Abstract
The ubiquitin–proteasome pathway plays a critical role in the intracellular trafficking of recombinant adeno-associated virus 2 (AAV2) vectors, which negatively impacts the transduction efficiency of these vectors. Because ubiquitination occurs on lysine (K) residues, we performed site-directed mutagenesis where we replaced each of 10 surface-exposed K residues (K258, K490, K507, K527, K532, K544, K549, K556, K665, and K706) with glutamic acid (E) because of similarity of size and lack of recognition by modifying enzymes. The transduction efficiency of K490E, K544E, K549E, and K556E scAAV2 vectors increased in HeLa cells in vitro up to 5-fold compared with wild-type (WT) AAV2 vectors, with the K556E mutant being the most efficient. Intravenous delivery of WT and K-mutant ssAAV2 vectors further corroborated these results in murine hepatocytes in vivo. Because AAV8 vectors transduce murine hepatocytes exceedingly well, and because some of the surface-exposed K residues are conserved between these serotypes, we generated and tested two single mutants (K547E and K569E), and one double-mutant (K547 + 569E) AAV8 vector. However, no significant increase in the transduction efficiency of any of these mutant AAV8 vectors was observed in murine hepatocytes in vivo. These studies suggest that although targeting the surface-exposed K residues is yet another strategy to improve the transduction efficiency of AAV vectors, phenotypic outcome is serotype specific.
Introduction
Adeno-associated virus 2 (AAV2) is a single-stranded DNA-containing, nonpathogenic human parvovirus.1 The use of recombinant AAV2 vectors has proven to be safe and, in a number of phase I/II clinical trials, has shown efficacy in gene therapy for at least five human diseases.2 However, the use of large vector doses often needed to achieve therapeutic benefits is also associated with the risk of the host immune response to the vector.3 For example, when recombinant AAV2 vectors were used in a clinical trial for the potential gene therapy of hemophilia B, the therapeutic level of expression of human factor IX (hF.IX) could not be achieved at low vector doses (1012–1013 vgs), and at a higher vector dose (1014 vgs), the therapeutic level of expression of hF.IX was achieved, but it was short-lived because of a cytotoxic T-cell response to AAV2 capsids.4–6 More recently, in a clinical trial with recombinant AAV8 vectors, therapeutic levels of expression of hF.IX were achieved, but an immune response to AAV8 capsid proteins was also observed.7,8 Thus, there is a clear need to develop AAV vectors that can be delivered at low doses, and yet achieve high transduction efficiency.9 In our previously published studies, we reported that phosphorylation of certain surface-exposed tyrosine (Y), serine (S), and/or threonine (T) residues negatively impacts the transduction efficiency of these vectors because of subsequent potential ubiquitination, followed by proteasomal degradation of AAV capsids.10,11 These studies led to the development of the next generation of Y-, S-, and T-mutant AAV2 vectors that were shown to efficiently transduce various cell types at significantly reduced vector doses.12–14
However, because ubiquitination occurs at lysine (K) residues,15 we reasoned that the elimination of surface-exposed K residues by site-directed mutagenesis might also lead to the development of AAV vectors with increased transduction efficiency at lower doses. To this end, we carried out site-directed mutagenesis of each of 10 surface-exposed K residues in the AAV2 capsid by substituting with glutamic acid (E) residues. We report here that four of these mutants, K490E, K544E, K549E, and K556E, showed an increase in transduction efficiency up to ∼5-fold in human HeLa cells in vitro, and up to ∼10-fold in murine hepatocytes in vivo. When the two best performers were combined on the same capsid, the transduction efficiency of the K544 + 556E double mutant was further increased by up to 12-fold in murine hepatocytes in vivo. The transduction efficiency of this double mutant was significantly higher than that of a tyrosine triple-mutant (Y730F+500 + 444F) AAV2 vector, previously reported to transduce murine hepatocytes efficiently.16
Because AAV8 serotype vectors have previously been shown to transduce murine hepatocytes exceedingly well,17,18 and because some of the surface-exposed K residues are also conserved in this serotype, we generated and tested two single mutants (K547E and K569E), and one double-mutant (K547+ 569E) AAV8 vectors. However, no significant increase in the transduction efficiency of any of these mutant AAV8 vectors over wild type (WT) was observed in murine hepatocytes in vivo. These studies suggest that although targeting the surface-exposed K residues is a yet another strategy that can be used to improve the transduction efficiency of AAV vectors, it is likely serotype specific, which has implications in the development of tissue-targeted vectors for use in human gene therapy.
Materials and Methods
Cells and reagents
Human embryonic kidney cell line, HEK293 cells, and human cervical carcinoma cell line, HeLa cells, were purchased from the American Type Culture Collection, and maintained as monolayer cultures in DMEM (Invitrogen) containing 10% fetal bovine serum (Sigma) and antibiotics (Lonza).19
Production of recombinant AAV vectors
Recombinant AAV2 vectors containing either self-complementary EGFP (scAAV2-EGFP) or single-stranded firefly luciferase gene (Fluc) (ssAAV2-Fluc and ssAAV8-Fluc) under the control of the chicken β-actin promoter (CBA) were packaged as described previously.20 HEK293 cells were transfected using polyethyleneimine (linear, MW 25,000; Polysciences, Inc.), and 72 hr posttransfection, cells were harvested and vectors were purified by iodixanol (Sigma) gradient centrifugation and ion exchange column chromatography (HiTrap SP HP and Q HP columns, 5 ml; GE Healthcare). Vector stocks were concentrated using centrifugal spin concentrators (Apollo; 150 kDa cutoff, 20 ml capacity, CLP). Ten microliters of purified vector stocks was incubated with DNase I (Invitrogen) at 37°C for 2 hr, and digested with Proteinase K (Invitrogen) at 55°C for an additional 2 hr. The reaction mixture was subjected to purification using phenol/chloroform, followed by chloroform extractions. DNA was precipitated using ethanol in the presence of 20 μg glycogen (Invitrogen). The titers of DNase I–resistant vector particles were determined by qPCR using the following primer pairs specific for the CBA promoter: F-5′-TCCCATAGTAACGCCAATAGG-3′, R-5′-CTTGGCATATGATACACTTGATG-3′, and SYBR GreenER PCR Master Mix (Invitrogen).
Site-directed mutagenesis of AAV capsid genes
A two-stage PCR strategy using Turbo Pfu Polymerase (Stratagene) was employed with plasmids pACGr2c2 and pACGr2c8 as described previously.14 In stage one, two PCR extension reactions were carried out in separate tubes for the forward and reverse PCR primers for three cycles. In stage two, the two reactions were combined and a PCR was carried out for an additional 15 cycles, followed by digestion with DpnI for 1 hr. Primers were designed to introduce changes from lysine (AAG) to glutamic acid (GAG) for each of the residues mutated. In some experiments, lysine residues were also mutated to arginine (CGA) residues.
Recombinant AAV vector transduction assays in vitro
Human HeLa cells were transduced with 2 × 103 vgs/cell with WT and mutant scAAV2-EGFP vectors and incubated for 48 hr. Transgene expression was evaluated as the total area of green fluorescence (pixel2) per visual field (mean ± SD) as described previously.16,21 Analysis of variance was used to compare the controls and the test results, which were determined to be statistically significant.
In vivo bioluminescence imaging of mice
The University of Florida Institutional Animal Care and Use Committee approved all animal experiments. All procedures were carried out in accordance with the principles of the National Research Council's Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize pain and suffering. C57BL/6 male mice (Jackson Laboratory) were injected at 10 weeks following intravenous delivery of 1 × 1010 vgs/animal of WT or mutant ssAAV2-Fluc or ssAAV8-Fluc vectors (n = 3). Two weeks postinjections, luciferase activity was analyzed using a Xenogen IVIS Lumina System (Caliper Life Sciences).22,23 Mice were anesthetized using 2% isofluorane and injected with luciferin substrate (Beetle luciferin; Caliper Life Sciences) intraperitoneally at a dose of 150 μg/g of body weight. Mice were placed in a light-tight chamber and images were obtained at 5 min after injection of the substrate. Image analyses were carried out using Living Image 3.2 software (Caliper Life Sciences), and relative signal intensities were determined.
Analysis of surface mutations on the AAV capsid
To localize the mutated amino acids on the AAV2 and AAV8 capsid surfaces, their VP3 monomer coordinates (RCSB PDB No. 1LP3 and 2QA0, respectively) were downloaded and visualized using the COOT program.24 The VP3 monomer coordinates were used to generate capsid surface icosahedral asymmetric unit “Roadmaps” to highlight the mutated amino acids using the RIVEM program.25 This application generates the image from a monomer in a standard icosahedral orientation. The VP3 monomers were also used to generate 60mer coordinates by icosahedral matrix multiplication in the Viperdb online server25 for creating capsid surface images, with the mutated residues highlighted, using the PyMOL program.26
Statistical analysis
Results are shown as mean ± SD. Differences between experimental groups were identified using a grouped-unpaired two-tailed distribution of Student's t-test, and p < 0.05 were considered statistically significant.
Results
Site-directed mutagenesis of surface-exposed lysine residues on the AAV2 capsid improves vector-mediated transgene expression in human cells in vitro
The AAV2 capsid contains 34 lysine (K) residues in the capsid viral protein 3 (VP3). Ten of these (K258, K490, K507, K527, K532, K544, K549, K556, K665, and K706) are surface exposed.27 Each of the 10 K residues was substituted with glutamic acid (E) by site-directed mutagenesis as described previously.12,14 Each of the mutants containing a self-complementary (sc) AAV-EGFP genome could be generated at titers similar to the WT AAV2 vectors. Each of the K-E mutant vectors was evaluated for transduction efficiency in HeLa cells. These results, shown in Fig. 1A and B, indicate that, of the 10 mutants, the K490E mutant transduced HeLa cells ∼3-fold more efficiently than its AAV2-WT counterpart. The transduction efficiency of the K544E, K549E, and K556E mutant vectors was increased by up to ∼10-fold. These data support the hypothesis that modification of lysine residues on the AAV2 capsid surface, which would eliminate the potential for ubiquitination, improves the transduction efficiency of these vectors.28
Figure 1.
Analysis of EGFP expression after transduction of HeLa cells with individual site-directed adeno-associated virus 2 (AAV2) capsid mutants. Each of the 10 surface-exposed lysine (K) residues in AAV2 capsid was substituted with glutamic acid (E) and evaluated for its efficiency to mediate transgene expression. (A) EGFP expression analysis at 48 hr postinfection at MOI of 2 × 103 vgs/cell of vscAAV2-EGFP vectors. (B) Quantitation of transduction efficiency of each of the lysine-mutant scAAV2 vectors. **p < 0.01 vs. WT AAV2. Color images available online at www.liebertpub.com/hgtb
Specific lysine-mutant scAAV2 vectors efficiently transduce primary murine hepatocytes in vivo
We also evaluated the transduction efficiency of the AAV2 lysine-mutant vectors packaged with scAAV2-EGFP vectors in C57BL/6 mice (n = 3 for each group). Animals were injected with 1 × 1010 vgs of each vector intravenously and the levels of expression of the EGFP gene were assessed in liver sections two weeks postinjection by fluorescence microscopy. These results, shown in Fig. 2A and B, document that the AAV2-K556E mutant was the most efficient, with an ∼10-fold increase in the transduction efficiency, followed by AAV2-K544E and AAV2-K527E mutants, which increased the transduction efficiency by ∼8-fold compared with WT AAV2 vectors. The transduction efficiency of the two other mutants, K490E and K549E, which were efficient in transducing HeLa cells in vitro (Fig. 1A and B), was not significantly improved in murine hepatocytes in vivo, the reason for which remains unclear.
Figure 2.
Analysis of EGFP expression in primary murine hepatocytes ex vivo following intravenous delivery of individual site-directed AAV2 capsid mutants in C57BL/6 mice in vivo. (A) EGFP expression analysis 2 weeks postinjection of 1 × 1010 vgs of scAAV2-EGFP vectors. (B) Quantification of transduction efficiency of each of the lysine-mutant scAAV2 vectors. **p < 0.01 vs. WT AAV2. Color images available online at www.liebertpub.com/hgtb
The transduction efficiency of specific lysine-mutant ssAAV2 vectors is also increased in murine hepatocytes in vivo
We next evaluated the transduction efficiency of the lysine-mutant ssAAV2 vectors in the same murine model in vivo. The WT and the two most efficient mutant vectors (K544E and K556E) were packaged with a single-stranded firefly luciferase (Fluc) AAV2 genome, and 1 × 1010 vgs of each vector was injected intravenously into C57BL/6 mice (n = 3 for each group). Levels of expression of the Fluc gene, assessed two weeks postinjection by bioluminescence imaging, showed that expression from each of the two mutant vectors was increased, respectively, by ∼6-fold and ∼8-fold compared with that from the WT ssAAV2 vectors (Fig. 3A and B). The level of transgene expression from the K544 + 556E-AAV2 double-mutant vector was ∼10-fold higher than that from the WT ssAAV2 vector, also significantly higher than the tyrosine triple-mutant (Y444 + 500 + 730F) vector, which we have previously reported to be highly efficient in transducing murine hepatocytes in vivo.16
Figure 3.
In vivo bioluminescence imaging of luciferase gene expression following tail vein injection of various lysine-mutant ssAAV2 vectors. C57BL/6 mice were injected with 1 × 1010 vgs/animal of the most efficient vectors expressing the firefly luciferase gene. Live images were obtained to determine the luciferase activity. The visual output corresponds to the number of photons emitted/sec/cm2 as a false color image, in which the maximum is represented by red and the minimum is represented by blue (A). The relative signal intensity is depicted in (B). **p < 0.01 and *p < 0.05 in comparison with WT AAV2 were considered as significant. Color images available online at www.liebertpub.com/hgtb
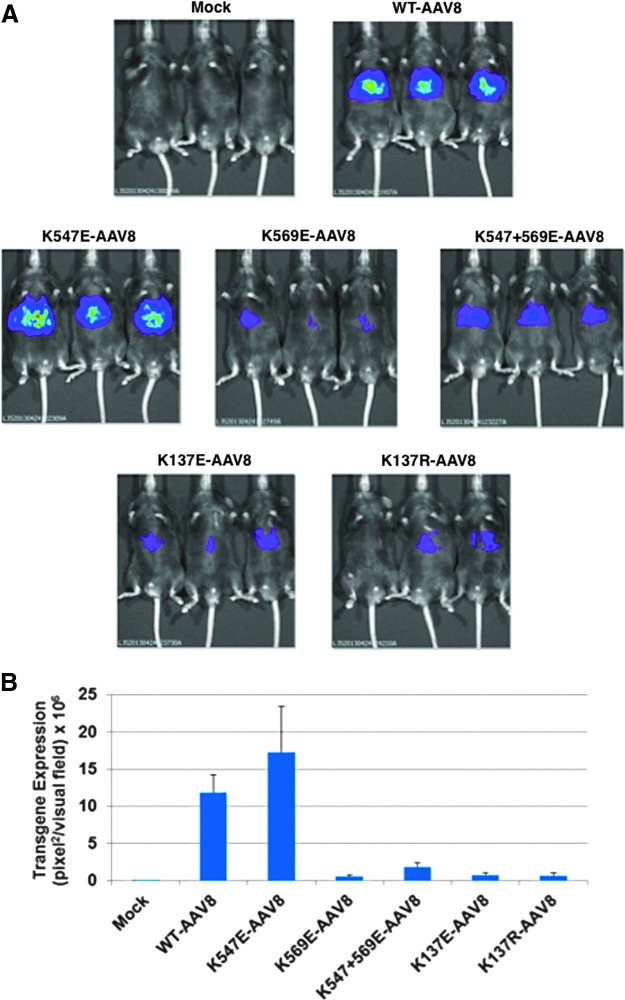
Lysine mutations have little effect on the transduction efficiency of ssAAV8 serotype vectors in murine hepatocytes in vivo
Because AAV8 serotype vectors are known to efficiently transduce primary murine hepatocytes in vivo,17 and have successfully been used in a phase I clinical trial for gene therapy of hemophilia B,7,8 we wished to evaluate whether the transduction efficiency of AAV8 vectors mutated at K residues could be also be further enhanced. The transduction efficiency of the WT and each of the two single mutants (K547E and K569E) and the double-mutant (K547 + 569E) vectors expressing the Fluc reporter gene was evaluated following tail vein injection with 1 × 1010 vgs per mouse (n = 3). As can be seen in Fig. 4A and B, consistent with previously published studies,29,30 the transduction efficiency of the WT ssAAV8 vectors was significantly higher than that of the WT ssAAV2 vectors (Fig. 3A and B). However, the transduction efficiency of the single mutant, K547E, was increased only by ∼1.5-fold, and in fact, the transduction efficiency of the K569E single mutant and the K547 + 569E double-mutant AAV8 vectors was significantly lower than that of the WT AAV8 vectors. These results for the K547E mutant corroborate our previously published studies showing that the effects AAV vector capsid modifications are amino acid specific, serotype specific, and cell and tissue type specific.13,31
Figure 4.
In vivo bioluminescence imaging of luciferase gene expression following tail vein injection of various lysine-mutant ssAAV8 vectors. Mice were injected with 1 × 1010 vgs/animal of the most efficient vectors expressing the firefly luciferase gene, and live images were taken and analyzed as described in the legend to Fig. 3. Maximum is represented in red and minimum is blue (A), and the relative signal intensity is shown in (B). Color images available online at www.liebertpub.com/hgtb
While our current studies were in progress, Jayandharan and colleagues28 reported that several lysine mutations (K527, K532, K544, and K490+K532) also increased the transduction efficiency of scAAV2-EGFP vectors ranging from ∼4-fold to ∼12-fold in primary murine hepatocytes in vivo. Subsequently, the same group also reported that a single lysine mutant (K137R) of scAAV8-EGFP vector increased the transduction efficiency by 11-fold,32 a claim that was not substantiated by a report by Xiao and colleagues.33 In view of these discrepancies, we generated two K137 mutants, K137E and K137R, in which we replaced the lysine (K) residue with glutamic acid (E), and both Jayandharan and colleagues and Xiao and colleagues replaced the K residue with the arginine (R) residue. In a side-by-side comparison, the transduction efficiency of both K137E and K137R mutants was observed to be significantly reduced compared with that of the WT ssAAV8 vectors.
Discussion
It is becoming increasingly clear that the use of recombinant AAV vectors composed of the naturally occurring WT AAV capsids might not be desirable, as AAV did not evolve to be used as a vector for the purposes of gene delivery. Nearly a decade and a half ago, our studies provided the first glimpse of one of the major obstacles that limit the transduction efficiency of AAV vectors, as ∼80% of these vectors failed to traffic to the nucleus.34 Englehardt and colleagues' elegant studies subsequently documented that a large fraction of these vectors became ubiquitinated, which led to their proteasomal-mediated degradation in the cytoplasm.35 In our quest to identify the cellular signal(s) that trigger potential ubiquitination of the AAV capsid, we identified that phosphorylation of specific surface-exposed tyrosine (Y), serine (S), and threonine (T) residues on AAV capsids mediates this process, which led to the creation of the next-generation AAV vectors in which each of these amino acids was mutagenized, and various permutations and combinations of these mutations were shown to significantly augment the transduction efficiency of these vectors, both in vitro and in vivo, presumably by preventing phosphorylation, subsequent potential ubiquitination, and proteasome-mediated degradation.12–14,16
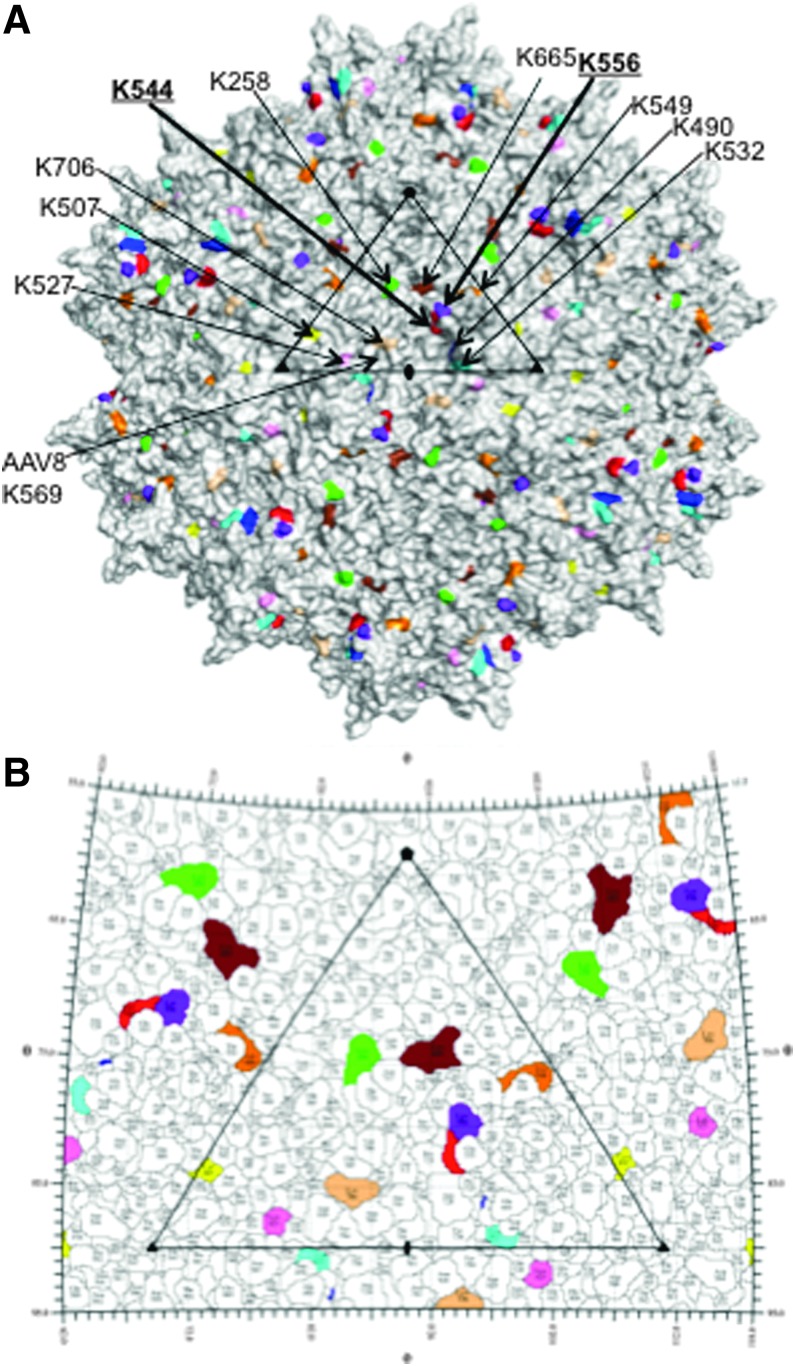
However, because ubiquitination of proteins is known to occur on lysine (K) residues, in our current studies, we mutagenized each of the 10 surface-exposed K residues on the AAV2 capsid, and documented a significant increase in the transduction efficiency of several of these mutant vectors, both in vitro and in vivo. This phenotype is presumably because of the circumvention of potential ubiquitination, followed by proteasome-mediated degradation.12–14,16 Not unexpectedly, the combination of the two most efficient mutations on a single capsid further augmented the transduction efficiency up to ∼10-fold, compared with that of the WT AAV2 vectors, for both single-stranded and self-complementary expression cassettes. Similar, if not identical, results were also reported by Gabriel et al.,28 who used computer software programs to predict the putative ubiquitination sites on the AAV2 capsid, and replaced the K residues with R residues. We, on the other hand, mutagenized only the surface-exposed K residues, which were replaced by E residues. Interestingly, AAV2 residues K544 and K556, the two most efficient mutants, are located adjacent to each other on the capsid surface on the 2/5-fold wall located between the depressions at the 2-fold and surrounding the 5-fold axes (Fig. 5A and B).36 Previous mutations of these two residues to alanine (i.e., K544A and K556A) had resulted in a minor reduction in transduction and a 2-fold increase, respectively, in HepG2 cells.37 These observations thus further point to a role for this capsid region in determining the transduction phenotype of AAV2.
Figure 5.
Capsid surface of AAV2. (A) Exterior capsid surface density image of AAV2 with the 10 surface-exposed lysine residues colored and labeled (K258 in green; K490 in blue; K507 in yellow; K527 in pink; K532 in cyan; K544 in red; K549 in orange; K556 in purple blue; K665 in brown; K706 in wheat). Residues K544 and K556, showing the highest in vivo transduction efficiency, are in bold underline. The viral asymmetric unit (AU) depicted as a triangle is formed by vertexes located at a fivefold axis (filled pentagon) and two threefold axes (filled triangle) with a twofold axis (filled oval) centered between the threefold axes. This image was generated using the PyMOL program.26 (B) Stereographic Roadmap25 of the AAV2 virus capsid showing a zoomed-in image of the viral AU [as described in (A)] viewed down the icosahedral twofold axis. The surface lysines are colored as in (A) with the surrounding residues in white. The black lines indicate the boundaries between the residues. The icosahedral axes are indicated as in Fig. 5A.
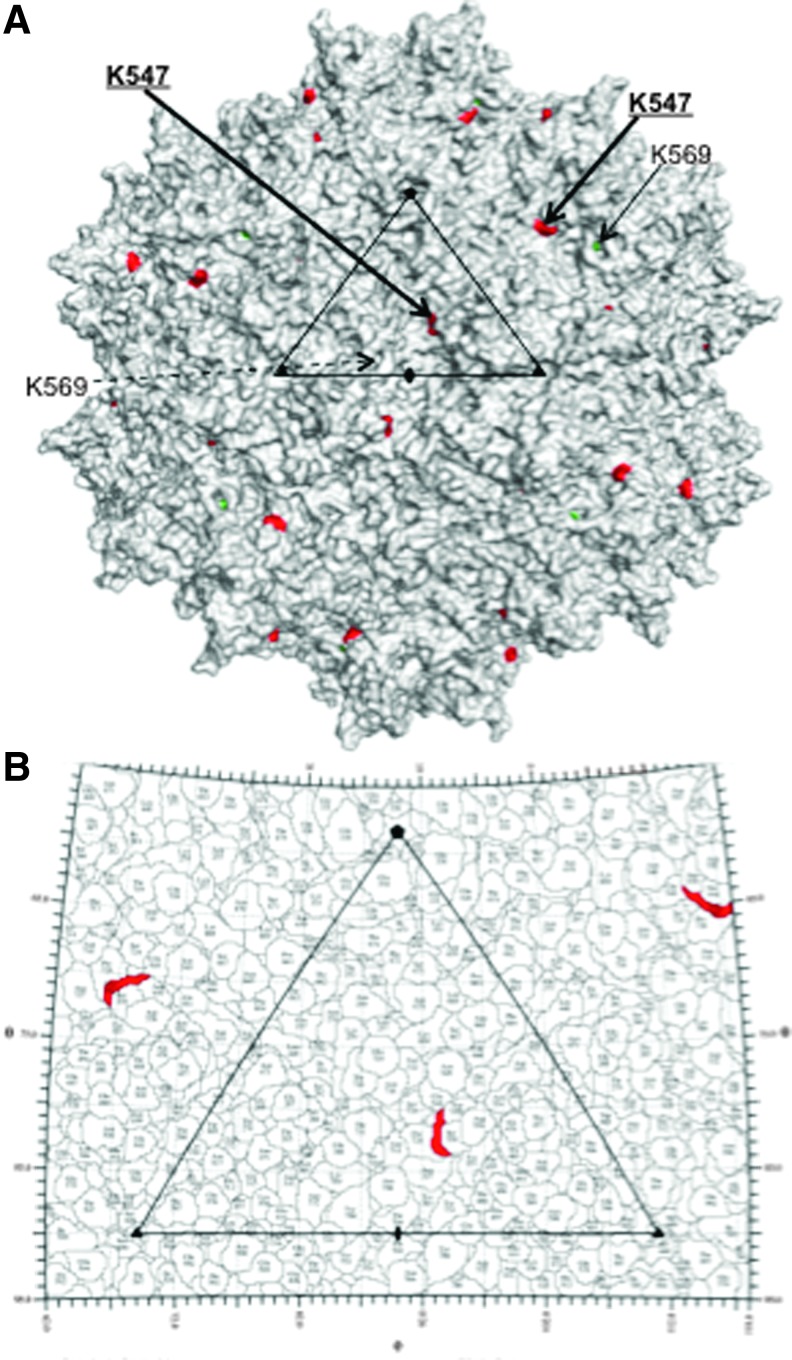
However, when these studies were extended to include a clinically relevant serotype vector, AAV8, mutations in two K residues, K547 and K569, had only a minor effect, even though K547 in AAV8 is spatially equivalent to AAV2 K544 (Figs. 5 and 6). Mutation of K569 reduced the transduction efficiency of these vectors, compared with the WT ssAAV8 vectors in murine hepatocytes in vivo. The minor increase in transduction observed for the K547E mutant compared with the effect in AAV2 may be because of the already high efficiency of WT AAV8 vectors in these cells.17,18 Although the precise underlying mechanism for the decrease in efficiency for the double mutant is not readily apparent, the K569, which is partially buried (Fig. 6), is located in a pH-sensitive region of the AAV8 capsid and is involved in hydrogen bonding interactions with highly conserved acidic residues, E566 and D531 (not shown). Thus, the K569E change may be abrogating pH transitions required for successful infection and negatively impacts the marginal increase in transduction by the K547E mutant in the context of the K547E+K569E double mutant.
Figure 6.
Capsid surface of AAV8. (A) Exterior capsid surface density image of AAV8 with the two lysine residues mutated colored and labeled (K547 [equivalent to AAV2 K544] in red and K569 in dark green). Residue K547 is in bold underline. The partially buried nature of K569 is evident in this image. The AU is depicted as in Fig. 5. This image was generated using the PyMOL program.26 (B) Stereographic Roadmap25 of the AAV8 virus capsid showing a zoomed-in image of the viral AU [as described in (A)] viewed down the icosahedral twofold axis. The surface representation of K547 is shown in red. K569 is not surface seen in this 2D representation of the capsid surface. As in Fig. 5B, the black lines indicate the boundaries between the residues. The icosahedral axes are indicated as in Fig. 5A.
Site-directed mutagenesis of several surface-exposed Y, S, and T residues in AAV8 capsids also did not lead to increased transduction of murine hepatocytes in vivo (unpublished data). This is, perhaps, not surprising because the WT AAV8 vectors have previously been shown to transduce murine hepatocytes extremely efficiently as already stated.17,18 It is of interest to note that, in our previously published studies, the transduction efficiency of the Y-mutant AAV8 vectors was found to be up to 17-fold higher in the rat striatum.38 In this context, it is also noteworthy that, when Finn et al.39 used proteasome inhibitors, they also observed a significant increase in the transduction efficiency of AAV2, but not AAV8 vectors. In our more recent studies, performed in collaboration with several investigators, the transduction efficiency of various Y- and T-mutant AAV8 vectors was indeed shown to be increased in the mouse retina, pancreas, and brain tissues (unpublished results). Thus, further studies are warranted to examine whether the underlying mechanisms of AAV8 vector trafficking, phosphorylation, potential ubiquitination, and vector-mediated transgene expression are different from those of AAV2 in various cells and tissues.
While our current studies were in progress, two independent groups reported conflicting results on K-mutant AAV8 vectors in murine hepatocytes in vivo.32,33 Whereas Jayandharan and colleagues32 reported that a single lysine mutant (K137) of scAAV8-EGFP vector increased the transduction efficiency by 11-fold in hepatocytes in C57BL/6 mice, Xiao and colleagues,33 using the same K137R-AAV8 mutant vectors expressing either the LacZ or the Fluc reporter genes, failed to observe a similar increase in C57BL/6, Balb/C, or ICR mice. These residues are at the exact juncture of VP1u and the VP1/2 common region. It is also a highly conserved residue with differences between the residues in the adjacent sites. Thus, it could be that mutating them affects other functions in addition to potential ubiquitination. Because the basis of the difference between the two groups, both of them having replaced the K residue with an R residue, could not be easily explained, and because in our studies we replaced the K residues with E residues, we evaluated the transduction efficiency of both K137R- and K137E-mutant AAV8 vectors, and observed that the transduction efficiency of both mutant vectors was significantly reduced compared with that of the WT ssAAV8 vectors in C57BL/6 mice.
The fact that three independent groups have obtained three seemingly disparate sets of results using similar, if not identical, AAV vectors, transgene cassettes, and experimental procedures highlights the inherently variable nature of such studies with experimental animals. We also concur with Xiao and colleagues33 and their conclusion that additional variables, such as the sensitivity of the reporter genes, the vector titers, and the vector potency, could also be major factors. These results, nonetheless, underscore the need to exercise caution in relying on, and interpreting data from, experimental rodent models, as well as the need for pursuing additional studies using nonhuman primate models to delineate the role of each of the critical Y, S, T, and K residues to get at the underlying molecular mechanism of the observed differences in the transduction efficiency of these mutant AAV8 vectors in various tissues in general and hepatocytes in particular. Ultimately, however, the value of these capsid modifications toward the development of optimized AAV vectors will only be validated by phase I clinical trials in humans.
Acknowledgments
We thank Drs. R. Jude Samulski and Xiao Xiao for their kind gifts of recombinant AAV plasmids. We also thank Dr. Sergei Zolotukhin for a critical review of this article. This research was supported in part by Public Health Service Grants R01 GM-082946 (to M.A.-M.), and P01 DK-058327 (Project 1), and R01 HL-097088 and R21 EB-015684 from the National Institutes of Health, and Special Award from the Bayer Hemophilia Foundation (to A.S.).
Author Disclosure
No competing financial interests exist.
References
- 1.Berns KI, Linden RM. The cryptic life style of adeno-associated virus. Bioessays 1995;17:237–245 [DOI] [PubMed] [Google Scholar]
- 2.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nat Rev Genet 2011;12:341–355 [DOI] [PubMed] [Google Scholar]
- 3.Mingozzi F, High KA. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013;122:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 5.Mingozzi F, Maus MV, Hui DJ, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med 2007;13:419–422 [DOI] [PubMed] [Google Scholar]
- 6.Pien GC, Basner-Tschakarjan E, Hui DJ, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest 2009;119:1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang LN, Wang Y, Lu Y, et al. Pristimerin enhances recombinant adeno-associated virus vector-mediated transgene expression in human cell lines in vitro and murine hepatocytes in vivo. J Integr Med 2014;12:20–34 [DOI] [PubMed] [Google Scholar]
- 10.Zhong L, Zhao W, Wu J, et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther 2007;15:1323–1330 [DOI] [PubMed] [Google Scholar]
- 11.Zhong L, Li B, Jayandharan G, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology 2008;381:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA 2008;105:7827–7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aslanidi GV, Rivers AE, Ortiz L, et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: The final threshold? PLoS One 2013;8:e59142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aslanidi GV, Rivers AE, Ortiz L, et al. High-efficiency transduction of human monocyte-derived dendritic cells by capsid-modified recombinant AAV2 vectors. Vaccine 2012;30:3908–3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem 2012;81:203–229 [DOI] [PubMed] [Google Scholar]
- 16.Markusic DM, Herzog RW, Aslanidi GV, et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther 2010;18:2048–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malato Y, Naqvi S, Schurmann N, et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J Clin Invest 2011;121:4850–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakai H, Fuess S, Storm TA, et al. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol 2005;79:214–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondal J, Bishayee K, Panigrahi AK, et al. Low doses of ethanolic extract of Boldo (Peumus boldus) can ameliorate toxicity generated by cisplatin in normal liver cells of mice in vivo and in WRL-68 cells in vitro, but not in cancer cells in vivo or in vitro. J Integr Med 2014;12:425–438 [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Joo KI, Wang P. Endocytic processing of adeno-associated virus type 8 vectors for transduction of target cells. Gene Ther 2013;20:308–317 [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Kim YJ, Ji M, et al. Enhancing gene delivery of adeno-associated viruses by cell-permeable peptides. Mol Ther Methods Clin Dev 2014;1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu Y, Lin S, Li JL, et al. Altered LKB1/CREB-regulated transcription co-activator (CRTC) signaling axis promotes esophageal cancer cell migration and invasion. Oncogene 2012;31:469–479 [DOI] [PubMed] [Google Scholar]
- 23.Zhang YH, Wang Y, Yusufali AH, et al. Cytotoxic genes from traditional Chinese medicine inhibit tumor growth both in vitro and in vivo. J Integr Med 2014;12:483–494 [DOI] [PubMed] [Google Scholar]
- 24.Emsley P, Lohkamp B, Scott WG, et al. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 2010;66:486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrillo-Trip M, Shepherd CM, Borelli IA, et al. VIPER2: an enhanced and web API enabled relational database for structural virology. Nucl Acids Res 2009;37:D436–D442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bramucci E, Paiardini A, Bossa F, et al. PyMod: Sequence similarity searches, multiple sequence-structure alignments, and homology modeling within PyMOL. BMC Bioinform 2012;13 Suppl 4:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Q, Bu W, Bhatia S, et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci USA 2002;99:10405–10410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabriel N, Hareendran S, Sen D, et al. Bioengineering of AAV2 capsid at specific serine, threonine, or lysine residues improves its transduction efficiency in vitro and in vivo. Hum Gene Ther Methods 2013;24:80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zincarelli C, Soltys S, Rengo G, et al. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 2008;16:1073–1080 [DOI] [PubMed] [Google Scholar]
- 30.Ling C, Lu Y, Kalsi JK, et al. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum Gene Ther 2010;21:1741–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song L, Kauss MA, Kopin E, et al. Optimizing the transduction efficiency of capsid-modified AAV6 serotype vectors in primary human hematopoietic stem cells in vitro and in a xenograft mouse model in vivo. Cytotherapy 2013;15:986–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sen D, Gadkari RA, Sudha G, et al. Targeted modifications in adeno-associated virus serotype 8 capsid improves its hepatic gene transfer efficiency in vivo. Hum Gene Ther Methods 2013;24:104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao C, Li C, Zhao C, et al. K137R mutation on adeno-associated viral capsids had minimal effect on enhancing gene delivery in vivo. Hum Gene Ther Methods 2014;25:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen J, Qing K, Kwon HJ, et al. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J Virol 2000;74:992–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Z, Zak R, Luxton GW, et al. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol 2002;76:2043–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halder S, Ng R, Agbandje-McKenna M. Parvoviruses: structure and infection. Future Virol 2012;7:253–278 [Google Scholar]
- 37.Lochrie MA, Tatsuno GP, Christie B, et al. Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. J Virol 2006;80:821–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojano-Dirain C, Glushakova LG, Zhong L, et al. An animal model of PDH deficiency using AAV8-siRNA vector-mediated knockdown of pyruvate dehydrogenase E1alpha. Mol Genet Metab 2010;101:183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finn JD, Hui D, Downey HD, et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther 2010;18:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]