Abstract
Purpose: Tissue diagnosis of upper tract urothelial carcinoma (UTUC) is limited by variance in tumor sampling by standard ureteroscopic biopsy. Optical imaging technologies can potentially improve UTUC diagnosis, surveillance, and endoscopic treatment. We previously demonstrated in vivo optical biopsy of urothelial carcinoma of the bladder using confocal laser endomicroscopy (CLE). In this study, we evaluated a new 0.85-mm imaging probe in the upper urinary tract and demonstrated feasibility and compatibility with standard ureteroscopes to achieve in vivo optical biopsy of UTUC.
Materials and Methods: Fourteen patients scheduled for ureteroscopy of suspected upper tract lesions or surveillance of UTUC were recruited. After intravenous (IV) administration of fluorescein, CLE was performed using a 0.85-mm-diameter imaging probe inserted through the working channel of standard ureteroscopes. Acquired confocal video sequences were reviewed and analyzed. A mosaicing algorithm was used to compile a series of images into a single larger composite image. Processed CLE images were compared with standard histopathologic analysis.
Results: Optical biopsy of the UTUC using CLE was effectively achieved during standard ureteroscopy. There were no adverse events related to IV fluorescein administration or image acquisition. Confocal imaging of UTUC showed characteristic features similar to urothelial carcinoma of the bladder, including papillary structure, fibrovascular stalks, and pleomorphism. Lamina propria in normal areas of the renal pelvis and ureter was also identified.
Conclusions: We report an initial feasibility of CLE of UTUC. Pending further clinical investigation, CLE may become a useful adjunct to ureteroscopic biopsy, endoscopic ablation, and surveillance of UTUC.
Introduction
Upper tract urothelial carcinoma (UTUC) accounts for ∼5 to 10% of all urothelial carcinomas.1 While nephroureterectomy with bladder cuff excision remains the standard of care in the treatment of UTUC,2 renal-sparing endoscopic tumor ablation and subsequent ureteroscopic surveillance have emerged as a management option for patients with low-grade tumors where oncologic outcomes are comparable to radical treatment.3,4 Diagnosis of UTUC is typically established by ureteroscopic biopsy. Despite significant technologic advancements in endourology over the last two decades, ureteroscopic biopsy remains technically challenging and has several recognized limitations.5–7 Tissue quantity is insufficient for diagnosis in up to 25% of ureteroscopic biopsies.8 Variability in tumor sampling can contribute to the ∼30% rate in upgrading of low-grade UTUC from the time of endoscopic biopsy to definitive nephroureterectomy.5,7 Inaccurate diagnosis from ureteroscopic biopsy can negatively affect patient prognosis and prevent optimal clinical management.9
Several optical imaging technologies have been proposed to improve the diagnosis of UTUC, with the goal to better stratify patients for renal-sparing management options and long-term endoscopic surveillance. Feasibility studies have been reported using narrow band imaging,10 photodynamic diagnosis,11 and optical coherence tomography.12 Confocal laser endomicroscopy (CLE) utilizes miniaturized fiber-optic imaging probes to provide a high-resolution in vivo microscopic visualization of cellular architecture and morphology similar to histology. We previously established the feasibility of CLE in the lower urinary tract using 2.6-mm and 1.4-mm imaging probes and developed diagnostic criteria to enhance the detection of neoplastic bladder lesions.13–16
In this study, we investigated the clinical feasibility of CLE imaging in the upper tract with a new 0.85-mm probe. This smaller probe has been applied in the gastrointestinal tract for needle-based optical biopsy of pancreatic cysts17 that pose similar challenges of accessibility to areas of interest as those presented for UTUC in the upper urinary tract. The compatibility of the smaller probe with standard ureteroscopes could expand the utility of CLE to ureteroscopic biopsy and upper urinary tract surveillance for UTUC.
Materials and Methods
Instrumentation
CLE was performed with the Cellvizio clinical system (Mauna Kea Technologies, Paris, France). A 0.85-mm-outer-diameter fiber-optic probe was used for image acquisition. The 0.85-mm probe has a depth of tissue penetration of 50 μm, field of view of 320 μm, and spatial resolution of 3.5 μm. The probe was sterilized with the STERRAD system (Advanced Sterilization Products, Irvine, CA) before each use, and each probe could be sterilized up to 10 times.
Intraoperative confocal endomicroscopy during ureteroscopy
The study was conducted with approval from the Stanford University Institutional Review Board and Veterans Affairs Palo Alto Health Care System (VAPAHCS) Research and Development. Patients scheduled to undergo ureteroscopy for suspected UTUC or surveillance of UTUC were recruited. CLE imaging of the upper tract was performed through the working channel of a 6.9 French semi-rigid ureteroscope (Stryker, San Jose, CA), a 7.5 French flexible ureteroscope (Karl Storz Endoscopy, El Segundo, CA), or a 7.9 French flexible video ureteroscope (Olympus Corporation, Tokyo, Japan).
Following initial white light endoscopy, 0.5 to 1.0 mL of 10% sodium fluorescein (Akorn, Lake Forest, IL) was administered intravenously.16 CLE imaging was feasible within 2 to 3 minutes after intravenous (IV) fluorescein. For image acquisition, the probe tip was positioned perpendicularly to the tissue for en face contact. White light endoscopy and CLE images of normal and abnormal appearing urothelium were reviewed in real time and recorded for additional analysis offline. Video sequences from CLE imaging were collected at 12 frames per second. Imaged tissue locations were biopsied or surgically resected, then stained with hematoxylin and eosin (H&E) for corresponding histopathologic analysis.
Data analysis
Confocal video sequences acquired in vivo during ureteroscopy were processed, reviewed, and analyzed using the Cellvizio Viewer software version 1.6.1. Consecutive images were compiled into a single larger composite image of greater than two frames using a built-in mosaicing algorithm. Confocal images and corresponding H&E stains were reviewed with a surgical pathologist (Robert V. Rouse).
Results
Between June 2012 and November 2013, 14 patients (mean age 74 years, range 61–88 years) scheduled to undergo ureteroscopy at VAPAHCS were recruited. In vivo CLE imaging of the upper tract was performed in all patients. Upper tract imaging of one patient was performed during two different procedures, first for suspected UTUC in a solitary kidney and second during follow-up surveillance 4 months later. Patient characteristics and histopathologic diagnoses, where available, are described in Table 1.
Table 1.
Patient Characteristics and Diagnoses
| Case | Age | Sex | Indication for ureteroscopy | Clinical findings (action) | Histopathology | Grade |
|---|---|---|---|---|---|---|
| 1 | 79 | M | Suspected carcinoma | Distal ureteral stone (basketed) | — | — |
| 2 | 66 | M | Suspected carcinoma | Renal pelvis tumor (nephroureterectomy) | Urothelial carcinoma | High |
| 3 | 68 | M | Suspected carcinoma | Renal pelvis tumor (nephroureterectomy) | Urothelial carcinoma | High |
| 4 | 79 | M | Suspected carcinoma | Renal pelvis tumor (biopsy) | Urothelial carcinoma | Low |
| 5 | 66 | M | Suspected carcinoma | No lesions detected | — | — |
| 6 | 66 | M | Surveillance | No lesions detected | — | |
| 7 | 84 | M | Surveillance | No lesions detected | — | — |
| 8 | 80 | M | Suspected recurrence | No lesions detected (renal pelvis wash) | Atypical | — |
| 9 | 88 | M | Suspected carcinoma | Suspicious lesion in ureter (biopsy) | Denuded mucosa and inflammation | — |
| 10 | 61 | M | Suspected carcinoma | Renal pelvis tumor (biopsy) | Urothelial carcinoma | Low |
| 11 | 72 | M | Suspected carcinoma | Renal pelvis tumor (biopsy) | Atypical: prominent papillary cell clusters (renal pelvis)a | — |
| 12 | 70 | M | Suspected carcinoma | Ureteral tumor (biopsy) | Urothelial carcinoma | Low |
| 13 | 81 | M | Surveillance | Ureteral tumor (biopsy) | Urothelial carcinoma | Low |
| 14 | 80 | M | Suspected carcinoma | Ureteral tumor (biopsy) | Urothelial carcinoma | High |
| 15b | 80 | M | Surveillance | Ureteral and renal pelvis tumors (laser fulguration) | — | — |
Renal pelvis biopsy processed as cytology.
Cases 14 and 15 were the same patient.
M = male.
A total of 73 CLE video sequences were collected for analysis, with an average of 5 video sequences (range 1–12 sequences) per case. The average image acquisition time was 5 minutes (range 2–13 minutes) per case. The average duration of imaging per area was 66 seconds (range 12–292 seconds). There were no adverse events in relation to fluorescein administration or image acquisition.
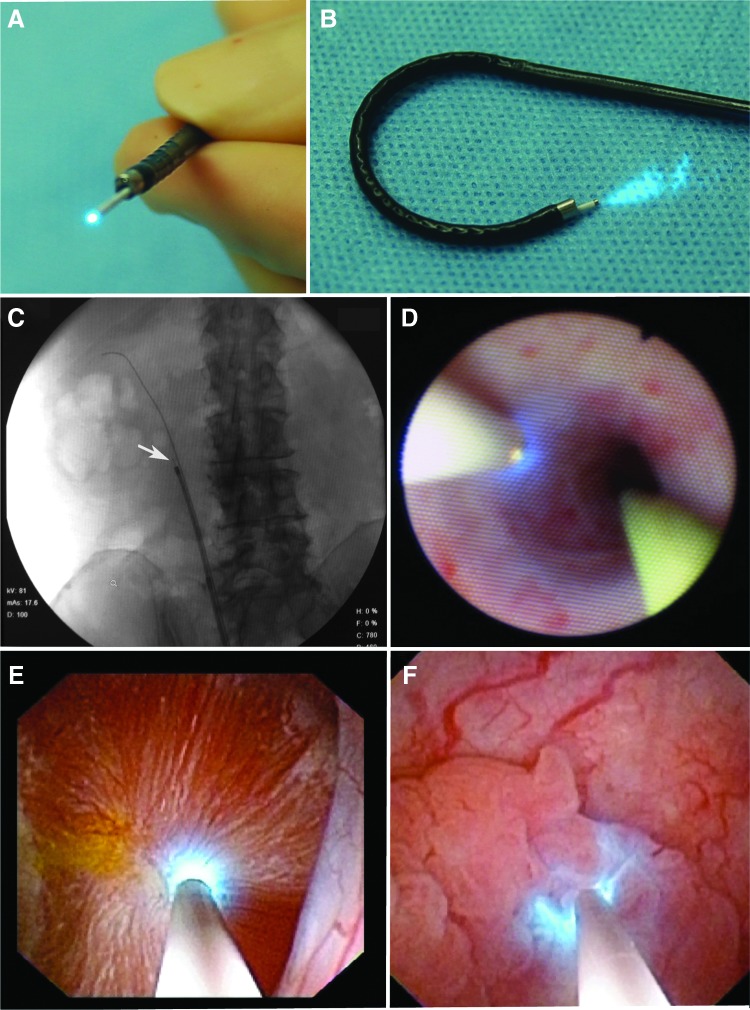
The 0.85-mm probe was found to be compatible with the working channels of all semirigid and flexible ureteroscopes tested (Fig. 1). A slight reduction of the irrigation flow rate comparable to insertion of a Holmium laser fiber was observed. The probe is visible under fluoroscopy (Fig. 1C). With the 0.85-mm imaging probe in place, access to all parts of the ureter and renal collecting system, as well as retroflexion of the flexible ureteroscope (Fig. 1B), remains feasible.
FIG. 1.
Compatibility of confocal imaging probe with a standard flexible ureteroscope. (A) The 0.85-mm probe within the working channel of the ureteroscope. (B) Retroflexion of the ureteroscope with the confocal probe in place. (C) Fluoroscopic view of the confocal probe (white arrow) in the ureteroscope in the right ureter. (D) White light view of the confocal probe in the ureter along a standard 0.035 inch guidewire. (E) Confocal laser endomicroscopy (CLE) imaging of normal renal calyx and (F) papillary tumor in the renal pelvis.
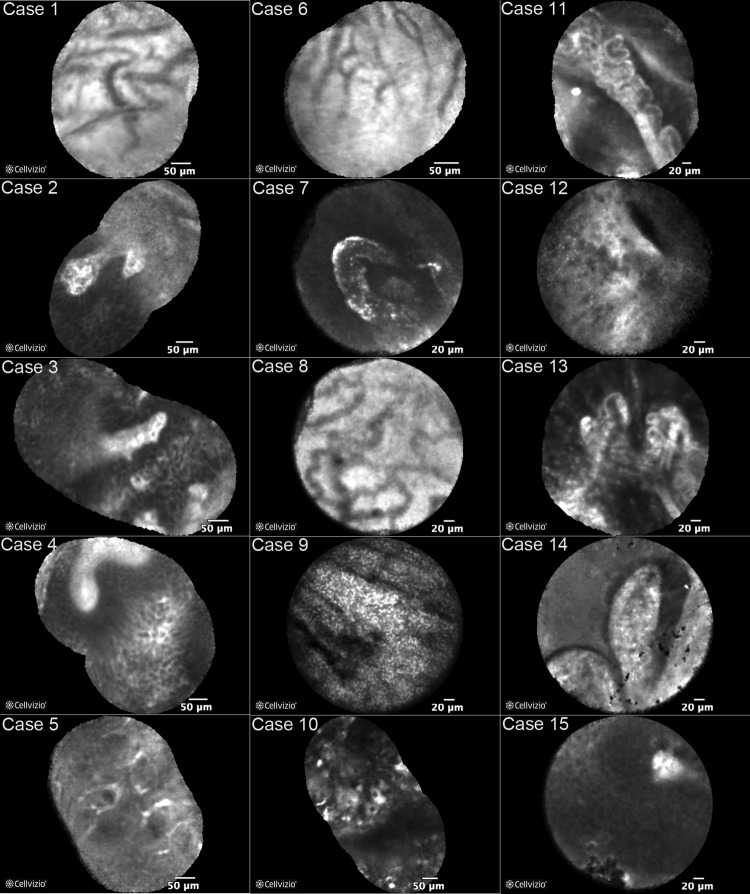
CLE imaging of normal urothelium, inflammation, and low-grade and high-grade UTUC was performed using the 0.85-mm imaging probe. Features similar to histopathology could be identified in the CLE images (Fig. 2). Representative mosaic images (average 9 frames per mosaic, range 2–29 frames) from each case are shown in Figure 3. A representative video of a papillary tumor (case 2) is shown in Supplementary Video S1 (Supplementary Data are available online at www.liebertpub.com/end). Stitching together multiple frames from a CLE video sequence into a mosaic image increased the field of view and aided in image interpretation. Previously reported CLE diagnostic criteria for high-grade urothelial carcinoma of the bladder include papillary features, pleomorphic cells with poor cohesion, and fibrovascular stalks.16 These features were effectively identified using the 0.85-mm probe in high-grade papillary tumors of the renal pelvis (Figs. 2 and 3, cases 2, 3, and 14). Characteristic low-grade CLE features such as densely packed monomorphic cells and fibrovascular stalks were also identified in low-grade papillary tumors of the renal pelvis (Fig. 3, cases 4, 10, 12, and 13). Imaging of normal upper tract urothelium consistently allowed visualization of lamina propria in both the ureter and the renal pelvis. In one case, a structure likely to represent Randall's plaque, a subepithelial calcification of the renal papilla, was visualized (Fig. 3, case 7).
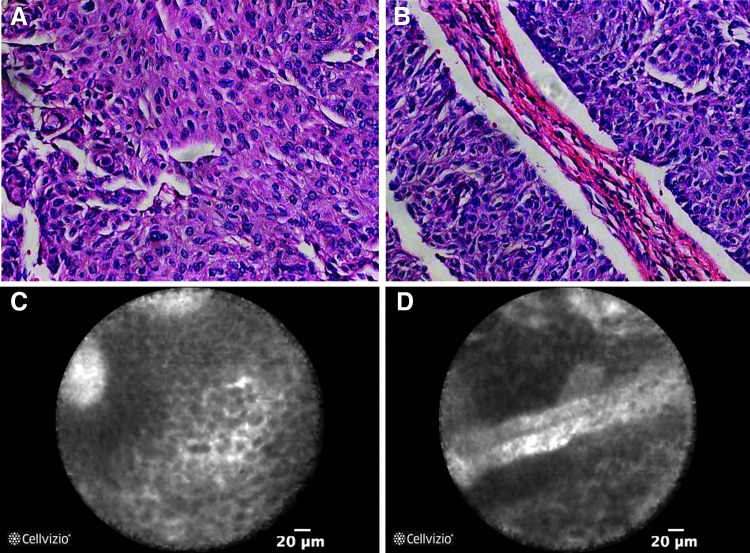
FIG. 2.
In vivo CLE and corresponding hematoxylin and eosin (H&E) images of high-grade upper tract urothelial carcinoma (UTUC) in the renal pelvis. CLE images are reminiscent of H&E with the visualization of pleomorphic cells and papillary features (A, C) and fibrovascular stalks (B, D).
FIG. 3.
Mosaic CLE images from each case. CLE images of normal urothelium showing lamina propria are shown for cases 1, 5, 6, and 8 where no suspicious lesions were visualized. No suspicious lesions were detected in case 7, however, a structure likely to be Randall's plaque was visualized. The image for case 9 was taken from a region of inflammation. Images from cases 4, 10, 12, and 13 are from low-grade papillary tumors and cases 2, 3, and 14 from high-grade papillary tumors. Images from cases 11 and 15 were taken from lesions suspicious for UTUC; however, pathologic correlation was inconclusive (case 11) or unavailable (case 15).
Discussion
We report a feasibility study of optical biopsy of UTUC using CLE. The 0.85-mm CLE probe used in this study is compatible with standard semirigid and flexible ureteroscopes, offering simultaneous confocal microscopy and white light endoscopy. In vivo microscopy images seen in the upper tract are similar to those collected from the lower tract. Even though the 0.85-mm probe has a lower resolution than the 2.6-mm probe previously used in the bladder, the CLE images have sufficient resolution to identify microarchitectural and morphologic features characterized as diagnostic criteria for urothelial carcinoma. The ability to visualize real-time histopathologic features is an attractive concept that has the potential to help avoid unnecessary biopsies for some patients, while improving biopsy yield for others. For example, the CLE images from case 9 were indistinct and appeared similar to normal tissue, and histopathology of the biopsy found only denuded mucosa and acute and chronic inflammation. Conversely, CLE images from case 11 identified a fibrovascular stalk and papillary features, while the scant biopsy sample was processed as a cytology sample reported as “atypical” with a possible differential diagnosis of low-grade papillary carcinoma. Histopathology of a follow-up biopsy resulted in a definitive diagnosis of low-grade UTUC.
Optical biopsy in the upper tract using CLE may serve as a useful adjunct to white light ureteroscopy during the initial diagnosis of UTUC and subsequent surveillance in selected patients who are candidates for endoscopic management. Initially, the primary application of CLE for minimally invasive management of UTUC might be to reduce unnecessary biopsies and associated risk. In our upper tract CLE images, the most apparent difference is between neoplastic and benign tissue, a distinction that can be made in real time by the urologist. Eventually, CLE may be used to provide cellular diagnosis before optical ablation with laser. With refinement and validation of diagnostic criteria, trained urologists may be able to independently provide cellular diagnoses comparable to histopathology. Development of software for automated CLE image interpretation may be applied to further improve real-time diagnoses and reduce the learning curve for CLE.18
In the current study, we used IV fluorescein as the contrast agent, which enables efficient image acquisition within 2 to 3 minutes after fluorescein administration without additional washing steps. The characteristic fibrovascular stalks and morphologic features of urothelial carcinoma were well visualized after IV fluorescein in the upper tract. CLE imaging of normal mucosa in the ureter and renal pelvis demonstrated characteristic vasculature within the lamina propria, but the cellular features of normal urothelium (e.g., umbrella cells) were not well visualized. The inability to visualize the normal urothelial cells may be due to inefficient staining from IV fluorescein and the thin layer of urothelium. As we have previously shown in the lower tract, fluorescein may be introduced either intravenously or topically. Additional studies are needed to investigate the feasibility of topical fluorescein administration in the upper tract and a comparative analysis with IV route of administration.
Our feasibility study is limited by its small sample size and not intended to address the diagnostic accuracy of CLE for UTUC. Furthermore, image acquisition, interpretation, and precise imaging–pathologic correlation present challenges. Even though the probe is flexible, manipulation for steady en face contact can be difficult and the inability to achieve proper contact could limit the full interrogation of a region. Image acquisition is also sensitive to motion introduced by the patient (e.g., respiratory movements) or the operator. As CLE imaging focuses on discrete regions identified by white light, short video sequences may be sufficient for the identification of tumor features. Similar to CLE imaging elsewhere, there is a learning curve associated with image interpretation. In particular to the upper urinary tract, skill sets required for CLE imaging appears to be similar to laser ablation of tumor. In regards to image interpretation, we previously demonstrated moderate interobserver variance and the relative ease of learning the imaging criteria for bladder cancer in novice CLE users.14 Prior experiences of CLE for bladder cancer will likely be beneficial as the optical imaging features of urothelial carcinoma are similar. Finally, the inherent limitations of acquiring adequate tissue through endoscopic biopsy also limit precise imaging–pathologic correlation.
Despite current limitations, CLE is a promising technology that may aid in the conservative management of UTUC. A larger scale prospective study will be needed to assess the accuracy of distinguishing the benign urothelium from low-grade and high-grade UTUC. In addition, multimodal imaging with CLE and macroscopic imaging technologies, such as narrow band imaging, should be explored to facilitate characterization of challenging lesions of the upper urinary tract.
Conclusions
Optical biopsy using CLE in the upper tract is feasible and provides real-time in vivo microscopy with sufficient resolution to distinguish between benign tissue and urothelial carcinoma. Imaging was performed using a new 0.85-mm probe that fits into the working channel of standard flexible ureteroscopes. Pending further clinical investigation, CLE is a promising adjunct to white light endoscopy to improve ureteroscopic biopsy and facilitate the endoscopic management of UTUC.
Supplementary Material
Abbreviations Used
- CLE
confocal laser endomicroscopy
- H&E
hematoxylin and eosin
- IV
intravenous
- UTUC
upper tract urothelial carcinoma
Acknowledgments
This work was supported, in part, by the U.S. National Institute of Health R01 CA160986 to J.C.L. The authors thank Timothy Chang, Jen-Jane Liu, Ruchika Mohan, and John Lavelle for technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Roupret M, Babjuk M, Comperat E, et al. . European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol 2013;63:1059–1071 [DOI] [PubMed] [Google Scholar]
- 2.Lughezzani G, Sun M, Perrotte P, et al. . Should bladder cuff excision remain the standard of care at nephroureterectomy in patients with urothelial carcinoma of the renal pelvis? A population-based study. Eur Urol 2010;57:956–962 [DOI] [PubMed] [Google Scholar]
- 3.Gadzinski AJ, Roberts WW, Faerber GJ, Wolf JS., Jr. Long-term outcomes of immediate versus delayed nephroureterectomy for upper tract urothelial carcinoma. J Endourol 2012;26:566–573 [DOI] [PubMed] [Google Scholar]
- 4.Roupret M, Traxer O, Tligui M, et al. . Upper urinary tract transitional cell carcinoma: Recurrence rate after percutaneous endoscopic resection. Eur Urol 2007;51:709–713; discussion 714 [DOI] [PubMed] [Google Scholar]
- 5.Smith AK, Stephenson AJ, Lane BR, et al. . Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: Implications for conservative management. Urology 2011;78:82–86 [DOI] [PubMed] [Google Scholar]
- 6.Straub J, Strittmatter F, Karl A, Stief CG, Tritschler S. Ureterorenoscopic biopsy and urinary cytology according to the 2004 WHO classification underestimate tumor grading in upper urinary tract urothelial carcinoma. Urol Oncol 2013;31:1166–1170 [DOI] [PubMed] [Google Scholar]
- 7.Wang JK, Tollefson MK, Krambeck AE, Trost LW, Thompson RH. High rate of pathologic upgrading at nephroureterectomy for upper tract urothelial carcinoma. Urology 2012;79:615–619 [DOI] [PubMed] [Google Scholar]
- 8.Tavora F, Fajardo DA, Lee TK, et al. . Small endoscopic biopsies of the ureter and renal pelvis: Pathologic pitfalls. Am J Surg Pathol 2009;33:1540–1546 [DOI] [PubMed] [Google Scholar]
- 9.Cutress ML, Stewart GD, Tudor EC, et al. . Endoscopic versus laparoscopic management of noninvasive upper tract urothelial carcinoma: 20-year single center experience. J Urol 2013;189:2054–2060 [DOI] [PubMed] [Google Scholar]
- 10.Traxer O, Geavlete B, de Medina SG, Sibony M, Al-Qahtani SM. Narrow-band imaging digital flexible ureteroscopy in detection of upper urinary tract transitional-cell carcinoma: Initial experience. J Endourol 2011;25:19–23 [DOI] [PubMed] [Google Scholar]
- 11.Somani BK, Moseley H, Eljamel MS, Nabi G, Kata SG. Photodynamic diagnosis (PDD) for upper urinary tract transitional cell carcinoma (UT-TCC): Evolution of a new technique. Photodiagnosis Photodyn Ther 2010;7:39–43 [DOI] [PubMed] [Google Scholar]
- 12.Bus MT, Muller BG, de Bruin DM, et al. . Volumetric in vivo visualization of upper urinary tract tumors using optical coherence tomography: A pilot study. J Urol 2013;190:2236–2242 [DOI] [PubMed] [Google Scholar]
- 13.Adams W, Wu K, Liu JJ, Hsiao ST, Jensen KC, Liao JC. Comparison of 2.6- and 1.4-mm imaging probes for confocal laser endomicroscopy of the urinary tract. J Endourol 2011;25:917–921 [DOI] [PubMed] [Google Scholar]
- 14.Chang TC, Liu JJ, Hsiao ST, et al. . Interobserver agreement of confocal laser endomicroscopy for bladder cancer. J Endourol 2013;27:598–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonn GA, Jones SN, Tarin TV, et al. . Optical biopsy of human bladder neoplasia with in vivo confocal laser endomicroscopy. J Urol 2009;182:1299–1305 [DOI] [PubMed] [Google Scholar]
- 16.Wu K, Liu JJ, Adams W, et al. . Dynamic real-time microscopy of the urinary tract using confocal laser endomicroscopy. Urology 2011;78:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konda VJ, Meining A, Jamil LH, et al. . A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy 2013;45:1006–1013 [DOI] [PubMed] [Google Scholar]
- 18.Andre B, Vercauteren T, Buchner AM, Krishna M, Ayache N, Wallace MB. Software for automated classification of probe-based confocal laser endomicroscopy videos of colorectal polyps. World J Gastroenterol 2012;18:5560–5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.