Abstract
Bach1, among the genes encoded on chromosome 21, is a transcription repressor, which binds to antioxidant response elements (AREs) of DNA thus inhibiting the transcription of specific genes involved in the cell stress response including heme oxygenase-1 (HO-1). HO-1 and its partner, biliverdin reductase-A (BVR-A), are up-regulated in response to oxidative stress (OS) in order to protect cells against further damage. Since OS is an early event in Down syndrome (DS) and might contribute to the development of multiple deleterious DS phenotypes, including AD pathology, we investigated the status of the Bach1/HO-1/BVR-A axis in DS and its possible implications for AD development. In the present study, we showed increased total Bach1 protein levels in the brain of all DS cases coupled with reduced induction of brain HO-1. Furthermore, increased OS could on one hand overcome the inhibitory effects of Bach1 and on the other hand, promote BVR-A impairment. Our data show that the development of AD in DS subjects is characterized by (i) increased Bach1 total and poly-ubiquitination; (ii) increased HO-1 protein levels; and (iii) increased nitration of BVR-A followed by reduced activity. To corroborate our findings we analyzed Bach1, HO-1 and BVR-A status in the Ts65Dn mouse model at 3 (young) and 15 (old) months of age. The above data support the hypothesis that the dysregulation of HO-1/BVR-A system contributes to the early increase of OS in DS and provide potential mechanistic paths involved in the neurodegenerative process and AD development.
Keywords: Biliverdin reductase, heme oxygenase, oxidative stress, trisomy 21, Bach1
INTRODUCTION
Trisomy of human chromosome 21 (HSA21) causes Down syndrome (DS), one of the most common chromosomal abnormalities in children. As a consequence, persons with DS exhibit complex phenotypes such as intellectual disability, congenital heart defects, and characteristic facial and physical features. A large series of proteins have been found to be abnormal in adult DS brain, but mechanisms elucidating how three copies of normal genes on HSA21 segments can lead to altered protein expression in DS over time and to complex metabolic and developmental aberrations are limited. In addition, in the brains of DS individuals the accumulation of amyloid beta (Aβ)-peptide can be observed as early as 8–12 years of age, and this accumulation increases during the lifespan [1, 2], resulting in Alzheimer disease (AD) pathology generally by the fourth decade of life.
Oxidative damage is also an early event in DS and might contribute to the development of deleterious DS phenotypes, including abnormal brain development and AD [3–6]. Increased oxidative damage result in increased lipid peroxidation, oxidative DNA damage and peripheral nitrosative stress [7–12], indicating that DS is associated with a “pro-oxidant state.” Among the most relevant contributory factors to oxidative stress (OS)/nitrosative stress (NS)-induced neurotoxicity, CuZn superoxide dismutase (SOD1), amyloid precursor protein (APP), the transcription factor Ets-2, S100B and carbonyl reductase, are encoded on HSA21. Furthermore, recent studies on fetal DS showed increased levels of Bach1 protein levels in the brain, suggesting that the repression of HO-1 transcription could significantly contribute to the increased oxidative stress found in DS [13].
According to “the amplified developmental instability hypothesis”, it is likely that the array of phenotypic features of DS does not involve exclusively specific genes on HSA21, but rather elevated activity of sets of genes, regardless of their identity, which lead to a decrease in genetic stability or homeostasis [14–17].
Lubec and colleagues carried-out a series of studies in order to challenge this hypothesis and to elucidate the impact of HSA21 trisomy on DS pathology [18–23]. Among HSA21 gene candidates, Bach1, a basic leucine zipper protein belonging to the cap’n’collar (CNC) family, is a transcription repressor encoded on HSA21. Under physiologic conditions, Bach1 forms heterodimers with small Maf proteins (i.e., MafK, MafF and MafG) that bind to the Maf recognition element [24], such as antioxidant response elements (AREs) in the nucleus, thus repressing transcription of certain genes controlled by ARE. When intracellular heme levels increase, as under pro-oxidant condition, nuclear Bach1 binds heme, changes its conformation, dissociates from ARE and allows transcription factors to bind and activate the expression of oxidative stress-responsive genes [25] such as NAD(P)H: Quinone oxidoreductase-1 (NQO1), glutathione S-transferase, glutamate-cysteine ligase, and heme oxygenase-1 [26].
Heme oxygenase (HO) catalyzes the first and rate-limiting enzymatic step of heme degradation, producing equimolar amounts of carbon monoxide (CO), iron (II) and biliverdin [27] [28, 29]. This latter is then converted into the powerful antioxidant bilirubin via the activity of cytosolic biliverdin reductase (BVR). Two genetically distinct HO isozymes, HO-1 and HO-2, are known. HO-2 represents the constitutive isoform primarily expressed in brain and testis [30]. By contrast, the inducible isoform HO-1, which exhibits low basal expression levels in most cells and tissues, is highly up-regulated by a wide variety of OS stimuli. HO has been suggested to function as a defense system against oxidative stress, since biliverdin or bilirubin produced locally in the body may act as physiological antioxidants [28, 31].
Similar to HO, two isoforms of BVR have been described and named BVRA and BVR-B [32–34]. Both these enzymes generate BR, but only BVR-A reduces BV-alpha into the powerful antioxidant and anti-nitrosative molecule BR-IX-alpha (hereafter BR) [35–41]. BVR-A is also a serine/threonine/tyrosine (Ser/Thr/Tyr) kinase that interacts with members of the MAPK family, in particular the extracellular signal-regulated kinases 1/2 (ERK1/2) that once translocated into the nucleus regulates the expression of oxidative-stress-responsive genes such as HO-1 or inducible nitric oxide synthase (iNOS) [32, 42]. Recent studies [43–45] showed that in AD and amnestic mild cognitive impairment (aMCI) both HO-1 and BVR-A undergo oxidative post-translational modifications, resulting in a further reduction of their antioxidant/neuroprotective activity, suggesting the potential involvement of altered HO-1/BVR-A system in AD pathogenesis and progression [46].
Based on previous studies [43–45] we believe that the analysis of the entire HO/BVR-A system better explains the impact that a dysfunction of HO-1 and/or BVR-A expression/activity has on OS/NS levels. In this context, considering the importance of Bach1 in the transcription of HO-1 gene, the current study tested the hypothesis that HSA21 trisomy leads to elevated Bach1 expression with consequent effects on the HO/BVR-A system. We further hypothesize that the impairment of the HO-1/BVR-A system is a common pathway of neurodegeneration, both in DS and AD cases.
METHODS
Subjects
DS and young or older control cases (without AD neuropathology) were obtained from the University of California-Irvine-ADRC Brain Tissue Repository, the Eunice Kennedy Shriver NICHD Brain and Tissue Bank for Developmental Disorders, and the University of Kentucky Alzheimer’s Disease Center. Table 1 shows the characteristics of the cases studied. DS cases were divided into two groups, with or without sufficient pathology for a neuropathological diagnosis of AD. All cases with both DS and AD were over the age of 40 years. Thus for the current study, controls were split into two groups, either less than or equal to 40 years or older than 40 years at death. The post mortem interval (PMI) was different across groups with DS/AD having a significantly shorter PMI with respect to the other groups (Table 1). The subsample used in this study was selected in order to maintain homogenous age and gender inside the groups and is part of the entire cohort used in a previous experiment to investigate Aβ and total oxidation as a function of age in DS [9, 11, 47, 48]
Table 1.
Autopsy case demographics. A complete set of information is reported as supplementary material.
| Groups | n | Gender (M/F) | Age (avg. ± SD) |
PMI (avg. ± SD) |
|---|---|---|---|---|
| Control Y | 8 | 4/4 | 24.8 ± 11.66 | 12.1 ± 4.71 |
| DS | 8 | 6/2 | 23.3 ± 16.83 | 13.4 ± 2.13 |
| Control O | 8 | 5/3 | 57.2 ± 7.63 | 11.3 ± 6.90 |
| DS/AD | 8 | 5/3 | 59.0 ± 3.20 | 4.38 ± 1.57* |
Significantly shorter than the other 3 groups.
Mouse colonies
Mice were generated by repeatedly backcrossing Ts65Dn trisomic (TS) females with (C57BL/6JxC3H/ HeJ) F1 hybrid males, the parental generation were purchased from the Jackson Laboratories. These breeding pairs produce litters containing both trisomic (Ts65Dn) and disomic (2N) offspring. The pups were genotyped for determine the trisomy using standard PCR, as described by Reinholdt et al. [49]. In addition, in all the animals the recessive retinal degeneration 1 mutation (Pdeb rd1), which is segregating in the colony and results in blindness in homozygotes, was detected by using a standard PCR [50]. Mice were housed in clear plexiglass cages (20×22×20 cm) under standard laboratory conditions with a temperature of 22 ± 2°C and 70% humidity, a 12-hour light/dark cycle and free access to food and water. Mice have been sacrificed at 3 and at 15 months of age and frontal cortex region has been collected (Table 2). All the experiments were performed in strict compliance with the Italian National Laws (DL 116/92), the European Communities Council Directives (86/609/EEC). All efforts were made to minimize the number of animals used and their suffering.
Table 2.
Mice demographics
| Groups | n | Months of age (avg. ± SD) |
Weight (avg. ± SD) |
|---|---|---|---|
| Euploid 3M | 6 | 3.0 ± 0.1 | 23.6 ± 1.3 |
| Ts65Dn 3M | 6 | 2.96 ± 0.1 | 25.0 ± 2.0 |
| Euploid 15M | 6 | 15.0 ± 0.1 | 39.4 ± 7.0 |
| Ts65Dn 15M | 6 | 15.0 ± 0.1 | 42.5 ± 3.3 |
Sample preparation for WB and IP
Frontal cortex tissue from human controls (young and old), DS, and DS/AD brain (n= 8 per group), and from mouse brain of Ts65Dn and euploid littermates at 3 and 15 months of age (n=6 per group), were thawed in RIPA buffer (pH 7.4) containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.25 % sodium deoxycholate,1mM EDTA, 0,1% SDS, 1mM PMSF, 1 mM NaF and 1 mM Na3VO4. Brains were homogenized by 20 passes of a Wheaton tissue homogenizer, and the resulting homogenate was centrifuged at 14,000 ×g for 10 min to remove cellular debris. The supernatant was extracted to determine the total protein concentration by the Bradford assay (Pierce, Rockford, IL).
Western Blot
For Western blots, 30 µg of proteins (CTR and DS) were separated by 12% and 7.5% SDS–PAGE using Criterion Gel TGX and blotted into a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Before blotting the gel image analyzed for total protein load was acquired to normalize blot analysis. Membranes were blocked with 3% bovine serum albumin in T-TBS and incubated for 90 min at room temperature with primary antibodies: anti-Bach1, anti-ubiquitin (Santa Cruz Biotechnology, Dallas, TX, USA), anti-poly-ubiquitin (K63-linkage-specific), anti-HO-1, anti-HO-2 (Enzo Life Science, Farmingdale, NY, USA) anti-NQO1, anti-BVR-A (Sigma- Aldrich, St Louis, MO, USA), anti-β-actin and anti-3NT (Millipore Billerica, MA, USA). After three washes with T-TBS, the membranes were incubated for 60 min at room temperature with anti-rabbit/mouse IgG (1:5000; Sigma–Aldrich, St Louis, MO, USA) secondary antibody conjugated with horseradish peroxidase. Membranes were developed with the Super Signal West Pico chemiluminescent substrate (Thermo Scientific, Waltham, MA, USA) acquired with Chemi-Doc MP (Bio-Rad, Hercules, CA, USA) and analyzed using Image Lab software (Bio-Rad, Hercules, CA, USA) that permit the normalization of a specific protein signal with the β-actin signal in the same lane or total proteins load.
Immunoprecipitation
The immunoprecipitation procedure was performed as previously described with modifications [45]. Briefly, 150 µg of protein extracts were dissolved in 500 µl of RIPA buffer (10 mM Tris, pH 7.6; 140 mM NaCl; 0.5% NP40 including protease inhibitors) and then incubated with 1 µg of primary antibody at 4°C overnight. Immunocomplexes were collected by using protein A/G beads suspension for 2 h at 4°C and washed five times with immunoprecipitation buffer. Immunoprecipitated protein was recovered by resuspending the pellets in reducing SDS buffers and subjected to electrophoresis on 12% gels followed by Western blot analysis.
BVR-A reductase activity assay
To test BVR activity in frontal cortex of CTR and DS cases, and of euploid and Ts65Dn mice, the biliverdin reductase assay kit (Sigma-Aldrich, St Louis, MO, USA) was used following the standard protocol with minor changes. Briefly, 150 µg of protein samples were prepared for the assay and loaded in a 96 well plate. Furthermore, BVR positive control solution (2.5, 5, 10, 15 20 µl) was included in the assay for generation of a standard curve. Then, 50 µl of assay buffer and 150 µl of working solution (containing NAPDH, substrate solution and assay buffer) were added to standards and samples on the plate. The plate was placed on the UV-VIS plate reader at 37° C and read every minute for ten minutes. The reading after 5 min, that presented a linear reaction rate, was chosen for BVR activity calculation.
Real-Time RT-PCR
RNA preparation from total brain was performed using RNeasy Plus Mini kit (QIAGEN, Milan, Italy), according to the manufacturer’s instructions. For each sample 500 ng of RNA were retrotranscribed into cDNA for 1h at 42°C by Superscript III (Life Technologies, Logan, UT, USA) according to the manufacturer's instruction using both random primers and OligodT. The quantitative real-time PCR was performed on the i-Cycler iQ detection system (BioRad Laboratories Inc., Milan, Italy), using the kit for SYBR Green (KAPA Biosystem, Wilmington, MA, USA), according to the manufacturer instruction. For each reaction 5 ng of cDNA were used. Specific sequences were amplified in separate tubes for HO-1, NQO1 and human β-Actin gene primers as a reference, using the following protocol: 95°C for 3 min, followed by 40 cycles of 95°C for 5 min, 60°C for 30 min. The increase in fluorescence was measured in real time during the extension and the relative expression of the transcripts was quantified with the 2−ΔCt method [25, 51, 52]. Primers for β-Actin were: Act-up (5'-ACCACACCTTCTACAATGAGCTGCGTG-3') and Act-down (5'-CACAGCTTCTCCTTAATGTCACGCACG-3'). RNA and PCR mixtures were prepared and kept in separate rooms.
Statistical analysis
Data are expressed as mean ± SD per group. All statistical analyses were performed using a non-parametric one-way ANOVA with post hoc Bonferroni t-tests. p<0.05 (*) was considered significant. Since the DS/AD group PMI is significantly lower in comparison to the other three groups we performed linear regression analyses between each experimental marker vs. PMI in order to test if our results were affected by variable PMI. Moreover, we correlated experimental data from CTR group (CTRY and CTRO) and DS group (DS and DS/AD) with age to analyze the effects of age variations. Finally, to determine how our data were affected by genotype (DS) and age and the interaction of such factors, a 2-way ANOVA analysis was performed. All statistical analysis was performed using GraphPad Prism 5.0 software.
RESULTS
Bach1 protein levels and ubiquitination
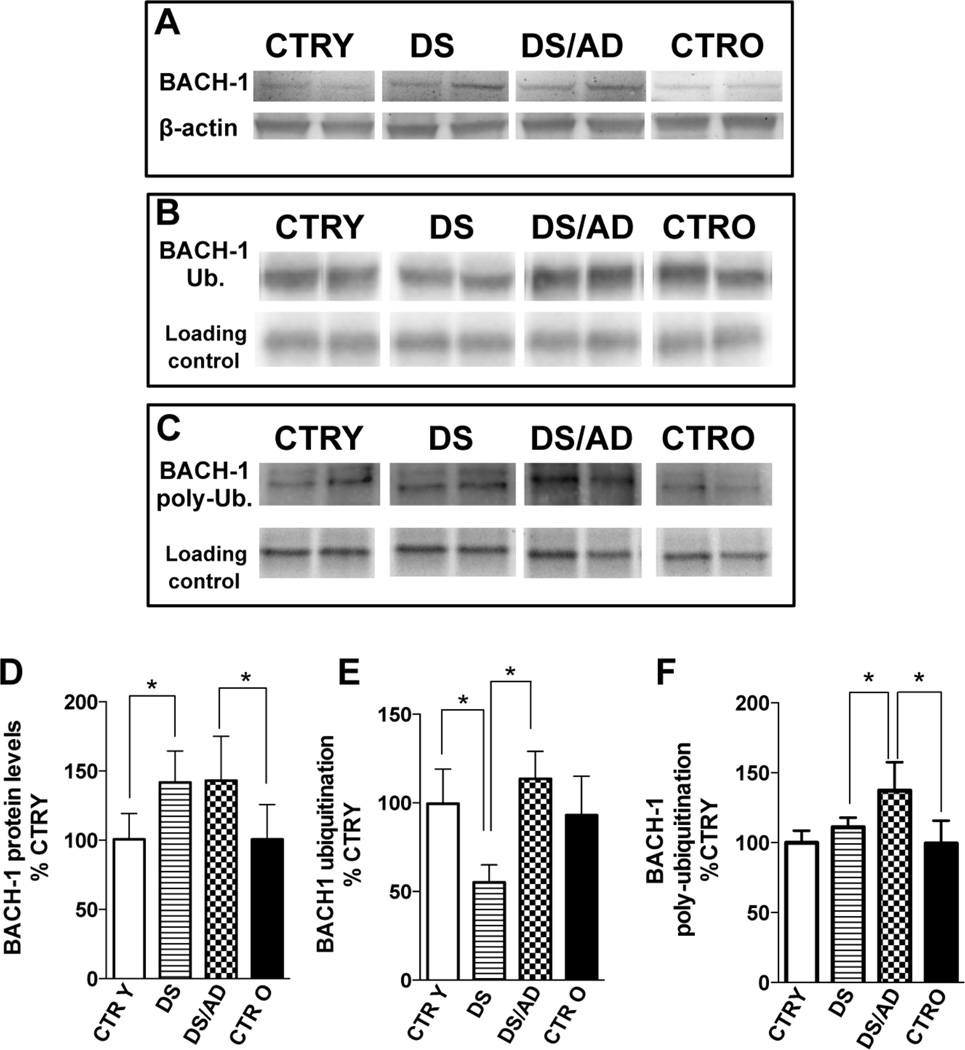
In order to clarify the role of Bach1 in the regulation of HO-1 during the progression of DS neuropathology, total Bach1 protein levels were evaluated (Fig. 1A&D). The first analysis concerned the increased expression levels, genotype dependent, in DS compared to CTRY (about 1.5-fold) and in DS/AD compared to CTRO (about 1.5-fold). Consistent with these observations, a 2-way ANOVA analysis showed that genotype significantly accounts for 44.2% (Table 3, p<0.0001) of total variance, while age is not significant. The lack of age-associated effects on Bach1 levels is confirmed by the results of the linear regression analysis that showed an absence of any association between Bach1 protein levels and chronological age either in CTR or DS cases (Sup. Fig.1). Moreover, no differences in Bach1 levels were observed between young controls (CTRY, <40 years) and old controls (CTRO, >40 years). Similarly, the effect of PMI, which conceivably could represent a significant contributor to the evaluation of OS/NS-induced proteins, did not contribute to variability in Bach1 (Sup. Fig.1).
Figure 1. Bach1 protein levels and ubiquitination in the brain of DS and DS/AD cases.
Human frontal cortex of DS (n=8) and DS/AD (n=8) with respect to matched controls, i.e., control young (CTR Y, <40 years, n=8) and control old (CTR O, >40 years, n=8), has been analyzed to evaluate Bach1 protein levels and ubiquitination. For total protein levels samples were analyzed by WB using anti-Bach1 antibody (panel A and C), while for protein total and poly-ubiquitination, samples were immunoprecipitated with anti-Bach1 antibody and analyzed by WB using anti polyubiquitin antibody (panel B&E and D&F). All the gel images show two representative bands for each group. The bar graph were built on all the samples values normalized on CTRY that was set at 100%; Means ± SD are reported, *P<0.05.
Table 3.
2-way ANOVA analysis to account for age and genotype effects on experimental data. Bold values indicate p<0.05
| PROTEIN | 2-way ANOVA | ||||
|---|---|---|---|---|---|
| Age (Young vs. Old) | Genotype (DS vs. DS/AD) | Interaction | |||
| % of tot var. | p value | % of tot var. | p value | ||
| BACH-1 | 0.1 | 0.94 | 44.2 | <0.0001 | 0.93 |
| HO-1 | 25.62 | 0.011 | 2.58 | 0.26 | 0.756 |
| NQO1 | 63.29 | 0.0001 | 5.01 | 0.11 | 0.099 |
| BVR-A | 4.61 | 0.16 | 49.82 | 0.0001 | 0.71 |
| BVR-A activity | 33.89 | 0.012 | 0.98 | 0.62 | 0.057 |
| 3NT-BVR-A | 93.80 | 0.0001 | 0.70 | 0.081 | 0.014 |
| HO-2 | 18.9 | 0.02 | 5.86 | 0.17 | 0.012 |
To determine the involvement of ubiquitin-mediated protein degradation [53] on Bach1 protein levels, Bach1 total ubiquitination and poly-ubiquitination levels were evaluated. Decreased levels (~2-fold) of total ubiquitination of Bach1 were observed in DS compared to CTRY and in DS compared to DS/AD (Fig.1B&E). Increased levels of poly-ubiquitinated Bach1 (1.4-fold) were found between DS/AD vs. DS and DS/AD vs. CTRO (Fig.1C&F). In addition, no differences were observed between CTRY and CTRO for both total and poly-ubiquitination, thus excluding once again an age contribution.
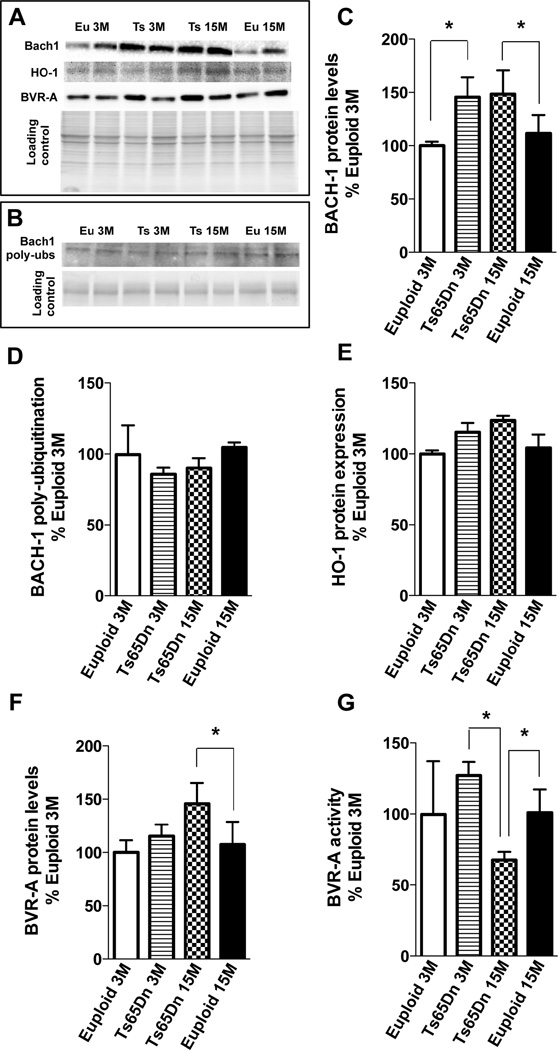
The analysis of Bach1 protein levels and ubiquitination has also been investigated in Ts65Dn mice at 3 months (young group) and at 15 months (old group) of age compared to age-matched euploid animals. Previous studies on this mouse model suggested the triplication of this transcription factor due to trisomy condition as well as in humans [54]. Our data confirm that in both young and old Ts65Dn mice groups compared to euploid animals demonstrate an increase of Bach1 expression of about 1.45-fold and 1.5-fold respectively (Fig. 5A&C). Regarding poly-ubiquitination of Bach1 no differences were found between Ts65Dn and euploid animals (Fig. 5B&D) thus suggesting differential mechanisms of Bach1 levels regulation between mice and humans.
Figure 5. Bach1 protein and poly-ubiquitination levels, HO-1 protein levels and BVR-A protein levels and activity in Ts65Dn mouse model at 3 and 15 months of age compared to age-matched euploid animals.
Proteins total levels were analyzed by WB using anti-Bach1 antibody (panel A and C), anti-HO-1 antibody (panel A and E) and anti-BVR-A antibody (panel A and F), while for protein ubiquitination samples were immunoprecipitated with anti-Bach1 antibody and analyzed by WB using anti (poly-)ubiquitin antibody (panel B&D). BVR-A reductase activity (panel G) was evaluated by enzymatic assay as described in materials and methods section. All the gel images show two representative bands for each group. The bar graph were built on all the samples values normalized on CTRY that was set at 100%; Means ± SD are reported, *P<0.05.
HO-1, HO-2 and NQO1 expression levels
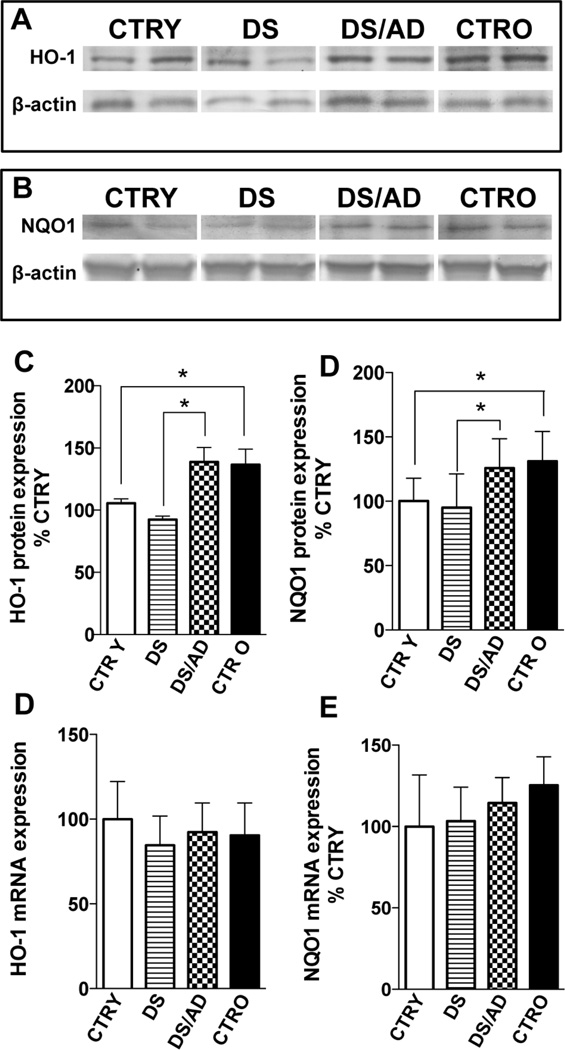
As cited above, Bach1 inhibits HO-1 transcription by binding to AREs. Given our observation that Bach1 is increased in DS cases, we hypothesized that HO-1 induced transcription would be inhibited in the same groups. To determine if changes in Bach1 protein levels inhibit HO-1 expression, HO-1 mRNA and protein levels were analyzed. As showed in Fig. 2D, HO-1 mRNA levels show no significant difference across the 4 groups, while differences are seen when compared to AD samples (Sup.Fig.3). Similarly, no overall differences were found for HO-1 protein levels between CTR and DS groups as result of genotype effects.
Figure 2. Heme oxygenase-1 (HO-1) and NAD(P)H:Quinone oxidoreductase-1 (NQO1) protein levels and mRNA expression levels in the brains of DS and DS/AD cases.
HO-1 and NQO1 protein levels and mRNA expression levels in the brain of DS (n=8) and DS/AD (n=8) with respect to their matched controls, i.e., control young (CTR Y, <40 years, n=8) and control old (CTR O, >40 years, n=8), respectively. For total protein levels samples were analyzed by WB using anti-HO-1 antibody (panel A and C) and anti-NQO1 antibody (panel B and D). mRNA levels were evaluated by real time PCR as described in materials and methods section. Representative gels of 2 independent samples per group are shown. The bar graph were built on all the samples values normalized on CTRY that was set at 100%; Means ± SD are reported, *P<0.05.
The analysis of DS subjects revealed no significant changes for HO-1 protein levels with respect to age-matched controls (DS vs. CTRY and DS/AD vs. CTRO). Conversely, an age-dependent increase of HO-1 protein levels was observed with a significant increase of about 1.4-fold in DS/AD compared to DS and of about 1.3-fold in CTRO compared to CTRY (Fig.2A&C) consistent with previous findings [55]. Increased HO-1 protein levels with age also were supported by the positive and significant linear regression analysis with age found in both CTR and DS groups (Sup. Fig.2). No association between PMI differences and HO-1 was found (R2=0.01, p=0.53). A two-way ANOVA analysis confirms the linear regression data by showing that age (unlike in the Bach1 analyses) significantly accounts for 25.6% (p=0.011) of the total variance, while genotype had no significant effect on HO-1 protein levels (Table 3). The analysis of HO-1 protein levels in Ts65Dn model compared with euploid counterpart shows no significant increase between Tg and euploid animals neither between groups at different age (Fig. 5A&E) supporting the role of Bach1 in repressing HO-1 induction.
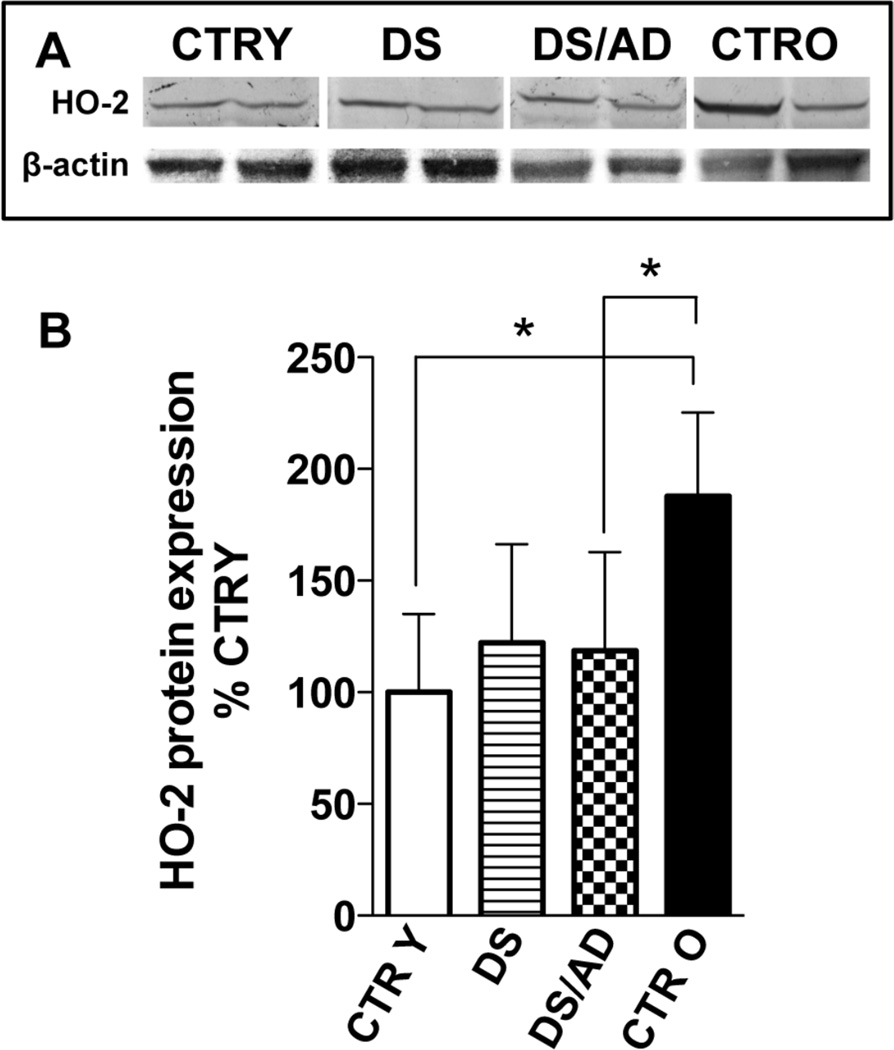
HO-2, the constitutive isoform of heme oxygenase is not regulated by Bach1 and is reduced in pathological conditions such as AD [46, 56, 57]. Our data show an age-dependent increase of the HO-2 protein levels in controls but not DS (Fig.3A&B). Linear regression analysis shows that HO-2 levels are not associated with PMI differences (R2= 0.00048, p= 0.98), while they are apparently associated with age but only in CTR subjects (R2= 0.47, p= 0.019, sup. Fig.5). A two-way ANOVA confirmed that the age-associated differences with regard to HO-2 expression accounts for 18.9% (p=0.02) of total variance. HO-2 in DS was similar to CTRY cases, but significantly lower HO-2 levels were observed (2-fold) in DS/AD compared to CTRO.
Figure 3. Heme oxygenase-2 protein levels in the brains of DS and DS/AD cases.
HO-2 protein levels in the brain of DS (n=8) and DS/AD (n=8) with respect to their matched controls, i.e., control young (CTR Y, <40 years, n=8) and control old (CTR O, >40 years, n=8), respectively. For total protein levels samples were analyzed by WB using anti-HO-2 antibody (panel A and B). Representative gels of 2 independent samples per group are shown. The bar graph were built on all the samples values normalized on CTRY that was set at 100%; Means ± SD are reported, *P<0.05.
To extend our results of the consequences of increased Bach1 in DS we analyzed mRNA and protein levels of NQO1, another well-known protein whose expression is regulated by Bach1 [58]. NQO1 is a phase II antioxidant enzyme that catalyzes the detoxification of quinones, which prevents the generation of reactive semiquinones, O2°, and H2O2 [59]. Our results show unaltered levels of NQO1 mRNA among the 4 groups (Fig.2E), consistent with HO-1 data. NQO1 levels are similar in DS overall compared to control cases overall. However an age-associated increase (about 1.3-fold, CTRY vs. CTRO) is found in control cases but not DS cases. The linear regression analysis supports the influence of age in the controls on NQO1 protein induction in CTR (R2= 0.53, p= 0.04) but not DS (R2= 0.31, p= 0.14) cases. In addition, a two-way ANOVA analysis confirms that age significantly accounts for 63.3% (p=0.0001) of the total variance, while genotype did not significantly affect NQO1 protein levels (Table 3). Similar to HO-1, DS/AD subjects showed a significant increase of about 1.3-fold (Fig. 2) in NQO1 protein levels compared to younger DS cases without AD pathology (Fig.2B&D).
BVR-A protein levels, nitration and reductase activity
BVR-A with HO is part of the heme degradation pathway in which this enzyme catalyzes the reduction of BV to BR [32, 46]. In addition, through its serine/threonine/tyrosine kinase activity BVR is able to modulate several signaling transduction pathways having neuroprotective effects [32]. To extend our knowledge about the role of HO/BVR-A system in the progression of DS pathology, BVR-A protein levels and activity were evaluated. Interestingly, a significant increase of BVR-A protein levels was found in DS (1.4-fold vs. CTRY) and DS/AD (1.5-fold vs. CTRO) cases (Fig. 4A&C). Furthermore, BVR-A protein levels are not associated with age as the same levels were found in CTRY and CTRO. Linear regression analyses support these observations by showing that BVR-A levels are independent of PMI (R2= 0.09, p= 0.66) as well as from age in both CTR (R2= 0.04, p= 0.53) and DS (R2= 0.001, p= 0.92) groups. Furthermore, a two-way ANOVA analysis indicates that, with regard to BVR-A protein levels, genotype significantly accounts for 49.8% (p<0.0001) of the total variance, while age is not significantly involved (Table 3). The analysis of 3- and 15-month old Ts65Dn mice and their euploid counterpart support the above data on BVR-A in DS cases, showing a slight but not significant increase in the young group of mice, as seen in young DS, and a consistent increase of about 1.5-fold in the aged group (Fig. 5A&F). Regarding BVR-A levels, the discrepancy about data obtained in young DS and in young Tg mice in respect to their controls might be due to different thresholds of OS/NS.
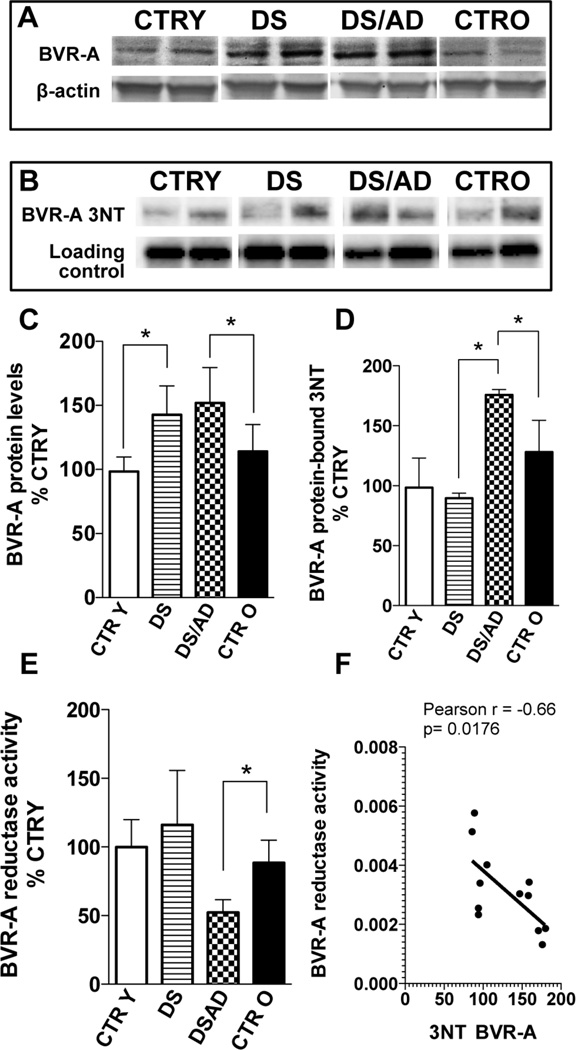
Figure 4. Biliverdin reductase-A (BVR-A) protein levels, activity and nitrosative post-translational modifications (3-NT) in the brains of DS and DS/AD cases.
BVR-A protein levels, reductase activity (expressed as bilirubin production) and nitrosative post-translational modifications in the brain of DS (n=8) and DS/AD (n=8) with respect to their matched controls, i.e., control young (CTR Y, <40 years, n=8) and control old (CTR O, >40 years, n=8), respectively. Protein levels were analyzed by WB using anti-BVR-A antibody (panel A and C), while for protein-bound 3NT levels samples were immunoprecipitated with anti-BVR-A antibody and analyzed by WB using anti-3NT antibody (panel B and D). BVR-A reductase activity (panel E) was evaluated by enzymatic assay as described in materials and methods section. In panel F the correlation analysis between 3-NT and BVR-A reductase activity data is shown. All the gel images show two representative bands for each group. The bar graph were built on all the samples values normalized on CTRY that was set at 100%; Means ± SD are reported, *P<0.05.
To test if increased BVR-A protein levels were paralleled with an increase in activity, BVR-A reductase activity was evaluated. DS cases showed similar BVR-A activity levels compared to CTRY (Fig.4E). Conversely, DS/AD subjects showed a significantly reduced activity when compared with CTRO (about 1.8-fold). In addition, DS/AD subjects had reduced BVR-A reductase activity compared to DS (~2.2-fold decrease), thus highlighting that AD pathology is associated with reduced BVR-A function. In support of these data, a linear regression analysis revealed a significant negative association between age and BVR-A activity (Sup. Fig.4). BVRA activity is independent of PMI (R2= 0.21, p= 0.055; Sup. Fig.4). Further, our data demonstrate no significant differences with age in CTR subjects, thus excluding age-associated effects (Fig.4; Sup. Fig.4). Overall, a two-way ANOVA shows that age significantly accounts for 33.9% (p=0.012; Sup. Fig.4) of total variance in BVR-A activity while genotype did not significantly influence BVR-A activity.
To determine if changes of BVR-A activity were dependent on OS/NS-induced post-translational modifications as previously reported [43–45, 60], 3-NT levels of BVR-A (3-NT-BVR-A) were evaluated. As shown in Fig. 4B&D, BVR-A nitration was comparable between DS and CTRY, while a significant increase (about 1.4-fold) was observed in DS/AD subjects with respect to CTRO. Intriguingly, an increase of 3-NTBVR-A was observed in DS/AD compared to DS (about 1.8-fold). These last findings parallel with lower BVR-A activity (Fig.4F) and are consistent with the evidence that increased OS/NS levels reduce BVR-A activity as previously reported in AD and MCI subjects and aged beagles [43–46, 60]. There was no effect of PMI, which could affect OS/NS levels [61] (R2= 0.09, p= 0.32, Sup. Fig.4). Linear regression analysis supports the age-associated effects in terms of protein nitration in both CTR (R2=0.80;p=0.014) and DS (R2=0.69; p=0.04) cases (Sup. Fig. 5) A two-way ANOVA analysis suggests that age significantly accounts for 93.8% (p=0.0001) of total variance in 3-NT-BVR-A, while genotype did not influence BVR-A nitration (Table 3). Further, analysis on BVR-A activity has been performed on Ts65Dn mice model compared to age-matched euploid groups showing that the activity of BVR-A does not change significantly at 3 months of age between the groups of comparison but decreases at 15 month-old Ts65Dn when compared to both age-matched euploid animals (1.35-fold) and 3 month-old Ts65Dn (1.5-fold) (Fig. 5G)
DISCUSSION
The mechanism(s) relating HSA21 trisomy and neurodegeneration are not still completely elucidated, and recent data suggest the contribution of increased OS/NS, [48, 62–70]. Similar to AD [71–74], Aβ accumulation in DS is associated with enhanced formation of reactive oxygen species (ROS) in neurons leading to premature neuronal dysfunction and death [75–77]. DS individuals possess a higher risk of developing AD than the general population [3, 63]. Trisomy of HSA21 likely increases levels of the proteins involved in redox homeostasis such as SOD-1 that when overexpressed could promote increased levels of OS, as observed in DS [3, 7, 11, 47, 78, 79]. A current challenge is to identify the intracellular pathways that are altered as a consequence of genetic contributions to AD neuropathology in DS, with the aim of providing a mechanistic basis to understand the neurodegenerative process and facilitate the design of new treatments.
To investigate this hypothesis, we analyzed the Bach1/HO-1/BVR-A axis that represents one of the main systems through which cells counteract/prevent OS-induced intracellular damage [25, 46, 80–82]. Here we report for the first time that total Bach1 is overexpressed in the brain of young DS cases. Interestingly, DS/AD cases show the same levels of Bach1 protein with respect to younger DS without AD pathology. Since DS cases are both higher than their respective controls, a dominant effect of DS pathology/genotype in accordance with the “gene dosage hypothesis” can be proposed [83]. This evidence is also supported by the lack of any association between age and Bach1 protein levels in DS, suggesting that the observed increase of Bach1 expression in DS subjects is strictly linked to the trisomy. Bach1 plays an important role in the regulation of several proteins involved in the cell stress response by blocking their induction under physiological conditions. In turn, increased OS levels suppress the function of Bach1 [53, 84, 85] by promoting Bach1 nuclear export, poly-ubiquitination by the heme-responsive E3 ubiquitin-protein ligase HOIL-1, and subsequent degradation by the 26S proteasome pathway [53]. ROS can also directly target the sulfhydryl groups of Bach1 to inhibit its binding to AREs, thus promoting nuclear export and degradation [26].
Increased Bach1 expression levels are involved, overall, in repressing the induction of HO-1 in DS cases, thus reducing HO-1 overexpression in stress conditions as observed in AD [56] (Fig.2; Sup.Fig.3). To date, other genes mapped on HSA21, such as GABPA or UBC, which modulate NRF2 (trough Keap1) or the same Bach1, might be involved in the regulation of HO-1 expression, thus amplifying the effects of trisomy on the alteration of the heme degradation pathway. A further novelty reported in this study is represented by the different pattern of ubiquitination among DS and CTR cases. We measured Bach1 total (mono- and polyubiquitination) and poly-ubiquitination. Mono-ubiquitination is involved in modulating protein function, compartmentalization and interactions, while polyubiquitination is a signal of protein degradation. Our data demonstrate that in the brain of DS the total ubiquitination but not poly-ubiquitination of Bach1 is reduced compared to controls. This effect together with increased total Bach1 levels might contribute to regulate HO-1 levels in DS comparable to CTRY. The presence of AD pathology increases Bach1 poly-ubiquitination in DS to levels higher than those observed in age-matched controls (Fig.1F). Despite increased protein levels of Bach1 both in DS and DS/AD, the different pattern of poly-ubiquitination may account for the differences of HO-1 and NQO1 protein levels between DS/AD and DS cases (Fig.2C&D). Nevertheless, HO-1 and NQO1 are selectively increased in aged groups (DS/AD and CTRO) compared to young groups (DS and CTRY) as a function of age or OS (Fig.2). In addition, the significant differences found between DS and DS/AD individuals (Fig.1) suggest a potential compensatory effect between DS and AD pathology. We might speculate that the increased Bach1 poly-ubiquitination in DS/AD with respect to DS is a result of increased OS observed in these individuals. Overall, it appears that Bach1 levels, and therefore HO-1 expression, in DS might result from the combination of genotype-related Bach1overexpression and Bach1 poly-ubiquitination levels. This observation is strengthen by data obtained on Ts65Dn mice (young and old) compared to euploid controls that show increased Bach1 expression levels but not Bach1 poly-ubiquitination. Increased Bach1 levels in Tg animals tend to maintain HO-1 expression levels comparable to those of euploid animals, without any contribution of the ubiquitination process. Therefore, the lack of different Bach1 poly-ubiquitination between young and old mice is conceivably responsible of the lack of age-dependent increase of HO-1 levels between the same groups observed in human samples.
However, whether inhibition of Bach1 is required to support HO-1 transcription driven by other transcription factors is not clear and requires further analysis [86]. It is important to address that ubiquitination process in DS cases might be altered as result of the triplication of USP25, USP16 (ubiquitin proteases); UBE2G2 (ubiquitin conjugating enzyme); SMT3A (ubiquitin-like) genes and ZNF294 (Listerin E3 Ubiquitin Protein Ligase 1) [54], thus contributing to the alteration of ubiquitination/de-ubiquitination processes in trisomic individuals.
Interestingly, HO-2 protein levels apparently are not affected by DS, but rather by age only in the controls (Fig.3). Furthermore, the significantly lower levels of HO- 2 found in DS/AD with respect to CTRO is consistent with previous studies in sporadic AD and MCI showing reduced HO-2 protein levels in the brain undergoing neurodegeneration [56, 57].
Another novelty of the present study is the differential regulation of BVR-A protein levels that are increased in both DS and DS/AD brain. This observation suggest that the DS genotype is associated with higher BVR-A conceivably as a consequence of early increased OS in DS brain [3, 15, 33, 87–89]. These notions parallel with previous findings in brain of MCI and AD cases showing an association of HO-1 and BVR-A protein levels with OS [45, 56]. Considering the pleiotropic functions of BVR-A in the maintenance of cellular homeostasis [32], it is plausible that the induction of BVR-A is a process more sensitive to OS changes and precedes that of HO-1, as previously suggested [44, 45].
However, increased and prolonged nitrosative stress levels exert a critical detrimental effect on BVR-A function, modulating its catalytic activity. Indeed, we show a negative correlation between the significant increase of protein-bound 3-NT and decreased BVR-A activity in the brain of DS/AD when compared to either CTRO and DS (Fig.4) [43–46, 60]. Overall, BVR-A appears to respond to increased OS in young DS individuals (increased expression) but at the same time is highly susceptible to oxidative damage, which compromises its functional activity. The results obtained on Tg mice show, as in humans, an increase of BVR-A expression levels coupled with decreased levels of BVR-A reductase activity in the aged, but not in the young, trisomic group suggesting again the involvement of OS/NS in the regulation of BVR-A protein functionality.
To summarize, the results of the current study show that a dysfunction of the HO/BVR-A system occurs in DS and that this phenomenon appears to be an early event that may be linked to increased OS. Bach1, encoded on HSA21, is overexpressed in DS humans and mouse models of the disease playing a key role in the regulation of HO-1 protein expression, but not BVR-A. The further increase of OS over time could be responsible for the nitration and subsequent impairment of BVR-A activity, thus negatively affecting both the production of bilirubin and the BVR-A-mediated cell stress response (Fig.6). Taken together, this series of changes could impact the progression of the neuropathology from DS to DS/AD. The implication of these results for understanding the molecular mechanisms leading to OS-mediated neurotoxicity are interesting, and offer a molecular bridge connecting DS and AD. Two major theories, the “gene dosage hypothesis and the “amplified developmental instability hypothesis” have been proposed to explain the high susceptibility of DS population to AD. Our study suggests that the triplication of genes encoded on HSA21 are directly involved in the appearance of harmful conditions such as increased OS/NS which over time contributes to the early development of AD pathology in DS individuals. The understanding of such altered molecular mechanisms and their impact on DS brain could lead to novel therapies to prevent or treat AD, and research on this topic is ongoing in our laboratory.
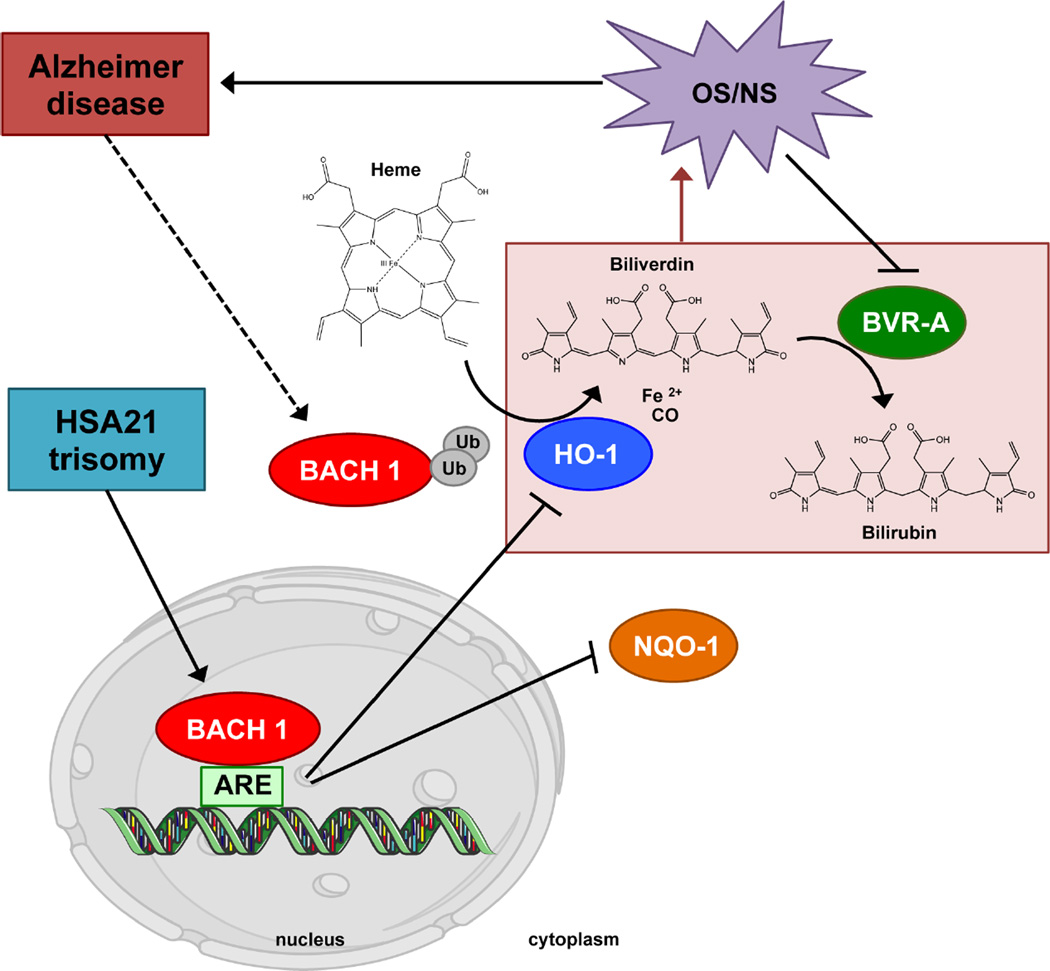
Figure 6. Proposed scenario of Bach1 modulation and downstream signaling in DS pathology.
HSA21 trisomy leads to Bach1 repressor of transcription overexpression, which, in the nucleus, forms heterodimers with small Maf proteins and bind to antioxidant response elements (AREs), repressing transcription of HO-1 and NQO1 genes. DS individuals are characterized by early increase of oxidative/nitrosative stress (OS/NS), and the inhibition of HO-1 induction both contributes to BVR-A activity by increasing protein nitration. The HO-1/BVR-A pathway, when impaired, is not able to exert its neuroprotective function thus favoring the progression of AD pathology in DS individuals. Finally, the development of AD pathology in DS individuals leads the ubiquitination of Bach1 partially recovering HO-1 induction but not the system functionality.
Supplementary Material
ACKNOWLEDGMENTS
This work was partially supported by fondi di Ateneo Sapienza to M.P. and F.D.D., NIH grant to D.A.B. [AG-05119] and funding from the People Program (Marie Curie Actions) of the European Union's Seventh Framework Program (FP7/2007–2013) under REA grant agreement n° 624341 to E.B. Brain tissue was acquired by E.H. under funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute on Aging Grant #NIH 1RO1HD064993-01. Autopsy tissue was obtained from the UCI-ADRC (P50AG16573), from the UK ADC (P30AG28383) and from the NICHD Brain and Tissue Bank for Developmental Disorders of the University of Maryland, Baltimore, MD, contract HHSN275200900011C (N01HD90011). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
All authors state that they have no conflicts of interest.
REFERENCES
- 1.Leverenz JB, Raskind MA. Early amyloid deposition in the medial temporal lobe of young Down syndrome patients: a regional quantitative analysis. Exp Neurol. 1998;150:296–304. doi: 10.1006/exnr.1997.6777. [DOI] [PubMed] [Google Scholar]
- 2.Wegiel J, Wisniewski HM, Dziewiatkowski J, Popovitch ER, Tarnawski M. Differential susceptibility to neurofibrillary pathology among patients with down syndrome. Dementia. 1996;7:135–141. doi: 10.1159/000106868. [DOI] [PubMed] [Google Scholar]
- 3.Perluigi M, Butterfield DA. Oxidative Stress and Down Syndrome: A Route toward Alzheimer-Like Dementia. Curr Gerontol Geriatr Res. 2012;2012:724904. doi: 10.1155/2012/724904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lott IT. Neurological phenotypes for Down syndrome across the life span. Prog Brain Res. 2012;197:101–121. doi: 10.1016/B978-0-444-54299-1.00006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attar H, Bedard K, Migliavacca E, Gagnebin M, Dupre Y, Descombes P, Borel C, Deutsch S, Prokisch H, Meitinger T, Mehta D, Wichmann E, Delabar JM, Dermitzakis ET, Krause KH, Antonarakis SE. Extensive natural variation for cellular hydrogen peroxide release is genetically controlled. PLoS One. 2012;7:e43566. doi: 10.1371/journal.pone.0043566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenti D, de Bari L, De Filippis B, Henrion-Caude A, Vacca RA. Mitochondrial dysfunction as a central actor in intellectual disability-related diseases: An overview of Down syndrome, autism, Fragile X and Rett syndrome. Neurosci Biobehav Rev. 2014 doi: 10.1016/j.neubiorev.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Perluigi M, di Domenico F, Fiorini A, Cocciolo A, Giorgi A, Foppoli C, Butterfield DA, Giorlandino M, Giorlandino C, Schinina ME, Coccia R. Oxidative stress occurs early in Down syndrome pregnancy: A redox proteomics analysis of amniotic fluid. Proteomics Clin Appl. 2011;5:167–178. doi: 10.1002/prca.201000121. [DOI] [PubMed] [Google Scholar]
- 8.Butterfield DA, Di Domenico F, Swomley AM, Head E, Perluigi M. Redox proteomics analysis to decipher the neurobiology of Alzheimer-like neurodegeneration: overlaps in Down's syndrome and Alzheimer's disease brain. Biochem J. 2014;463:177–189. doi: 10.1042/BJ20140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cenini G, Dowling AL, Beckett TL, Barone E, Mancuso C, Murphy MP, Levine H, 3rd, Lott IT, Schmitt FA, Butterfield DA, Head E. Association between frontal cortex oxidative damage and beta-amyloid as a function of age in Down syndrome. Biochim Biophys Acta. 2012;1822:130–138. doi: 10.1016/j.bbadis.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jovanovic SV, Clements D, MacLeod K. Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radic Biol Med. 1998;25:1044–1048. doi: 10.1016/s0891-5849(98)00137-3. [DOI] [PubMed] [Google Scholar]
- 11.Di Domenico F, Pupo G, Tramutola A, Giorgi A, Schinina ME, Coccia R, Head E, Butterfield DA, Perluigi M. Redox proteomics analysis of HNE-modified proteins in Down syndrome brain: clues for understanding the development of Alzheimer disease. Free Radic Biol Med. 2014;71C:270–280. doi: 10.1016/j.freeradbiomed.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ripoll C, Dairou J, Stora S, Delabar JM, Janel N. Plasma nitrate levels are increased in adult Down syndrome patients. Biomarkers. 2013;18:373–374. doi: 10.3109/1354750X.2013.783117. [DOI] [PubMed] [Google Scholar]
- 13.Ferrando-Miguel R, Cheon MS, Yang JW, Lubec G. Overexpression of transcription factor BACH1 in fetal Down syndrome brain. J Neural Transm Suppl. 2003:193–205. doi: 10.1007/978-3-7091-6721-2_17. [DOI] [PubMed] [Google Scholar]
- 14.Patterson D. Molecular genetic analysis of Down syndrome. Hum Genet. 2009;126:195–214. doi: 10.1007/s00439-009-0696-8. [DOI] [PubMed] [Google Scholar]
- 15.Iannello RC, Crack PJ, de Haan JB, Kola I. Oxidative stress and neural dysfunction in Down syndrome. J Neural Transm Suppl. 1999;57:257–267. doi: 10.1007/978-3-7091-6380-1_17. [DOI] [PubMed] [Google Scholar]
- 16.Lubec G, Engidawork E. The brain in Down syndrome (TRISOMY 21) J Neurol. 2002;249:1347–1356. doi: 10.1007/s00415-002-0799-9. [DOI] [PubMed] [Google Scholar]
- 17.Patterson D, Costa AC. Down syndrome and genetics - a case of linked histories. Nat Rev Genet. 2005;6:137–147. doi: 10.1038/nrg1525. [DOI] [PubMed] [Google Scholar]
- 18.Cheon MS, Dierssen M, Kim SH, Lubec G. Protein expression of BACE1, BACE2 and APP in Down syndrome brains. Amino Acids. 2008;35:339–343. doi: 10.1007/s00726-007-0618-9. [DOI] [PubMed] [Google Scholar]
- 19.Cheon MS, Shim KS, Kim SH, Hara A, Lubec G. Protein levels of genes encoded on chromosome 21 in fetal Down syndrome brain: Challenging the gene dosage effect hypothesis (Part IV) Amino Acids. 2003;25:41–47. doi: 10.1007/s00726-003-0009-9. [DOI] [PubMed] [Google Scholar]
- 20.Cheon MS, Kim SH, Ovod V, Kopitar Jerala N, Morgan JI, Hatefi Y, Ijuin T, Takenawa T, Lubec G. Protein levels of genes encoded on chromosome 21 in fetal Down syndrome brain: challenging the gene dosage effect hypothesis (Part III) Amino Acids. 2003;24:127–134. doi: 10.1007/s00726-002-0340-6. [DOI] [PubMed] [Google Scholar]
- 21.Cheon MS, Bajo M, Kim SH, Claudio JO, Stewart AK, Patterson D, Kruger WD, Kondoh H, Lubec G. Protein levels of genes encoded on chromosome 21 in fetal Down syndrome brain: challenging the gene dosage effect hypothesis (Part II) Amino Acids. 2003;24:119–125. doi: 10.1007/s00726-002-0337-1. [DOI] [PubMed] [Google Scholar]
- 22.Cheon MS, Kim SH, Yaspo ML, Blasi F, Aoki Y, Melen K, Lubec G. Protein levels of genes encoded on chromosome 21 in fetal Down syndrome brain: challenging the gene dosage effect hypothesis (Part I) Amino Acids. 2003;24:111–117. doi: 10.1007/s00726-002-0336-2. [DOI] [PubMed] [Google Scholar]
- 23.Ferrando-Miguel R, Cheon MS, Lubec G. Protein levels of genes encoded on chromosome 21 in fetal Down Syndrome brain (Part V): overexpression of phosphatidyl-inositol-glycan class P protein (DSCR5) Amino Acids. 2004;26:255–261. doi: 10.1007/s00726-004-0065-9. [DOI] [PubMed] [Google Scholar]
- 24.Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Sakoda E, Igarashi K, Sun J, Kurisu K, Tashiro S. Regulation of heme oxygenase-1 by transcription factor Bach1 in the mouse brain. Neurosci Lett. 2008;440:160–165. doi: 10.1016/j.neulet.2008.04.082. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa M, Numazawa S, Yoshida T. Redox regulation of the transcriptional repressor Bach1. Free Radic Biol Med. 2005;38:1344–1352. doi: 10.1016/j.freeradbiomed.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 29.Mancuso C, Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Curr Drug Metab. 2009;10:579–594. doi: 10.2174/138920009789375405. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi G, Yoshida T, Noguchi M. Heme oxygenase and heme degradation. Biochem Biophys Res Commun. 2005;338:558–567. doi: 10.1016/j.bbrc.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Maines MD. The heme oxygenase system and its functions in the brain. Cell Mol Biol (Noisy-le-grand) 2000;46:573–585. [PubMed] [Google Scholar]
- 32.Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Maines MD. New insights into biliverdin reductase functions: linking heme metabolism to cell signaling. Physiology (Bethesda) 2005;20:382–389. doi: 10.1152/physiol.00029.2005. [DOI] [PubMed] [Google Scholar]
- 34.Pereira PJ, Macedo-Ribeiro S, Parraga A, Perez-Luque R, Cunningham O, Darcy K, Mantle TJ, Coll M. Structure of human biliverdin IXbeta reductase, an early fetal bilirubin IXbeta producing enzyme. Nat Struct Biol. 2001;8:215–220. doi: 10.1038/84948. [DOI] [PubMed] [Google Scholar]
- 35.Stocker R, Ames BN. Potential role of conjugated bilirubin and copper in the metabolism of lipid peroxides in bile. Proc Natl Acad Sci U S A. 1987;84:8130–8134. doi: 10.1073/pnas.84.22.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 37.Mancuso C, Bonsignore A, Di Stasio E, Mordente A, Motterlini R. Bilirubin and S-nitrosothiols interaction: evidence for a possible role of bilirubin as a scavenger of nitric oxide. Biochem Pharmacol. 2003;66:2355–2363. doi: 10.1016/j.bcp.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Mancuso C, Bonsignore A, Capone C, Di Stasio E, Pani G. Albumin-bound bilirubin interacts with nitric oxide by a redox mechanism. Antioxid Redox Signal. 2006;8:487–494. doi: 10.1089/ars.2006.8.487. [DOI] [PubMed] [Google Scholar]
- 39.Mancuso C, Barone E, Guido P, Miceli F, Di Domenico F, Perluigi M, Santangelo R, Preziosi P. Inhibition of lipid peroxidation and protein oxidation by endogenous and exogenous antioxidants in rat brain microsomes in vitro. Neurosci Lett. 2012;518:101–105. doi: 10.1016/j.neulet.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 40.Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 41.Barone E, Trombino S, Cassano R, Sgambato A, De Paola B, Di Stasio E, Picci N, Preziosi P, Mancuso C. Characterization of the S-denitrosylating activity of bilirubin. J Cell Mol Med. 2009;13:2365–2375. doi: 10.1111/j.1582-4934.2008.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibbs PE, Miralem T, Lerner-Maramarosh N, Tudor C, Maines MD. Formation of a ternary complex of human biliverdin reductase/ PKC-delta/ERK2 is essential for ERK2-mediated activation of Elk1, NF-kappaB and iNOS. J Biol Chem. 2011 doi: 10.1074/jbc.M111.279612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barone E, Mancuso C, Di Domenico F, Sultana R, Murphy MP, Head E, Butterfield DA. Biliverdin reductase-A: a novel drug target for atorvastatin in a dog pre-clinical model of Alzheimer disease. J Neurochem. 2012;120:135–146. doi: 10.1111/j.1471-4159.2011.07538.x. [DOI] [PubMed] [Google Scholar]
- 44.Barone E, Di Domenico F, Cenini G, Sultana R, Coccia R, Preziosi P, Perluigi M, Mancuso C, Butterfield DA. Oxidative and nitrosative modifications of biliverdin reductase-A in the brain of subjects with Alzheimer's disease and amnestic mild cognitive impairment. J Alzheimers Dis. 2011;25:623–633. doi: 10.3233/JAD-2011-110092. [DOI] [PubMed] [Google Scholar]
- 45.Barone E, Di Domenico F, Cenini G, Sultana R, Cini C, Preziosi P, Perluigi M, Mancuso C, Butterfield DA. Biliverdin reductase--a protein levels and activity in the brains of subjects with Alzheimer disease and mild cognitive impairment. Biochim Biophys Acta. 2011;1812:480–487. doi: 10.1016/j.bbadis.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barone E, Di Domenico F, Mancuso C, Butterfield DA. The Janus face of the heme oxygenase/biliverdin reductase system in Alzheimer disease: it's time for reconciliation. Neurobiol Dis. 2014;62:144–159. doi: 10.1016/j.nbd.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Domenico F, Coccia R, Cocciolo A, Murphy MP, Cenini G, Head E, Butterfield DA, Giorgi A, Schinina ME, Mancuso C, Cini C, Perluigi M. Impairment of proteostasis network in Down syndrome prior to the development of Alzheimer's disease neuropathology: redox proteomics analysis of human brain. Biochim Biophys Acta. 2013;1832:1249–1259. doi: 10.1016/j.bbadis.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perluigi M, Pupo G, Tramutola A, Cini C, Coccia R, Barone E, Head E, Butterfield DA, Di Domenico F. Neuropathological role of PI3K/Akt/mTOR axis in Down syndrome brain. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbadis.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reinholdt LG, Ding Y, Gilbert GJ, Czechanski A, Solzak JP, Roper RJ, Johnson MT, Donahue LR, Lutz C, Davisson MT. Molecular characterization of the translocation breakpoints in the Down syndrome mouse model Ts65Dn. Mamm Genome. 2011;22:685–691. doi: 10.1007/s00335-011-9357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowes C, Li T, Frankel WN, Danciger M, Coffin JM, Applebury ML, Farber DB. Localization of a retroviral element within the rd gene coding for the beta subunit of cGMP phosphodiesterase. Proc Natl Acad Sci U S A. 1993;90:2955–2959. doi: 10.1073/pnas.90.7.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foppoli C, De Marco F, Blarzino C, Perluigi M, Cini C, Coccia R. Biological response of human diploid keratinocytes to quinone-producing compounds: role of NAD(P)H:quinone oxidoreductase 1. Int J Biochem Cell Biol. 2005;37:852–863. doi: 10.1016/j.biocel.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Raina AK, Templeton DJ, Deak JC, Perry G, Smith MA. Quinone reductase (NQO1), a sensitive redox indicator, is increased in Alzheimer's disease. Redox Rep. 1999;4:23–27. doi: 10.1179/135100099101534701. [DOI] [PubMed] [Google Scholar]
- 53.Zenke-Kawasaki Y, Dohi Y, Katoh Y, Ikura T, Ikura M, Asahara T, Tokunaga F, Iwai K, Igarashi K. Heme induces ubiquitination and degradation of the transcription factor Bach1. Mol Cell Biol. 2007;27:6962–6971. doi: 10.1128/MCB.02415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gardiner K, Davisson M. The sequence of human chromosome 21 and implications for research into Down syndrome. Genome Biol. 2000;1 doi: 10.1186/gb-2000-1-2-reviews0002. REVIEWS0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirose W, Ikematsu K, Tsuda R. Age-associated increases in heme oxygenase-1 and ferritin immunoreactivity in the autopsied brain. Leg Med (Tokyo) 2003;5(Suppl 1):S360–S366. doi: 10.1016/s1344-6223(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 56.Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, Butterfield DA. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic Biol Med. 2012;52:2292–2301. doi: 10.1016/j.freeradbiomed.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, Kanski J, Pennisi G, Mancuso C, Stella AM, Butterfield DA. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer's disease. Antioxid Redox Signal. 2006;8:1975–1986. doi: 10.1089/ars.2006.8.1975. [DOI] [PubMed] [Google Scholar]
- 58.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 59.Dinkova-Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Domenico F, Barone E, Mancuso C, Perluigi M, Cocciolo A, Mecocci P, Butterfield DA, Coccia R. HO-1/BVR-a system analysis in plasma from probable Alzheimer's disease and mild cognitive impairment subjects: a potential biochemical marker for the prediction of the disease. J Alzheimers Dis. 2012;32:277–289. doi: 10.3233/JAD-2012-121045. [DOI] [PubMed] [Google Scholar]
- 61.Chandana R, Mythri RB, Mahadevan A, Shankar SK, Srinivas Bharath MM. Biochemical analysis of protein stability in human brain collected at different post-mortem intervals. Indian J Med Res. 2009;129:189–199. [PubMed] [Google Scholar]
- 62.Ball MJ, Nuttall K. Neurofibrillary tangles, granulovacuolar degeneration, and neuron loss in Down Syndrome: quantitative comparison with Alzheimer dementia. Ann Neurol. 1980;7:462–465. doi: 10.1002/ana.410070512. [DOI] [PubMed] [Google Scholar]
- 63.Bush A, Beail N. Risk factors for dementia in people with down syndrome: issues in assessment and diagnosis. Am J Ment Retard. 2004;109:83–97. doi: 10.1352/0895-8017(2004)109<83:RFFDIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 64.Lott IT, Head E. Down syndrome and Alzheimer's disease: a link between development and aging. Ment Retard Dev Disabil Res Rev. 2001;7:172–178. doi: 10.1002/mrdd.1025. [DOI] [PubMed] [Google Scholar]
- 65.Lott IT. Down's syndrome, aging, and Alzheimer's disease: a clinical review. Ann N Y Acad Sci. 1982;396:15–27. doi: 10.1111/j.1749-6632.1982.tb26840.x. [DOI] [PubMed] [Google Scholar]
- 66.Butterfield DA, Swomley AM, Sultana R. Amyloid beta-peptide (1–42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal. 2013;19:823–835. doi: 10.1089/ars.2012.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cocciolo A, Di Domenico F, Coccia R, Fiorini A, Cai J, Pierce WM, Mecocci P, Butterfield DA, Perluigi M. Decreased expression and increased oxidation of plasma haptoglobin in Alzheimer disease: Insights from redox proteomics. Free Radic Biol Med. 2012;53:1868–1876. doi: 10.1016/j.freeradbiomed.2012.08.596. [DOI] [PubMed] [Google Scholar]
- 68.Perluigi M, Swomley AM, Butterfield DA. Redox proteomics and the dynamic molecular landscape of the aging brain. Ageing Res Rev. 2014;13C:75–89. doi: 10.1016/j.arr.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Sultana R, Butterfield DA. Oxidative modification of brain proteins in Alzheimer's disease: perspective on future studies based on results of redox proteomics studies. J Alzheimers Dis. 2013;33(Suppl 1):S243–S251. doi: 10.3233/JAD-2012-129018. [DOI] [PubMed] [Google Scholar]
- 70.Perluigi M, Coccia R, Butterfield DA. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: a toxic combination illuminated by redox proteomics studies. Antioxid Redox Signal. 2012;17:1590–1609. doi: 10.1089/ars.2011.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer's disease. Neuroscience. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 72.Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 73.Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 74.Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- 75.Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- 76.Kedziora J, Bartosz G. Down's syndrome: a pathology involving the lack of balance of reactive oxygen species. Free Radic Biol Med. 1988;4:317–330. doi: 10.1016/0891-5849(88)90052-4. [DOI] [PubMed] [Google Scholar]
- 77.Lott IT, Head E, Doran E, Busciglio J. Beta-amyloid, oxidative stress and down syndrome. Curr Alzheimer Res. 2006;3:521–528. doi: 10.2174/156720506779025305. [DOI] [PubMed] [Google Scholar]
- 78.Perluigi M, Di Domenico F, Buttterfield DA. Unraveling the complexity of neurodegeneration in brain of subjects with down syndrome: insights from proteomics. Proteomics Clin Appl. 2013 doi: 10.1002/prca.201300066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perluigi M, Butterfield DA. The identification of protein biomarkers for oxidative stress in Down syndrome. Expert Rev Proteomics. 2011;8:427–429. doi: 10.1586/epr.11.36. [DOI] [PubMed] [Google Scholar]
- 80.Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J Gerontol A Biol Sci Med Sci. 2004;59:478–493. doi: 10.1093/gerona/59.5.m478. [DOI] [PubMed] [Google Scholar]
- 81.Ota K, Dohi Y, Brydun A, Nakanome A, Ito S, Igarashi K. Identification of senescence-associated genes and their networks under oxidative stress by the analysis of Bach1. Antioxid Redox Signal. 2011;14:2441–2451. doi: 10.1089/ars.2010.3574. [DOI] [PubMed] [Google Scholar]
- 82.Palozza P, Serini S, Curro D, Calviello G, Igarashi K, Mancuso C. beta-Carotene and cigarette smoke condensate regulate heme oxygenase-1 and its repressor factor Bach1: relationship with cell growth. Antioxid Redox Signal. 2006;8:1069–1080. doi: 10.1089/ars.2006.8.1069. [DOI] [PubMed] [Google Scholar]
- 83.Blouin JL, Duriaux Sail G, Guipponi M, Rossier C, Pappasavas MP, Antonarakis SE. Isolation of the human BACH1 transcription regulator gene, which maps to chromosome 21q22.1. Hum Genet. 1998;102:282–288. doi: 10.1007/s004390050692. [DOI] [PubMed] [Google Scholar]
- 84.Ogawa K, Sun J, Taketani S, Nakajima O, Nishitani C, Sassa S, Hayashi N, Yamamoto M, Shibahara S, Fujita H, Igarashi K. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J. 2001;20:2835–2843. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hira S, Tomita T, Matsui T, Igarashi K, Ikeda-Saito M. Bach1, a heme-dependent transcription factor, reveals presence of multiple heme binding sites with distinct coordination structure. IUBMB Life. 2007;59:542–551. doi: 10.1080/15216540701225941. [DOI] [PubMed] [Google Scholar]
- 86.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 87.Gibbs PE, Miralem T, Maines MD. Characterization of the human biliverdin reductase gene structure and regulatory elements: promoter activity is enhanced by hypoxia and suppressed by TNF-alpha-activated NF-kappaB. FASEB J. 2010;24:3239–3254. doi: 10.1096/fj.09-144592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Panahian N, Huang T, Maines MD. Enhanced neuronal expression of the oxidoreductase--biliverdin reductase--after permanent focal cerebral ischemia. Brain Res. 1999;850:1–13. doi: 10.1016/s0006-8993(99)01726-6. [DOI] [PubMed] [Google Scholar]
- 89.Panahian N, Maines MD. Assessment of induction of biliverdin reductase in a mouse model of middle cerebral artery occlusion. Brain Res Brain Res Protoc. 2000;6:53–70. doi: 10.1016/s1385-299x(00)00039-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.