013) Quantitative DNA methylation analysis improves epigenotype-phenotype correlations in Beckwith-Wiedemann syndrome. Epigenetics 8:1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Tucker MA. (1998) Risk of cancer during the first four years of life in children from the Beckwith-Wiedemann Syndrome Registry. J Pediatr 132(3 Pt 1):398–400 [DOI] [PubMed] [Google Scholar]
- Dias RP, et al. (2013) Comparison of the clinical scoring systems in Silver-Russell syndrome and development of modified diagnostic criteria to guide molecular genetic testing. J Med Genetics 50:635–639 [DOI] [PubMed] [Google Scholar]
- Docherty LE, et al. (2013) Clinical presentation of 6q24 transient neonatal diabetes mellitus (6q24 TNDM) and genotype-phenotype correlation in an international cohort of patients. Diabetologia 56:758–762 [DOI] [PubMed] [Google Scholar]
- Eggermann T, et al. (2014) Additional molecular findings in 11p15-associated imprinting disorders: an urgent need for multi-locus testing. J Mol Med (Berl) 92:769–777 [DOI] [PubMed] [Google Scholar]
- Elliott M, Maher ER. (1994) Beckwith-Wiedemann syndrome. J Med Genet 31:560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton TR. (2003) A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston V, et al. (2001) Analysis of the methylation status of the KCNQ1OT and H19 genes in leukocyte DNA for the diagnosis and prognosis of Beckwith-Wiedemann syndrome. Eur J Hum Genet 9:409–418 [DOI] [PubMed] [Google Scholar]
- Gicquel C, et al. (2005) Epimutation of the telomeric imprinting center region on chromosome 11p15 in Silver-Russell syndrome. Nat Genet 37:1003–1007 [DOI] [PubMed] [Google Scholar]
- Gonzalgo ML, Liang G. (2007) Methylation-sensitive single-nucleotide primer extension (Ms-SNuPE) for quantitative measurement of DNA methylation. Nat Protoc 2:1931–1936 [DOI] [PubMed] [Google Scholar]
- Ibrahim A, et al. (2014) Methylation analysis and diagnostics of Beckwith-Wiedemann syndrome in 1,000 subjects. Clin Epigenetics 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KY, et al. (1994) Cognitive abilities associated with the Silver-Russell syndrome. Arch Dis Child 71:490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DJG, et al. (2008) Hypomethylation of multiple imprinted loci in individuals with transient neonatal diabetes is associated with mutations in ZFP57. Nat Genet 40:949–951 [DOI] [PubMed] [Google Scholar]
- Mackay DJG, et al. (2015) Multilocus methylation defects in imprinting disorders. Biomol Concepts 6:47–57 [DOI] [PubMed] [Google Scholar]
- Mussa A, et al. (2013) Prevalence of Beckwith-Wiedemann syndrome in North West of Italy. Am J Med Genet A 161:2481–2486 [DOI] [PubMed] [Google Scholar]
- Netchine I, et al. (2007) 11p15 imprinting center region 1 loss of methylation is a common and specific cause of typical Russell-Silver syndrome: clinical scoring system and epigenetic-phenotypic correlations. J Clin Endocrinol Metab 92:3148–3154 [DOI] [PubMed] [Google Scholar]
- Ounap K, et al. (2004) Two sisters with Silver-Russell phenotype. Am J Med Genet A 131:301–306 [DOI] [PubMed] [Google Scholar]
- Peñaherrera MS, et al. (2010) Methylation profiling in individuals with Russell-Silver syndrome. Am J Med Genet A 152A:347–355 [DOI] [PubMed] [Google Scholar]
- Price SM, et al. (1999) The spectrum of Silver-Russell syndrome: a clinical and molecular genetic study and new diagnostic criteria. J Med Genet 36:837–842 [PMC free article] [PubMed] [Google Scholar]
- Schneid H, et al. (1993) Parental allele specific methylation of the human insulin-like growth factor II gene and Beckwith-Wiedemann syndrome. J Med Genet 30:353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple IK, Mackay DJG, Docherty LE. (2005, updated 2015) Diabetes mellitus, 6q24-related transient neonatal. In GeneReviews. Available at: http://www.ncbi.nlm.nih.gov/books/NBK1534, accessed May20, 2015
- Uibo O, et al. (2010) Lastehaiguste propedeutika algtõed. Tartu Ülikooli Kirjastus, Tartu [Google Scholar]
- Vals M-A, et al. (2014) Coffin-Siris syndrome with obesity, macrocephaly, hepatomegaly and hyperinsulinism caused by a mutation in the ARID1B gene. Eur J Hum Genet 22:1327–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vals M-A, et al. (2015) Familial 1.3Mb 11p15.5–15.4 duplication in three generations causing Silver-Russell and Beckwith-Wiedemann syndromes. Mol Syndromol 6:147–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksberg R, Shuman C, Beckwith JB. (2010) Beckwith-Wiedemann syndrome. Eur J Hum Genet 18:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksberg R, et al. (1993) Disruption of insulin-like growth factor 2 imprinting in Beckwith-Wiedemann syndrome. Nat Genet 5:143–150 [DOI] [PubMed] [Google Scholar]