Abstract
The present prognosis for the recovery of voluntary control of movement in patients diagnosed as motor complete is generally poor. Herein we introduce a novel and noninvasive stimulation strategy of painless transcutaneous electrical enabling motor control and a pharmacological enabling motor control strategy to neuromodulate the physiological state of the spinal cord. This neuromodulation enabled the spinal locomotor networks of individuals with motor complete paralysis for 2–6 years American Spinal Cord Injury Association Impairment Scale (AIS) to be re-engaged and trained. We showed that locomotor-like stepping could be induced without voluntary effort within a single test session using electrical stimulation and training. We also observed significant facilitation of voluntary influence on the stepping movements in the presence of stimulation over a 4-week period in each subject. Using these strategies we transformed brain–spinal neuronal networks from a dormant to a functional state sufficiently to enable recovery of voluntary movement in five out of five subjects. Pharmacological intervention combined with stimulation and training resulted in further improvement in voluntary motor control of stepping-like movements in all subjects. We also observed on-command selective activation of the gastrocnemius and soleus muscles when attempting to plantarflex. At the end of 18 weeks of weekly interventions the mean changes in the amplitude of voluntarily controlled movement without stimulation was as high as occurred when combined with electrical stimulation. Additionally, spinally evoked motor potentials were readily modulated in the presence of voluntary effort, providing electrophysiological evidence of the re-establishment of functional connectivity among neural networks between the brain and the spinal cord.
Key words: : motor complete paralysis, neuronal network, transcutaneous spinal cord stimulation, voluntary movements
Introduction
It is becoming increasingly evident that the spinal circuitries below a paralyzing injury have a functional potential that far exceeds what has been thought possible.1–3 From the results of recent studies in mice and rats with motor complete paralysis we know that the lumbosacral spinal cord can be neuromodulated electrically and pharmacologically to enable motor control, including full weight-bearing stepping.1,3,4 Recently we reported that four human patients having been motor complete for 2 or more years regained the ability to stand and voluntarily generate leg movements in the supine position with the aid of epidural stimulation.5,6 Although the subjects were able to generate flexor movements with considerable precision, minimal locomotor-like movements were generated.6 Given these observations that the lumbosacral spinal circuitry can be neuromodulated in completely paralyzed individuals with the surgically invasive electrical epidural stimulation strategy, we then asked whether similar improvement in motor functions could be realized using noninvasive strategies to neuromodulate the spinal cord networks.
We previously demonstrated that the lumbosacral neuronal circuitry could be induced to generate locomotor-like movements in healthy, uninjured individuals with electromagnetic spinal cord stimulation.7 We then demonstrated a similar response when using transcutaneous spinal cord stimulation having a unique waveform that minimizes pain and discomfort.8,9 These observations led us to examine whether the lumbosacral circuitry could be modulated electrically via transcutaneous electrodes sufficiently to enable subjects with complete paralysis to generate step-like movements.
Herein we report the effectiveness of two novel noninvasive neuromodulatory strategies in regaining some voluntary locomotor-like leg movements after motor complete paralysis. We developed a method of electrically activating the spinal circuitry via electrodes placed on the skin overlying the lower thoracic, lumbosacral, and coccygeal vertebrae. We also demonstrate for the first time the interactive effects of administering a monoaminergic drug in combination with transcutaneous stimulation on the ability to voluntarily generate stepping-like movements of the lower limbs. Finally, we provide electrophysiological evidence of functional connectivity between the brain and the spinal cord and how this connectivity is modulated with voluntary effort in motor complete paralyzed subjects.
Methods
Our hypothesis was that functional brain-to-spinal cord connectivity in completely paralyzed individuals could be facilitated with electrical and/or pharmacological neuromodulation when presented concomitantly with training of the neural networks that control stepping-like movements. To assess the effectiveness of these interventions, we conducted a series of weekly training-testing sessions over a period of ∼18 weeks (Fig. 1). The subjects were tested while lying on their left side with the upper leg supported directly in the area of the shank and the lower leg placed on a rotating brace attached to a horizontal board supported by vertical ropes secured to the ceiling as described previously.7,10 By using this supportive device, the subject's legs were placed in a gravity-neutral position. Transcutaneous electrical stimulation was delivered using a 2.5 cm round electrode (Axelgaard, Fallbrook, CA) placed midline on the skin between spinous processes T11 and T12 (simply T11) or over coccyx 1 (Co1) as a cathode and two 5.0 × 10.2 cm2 rectangular plates (Axelgaard, Fallbrook, CA) placed symmetrically on the skin over the iliac crests as anodes. The cathode electrode was manually placed on the skin with a constant pressure to maintain consistent conductive contact to generate the most robust oscillatory movement of the lower extremity. For electrical stimulation we used monopolar rectangular stimuli (1 msec duration) filled with a carrier frequency of 10 kHz and at an intensity ranging from 80 to 180 mA (30 Hz at T11 and 5 Hz at Co1). The intensity of stimulation selected was adjusted to effect in each subject to induce stepping-like movements.
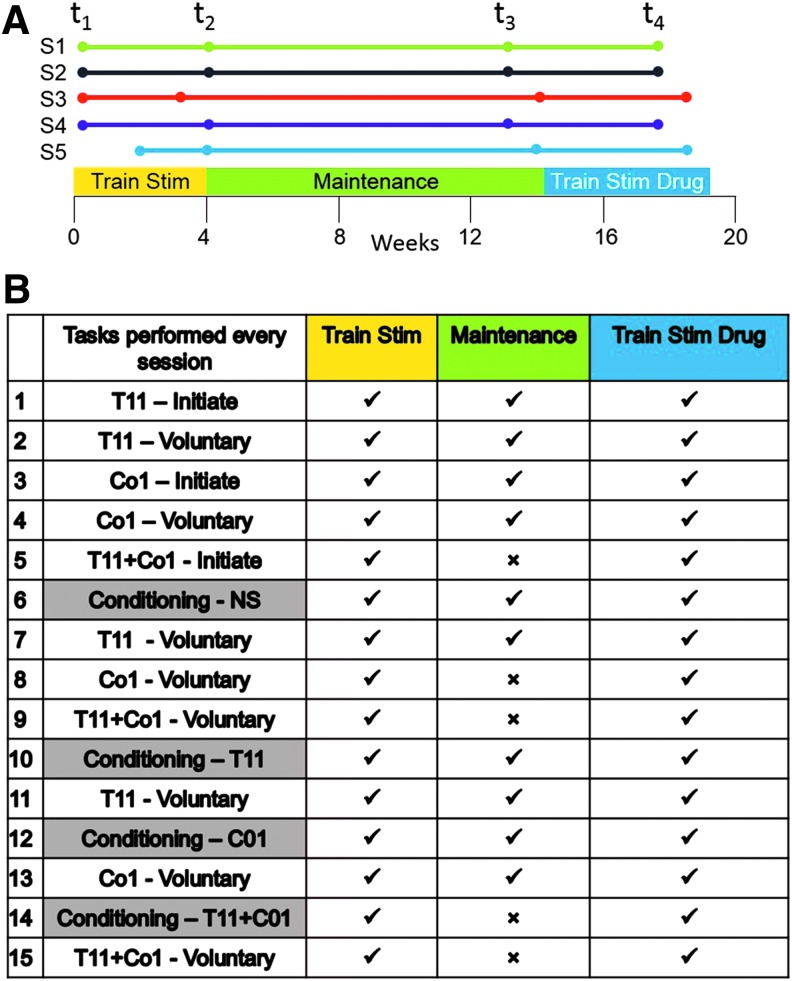
FIG. 1.
(A) Experimental time line. The experimental plan was divided into three segments. During the first 4 weeks (Pre-Train, t1 to Post-Train, t2; Train Stim) the subjects were trained with painless cutaneous enabling motor control at T11, Co1, and T11+Co1 plus conditioning (see Fig. 1B). From t2 to t3 (Maintenance) the same procedures were followed but without the conditioning oscillation with stimulation at both sites simultaneously. This 10-week Maintenance phase of the experimental protocol was designed to establish a stable functional baseline to more clearly differentiate the effects of painless cutaneous enabling motor control plus pharmacological enabling motor control from painless cutaneous enabling motor control alone. The final 4-week phase (Pre-Drug, t3 to Post-Drug, t4; Train Stim Drug) consisted of painless cutaneous enabling motor control plus conditioning as performed in the first phase with the addition of pharmacological enabling motor control. (B) Table summarizing the protocol of sequence of tasks performed during the study, while the subject was placed in a gravity-neutral position. EMG and limb kinematics were recorded when stimulated with the cathode electrode placed at T11, at Co1, or at both sites simultaneously (T11+Co1). At each stimulation site initiation (Initiate = response to stimulation alone) was first recorded followed by efforts to voluntarily oscillate (Voluntary) the limbs in a stepping-like fashion (rows 1–5). The lower limbs then were passively moved (Conditioning) in a stepping-like fashion for 3 min with and 3 min without stimulation (rows 6–15). After each of these conditioning procedures the subject was asked to oscillate the limbs without stimulation. ✓, tasks performed every session; ×, tasks not performed during the Maintenance phase that was designed to establish a new baseline prior to initiation of the drug treatment, that is, buspirone; S1–S5, Subjects 1–5. Color image is available online at www.liebertpub.com/neu
Each session from Pre-Train (t1) to Post-Train (t2) consisted of stimulation at the level of T11, Co1, and the combination of the two sites with and without simultaneous voluntary effort to move the lower limb in a stepping-like motion (Fig 1). In addition, each session consisted of passively oscillating the limbs in a step-like fashion for 3 min without and 3 min with continuous stimulation at each of the three stimulation sites noted above. These passive oscillations are referred to as “conditioning” (see details below). From Post-Train (t2) to Pre-Drug (t3) the same procedures were followed, but without the conditioning oscillation with stimulation at both sites simultaneously. This reduced level of oscillations was designed to maintain and sustain a plateau performance prior to the initiation of the drug phase of the intervention. Over the 18-week period we performed four detailed recording-assessment sessions (t1–t4; Fig. 1A) reflecting the quality of the stepping-like motions. In the first 4 weeks Subjects 1–4 (S1–S4) performed all four training-testing sessions, whereas Subject 5 (S5) performed only two training-testing sessions at 3 and 4 weeks. To determine the effects of pharmacological enabling motor control plus painless cutaneous enabling motor control the subjects were trained once per week over a period of 4 weeks (t3–t4). To determine the potential for generating locomotor-like stepping all training and periodic testing (t1–t4) were performed in an apparatus designed to minimize gravitational effects (Fig. 2A).7,10
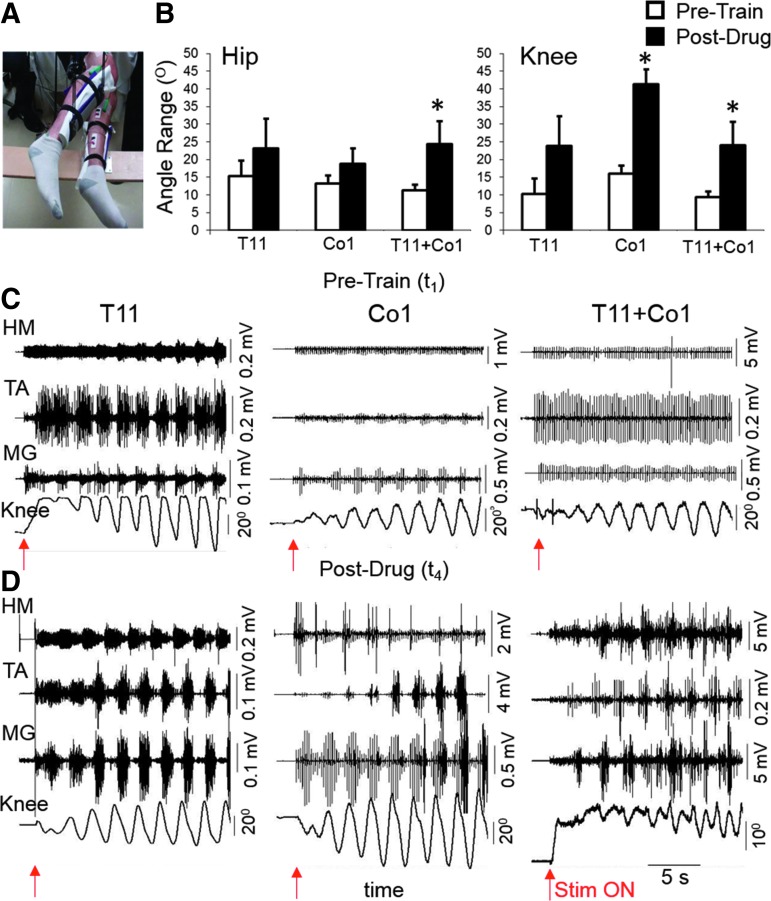
FIG. 2.
Facilitation of stepping-like movements during noninvasive T11 and/or Co1 transcutaneous stimulation. (A) Position of the legs of a paralyzed subject when in the gravity-neutral apparatus. (B) Mean ± SEM (n = 5 subjects) angular displacements of the hip and knee at the Pre-Train (t1) and Post-Drug (t4) phases. HM, TA, and MG raw EMG and angular displacement at the knee during leg movements in the presence of stimulation at T11, Co1, or T11+Co1 at the Pre-Train ((C), t1) and Post-Drug ((D), t4) phases are shown. Red arrows indicate the time the stimulation was initiated. *, significantly different from Pre-Train at p < 0.05. EMG, electromyogram; HM, medial hamstring; MG, medial gastrocnemius; SEM, standard error of mean; TA, tibialis anterior. Color image is available online at www.liebertpub.com/neu
Angular displacements at the hip and knee joints in both legs were recorded with goniometers (Biometrics Ltd., Ladysmith, VA). The subjects had access to visual feedback via a mirror placed so that movement of the legs could be observed. Bipolar surface electrodes were placed bilaterally on the soleus, medial gastrocnemius (MG), tibialis anterior (TA), medial hamstring (HM), and vastus lateralis (VL) muscles.11,12 Electromyogram (EMG) signals were amplified (Konigsberg Instruments, Pasadena, CA) differentially (bandwidth of 10 Hz to 10 kHz) and acquired at 10 KHz using a 16-channel hard-wired A/D board and customized LabVIEW software (National Instruments, Austin, TX). To minimize artifacts from the stimulation, the EMG signals were passed through a band-pass filter (30–200 Hz). The filtered EMG signals were analyzed off-line to compute the amplitude, duration, and timing of individual bursts.
The conditioning treatment consisted of imposing passive, continuous, anterior-posterior limb movements in an oscillatory stepping-like pattern (∼20 cycles/min) for 3 min without stimulation, followed by 3 min with sub-motor threshold stimulation either at T11, Co1, or T11+Co1 with the subject's legs supported in the gravity-neutral apparatus. The total time for each testing or training session was ∼45 min. We also tested whether the conditioning treatment alone within a single training session could facilitate the ability to generate knee oscillations without and with stimulation at the end of the study (t4 in Fig. 1).
For the final 4 weeks of the study all subjects were informed that they would receive either a placebo or the serotoninergic agonist buspirone. All subjects were given buspirone 7.5 mg orally twice daily for the last 4 weeks (t3–t4) (Fig. 1B).
The University of California, Los Angeles Institutional Review Board approved all procedures. Subjects were enrolled based on the enrollment criteria of traumatic cervical or thoracic injury, AIS B, greater than one year from injury, and stable motor function as documented by sequential clinical exams. All subjects signed voluntary written consent forms to participate in these studies. The clinical profiles of the subjects upon enrollment into this study are as follows: S1 is a 42-year-old male who suffered a T3–T4 spinal cord injury (SCI) after a steer wrestling accident and was 24 months from initial injury; S2 is a 20-year-old male who suffered a C5–C6 SCI sustained during a football game and was 36 months from initial injury; S3 is a 20-year-old male who suffered a C6 SCI after a motor vehicle accident and was 36 months from initial injury; S4 is a 19-year-old male who suffered a C7 SCI during a swimming accident and was 24 months from injury; and S5 is a 56-year-old male who suffered a T3–T4 SCI after a bicycle accident and was 72 months from injury. Magnetic resonance imaging was obtained in all subjects to confirm the location and extent of the injury (Supplementary Fig. 1; see online supplementary material at http://www.liebertpub.com).
The ability to facilitate voluntarily generated locomotor-like movements during spinal cord painless cutaneous enabling motor control when the legs were placed in a gravity-neutral position was determined. After instructing each subject to oscillate the limbs as if stepping, we asked the subject to relax for a period of 15–20 sec before we initiated stimulation with the cathode at the T11 and/or Co1 vertebral level. Then, we asked the subjects to attempt to voluntarily oscillate the legs in the presence of stimulation at T11, Co1, or T11+Co1 (Fig 1B).
Statistical analysis
All statistics were performed in SPSS version 19 (IBM Corp., Armonk, NY) with base, regression, advanced models, and categories packages. Several parameters were studied to thoroughly characterize the recovery of sensorimotor function. Such high dimensionality substantially complicates the extraction of relevant parameters to account for differences between experimental conditions. Reduction of such multi-dimensional datasets, however, can be achieved via multivariate statistical analysis such as principal component analysis (PCA).13,14 PCA is mathematically defined as an orthogonal linear transformation that transforms the original dataset to a new coordinate system, such that the variance is maximized on each new coordinate axis. Non-linear PCA (NL-PCA) was implemented using the CATPCA command with appropriate link functions to accommodate non-linear and non-normal distributional features for each variable.15 Herein, data were analyzed using the correlation method, which adjusts the mean of the data to 0 and the standard deviation to 1. The degree of similarities and differences, that is, the criteria of success of an intervention, between the subjects and conditions were evaluated as the difference in the factor coordinates of each observation (scores) on each PC axis. PC (object) scores were extracted across all end-points, subjects, and levels of the repeated measures. PC retention was determined by the eigenvalue >1 rule,16 and the face validity of PC loading patterns. PC1 scores then were carried forward as the principal outcomes for assessing the impact of the experimental manipulations by three-way repeated measures analysis of variance (ANOVA) (GLM command), with each subject serving as his own control. Significant interactions were followed up by interaction graphing with assessment of specific differences by one-way ANOVA and Tukey's post hoc. Significance was assessed at p < 0.05.
Results
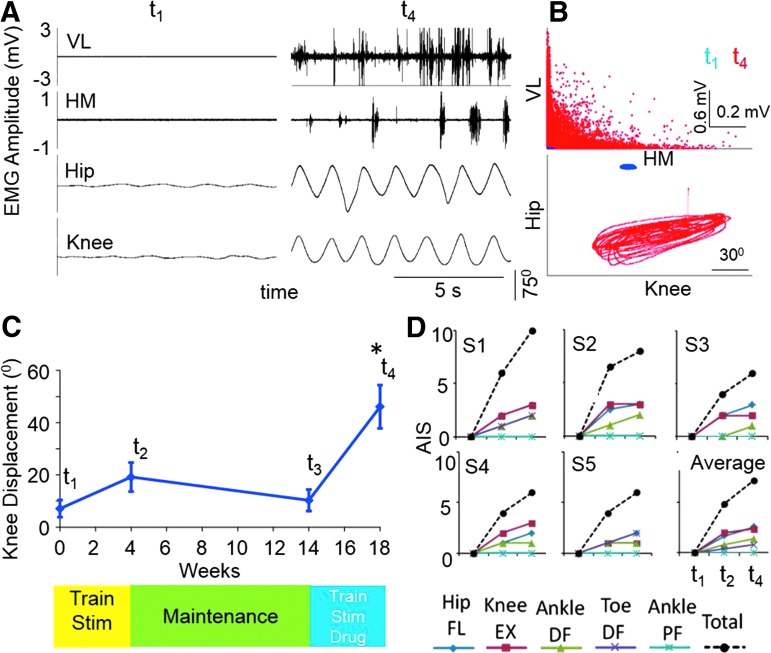
During the first testing session (Pre-Train, t1) some rhythmic hip and knee movements and corresponding EMG activity were induced during painless cutaneous enabling motor control without any voluntary effort when the legs were placed in a gravity-neutral position (Fig. 2A). Examples of the relative effects of spinal cord stimulation with the electrodes placed at T11 (30 Hz) or Co1 (5 Hz) vertebral levels and the combination of stimulation from both sites simultaneously on these leg movements in the same subject during Pre-Train (t1) and Post-Drug (t4) testing are shown in Figure 2C,D, respectively (data from S1). The mean amplitudes of the angular movements at the hip and knee joints during voluntary efforts with and without stimulation were consistently greater Post-Drug (t4) than Pre-Train (t1) (Fig. 2B). The magnitude of the differences in the EMG bursting patterns and the kinematics of the limb movements between the Pre-Train (t1) and Post-Drug (t4) phases was highly dependent on the site of stimulation (Fig. 2B–D).
Initially (Pre-Train, t1), none of the subjects could voluntarily initiate rhythmic stepping-like movements with any detectable rhythmic EMG activity of the leg muscles (Fig. 3A) and showed minimal knee displacement (Fig. 3B). When we asked the subjects to voluntarily attempt to oscillate the legs in the presence of stimulation at T11, Co1, or both simultaneously, we observed significantly greater movement amplitudes at the knee (Fig. 3B). After four training sessions (Fig. 1), the subjects had recovered a significantly greater level of voluntarily enhanced knee movement (compare t1 vs. t2) in the presence of stimulation at T11, Co1, and T11+Co1 (Fig. 3C). In addition, the average range of movement at the knee with voluntary effort in the presence of stimulation was significantly greater at t2 than at t1, demonstrating an improved recovery of a voluntary influence on knee movement after only four training sessions (compare Fig. 3B vs. Fig. 3C).
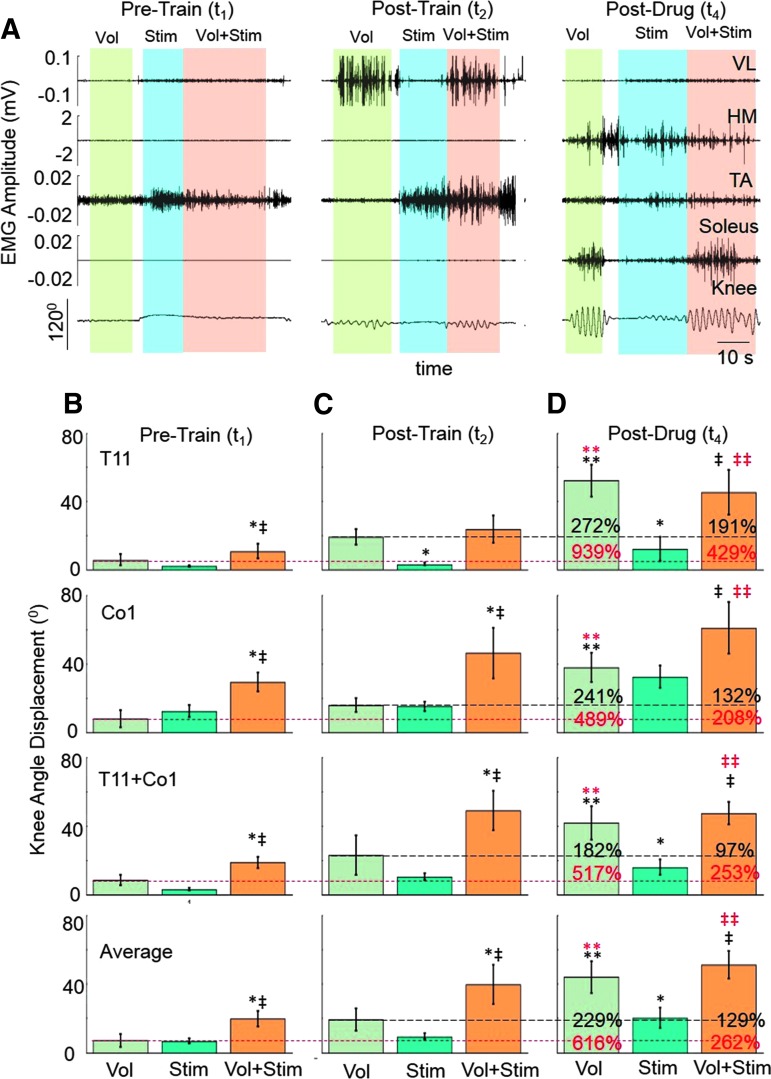
FIG. 3.
Voluntary control of leg movements enabled by electrical and pharmacological stimulation and training. (A) VL, HM, TA, and soleus raw EMG and angular displacement at the knee during leg oscillations with a voluntary effort alone (Vol), stimulation at T11 (Stim), and Vol+Stim at the Pre-Train (t1), Post-Train (t2), and Post-Drug (t4) phases. (B) Mean ± SEM (n = 5 subjects) knee angular displacements at the Pre-Train (t1), Post-Train (t2), and Post-Drug (t4) phases under each experimental condition described in (A) with stimulation at T11, Co1, or T11+Co1. The red and black dashed horizontal lines indicate the mean voluntary effort at t1 and t2, respectively. The percentiles at t4 reflect differences between t4 and t1 (red) and t4 and t2 (black), respectively. *, significantly different from Vol; ‡, significantly different from Stim; ** (red), significantly different from Vol at t1; ‡‡ (red), significantly different from Vol+Stim at t1; **, significantly different from Vol at t2; ‡‡, significantly different from Vol+Stim at t2; all at p < 0.05. EMG, electromyogram; HM, medial hamstring; SEM, standard error of mean; TA, tibialis anterior; VL, vastus lateralis. Color image is available online at www.liebertpub.com/neu
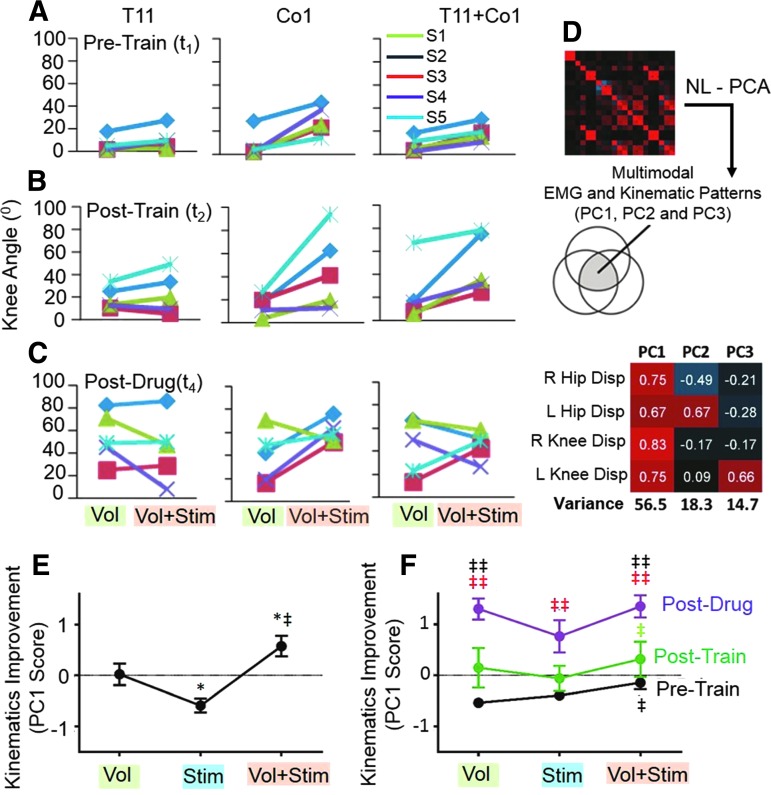
Although the effects of stimulation at different spinal levels and at different phases of the interventions varied among subjects, NL-PCA revealed a consistent linkage pattern between the hip and knee angular displacements (Fig. 4D–F). Based on PC1 there was an emergence of organized oscillations and significant improvement (Fig. 5C–E) in response to the electrical and pharmacological interventions in combination with training. The effectiveness of voluntary effort alone was similar to voluntary effort plus stimulation at the Post-Drug phase (t4) (Fig. 3D; Supplementary Video 1; see online supplementary material at http://www.liebertpub.com). The average range of voluntary movement at the knee Post-Drug (t4) was twofold greater than Pre-Drug/Post-Train (t2) and sixfold greater than at the initial test (t1) (Fig. 3B–D). These average differences in knee displacement are even more impressive when considering the variation in the responsiveness to stimulation as a function of subject specificity and the site of stimulation (Fig. 4A–C). For example, the angular knee displacement for S1 was lower with the combination of voluntary effort plus stimulation at T11 versus voluntary effort alone at the Post-Drug (t4) phase. This occurred at each of the stimulation sites for this subject. This also occurred with S4 with the stimulation sites involving T11 and T11+Co1.
FIG. 4.
Subject specific kinematics responses and NL-PCA. Mean angular displacements of the knee at the (A) Pre-Train, (B) Post-Train, and (C) Post-Drug phases for each subject during Vol and Vol+Stim. (D) NL-PCA to distill non-parametric multivariate kinematics cross-correlations into PC patterns. PC loading matrix depicts the relationship between variables and the extracted PC patterns. Positive loadings are red and inverse loadings are blue. Loading weights were used to calculate PC scores. (E) Three-way repeated measures ANOVA for testing the impact of Stim, Vol, and Vol+Stim on the improvement of the kinematics based on the PC1 score. (F) Interaction of Stim by Drug modulation on the improvement of the kinematics based on the PC1 score: significance was assessed by factorial three-way within subjects ANOVA followed by post hoc one-way ANOVA and Tukey's test. *, significantly different from Vol; ‡, significantly different from Stim; ‡‡ (red), significantly different from Pre-Train; ‡‡, significantly different from Post-Train; all at p < 0.05. ANOVA, analysis of variance; NL-PCA, non-linear principal component analysis; PC, principal component; S1–S5, Subjects 1–5.
FIG. 5.
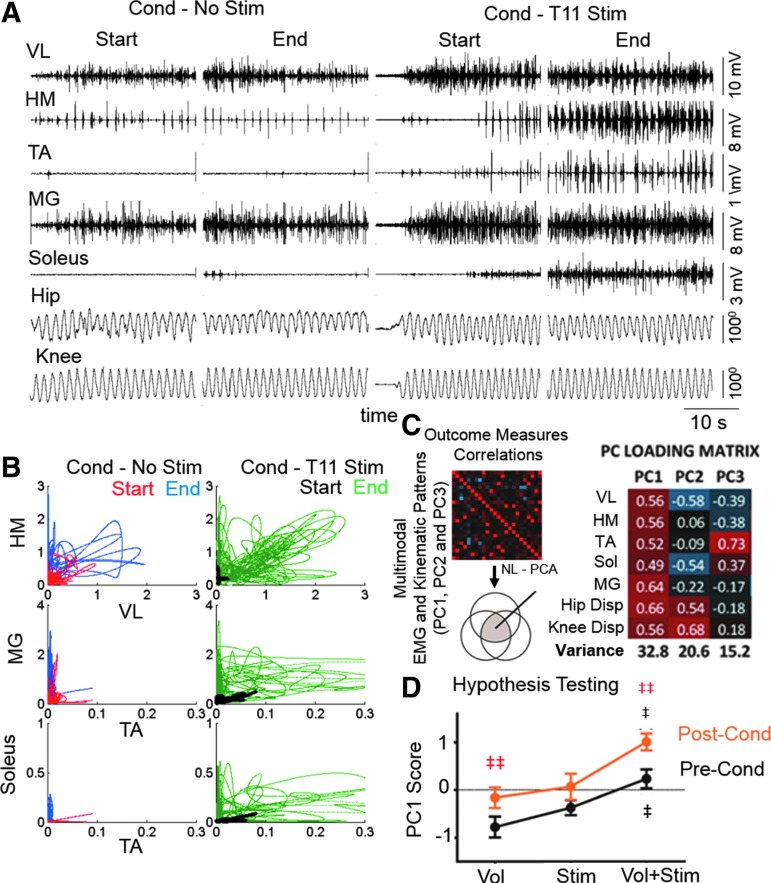
Effects of proprioceptive conditioning on voluntary leg movements. (A) An example of raw EMG and hip and knee angular displacements from the first (start) and last (end) 30 sec of a single conditioning bout without and with stimulation at T11. (B) Scatterplots between filtered EMG activities of antagonistic muscle pairs (VL vs. HM, TA vs. MG, and TA vs. Soleus) for the first 5 sec from each segment shown in (A). (C) NL-PCA was used to distill non-parametric multivariate kinematics cross-correlations into PC patterns. PC loading matrix depicts the relationship between variables and the extracted PC patterns. (D) Three-way repeated measures ANOVA of the impact of Vol, Stim, and Vol+Stim using PC1 outcome as the end-point. (E) Interaction of Stim by Conditioning modulation. ‡, significantly different from Stim; ‡‡ (red), significantly different Pre-Cond; all at p < 0.05. ANOVA, analysis of variance; EMG, electromyogram; HM, medial hamstring; MG, medial gastrocnemius; NL-PCA, non-linear principal component analysis; PC, principal component; SEM, standard error of mean; TA, tibialis anterior; VL, vastus lateralis.
The statistical analyses shown in Figures 3 and 4 led to the following conclusions: 1) the magnitude of the range in knee oscillations was increased with voluntary effort plus stimulation at t1 even within the first test session (Fig. 3B); 2) there was a further increase in the range of these oscillations after 4 weeks of pharmacological enabling motor control (t4) (Fig. 3D and Fig. 4F); and 3) there was notable subject-specificity for the stimulation site that evoked the greatest responses to the interventions at all phases (Fig. 4A–C).
All of the observations noted above demonstrate malleability of the physiological state of the spinal circuitry in response to both acute and chronic oscillations. These oscillations were initiated from supraspinal (initiated voluntarily) sources and by direct stimulation of the spinal circuitry. Recognizing this high degree of malleability we questioned whether the properties of these brain–spinal cord networks modulated by passively imposing stepping-like proprioception also could change the physiological state of locomotor-related neural networks. To study this issue we tested whether a passively imposed stepping-like pattern could induce a conditioning effect within a single training session.
We followed the changes in the EMG and kinematics output over the progression of a single 3-min conditioning session without electrical stimulation and then with electrical stimulation at T11 (Fig. 5). When we compared the EMG and kinematics responses during the first 30 sec and the final 30 sec of a 3-min conditioning bout, we observed a gradual increase in EMG amplitude during the initial 30 sec that remained elevated up to the final 30 sec with little or no change in the kinematics with and without electrical stimulation (Fig. 5A). The level of neuromodulation during conditioning combined with electrical stimulation was significantly greater than conditioning without electrical stimulation. Improved coordination of the motor pools for the knee and ankle flexors and extensors over these different 30-sec time periods under each condition also was evident (Fig. 5B). In addition to the reciprocal patterns shown in Figure 5B there were cyclic responses probably reflecting direct responses of stimulation artifact (see HM and VL EMG in the presence of T11 stimulation also seen in Fig. 5B).
NL-PCA revealed the following effects when comparing Pre-Cond (before conditioning) and Post-Cond (after conditioning): 1) the level of activation of the motor pools associated with the flexor and extensor muscles at the hip, knee, and ankle and the hip and knee displacement significantly increased with voluntary effort alone and with voluntary effort plus electrical stimulation beyond that observed prior to the conditioning (Fig. 5C–E); 2) this effect was significantly greater with voluntary effort plus electrical stimulation than with voluntary effort alone (Fig. 5E); and 3) significant levels of reciprocal modulation in concert with elevated EMG amplitudes were observed in the presence of stimulation (Fig. 5B).
An example of the magnitude of the overall (t1 vs. t4) effects on the stepping kinematics, EMG, and intralimb coordination in one subject is shown in Figure 6A. Note the rhythmic EMG bursting patterns and oscillatory movement at the hip and knee at t4 but not at t1. During these voluntary oscillations there was a clear pattern of reciprocity in the modulation of EMG amplitudes in the HM and VL and hip versus knee angular displacements (Fig. 6B). Whereas there was a significant effect of the combination of voluntary effort and stimulation (Fig. 3B), no significant effects with voluntary effort alone had emerged after four training sessions (t2) (Fig. 6C). After 4 weeks of electrical plus pharmacological intervention we observed a significant facilitation in the mean voluntary performance (without stimulation) of stepping-like movements (Fig. 6C; t4).
FIG. 6.
Voluntary performance (without spinal cord stimulation) at different phases of the study. (A) An example of VL and HM EMG activity and hip and knee displacements during voluntary leg oscillations without stimulation for Subject 1 during the Pre-Train (t1) and Post-Drug (t4) phases. (B) Pattern of reciprocity for EMG amplitudes of the HM and VL and kinematics coordination based on knee and hip movements. (C) Mean ± SEM knee displacement Pre-train (t1) and Post-train (t2), at the beginning (t2) and end (t3) of the maintenance phase, and the beginning (t3) and end (t4) of the drug phase. *, significantly different from t1-t3. (D) Clinical assessment according to AIS. AIS motor scores at t1, t2, and t4 for individual subjects (S1–S5) and the average for all subjects. EMG, electromyogram; EX, extension; DF, dorsiflexion; FL, flexion; HM, medial hamstring; PF, plantarflexion; S1–S5, Subject 1–Subject 5; SEM, standard error of mean; VL, vastus lateralis. Color image is available online at www.liebertpub.com/neu
To differentiate the pharmacological effects from the previous stimulation and training effects, we initiated a series of training sessions at t2 to establish a new baseline of voluntary performance. We did this by eliminating procedures within each training session that involved stimulation at both sites simultaneously (Fig. 1B). The establishment of this stimulation-trained baseline was demonstrated by the similarity in the voluntary control level at t2 and t3 (Fig. 6C). Thus, the results are consistent with there being an enhancement of voluntary control that can be attributed to the drug treatment when combined with training and stimulation based on the observation that t4 is greater than t3 combined with the similarity in voluntary control at t2 and t3.
We also assessed the progression in voluntary control (in the absence of spinal cord stimulation) using the standard clinical assessment scale (AIS; Fig. 6D). There was a progressive increase in movement at the hip, knee, and ankle during efforts to dorsiflex and plantarflex. No clinical movement (score of 0) was observed in any of the lower extremity joints at the Pre-Train phase (t1). An average improvement of 7 points was observed in this cohort during the Post-Drug (t4) condition. In the presence of these interventions this would be equivalent to the subjects being reclassified from AIS B to C. In general, the clinical assessments were consistent with the knee movements observed in the gravity-neutral apparatus.
Given our previous observations in animal and human subjects that the varying physiological states of the spinal circuits can be monitored in vivo while performing motor tasks,17,18 we used a similar approach to demonstrate electrophysiological evidence of supraspinally mediated neuromodulation of the spinal circuitry in the present subjects. To assess these interactions we recorded spinally evoked motor potentials generated in the TA and MG muscles when the subject was lying in the gravity-neutral apparatus. We asked the subject to swing the leg forward (flex) and backward (extend; shaded areas in Fig. 7A) and then forward again in the presence of continuous stimulation. Figure 7B,C shows examples of the malleability of the brain–spinal cord to muscle connections in response to different transcutaneous stimuli in the presence and absence of voluntary efforts to flex or extend the lower limbs.
FIG. 7.
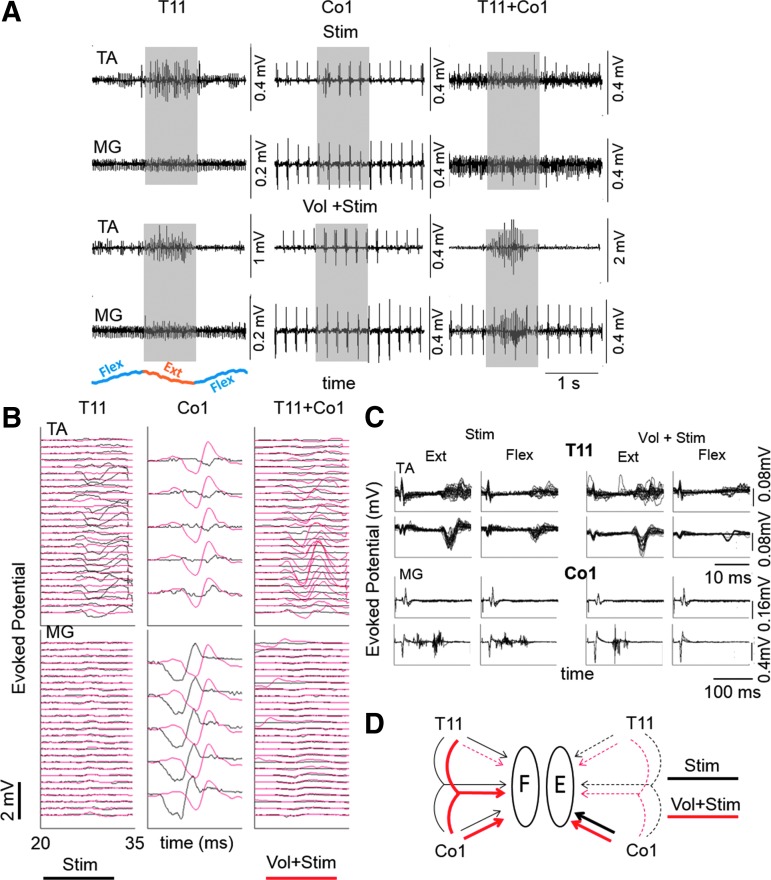
Modulation of evoked potentials with and without voluntary effort. (A) Raw EMG from the TA and MG muscles under the influence of stimulation at T11, Co1, and T11+Co1 with and without voluntary effort to oscillate the legs (flexion-extension-flexion) when placed in a gravity-neutral position. (B) Evoked potentials generated within the shaded areas in (A) without (black traces) and with (red traces) voluntary effort. The sequence of traces is triggered from the stimulation pulse with the lowest trace being the first and the top trace being the last response. (C) Overlay of multiple responses (T11, 0–33 msec and Co1, 0–200 msec) during flexion and extension without (Stim) and with (Vol+Stim) voluntary effort. (D) Schematic summary of the variations in 20–35 msec TA (Flexor-F) and MG (Extensor-E) responses (see B) reflects how spinally evoked potentials can be gated by a voluntary effort. The dotted lines reflect minimal or no evoked potential and the thin and thick solid lines indicate modest and high amplitude evoked potentials, respectively. Generally, Co1 stimulation evoked more consistent responses in both muscles than T11 or T11+Co1 stimulation. EMG, electromyogram; MG, medial gastrocnemius; TA, tibialis anterior.
By testing this brain–spinal connectivity when stimulating at the level of Co1 and/or T11 we could compare the influence of ascending pathways projecting rostrally versus the more proximal networks projecting caudally to the upper lumbar segments on voluntary movement. We observed clear inhibitory and excitatory patterns of responses in the TA and MG motor pools depending on the stimulation site. When stimulating at Co1, we observed a clear facilitation of the TA motor pool mediated by voluntary effort, whereas the MG motor pool was slightly suppressed with voluntary effort. Another consistent observation was the presence of a 2 msec delay of the response in the MG when there was a voluntary effort to extend the lower limb (Fig. 7B). The complexity of the functional brain–spinal connectivity was illustrated further by the suppression of any response in the MG when T11 and Co1 were stimulated simultaneously with or without voluntary effort. An evoked potential occurred only in the TA when there was a voluntary effort to extend. These interactive effects of stimulation at different sites with and without voluntary efforts to extend or flex are summarized graphically in Figure 7D. The key message derived from these observations is that some combination of electrical stimulation, pharmacological intervention, and training can open new functional connections among brain–spinal cord networks and that these functional connections are highly dynamic and interactive.
Figure 8 demonstrates spinally evoked motor potentials (Fig. 8A,B) and corresponding recruitment curves (Fig. 8C,D) during T11 stimulation in a supine position at the Pre-Train (t1) and at Post-Drug (t4) phases of the study, respectively. At corresponding stimulation intensities the magnitude of the responses was higher at Post-Drug (t4) (Fig. 8B,D) than Pre-Train (t1) (Fig. 8B,C). At the Post-Drug (t4) phase, the order of recruitment of different motor pools relative to each other had recovered based on their rostro-caudal alignment along the lumbosacral enlargement, as well as on the proximity of the recording EMG electrodes from the stimulation point, which is similar to the previous data obtained in experiments with epidural stimulation.19 That is, the evoked responses from the HM were characterized by lower thresholds and shorter latency compared with those from the TA and MG (Fig. 8B,D).
FIG. 8.
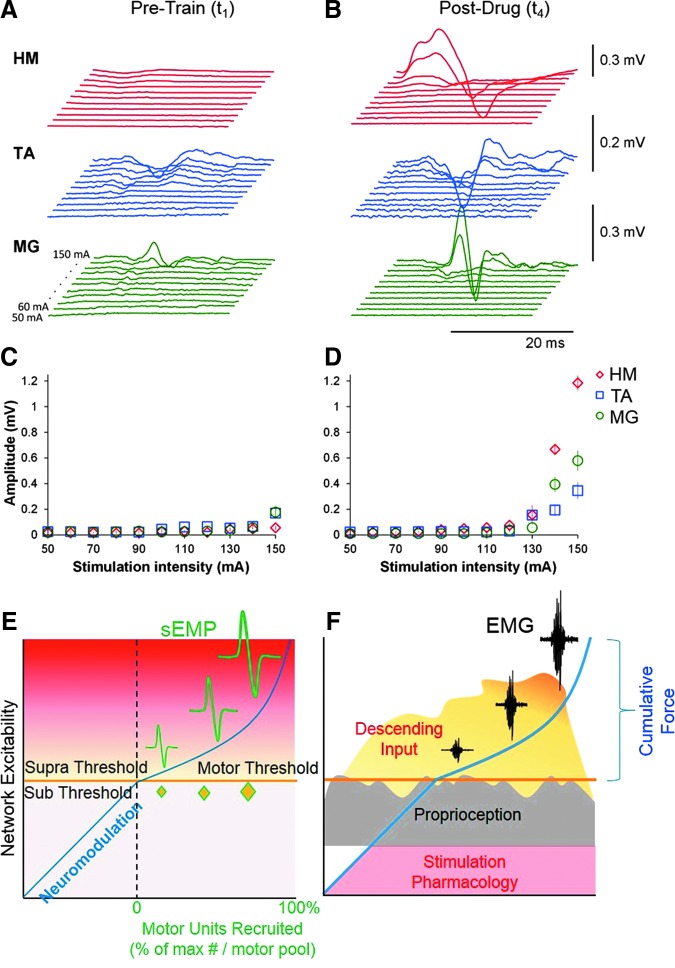
Spinally evoked motor potentials in one participant during transcutaneous electrical spinal stimulation delivered between the spinous processes of the T11 and T12 vertebrae. The average of three non-rectified responses in the HM, TA, and MG of the right leg at stimulation intensities from 50 to 150 mA (10 mA increments) at Pre-Train (A, t1) and Post-Drug (B, t4) phases of the study. Data are shown from the time window between 10 and 40 msec following the stimulus. Recruitment curves are shown for the HM, TA, and MG at stimulation intensities ranging from 50 to 150 mA at the Pre-Train (C, t1) and Post-Drug (D, t4) phases of the study. Note the increase in the response magnitudes at the higher intensities, especially at t4. (E) Schematic reflecting the increasing amplitudes of sEMP with an increasing intensity of stimulation and the generation of evoked motor potentials when the stimulation crosses motor threshold. Note the presence of motor units only after network excitability crosses the motor threshold. (F) Schematic representing the combination of various neuromodulation modalities including stimulation, pharmacology, proprioception, and descending input in the generation of the EMG bursting patterns and the corresponding force. EMG, electromyogram; HM, medial hamstring; MG, medial gastrocnemius; sEMP, spinally evoked motor potentials; TA, tibialis anterior. Color image is available online at www.liebertpub.com/neu
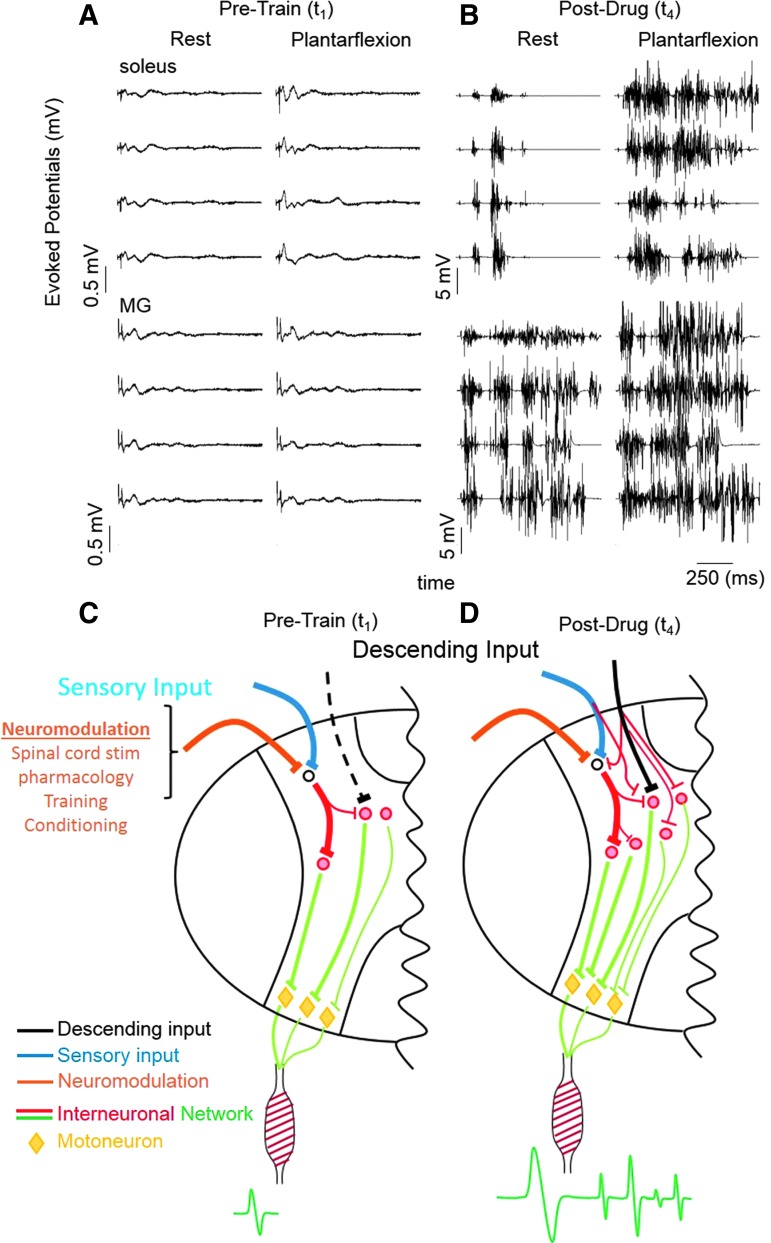
To characterize the specific neuromodulatory effects of descending motor control on the electrophysiological characteristics of the spinal circuitry that may have changed in response to motor training and to the pharmacological intervention, we compared the evoked potentials during single pulse stimulation (T11) delivered every 5 sec during rest and during a plantarflexion effort with the subjects in a supine position. This test was performed at Pre-Train (t1) and at the end of all intervention and training procedures (Post-Drug (t4)). At t1, an early response (latency of about 25–30 msec) was generated in each muscle tested during both rest and the plantarflexion effort, whereas no late responses (latency of about 100–1000 msec) were observed (Fig. 9A). Moreover, the amplitude of the early response did not change during the plantarflexion effort at t1. At t4, however, there was a dramatic increase in the amplitude of the early response as compared with t1 and a late response was present during both rest and the plantarflexion effort (Fig. 9B: note the difference in the ordinate scale between A and B). The pattern of these late responses are of particular interest given that multiple cyclic bursts occurred in both the soleus and gastrocnemius muscles during the plantarflexion effort following a single stimulation pulse. These findings are of particular interest given the mechanistic implications (Figs. 7–9; see Discussion).
FIG. 9.
Electrophysiological assessment of brain–spinal network interactions. MG and soleus EMG recorded during plantarflexion with and without a maximum voluntary effort with stimulation at T11 at t1 (A) and t4 (B). (C,D) show schematic diagrams of hypothetical mechanisms underlying the observed results of short and long latency responses of motor pools (see Discussion for details).
Discussion
Using completely noninvasive interventions, we have demonstrated recovery of function of locomotor networks within the lumbosacral spinal cord of human subjects that have been completely paralyzed for more than 2 years. First, we show that transcutaneous electrical stimulation of the lumbosacral spinal segments delivered distally to the lesion can induce coordinated stepping-like movements when the subject's lower limbs are supported in a gravity-neutral apparatus. This coordinated alternating bilateral motion was initiated and sustained by the subject without afferent input associated with weight bearing. Each tested subject could voluntarily move their limbs in a similar but more robust motion when a voluntary effort was combined with stimulation even at the earliest phases of testing and training. As weekly training sessions progressed the subjects became less dependent on the stimulation. In fact, in a number of instances, the stimulation seemed to interfere with the robustness of the stepping. During the 4 weeks of monoaminergic agonist treatment, the magnitude of voluntarily generated movements increased further with and without the presence of stimulation. Finally, the combination of stimulation, pharmacology, and training resulted in robust late responses during plantarflexion, suggesting a complex functional reorganization of the spinal circuitry (Fig. 9C,D).
We have developed a novel method of noninvasive transcutaneous spinal cord stimulation delivery using a special form of electrical pulses at a high frequency. This methodology has enabled us to neuromodulate the spinal locomotor networks and induce involuntary stepping-like movements in non-injured subjects when their legs were placed in a gravity-neutral apparatus.8,9 In addition, this transcutaneous spinal cord stimulation technique was used to induce multi-segmental reflexes in the leg muscles of non-injured11,20,21 as well as spinally injured subjects.12 Similarly, we have shown that transcutaneous electrical spinal cord stimulation applied to the lumbosacral enlargement can facilitate locomotor activity in decerebrated and spinal cats.22 Computer modeling suggests that transcutaneous spinal cord stimulation, as well as epidural stimulation, can activate similar afferent neurons projecting to the locomotor circuitry within the spinal cord.23 Therapeutic application of transcutaneous spinal cord stimulation has been used for modification of spasticity24 and for facilitation of stepping-like movements in incomplete spinal cord injured subjects25. All of these observations suggest that transcutaneous electrical spinal cord stimulation can effectively neuromodulate the spinal locomotor circuitry after SCI.
In the present study we induced stepping-like movements in completely paralyzed individuals using transcutaneous electrical spinal cord stimulation without any voluntary effort (Fig. 2). These findings are consistent with previous observations in uninjured participants when involuntary stepping-like movements were generated by transcutaneous spinal cord stimulation.9 Furthermore, the stimulation enabled the subjects to voluntarily facilitate stepping-like movements of the lower limbs within four treatment sessions. Finally, most subjects could perform stepping-like movements in the absence of any stimulation at the latter phases of the present study (Fig. 3A,D) and these movements were similar to those performed in the presence of stimulation (Figs. 3A, 4C, and 5). In essence, this effect was similar to that observed in our previous experiments with four spinal cord injured subjects implanted with epidural electrodes when they were instructed to move specific joints while lying in a supine position.6 Thus, in the present and the previous6 studies spinal cord electrical stimulation has enabled nine out of nine subjects with motor complete paralysis to move the lower limbs when they intended to do so, rather than by directly inducing a movement. The significance of this enabling effect is consistent with the results of animal studies. For example, at stimulation intensities 20% below the motor threshold the spinal networks in paralyzed rats were modulated to a physiological state that enabled proprioception and cutaneous input to initiate a fivefold increase in spontaneous cage activity and more normal EMG bursting patterns.26
We propose an elemental spinal network that could generate the observed output patterns with pharmacological and electrical spinal cord stimulation in the presence of proprioceptive input in our paralyzed subjects. Such a pathway architecture can conceptually account for all interactive observations made in the present study when guided by activity-dependent mechanisms (i.e., stimulation, pharmacology, conditioning, and training). In Figure 8E,F we illustrate progressively larger amplitudes in spinally evoked motor potentials with increasing current via transcutaneous stimulation, indicating an increased excitability of the sensorimotor pathways. This emphasizes the extensive modulation that can occur in the excitability of the spinal networks below the motor threshold and that once this threshold is reached more motor units are recruited.
It appears that the increased excitability of the interneuronal spinal networks that normally generate a largely random-stochastic pattern of excitation of the motoneurons can do so even after complete paralysis. Convergence of somatosensory and descending motor inputs combined with electrical and pharmacological neuromodulation of interneurons, and perhaps to some degree motoneurons, results in the re-emergence of not only “automated” locomotor movements but also voluntary control (Fig. 8F). Once the dormant neural networks are awoken, they can be engaged in a motor task and, therefore, trained given the learning potential of the spinal networks.27
These results raise new and exciting questions about the anatomical versus functional completeness of the injury, the potential to use spared but perhaps nonfunctional descending axons across the lesion site, and re-activation of dormant neuronal networks below the injury using noninvasive painless spinal cord stimulation. Descending supraspinal input, peripheral sensory input, and neuromodulatory inputs project to interneuronal networks (Fig. 9C) that, in turn, project to different motor pools that activate the appropriate muscles generating the EMG response. With repetitive training, there appears to be an emergence of new synaptic connections among the spinal interneurons projecting to motoneurons resulting in more “normal stochastic bursting patterns” from the motor pools compared with pre-trained dormant networks that may generate synchronized responses (Fig. 9C,D). This hypothesis is largely consistent with observations in spinal rats that have been trained with pharmacological facilitation and epidural stimulation.18
Together the most logical interpretation of our findings is that there are networks above, within, and below the lesion in a significant number of individuals with complete, chronic paralysis that can be transformed into a functional state by receiving a certain critical level of spinal activation. From a clinical perspective, an obvious and important question then arises: what exactly is a “significant number of individuals”? A number of anatomical28 as well as physiological29 reports have demonstrated the presence of some residual circuitry across the lesion of many individuals clinically classified as having been completely paralyzed for years. Combined, our previous results5,6 with epidural stimulation and the present findings with transcutaneous stimulation of the spinal cord indicate that nine of nine individuals with complete motor paralysis have regained some voluntary function.
We now know that there is a new potential for recovery of sensorimotor function after a severe SCI: that includes improved locomotor function with some voluntary control. The reported results combined with our previous reports in animals13,30 and humans5,6 make it virtually undeniable that significant levels of plasticity can occur in the nervous system of individuals even after years of complete paralysis due to SCI. These findings have significant implications for expanding the range of interventional strategies within an evolving clinical toolbox from which the best approach for a given individual can be selected.
Supplementary Material
Acknowledgments
This research was funded in part by NIH U01EB15521, R01EB007615 through NIBIB, NINDS, and NICHD, the Christopher & Dana Reeve Foundation, the Walkabout Foundation, and the F.M. Kirby Foundation. The research described was conducted in the UCLA Clinical and Translational Research Center (CTRC) that was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI grant number UL1TR000124. YPG is supported by RFBR grant number 13-04-12030. Partial support for data analysis was provided to YPG, VRE, DS, and PG from the Russian Scientific Fund project number 14-45-00024. D.C.L. is supported by the J. Yang and Family Foundation and is a 1999 Paul and Daisy Soros New American Fellow.
Author Disclosure Statement
VRE, YPG, DCL, PG, and RRR researchers on the study team hold shareholder interest in NeuroRecovery Technologies. VRE is president and chair of company's board of directors. VRE, YPG, DCL, and RRR hold certain inventorship rights on intellectual property licensed by the regents of the University of California to NeuroRecovery Technologies and its subsidiaries.
References
- 1.Edgerton V.R., Courtine G., Gerasimenko Y.P., Lavrov I., Ichiyama R.M., Fong A.J., Cai L.L., Otoshi C.K., Tillakaratne N.J., Burdick J.W., and Roy R.R. (2008). Training locomotor networks. Brain Res. Rev 57, 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossignol S., and Frigon A. (2011). Recovery of locomotion after spinal cord injury: some facts and mechanisms. Ann. Rev. Neurosci. 34, 413–440 [DOI] [PubMed] [Google Scholar]
- 3.Fong A.J., Roy R.R., Ichiyama R.M., Lavrov I., Courtine G., Gerasimenko Y., Tai Y.C., Burdick J., and Edgerton V.R. (2009). Recovery of control of posture and locomotion after a spinal cord injury: solutions staring us in the face. Prog. Brain Res. 175, 393–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichiyama R.M., Courtine G., Gerasimenko Y.P., Yang G.J., van den Brand R., Lavrov I.A., Zhong H., Roy R.R., and Edgerton V.R. (2008). Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J. Neurosci. 28, 7370–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harkema S., Gerasimenko Y., Hodes J., Burdick J., Angeli C., Chen Y., Ferreira C., Willhite A., Rejc E., Grossman R.G., and Edgerton V.R. (2011). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angeli C.A., Edgerton V.R., Gerasimenko Y.P., and Harkema S.J. (2014). Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137, 1394–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerasimenko Y., Gorodnichev R., Machueva E., Pivovarova E., Semyenov D., Savochin A., Roy R.R., and Edgerton V.R. (2010). Novel and direct access to the human locomotor spinal circuitry. J. Neurosci. 30, 3700–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorodnichev R.M., Pivovarova E.A., Pukhov A., Moiseev S.A., Savokhin A.A., Moshonkina T.R., Shcherbakova N.A., Kilimnik V.A., Selionov V.A., Kozlovskaia I.B., Edgerton V.R., and Gerasimenko Iu P. (2012). [Transcutaneous electrical stimulation of the spinal cord: non-invasive tool for activation of locomotor circuitry in human]. Fiziologiia cheloveka 38, 46–56 [PubMed] [Google Scholar]
- 9.Gerasimenko Y.P., Gorodnichev R., Puhov A., Moshonkina T., Savochin A., Selionov V.A., Roy R.R., Lu D.C., and Edgerton V.R. (2015). Initiation and modulation of locomotor circuitry output with multi-site transcutaneous electrical stimulation of the spinal cord in non-injured humans. J. Neurophysiol, 834–842 [DOI] [PubMed] [Google Scholar]
- 10.Gurfinkel V.S., Levik Y.S., Kazennikov O.V., and Selionov V.A. (1998). Locomotor-like movements evoked by leg muscle vibration in humans. Eur. J. Neurosci. 10, 1608–1612 [DOI] [PubMed] [Google Scholar]
- 11.Courtine G., Harkema S.J., Dy C.J., Gerasimenko Y.P., and Dyhre-Poulsen P. (2007). Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J. Physiol. 582, 1125–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dy C.J., Gerasimenko Y.P., Edgerton V.R., Dyhre-Poulsen P., Courtine G., and Harkema S.J. (2010). Phase-dependent modulation of percutaneously elicited multisegmental muscle responses after spinal cord injury. J. Neurophysiol. 103, 2808–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courtine G., Gerasimenko Y., van den Brand R., Yew A., Musienko P., Zhong H., Song B., Ao Y., Ichiyama R.M., Lavrov I., Roy R.R., Sofroniew M.V., and Edgerton V.R. (2009). Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nature Neurosci. 12, 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson A.R., Irvine K.A., Gensel J.C., Nielson J.L., Lin A., Ly J., Segal M.R., Ratan R.R., Bresnahan J.C., and Beattie M.S. (2013). Derivation of multivariate syndromic outcome metrics for consistent testing across multiple models of cervical spinal cord injury in rats. PLoS one 8, e59712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linting M., Meulman J.J., Groenen P.J., and van der Koojj A.J. (2007). Nonlinear principal components analysis: introduction and application. Psychol. Methods 12, 336–358 [DOI] [PubMed] [Google Scholar]
- 16.Kaiser H.F. (1960). The application of electronic computers to factor analysis. Ed. Psychol. Meas. 20, 141–151 [Google Scholar]
- 17.Lavrov I., Gerasimenko Y.P., Ichiyama R.M., Courtine G., Zhong H., Roy R.R., and Edgerton V.R. (2006). Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J. Neurophysiol. 96, 1699–1710 [DOI] [PubMed] [Google Scholar]
- 18.Gad P., Lavrov I., Shah P., Zhong H., Roy R.R., Edgerton V.R., and Gerasimenko Y. (2013). Neuromodulation of motor-evoked potentials during stepping in spinal rats. J. Neurophysiol. 110, 1311–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayenko D.G., Angeli C., Harkema S.J., Edgerton V.R., and Gerasimenko Y.P. (2014). Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J. Neurophysiol. 111, 1088–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minassian K., Persy I., Rattay F., Dimitrijevic M.R., Hofer C., and Kern H. (2007). Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 35, 327–336 [DOI] [PubMed] [Google Scholar]
- 21.Roy F.D., Gibson G., and Stein R.B. (2012). Effect of percutaneous stimulation at different spinal levels on the activation of sensory and motor roots. Exp. Brain Res. 223, 281–289 [DOI] [PubMed] [Google Scholar]
- 22.Musienko P.E., Bogacheva I.N., Savochin A.A., Kilimnik V.A., Gorskii O.V., Nikitin O.A., and Gerasimenko Ia P. (2013). [Non-invasive transcutaneous spinal cord stimulation facilitates locomotor activity in decerebrated and spinal cats]. Rossiiskii fiziologicheskii zhurnal imeni I.M. Sechenova/Rossiiskaia akademiia nauk 99, 917–927 [PubMed] [Google Scholar]
- 23.Ladenbauer J., Minassian K., Hofstoetter U.S., Dimitrijevic M.R., and Rattay F. (2010). Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans. Neural Syst. Rehabil. Eng. 18, 637–645 [DOI] [PubMed] [Google Scholar]
- 24.Hofstoetter U.S., McKay W.B., Tansey K.E., Mayr W., Kern H., and Minassian K. (2014). Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 37, 202–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofstoetter U.S., Hofer C., Kern H., Danner S.M., Mayr W., Dimitrijevic M.R., and Minassian K. (2013). Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed. Tech. (Berlin) [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26.Gad P., Choe J., Shah P., Garcia-Alias G., Rath M., Gerasimenko Y., Zhong H., Roy R.R., and Edgerton V.R. (2013). Sub-threshold spinal cord stimulation facilitates spontaneous motor activity in spinal rats. J. Neuroeng. Rehabil. 10, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Leon R.D., Tamaki H., Hodgson J.A., Roy R.R., and Edgerton V.R. (1999). Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J. Neurophysiol. 82, 359–369 [DOI] [PubMed] [Google Scholar]
- 28.Kakulas B.A. (1999). A review of the neuropathology of human spinal cord injury with emphasis on special features. J. Spinal Cord Med. 22, 119–124 [DOI] [PubMed] [Google Scholar]
- 29.Dimitrijevic M.R., McKay W.B., and Sherwood A.M. (1997). Motor control physiology below spinal cord injury: residual volitional control of motor units in paretic and paralyzed muscles. Adv. Neurol. 72, 335–345 [PubMed] [Google Scholar]
- 30.Musienko P., van den Brand R., Marzendorfer O., Roy R.R., Gerasimenko Y., Edgerton V.R., and Courtine G. (2011). Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. J. Neurosci. 31, 9264–9278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.