Abstract
The rabbit is a useful animal model for regenerative medicine. We previously developed pluripotent rabbit embryonic stem cell (rbESC) lines using fresh embryos. We also successfully cryopreserved rabbit embryos by vitrification. In the present work, we combined these two technologies to derive rbESCs using vitrified–thawed (V/T) embryos. We demonstrate that V/T blastocysts (BLs) can be used to derive pluripotent rbESCs with efficiencies comparable to those using fresh BLs. These ESCs are undistinguishable from the ones derived from fresh embryos. We tested the developmental capacity of rbESCs derived from V/T embryos by BL injection experiments and produced chimeric kits. Our work adds cryopreservation to the toolbox of rabbit stem cell research and applications and will greatly expand the available research materials for regenerative medicine in a clinically relevant animal model.
Introduction
The rabbit is a classical model animal species (Fan and Watanabe, 2003; Fan et al., 2015; Intawicha et al., 2009; Lin et al., 2011). It has a short gestation period (30–31 days) and large litter size (4–12/litter) and can be housed conveniently in an indoor facility. Compared to mice, rabbits are phylogenetically closer to humans (Fan and Watanabe, 2003). Because of the anatomical, physiological, genetic, and biochemical similarities between rabbits and humans, this species is used preferentially in pulmonary, cardiovascular, and metabolic studies (Fan and Watanabe, 2003).
Establishment of embryonic stem cell (ESC) lines from fresh rabbit embryos has been reported previously (Fang et al., 2006; Graves and Moreadith, 1993; Honda et al., 2008; Intawicha et al., 2009; Wang et al., 2007), including our own work (Xue et al., 2012). These rabbit ESC (rbESC) lines were derived from fertilized, parthenogenetically activated, as well as cloned embryos. Most of these cell lines were capable of self-renewal, remained undifferentiated in culture, formed embryoid bodies (EBs) containing all three primary germ layers upon induction, and generated positive teratomas after being transferred to immunocompromised mice. Following injection into blastocysts (BLs) and embryo transfer, some of these rbESCs formed coat color chimeras; unfortunately, none of them colonized into the germ line.
One barrier to the development of competent rbESC lines is the lack of embryos for researchers to perform derivation work. Although mouse and rat facilities are available in almost every major research institute, only a few laboratories have access to fresh rabbit embryos. Establishing a working protocol to use cryopreserved embryos for rbESC derivation would allow the transportation of these embryos to laboratories that do not have access to rabbit facilities so that more researchers could conduct experiments in this field. However, to our knowledge, to date there has been no report on establishing rbESCs using cryopreserved embryos.
Previously, we reported cryopreservation of different stages of rabbit embryos with satisfactory in vitro and in vivo results (Lin et al., 2011). We also established pluripotent rabbit ESCs using fresh embryos (Xue et al., 2012). In the present work, we report the derivation of pluripotent rbESC lines from vitrified–thawed (V/T) BLs.
Materials and Methods
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA), unless otherwise indicated.
Animal maintenance, hormone administration, and embryo collection
All animal maintenance, care, and use procedures were reviewed and approved by the University Committee on the Use and Care of Animals (UCUCA) of the University of Michigan or Nanjing Normal University. Sexually mature (6–18 months old) New Zealand white (NZW) female rabbits were superovulated using our routine regime (Du et al., 2009), consisting of two 3-mg, two 4-mg, and two 5-mg administrations of follicle-stimulating hormone (FSH; Folltropin, Bioniche Animal Health Canada, Belleville, Ontario, Canada) at intervals of 12 h, followed by 200 IU of human choriogonadotropin (hCG; Chorulon, Intervet Inc, Millsboro, DE, USA). Superovulated does were mated with fertile males and served as embryo donors.
Embryo collection and culture was performed as described previously (Lin et al., 2011). Dulbecco's Phosphate-Buffered Saline (DPBS; cat. no. 15240-013, Gibco, Grand Island, NY, USA) containing 0.1% polyvinyl alcohol (PVA; cat. no. P8136) (DPBS-PVP) was used for flushing embryos from the reproductive tract. Medium 199 (M199) with Earle's Salts, l-glutamine, 2.2 grams/liter sodium bicarbonate, and 25 mM HEPES (cat. no. 12340-014, Gibco) supplemented with 10% fetal bovine serum (FBS; cat. no. SH0070.03, Hyclone, Logan, UT, USA) was used as the standard manipulation medium. Zygotes were collected at 18–20 h postinsemination (hpi) from embryo donors and cultured in B2 medium (Laboratories CCD, Paris, France) supplemented with 2.5% FBS at 38.5°C in 5% CO2 and humidified air before they were used.
Vitrification and warming of embryos
Embryos of various stages were subjected to open pulled straw (OPS) vitrification and warming protocols, as we described previously (Lin et al., 2011). Solutions for vitrification were prepared as holding solution (HEPES-buffered DPBS containing 20% FBS and 50 μg/mL gentamicin), OPS-I [HEPES-buffered DPBS supplemented with 16% FBS, 10% ethylene glycol (EG), and 10% dimethyl sulfoxide (DMSO)], and OPS-II (HEPES-buffered DPBS supplemented with 0.6 M sucrose, 8% FBS, 20% EG, and 20% DMSO). Warming solutions were prepared as cryoprotective diluents I and II (CPD-I and CPD-II). CPD-I was prepared by mixing equal volumes of OPS-II and holding solution, and CPD-II was prepared by mixing OPS-I and holding solution in a 1:3 ratio. All solutions were warmed to 38.5°C before use. The OPS straws were either purchased from Minitube (cat. no. 19050/0025, Verona, WI, USA) or were fabricated. For manual pulling, French ministraws (0.25 mL) were heated slightly and hand pulled to a diameter half their original size. Later, three to five embryos were first equilibrated for 5 min in holding solution and then transferred to OPS-I for 2 min. Finally, embryos were washed through three drops of OPS-II for an average of 20 sec in each drop (total time in OPS-II was 1 min). Immediately after embryos were loaded, straws were immersed directly in liquid nitrogen and stored in liquid nitrogen for at least 1 month before being rewarmed.
For warming, the straws containing vitrified embryos were taken out of liquid nitrogen and directly immersed in prewarmed (38.5°C) CPD-I. The embryos were flushed into CPD-I by gently blowing on one end of the straw. One minute later, the embryos were transferred to CPD-II for 5 min and then washed twice in the holding solution for 5 min each. The warmed embryos were cultured in a standard in vitro culture condition as described above until they were used for rbESC derivation.
Rabbit ESC derivation, maintenance, and characterization
ESC derivation and maintenance
We used three culture conditions—two-inhibitor leukemia inhibitory factor (2i-LIF), FL, and iFLY—in the ESC derivation and maintenance experiments. The base medium (BASE) consisted of KnockOut DMEM (cat. no. 10829-018, Invitrogen) supplemented with 20% FBS, 0.1 mM nonessential amino acids, 0.1 mM β-mercaptoethanol (cat no. 444203, Millipore), 4 mM glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin. The 2i-LIF medium was constituted with NDiff N2B27 medium (cat. no. SCS-SF-NB-02Stem Cell Sciences Ltd.) supplemented with 10 ng/mL human LIF (hLIF; cat. no. LIF1010, Millipore), 3 μM CHIR99021 (cat. no. 04-0004, Stemgent), and 1 μM PD0325901 (cat. no. 04-0006, Stemgent). The FL medium was constituted with BASE medium supplemented with 10 ng/mL human recombinant basic fibroblast growth factor (bFGF; cat. no. 13256-029, Invitrogen) and 10 ng/mL hLIF (cat. no. LIF1005, Millipore). The iFLY medium was constituted with BASE medium supplemented with 100 ng/mL Noggin (cat. no. 03-0006, Stemgent), 10 ng/mL bFGF, 10 ng/mL hLIF, and 10 μM Y-27632 (cat. no. 04-0012, Stemgent).
Three groups of hatched BLs (hBLs) were used for ESC derivation: (1) Fresh hBLs; (2) hBLs derived from in vitro–cultured embryos that were V/T at the BL stage; and (3) hBLs derived from in vitro–cultured embryos that were V/T at one to two cells. Before seeding the embryos onto mitomycin C–treated mouse embryonic fibroblasts (MEFs), the zona pellucida (ZP) of each BL was removed mechanically under an inverted microscope. Five to seven days after plating, the cell outgrowths were picked and dissociated into small clumps before reseeding onto the new feeders in one well of a six-well plate. Passaging was performed by incubating the ESC-like colonies with StemPro Accutase (cat. no. A11105-01, Invitrogen) for 3 min followed by plating small cell clumps at the cell density of 1 × 103 cells/cm2 onto new feeders in a six-well culture dish. For cryopreservation, dissociated rbESCs were frozen in cryopreservation medium consisting of 90% FBS and 10% DMSO and stored in liquid nitrogen tanks.
After rbESC lines were established, we tested the effects of the following factors on rbESC maintenance: (1) bFGF; (2) Noggin, an inhibitor to the bone morphogenetic protein (BMP) signaling pathway; (3) 2i medium containing CHIR99021 [a glycogen synthase kinase-3 (GSK-3) inhibitor] and PD0325901 (an MEK inhibitor); and (4) Y27632, a Rho-associated coiled-coil–forming protein serine/threonine kinase (ROCK) inhibitor for prevention of apoptosis.
Alkaline phosphatase staining
The rbESC cultures were rinsed with DPBS prior to fixation in 4% paraformaldehyde (cat. no.P6148) solution. Five minutes after fixation at room temperature (RT), rbESCs were rinsed three times with DPBS and then stained with alkaline phosphatase (AP) solution for 30 min, as previously described (Intawicha et al., 2009). The AP staining buffer (100 mM Tris-HCl, 100 mM NaCl, 50 mM MgCl2, pH 9.5) consisted of 0.25 M Trizma maleate (cat. no. T3128), 0.008 M MgCl2 (cat. no. M-8266), 0.17 g/L Fast-Red TR salt (cat. no. F8764), and 0.4 grams/liter α-naphthyl phosphate (N7255). The stained cells were washed with DPBS and observed under an inverted microscope.
Immunocytochemical staining for ESC-specific markers
Immunostaining was performed to characterize ESC-specific markers, including SSEA4, Oct4, Nanog, and Sox2. Cells were either stained directly after DPBS washing (for cell-surface antigen) or fixed at room temperature with 4% paraformaldehyde for 10 min and permeated with 0.2% Triton X-100 (cat. no. T8787) and 0.1% Tween 20 (cat. no. P2287) in phosphate-buffered saline (PBS) for at least 10 min after washing with DPBS. Cells were then incubated with 2% bovine serum albumin (BSA; cat. no. A9647) in DPBS with 0.05% Tween 20 (DPBST) for 30 min to block nonspecific binding, followed by a primary antibody (anti-Sox2, MAB 2018, R & D Systems; anti-SSEA4, Chemicon MAB 4304, Millipore; anti-Oct4, Chemicon MAB 4401, Millipore; anti-Nanog, ab21603, Abcam, Cambridge, MA; anti-Gata4, sc25310, Santa Cruz Biotechnology, Dallas, TX; anti-βIII-Tubulin, T8578) for 1 h. All antibodies were diluted in blocking solution (1:200, Oct4; 1:200, SSEA4; 1:100, Nanog; 1:50, Sox2; 1:100, Gata4; 1:500, anti-βIII-Tubulin) and incubated with samples at 4°C overnight after an additional three washes (15 min/wash) with DPBST.
After incubation with the primary antibody, the rbESCs were washed again three times with DPBST and then incubated with the secondary antibodies [Sox2, fluorescein isothiocyanate (FITC)-labeled donkey anti-mouse immunoglobulin G (IgG), 1:500, A21202, Invitrogen; SSEA4, Alexa Fluor 594 goat anti-mouse IgG (H+L), 1:500, A11005, Invitrogen; Oct4, FITC-labeled donkey anti–mouse IgG, 1:400, A21202Invitrogen; Nanog, FITC-conjugated goat anti-rabbit IgG, F98871:300; Gata4, FITC-labeled donkey anti-mouse IgG, A21202, 1:500, Invitrogen; anti-βIII-Tubulin, Alexa Fluor 594 goat anti-mouse IgG (H+L), 1:500, A11005, Invitrogen) for 60 min. Finally, the cells were mounted with Vectashield Mounting Medium (cat. no. H1500, Vector Laboratories, Inc., Burlingame, Canada) for nuclear staining of 4′,6-diamidino-2-phenylindole (DAPI) fluorescence.
Karyotyping
We performed karyotyping on two representative cell lines—F6-4 (derived from a fresh BL) and V7-1 (derived from a V/T BL)—under the iFLY condition. For each line, we performed two experimental replicates. The cells were incubated in growth medium supplemented with 100 ng/mL colcemid (KaryoMax Colcemid Solution, cat. no. 15212-012, Invitrogen,) for 2–3 h at 38.5°C in 5% CO2. Then cells were trypsinized and pelleted at 1500 rpm for 5 min, resuspended in 6 mL of 75 mM KCl, and held in a 37°C water bath for 10 min. The cells were centrifuged and fixed in acetic acid/methanol (vol/vol 1:3) for 10 min. The centrifugation and fixing steps were repeated three times. For slide preparation, the cell pellet was resuspended in 0.5 mL of acid/methanol solution, and one drop of cell suspension was dropped onto a prechilled clean microscope slide. The chromosomes were stained with 5% Giemsa (cat. no. 10092013, Invitrogen) for 15 min. The chromosomes were examined at 1000× magnification under oil.
In vitro differentiation of rbESCs
The putative rbESC lines were cultured beyond passage 10 before induction of differentiation. The rbESCs were digested with StemPro Accutase (Invitrogen) for 3 min at 37°C and plated on an extra-low-cluster plate (Corning Inc., NY, USA) for 5–7 days to induce formation of EBs. Expression of the cell markers for the three germ layers in EBs was assessed by RT-PCR.
RT-PCR
Reverse transcription (RT)-PCR was used to detect the expression of Oct4, Sox2, Nanog, c-Myc, Klf4, and Dppa5 for rbESCs and Gata4, Desmin, Brachyury, Pax6, and Nestin for derived EBs. GAPDH was used as a control of each RT-PCR. The primers are shown in Table 1.
Table 1.
Primer Sequences for RT-PCR Analysis
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) | Product size (bp) |
|---|---|---|---|
| Oct4 | CCTTCGCAGGGGGGCCTA | CATGGGGGAGCCCAGAGCA | 673 |
| Sox2 | CCGCTACGACGTAGCGCG | CGAGCCCATGGAGCCGAGC | 133 |
| Nanog | CCCAGCTGTGTGTGTGCTCAA | CCAGGCTTGGGAGTACCAGG | 382 |
| c-Myc | GACGCGTCCTTCTCCCCCA | CTCTGGTTACCATGTCACCG | 112 |
| Klf4 | CCACAGACCTGGAAAGTGGT | GGAAGACGAGGATGAAGCTG | 187 |
| Dppa5 | GACCTGAAAGATCCCGAGGT | GTAGGAGCCGTAAACCACCA | 165 |
| Desmin | AGCAGGAGATGATGGAATAC | TCCAGCTTCCGGTAGG | 281 |
| Gata4 | CTCAGAAGGCAGAGAGTGTG | CCGCATTGCAAGAGGCCTGG | 321 |
| Nestin | AGGGGGAAGAGGAAGAGGAGGAGA | TGCTGCAGCCCGTTCACCACA | 394 |
| Brachyury | GCTTCCCCGAGACGCAGTTCAT | TGTCGGGGTAGGTTGGAGTGTTT | 360 |
| Fgf5 | TGCAAGTTCAGGGAGCGATT | CCTCGTTTGTTCAAGGCCAC | 110 |
| Rex1 | CGGCATTCCAAGGCGTTC | CCACCCTCCTTTCTCACGAC | 227 |
| Pax6 | GTCTACCAACCAATCCCGCA | TGTGAGGGCTGTGTCTGTTC | 87 |
| GAPDH | GGAGCCAAACGGGTCATCATCC | GAGGGGCCATCCACAGTCTTCT | 233 |
Rabbit ESCs and EBs derived from ESCs were collected and subjected to RNA isolation immediately or stored at −80°C for a few days and then subjected to RNA isolation. Total RNA was extracted with the Qiagen RNeasy Mini Kit (Qiagen, CA, USA). The RNA preparations were treated with RNase-free DNase I during preparation to remove possible contaminating DNA. The isolated RNA was eluted with RNase-free water and used for RT-PCR immediately.
Reverse transcription was carried out using 2.5 units of reverse transcriptase in the presence of 1 unit of RNase inhibitor and 5 μM oligo(dT) or random hexamers with approximately 0.5 μg of total RNA in a final volume of 20 μL. The solution was incubated at 42°C for 15 min to generate single-stranded complemetary DNA (cDNA). Four microliters of the reverse transcription products from rbESCs or EBs were used in the subsequent PCR to amplify transcripts of six pluripotent marker genes for ESCs (Oct4, Sox2, Nanog, c-Myc, Klf4, and Dppa5) or five germ layer marker genes for EBs (Desmin, Gata4, Nestin, Pax6, and Brachyury). The glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH) was amplified by RT-PCR in all RNA samples as an internal positive control. The PCR reaction was carried out in a total volume of 20 μL containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 0.2 mM deoxynucleotides (dNTPs), 0.2 mM oligonucleotide primers, and 0.4 unit Taq DNA polymerase (Invitrogen). The PCR was initiated at 95°C for 2 min, followed by 35 cycles of 30 sec at 95°C, 30 sec at 57°C, and 30 sec at 72°C. All RT-PCR reactions including the appropriate internal positive and negative controls were repeated twice. A volume of 10 μL of PCR products was analyzed on a 1.5% agarose gel. All PCR reactions were performed in a Hybaid Px2 Thermal Cycler.
Generation of chimeric rabbits
The rbESC line V7-1 derived from the iFLY condition using V/T BLs and line FM-5 derived from the FL condition using fresh embryos were transduced with green fluorescent protein (GFP) by the FuGENE system using a commercial kit (cat. no. 04-709-705-001, Roche). Mechanical colony pick-up was performed to purify and isolate the GFP-positive cells. For BL injection experiment, we used the ESCs from the colonies with a high GFP signal. BLs derived from wild type (WT) rabbits were used as host embryos. For each host embryo, five to 10 GFP-positive rbESCs were injected. After injection, embryos were transferred to synchronized recipient females (10–20/recipient).
Statistical analysis
Cell number was presented as means ± standard error of the means (SEM). Means were compared using the general linear model (SPSS 11.0, Chicago, IL, USA). Statistical significance was considered when a p value was less than 0.05.
Results
bFGF is indispensable for the derivation and maintenance of rbESC lines
Previously, we had developed several rbESC lines using fresh embryos in a FL culture system containing bFGF and LIF (Xue et al., 2012). For easy identification, in this article, a cell line whose name begins with F is derived from fresh embryos, whereas one beginning with V is derived from V/T embryos.
We confirmed that bFGF is an indispensable factor for the derivation and maintenance of our rbESC lines. We were able to achieve 16.7% rbESC derivation efficiency from seeded BLs with bFGF (10 ng/mL) supplementation, in contrast to the total failure when bFGF was not used (Fig. 1A). Furthermore, withdrawal of bFGF (10 ng/mL) in the culture medium of rbESCs established under the FL condition led to immediate differentiation of the putative rbESCs in line FM5 (Fig. 1B) and all other lines that were tested, indicating the LIF alone is not sufficient to maintain rbESC cultures. These results show that bFGF is indispensable for the derivation and maintenance of rbESC lines.
FIG. 1.
Optimization of culture system for rbESCs. (A) Effects of bFGF on the derivation efficiency of rbESCs. (B) Effects of bFGF on the maintenance of rbESCs that are derived using the FL system. (Left) rbESC line FM5 cultured with bFGF supplementation. (Right) rbESC line FM5 upon withdrawal of bFGF in the culture medium. (C) 2i-LIF medium failed to derive putative rbESC lines. (Left) seeded blastocysts; (middle) day 2 (D2) after seeding; (right) D4 after seeding. (D) Dosage curve of Y-27632 on the growth of rbESC line FM5. (E) Effects of Noggin on rbESC line FM5. (F) RT-PCR of typical pluripotent marker genes on line FM5 two passages after switching to the iFLY culture conditions versus those remaining in the FL conditions. (G) Effects of iFLY system on the growth of rbESC line FM5. Scale bars, 200 μm. a,b,c,d Different letters in D or in the same column of G indicate statistical difference (p < 0.05).
Effects of the murine 2i-LIF system on the derivation of rbESC lines
We examined the effects of the 2i-LIF system containing CHIR99021 (a GSK-3 inhibitor), PD0325901 (an MEK inhibitor), and LIF, which was proven to be effective in the derivation and maintenance of authentic mouse and rat pluripotent stem cells (PSCs) (Buehr et al., 2008; Nichols et al., 2009; Ying et al., 2008). The rabbit BLs were seeded onto MEF feeder cells (Fig. 1C, left) in the medium supplemented with 2i and LIF. Although domed structure was observed on days 2 (D2) after seeding (Fig. 1C, middle), which indicates capturing of putative ground state cell populations, cell death soon started and became severe on D4 (Fig. 1C, right). No rbESC lines were derived from the murine 2i-LIF system, suggesting that this system is not universally effective in mammalian species.
Y-27632 improves the growth of rbESCs
Y-27632 is a potent inhibitor of ROCK. Supplementation of Y-27632 enhances survival of hESCs by preventing dissociation-induced apoptosis, thus increasing their cloning efficiency (Watanabe et al., 2007). We also observed compromised survival when rbESCs were dissociated to single cells. We applied Y-27632 at serial concentrations (0, 5, 10, 20, 40, and 100 μM) on cell line FM5 to determine its optimal concentration on rbES cells (Fig. 1D). Supplementation of Y-27632 at 10–20 μM showed obvious beneficial effects on growth (Fig. 1D). Y-27632 at 100 μM appears to impact the cell growth negatively, likely due to its toxic effects at high concentration. The optimal dosage was thus determined at 10 μM.
Effects of Noggin on the maintenance of rbESC lines
Noggin is an effective inhibitor to the BMP signaling pathway. Early work showed that Noggin supplementation at 100 ng/mL inhibited the differentiation of rbESCs (Wang et al., 2008). We also tested the effects of Noggin on the rbESC line FM5 that was originally derived in the FL system using fresh embryos. Consistent with a previous report (Wang et al., 2008), adding Noggin to the FL system appeared to improve cell growth and the number of Oct4-positive populations (Fig. 1E).
Derivation of rbESC lines from V/T embryos in the iFLY system
On the basis of these findings, we added Noggin and Y-27632 to the FL system and constructed a new system, called iFLY, reflecting its composition of an inhibitor to the BMP pathway (Noggin), bFGF, LIF, and Y-27632. We first tested the effects of iFLY on the rbESC line FM5, which was originally derived using fresh embryos in the FL system. The iFLY culture system dramatically promoted proliferation of rbESCs (Fig. 1G). RT-PCR of typical pluripotent marker genes of line FM5 cells in the FL system versus the iFLY system after two passages (Fig. 1F) showed that the iFLY system enhanced the expression of Oct4, Sox2, Nanog, c-Myc, Klf4, Dappa5, and Rex1. The expression of Fgf5 is also higher under the iFLY system than in the original FL system.
We next applied the iFLY system to derive new rbESC lines. To expand the available materials for rbESC research and applications, we used both fresh and V/T embryos. Using our well-established embryo cryopreservation (Lin et al., 2011) and rbESC derivation protocols (Xue et al., 2012) (Fig. 2A), we derived a total of 13 rbESC lines from V/T embryos and five from fresh BLs (Fig. 2B). Satisfactory derivation efficiency (calculated as the ratio of number of AP+/Oct+ lines over total starting number of embryos) was achieved when embryos were V/T at the BL stage (12.5%, n = 80), comparable to those of fresh BLs (10.2%, n = 49) in the present experiment and in previous studies ranging from 9.7% to 12.1% (Honda et al., 2008; Xue et al., 2012), and higher than those V/T at one- to two-cell stages (1.5%, n = 200). This is consistent with our previous report that embryos of eight-cell stage or beyond have high cryotolerance, whereas embryos prior to the eight-cell stage have poor survival rates after V/T (Lin et al., 2011).
FIG. 2.
Derivation and characterization of rbESC lines derived and maintained in the iFLY system. (A) V/T blastocysts were used for rbESC derivation. (B) Derivation efficiency using fresh or V/T embryos at different stages. Overall derivation efficiency was calculated as the ratio of number of AP+/Oct4+ lines over total starting number of embryos. (C) Immunostaining of rbESC line F6-4 (derived from fresh BL) and V7-1 (derived from vitrified-thawed BL). (D) RT-PCR results on rbESC lines F6-4 and V7-1. (E) Karyotyping results of rbESC lines F6-4 and V7-1. (F) EB formation of rbESC line V7-1. (G) Differentiation of V7-1 rbESCs into endoderm-derived cells (Gata4) and ectoderm-derived neuron cells (βIII-Tubulin) after implantation of EBs on culture plate. (H) V7-1–derived EBs expressed all three primary germ layer markers (Gata4 for endoderm, Desmin and Brachyury for mesoderm, Nestin and Pax6 for ectoderm), but not the pluripotency genes Oct4, Sox2, or Nanog on D5. GAPDH was used as the internal control. Scale bars, 100 μm.
We performed experiments to characterize the rbESC line F6-4 (derived from fresh BLs) and V7-1 (derived from V/T BLs), both derived under the iFLY condition. Both lines displayed typical ESC surface markers (SSEA4, Oct4, Nanog, Sox2; Fig. 2C), expressed pluripotent genes (Oct4, Sox2, Nanog, c-Myc, Klf4, Rex1, and Dppa5, Fig. 2D), and possessed normal ploidy (Fig. 2E).
We next investigated the in vitro differentiation capacity of line V7-1. Line V7-1 formed EBs in the presence of serum in suspension culture (Fig. 2F). RT-PCR results showed that these EBs expressed the marker genes for all three germ layers (Gata4 for endoderm, Desmin and Brachyury for mesoderm, Nestin and Pax6 for ectoderm), but not the pluripotent genes (i.e., Oct4, Sox2, and Nanog) (Fig. 2H). Immunostaining confirmed that differentiated V7-1 cells have formed endoderm-derived cells (Gata4) and ectoderm-derived neuron cells (βIII-Tubulin) (Fig. 2G).
Production of chimeric rabbits
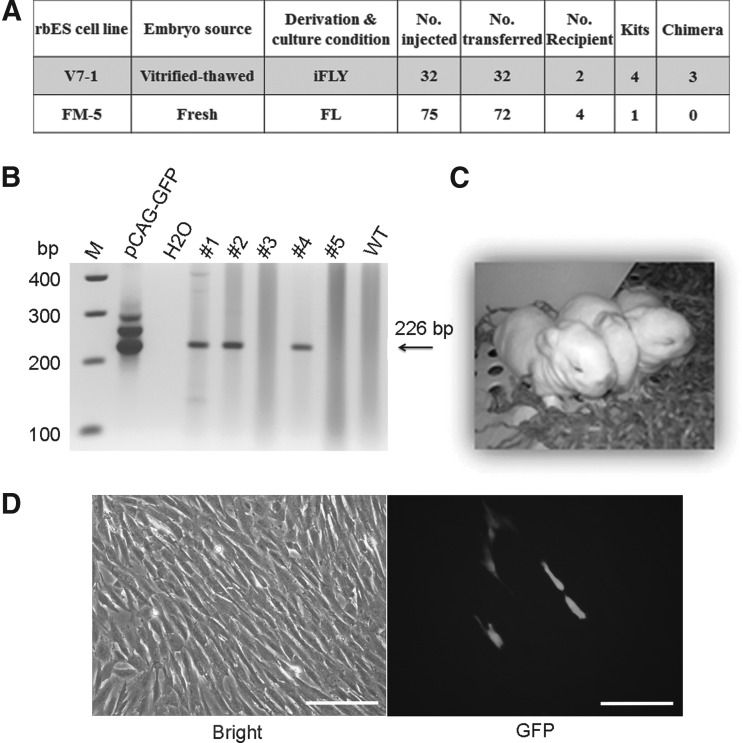
To evaluate the in vivo developmental capacity of the rbESCs, we performed an ESC injection experiment. A total of 32 embryos were injected with cells from line V7-1 (derived from the iFLY condition using V/T BLs); 75 embryos were injected with cells from line FM-5 (derived from the FL condition using fresh embryos) (Fig. 3A). After injection, embryos were transferred to synchronized recipient females. Newborn kits were sampled and genotyped by PCR assays. Only one kit (no. 5) was born from the FM-5 group, which tested negative for chimerism by PCR (Fig. 3B). In the V7-1 group, four kits were born and three survived (Fig. 3C). Three of the four term kits (nos. 1, 2, and 4) were identified as chimeric animals by PCR, containing GFP sequences in the genome (Fig. 3B). Culture of their ear skin cells confirmed the existence of GFP-positive cells in one live animal (no. 2), albeit at a very low percentage (Fig. 3D). Likely due to such low frequency, neither of these two live animals was proven to transmit the GFP-positive cells to their germ cells after two rounds of breeding.
FIG. 3.
Production of chimeric rabbits using rbESCs derived and maintained in the iFLY system. (A) Blastocyst injection results. (B) Genotyping gel showing that three kits (#1, 2, and 4) contain the GFP transgene in their genome. (Arrow) The expected band size at 226 bp. M, molecular marker; pCAG-GFP, GFP plasmid used to transduce rbESCs; #1–5, kit numbering; WT, wild-type control animals. (C) Three live kits produced from the embryos injected with cells from line V7-1. (D) Ear skin cell culture (left) of one animal (kit #2). Signals (right) indicate presence of GFP-positive cells, confirming the chimeric status of this animal. Scale bars, 100 μm.
Discussion
In the present work, we showed that V/T BLs can be used for rbESC derivation at efficiencies comparable to those using fresh BLs, consistent with our previous work that V/T BLs supported similar term development rates to those of fresh ones (Lin et al., 2011), confirming that rabbit BLs are tolerant to vitrification and thawing. We speculate that the major reason is the relatively large number of cells at the BL stage. Obviously, damage to one blastomere is much more detrimental to a pronuclear or two-celled embryo than to a BL. The high surface-to-volume ratio in the BLs (vs. that in the one- to two cell–stage embryos) also likely contributes to the high success rates of V/T at this embryonic stage. Importantly, rbESCs derived from V/T embryos are indistinguishable from those derived from fresh embryos. Hence, our work verifies that vitrification can be readily added to rbESC derivation protocols, enhancing the chance of deriving germ line–transmitting rbESCs by expanding the available research materials. It is noted, however, that the derivation efficiency dropped dramatically when V/T one- to two-cell stage embryos were used because few reached the BL stage due to the compromised cryotolerance of these early-stage embryos.
A limited number of groups have worked to derive rabbit PSCs (Fang et al., 2006; Graves and Moreadith, 1993; Honda et al., 2008; Honda et al., 2013; Intawicha et al., 2009; Jiang et al., 2014; Osteil et al., 2013; Tancos et al., 2012; Teramura et al., 2013; Wang et al., 2007; Wang et al., 2008; Xue et al., 2012). Capturing the ground state (interchangeably referred to as “authentic,” “naïve,” and “germ line transmitting” in this article) of rbESCs has been a challenge. Previously, we reported the establishment of pluripotent rabbit rbESC lines using the FL system (Xue et al., 2012). These cells, as with all other rabbit ESCs and induced pluripotent stem cells (iPSCs) reported to date, share typical properties of primed stem cells, and all failed to transmit to the germ cells (Honda et al., 2013; Jiang et al., 2014; Osteil et al., 2013; Tancos et al., 2012; Wang et al., 2008). Recently, rabbit PSCs with naïve-like domed morphology were reported, yet none of them had proven germ line transmission (Honda et al., 2013; Jiang et al., 2014; Osteil et al., 2013).
On the other hand, authentic mouse and rat ESCs and iPSCs, which are characterized by their dependency on leukemia inhibitory factor/signal transducer and activator of transcription 3 (LIF/STAT3) signaling pathway and independency on FGFs for maintenance of pluripotency, can be derived efficiently and maintained in 2i/3i-LIF conditions containing LIF and inhibitors to GSK3, MEK, and/or FGF pathways, readily contribute to the germ line cells (Blair et al., 2011; Buehr et al., 2008; Leitch et al., 2010; Nichols et al., 2009; Ying et al., 2008). To date, the mouse and rat remain the only mammalian species in which germ line–transmitting PSCs are available, despite the large amount of work by many groups racing to derive naïve PSCs in a number of nonrodent species, raising the question of whether mESC-like naïve PSCs exist in nonrodent species.
Many, including us, have tested the 2i/3i-LIF system in nonrodent species, hoping to repeat the success of the development of authentic rat ESCs. Although we were not successful in deriving any rbESC lines using this system, we note that promising dome-shaped structures appeared transiently on D2 after seeding using the 2i-LIF medium (Fig. 1C). This suggests that 2i-LIF may be used to capture the initial ground state at this time point. Future work is needed to optimize culture conditions for sustaining the ground state.
Unexpectedly, it was shown recently that deriving and maintaining naïve human and monkey PSCs require bFGF (Fang et al., 2014; Gafni et al., 2013; Theunissen et al., 2014), which for a long time has been considered as a marker for primed PSCs on the basis of the knowledge gained from mouse and rats. Here, we have demonstrated that bFGF is also indispensable for the derivation as well as the maintenance of rbESCs. Without bFGF supplementation, no putative rbESCs were derived. Withdrawal of bFGF from the culture medium caused immediate differentiation of the rbESCs. Consistently, rabbit PSCs with domed-shaped morphology were derived and maintained using medium supplemented with bFGF (Honda et al., 2013; Jiang et al., 2014; Osteil et al., 2013). This evidence shows similar bFGF requirements of human and rabbit PSCs, suggesting that rabbits may represent a good animal model for human embryology and regenerative medicine. In support of this notion, it has been shown that, compared to mouse embryos, rabbit embryos have a more similar pattern of preimplantational development and lineage formation to that of human embryos. For example, in human and rabbit embryos, Oct4 expression was present in both the inner cell mass (ICM) and the trophectoderm (TE) cells (Berg et al., 2011; Chen et al., 2012a; Chen et al., 2012b; Hansis et al., 2000; Kirchhof et al., 2000), whereas in mice, Oct4 is expressed restrictively in the ICM, but not in the TE (Dietrich and Hiiragi, 2007; Ovitt and Scholer, 1998; Palmieri et al., 1994; Pesce et al., 1998; Yeom et al., 1996).
Inhibitors to multiple signaling pathways, other than MEK and GSK3β, have been explored in nonrodent species. Recent work found that inhibitors to c-Jun N-terminal protein kinase (JNK), P38, protein kinase C (PKC), ROCK, BMP, B-Raf proto-oncogene serine/threonine kinase (BRAF), and SRC are beneficial for naïve human PSCs (Gafni et al., 2013; Theunissen et al., 2014). In the present work, we have demonstrated that inhibitors to the BMP pathway (Noggin) and apoptosis (Y-27632) had beneficial effects on the maintenance of rbESC lines.
In sum, we have demonstrated that pluripotent rbESCs can be established efficiently from both fresh and V/T embryos. Chimeric offspring were generated using rbESCs derived from V/T embryos. Supplementation of bFGF, Y-27632, and Noggin are beneficial for the maintenance of rbESCs. Our work adds a convenient tool to the animal stem cell research field and sheds light on deriving germ line–competent ESCs in an important, yet understudied, model animal species.
Acknowledgments
This study was supported by the National Institutes of Health (grant no. R44RR023774 to J.X. and F.D.), Premium Science and Technology Foundation of Jiangsu Province of China, the National Natural Science Foundation of China (grant no. 31340041 and 31471388 to F.D.), and Major Program of Natural Science Research of Jiangsu Higher Education Institutions of China (grant no. 14KJA180003 to F.D.).
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Berg D.K., Smith C.S., Pearton D.J., Wells D.N., Broadhurst R., Donnison M., and Pfeffer P.L. (2011). Trophectoderm lineage determination in cattle. Dev. Cell 20, 244–255 [DOI] [PubMed] [Google Scholar]
- Blair K., Wray J., and Smith A. (2011). The liberation of embryonic stem cells. PLoS Genet. 7, e1002019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehr M., Meek S., Blair K., Yang J., Ure J., Silva J., McLay R., Hall J., Ying Q.L., and Smith A. (2008). Capture of authentic embryonic stem cells from rat blastocysts. Cell 135, 1287–1298 [DOI] [PubMed] [Google Scholar]
- Chen C.-H., Chang W.-F., Liu C.-C., Su H.-Y., Shyue S.-K., Cheng W.T.K., Chen Y.E., Wu S.-C., Du F., Sung L.-Y., and Zu J. (2012a). Spatial and temporal distribution of Oct-4 expression and H4K5 acetylation in rabbit embryos. Reprod. Biomed. Online, doi: 10.1016/j.rbmo.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.H., Xu J., Chang W.F., Liu C.C., Su H.Y., Eugene Chen Y., Du F., and Sung L.Y. (2012b). Dynamic profiles of Oct-4, Cdx-2 and acetylated H4K5 in in-vivo-derived rabbit embryos. Reprod. Biomed. Online 25, 358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J.E., and Hiiragi T. (2007). Stochastic patterning in the mouse pre-implantation embryo. Development 134, 4219–4231 [DOI] [PubMed] [Google Scholar]
- Du F., Xu J., Zhang J., Gao S., Carter M.G., He C., Sung L.Y., Chaubal S., Fissore R.A., Tian X.C., Yang X., and Chen Y.E. (2009). Beneficial effect of young oocytes for rabbit somatic cell nuclear transfer. Cloning Stem Cells 11, 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., and Watanabe T. (2003). Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol. Ther. 99, 261–282 [DOI] [PubMed] [Google Scholar]
- Fan J., Kitajima S., Watanabe T., Xu J., Zhang J., Liu E., and Chen Y.E. (2015). Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 146, 104–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R., Liu K., Zhao Y., Li H., Zhu D., Du Y., Xiang C., Li X., Liu H., Miao Z. Z.hang X., Shi Y., Yang W., Xu J., and Deng H. (2014). Generation of naive induced pluripotent stem cells from rhesus monkey fibroblasts. Cell Stem Cell 15, 488–496 [DOI] [PubMed] [Google Scholar]
- Fang Z.F., Gai H., Huang Y.Z., Li S.G., Chen X.J., Shi J.J., Wu L., Liu A., Xu P., and Sheng H.Z. (2006). Rabbit embryonic stem cell lines derived from fertilized, parthenogenetic or somatic cell nuclear transfer embryos. Exp. Cell Res. 312, 3669–3682 [DOI] [PubMed] [Google Scholar]
- Gafni O., Weinberger L., Mansour A.A., Manor Y.S., Chomsky E., Ben-Yosef D., Kalma Y., Viukov S., Maza I., Zviran A., Rais Y., Shipony Z., Mukamel Z., Krupalnik V., Zerbib M., Geula S., Caspi I., Schneir D., Shwartz T., Gilad S., Amann-Zalcenstein D., Benjamin S., Amit I., Tanay A., Massarwa R., Novershtern N., and Hanna J.H. (2013). Derivation of novel human ground state naive pluripotent stem cells. Nature 504, 282–286 [DOI] [PubMed] [Google Scholar]
- Graves K.H., and Moreadith R.W. (1993). Derivation and characterization of putative pluripotential embryonic stem cells from preimplantation rabbit embryos. Mol. Reprod. Dev. 36, 424–433 [DOI] [PubMed] [Google Scholar]
- Hansis C., Grifo J.A., and Krey L.C. (2000). Oct-4 expression in inner cell mass and trophectoderm of human blastocysts. Mol. Hum. Reprod. 6, 999–1004 [DOI] [PubMed] [Google Scholar]
- Honda A., Hirose M., Inoue K., Ogonuki N., Miki H., Shimozawa N., Hatori M., Shimizu N., Murata T., Hirose M., Katayama K., Wakisaka N., Miyoshi H., Yokoyama K.K., Sankai T., and Ogura A. (2008). Stable embryonic stem cell lines in rabbits: Potential small animal models for human research. Reprod. Biomed. Online 17, 706–715 [DOI] [PubMed] [Google Scholar]
- Honda A., Hatori M., Hirose M., Honda C., Izu H., Inoue K., Hirasawa R., Matoba S., Togayachi S., Miyoshi H., and Ogura A. (2013). Naive-like conversion overcomes the limited differentiation capacity of induced pluripotent stem cells. J. Biol. Chem. 288, 26157–26166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intawicha P., Ou Y.W., Lo N.W., Zhang S.C., Chen Y.Z., Lin T.A., Su H.L., Guu H.F., Chen M.J., Lee K.H., Chiu Y.T., and Ju J.C. (2009). Characterization of embryonic stem cell lines derived from New Zealand white rabbit embryos. Cloning Stem Cells 11, 27–38 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Kou Z., Wu T., An W., Zhou R., Wang H., Gao Y., and Gao S. (2014). Xist deficiency and disorders of x-inactivation in rabbit embryonic stem cells can be rescued by transcription-factor-mediated conversion. Stem Cells Dev. 23, 2283–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof N., Carnwath J.W., Lemme E., Anastassiadis K., Scholer H., and Niemann H. (2000). Expression pattern of Oct-4 in preimplantation embryos of different species. Biol. Reprod. 63, 1698–1705 [DOI] [PubMed] [Google Scholar]
- Leitch H.G., Blair K., Mansfield W., Ayetey H., Humphreys P., Nichols J., Surani M.A., and Smith A. (2010). Embryonic germ cells from mice and rats exhibit properties consistent with a generic pluripotent ground state. Development 137, 2279–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.A., Chen C.H., Sung L.Y., Carter M.G., Chen Y.E., Du F., Ju J.C., and Xu J. (2011). Open-pulled straw vitrification differentiates cryotolerance of in vitro cultured rabbit embryos at the eight-cell stage. Theriogenology 75, 760–768 [DOI] [PubMed] [Google Scholar]
- Nichols J., Jones K., Phillips J.M., Newland S.A., Roode M., Mansfield W., Smith A., and Cooke A. (2009). Validated germline-competent embryonic stem cell lines from nonobese diabetic mice. Nat. Med. 15, 814–818 [DOI] [PubMed] [Google Scholar]
- Osteil P., Tapponnier Y., Markossian S., Godet M., Schmaltz-Panneau B., Jouneau L., Cabau C., Joly T., Blachere T., Gocza E., Bernat A., Yerle M., Acloque H., Hidot S., Bosze Z., Duranthon V., Savatier P., and Afanassieff M. (2013). Induced pluripotent stem cells derived from rabbits exhibit some characteristics of naive pluripotency. Biol. Open 2, 613–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovitt C.E., and Scholer H.R. (1998). The molecular biology of Oct-4 in the early mouse embryo. Mol. Hum. Reprod. 4, 1021–1031 [DOI] [PubMed] [Google Scholar]
- Palmieri S.L., Peter W., Hess H., and Scholer H.R. (1994). Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev. Biol. 166, 259–267 [DOI] [PubMed] [Google Scholar]
- Pesce M., Wang X., Wolgemuth D.J., and Scholer H. (1998). Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech.Dev. 71, 89–98 [DOI] [PubMed] [Google Scholar]
- Tancos Z., Nemes C., Polgar Z., Gocza E., Daniel N., Stout T.A., Maraghechi P., Pirity M.K., Osteil P., Tapponnier Y., Markossian S., Godet M., Afanassieff M., Bosze Z., Duranthon V., Savatier P., and Dinnyes A. (2012). Generation of rabbit pluripotent stem cell lines. Theriogenology 78, 1774–1786 [DOI] [PubMed] [Google Scholar]
- Teramura T., Sugimoto H., Frampton J., Kida Y., Nakano M., Kawakami M., Izumi H., Fukunaga N., Onodera Y., Takehara T. and others. (2013). Generation of embryonic stem cell lines from immature rabbit ovarian follicles. Stem cells and development 22, 928–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen T.W., Powell B.E., Wang H., Mitalipova M., Faddah D.A., Reddy J., Fan Z.P., Maetzel D., Ganz K., Shi L., Fukuda K., and Hosoi Y. (2014). Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15, 471–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Tang X., Niu Y., Chen H., Li B., Li T., Zhang X., Hu Z., Zhou Q., and Ji W. (2007). Generation and characterization of rabbit embryonic stem cells. Stem Cells 25, 481–489 [DOI] [PubMed] [Google Scholar]
- Wang S., Shen Y., Yuan X., Chen K., Guo X., Chen Y., Niu Y., Li J., Xu R.H., Yan X., Zhou Q., and Ji W. (2008). Dissecting signaling pathways that govern self-renewal of rabbit embryonic stem cells. J. Biol. Chem. 283, 35929–35940 [DOI] [PubMed] [Google Scholar]
- Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., Takahashi J.B., Nishikawa S., Muguruma K., and Sasai Y. (2007). A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686 [DOI] [PubMed] [Google Scholar]
- Xue F., Ma Y., Chen Y.E., Zhang J., Lin T.A., Chen C.H., Lin W.W., Roach M., Ju J.C., Yang L., Du F., and Xu J. (2012). Recombinant rabbit leukemia inhibitory factor and rabbit embryonic fibroblasts support the derivation and maintenance of rabbit embryonic stem cells. Cell. Reprogram. 14, 364–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeom Y.I., Fuhrmann G., Ovitt C.E., Brehm A., Ohbo K., Gross M., Hubner K., and Scholer H.R. (1996). Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 122, 881–894 [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., and Smith A. (2008). The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]