Abstract
We present a case of a middle-aged male who manifested with low-grade fever and lower back pain. MRI and bone scan of the spine were suggestive of vertebral osteomyelitis. Blood cultures were persistently positive for Enterococcus faecalis and echocardiogram revealed tricuspid valve endocarditis. There was no history of IV drug use and urine toxicology was negative. EKG showed Mobitz type II AV block and a transesophageal echocardiogram revealed no valve ring or septal abscesses. The heart block persisted despite antibiotic therapy and an epicardial pacemaker was placed. This is a rare presentation of high-grade AV block with tricuspid endocarditis in the absence of echocardiographic evidence of perivalvular extension of infection. Also, unique in this case is the finding of E. faecalis hematogenous vertebral osteomyelitis.
Keywords: tricuspid valve endocarditis, Mobitz type II AV block, Enterococcus faecalis, vertebral osteomyelitis
Tricuspid valve endocarditis (TVE) historically accounts for about 2.5–3% of all cases of infective endocarditis (1). It occurs predominantly in intravenous drug abusers and in patients with pacemakers, central venous lines, or congenital heart disease (1). It may be complicated with septicemia, tricuspid valve regurgitation, right heart failure, and pulmonary emboli phenomena (occur in 80% of these cases and vary from infiltrates, which may cavitate, and pleural effusions involving the lower lobes) (1). It is rarely associated with conduction abnormalities (2). Hematogenous osteomyelitis may be observed in association with infective endocarditis; however, this is uncommon when the isolated organism is Enterococcus spp. (3).
Case presentation
A 62-year-old African American man with a history of hypertension, coronary artery disease, compensated congestive heart failure, and paroxysmal atrial flutter presented to the outpatient clinic with a complaint of progressively worsening lower back pain of 2-month duration. He denied fever, night sweats, weight loss, or any neurological deficits.
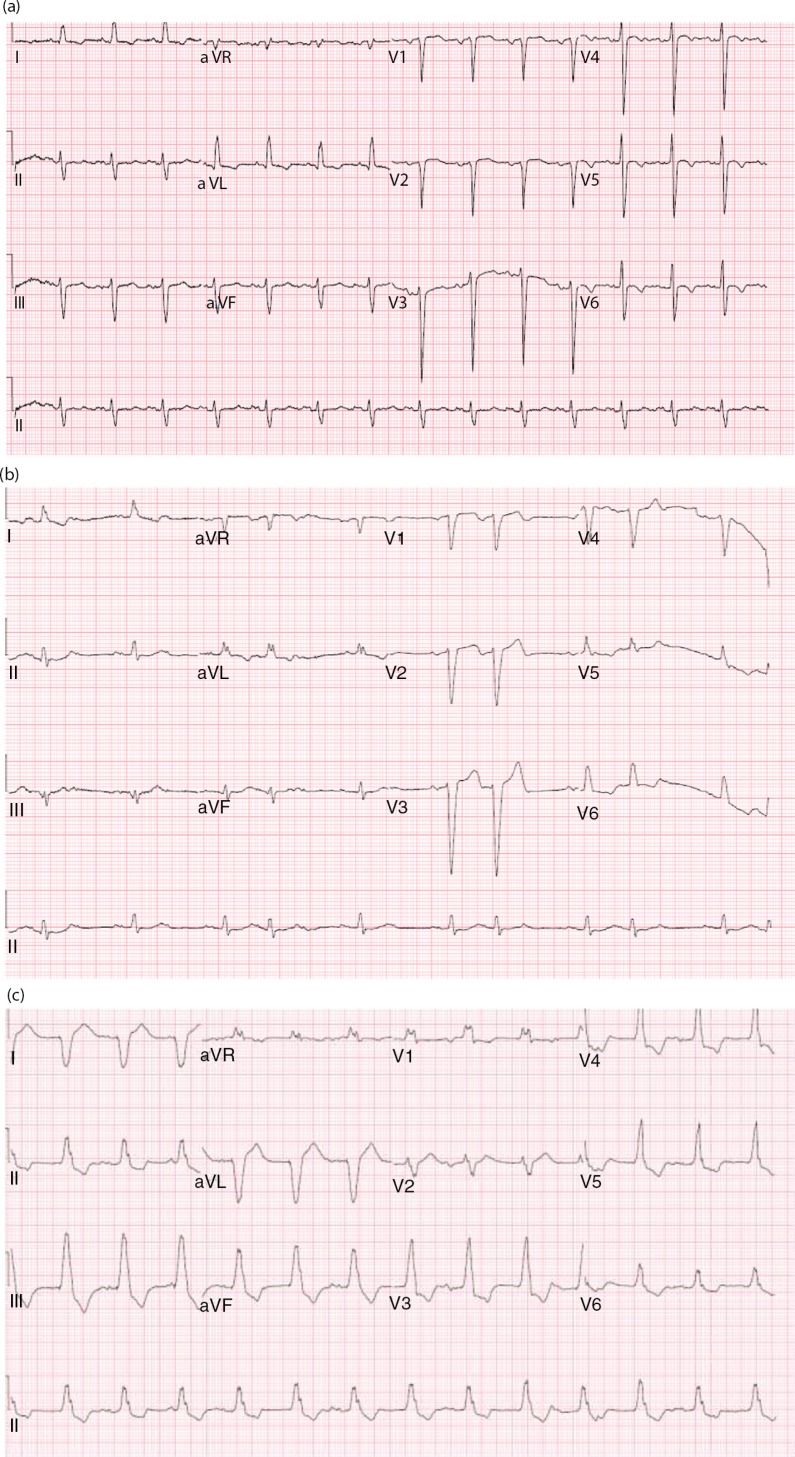
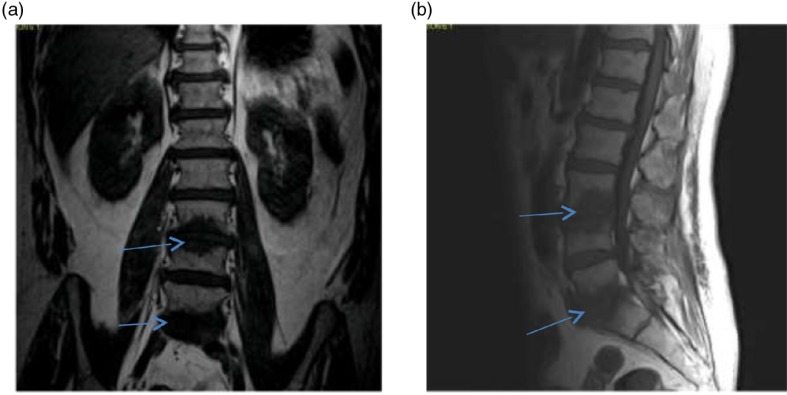
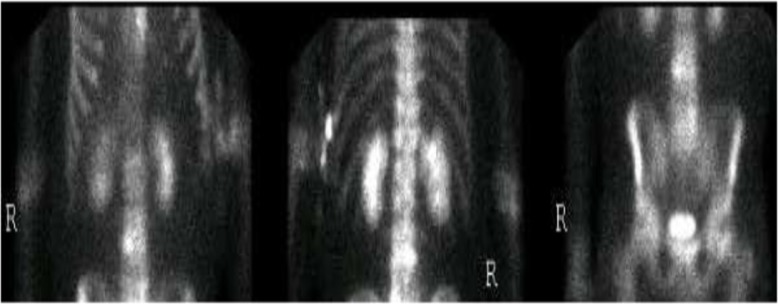
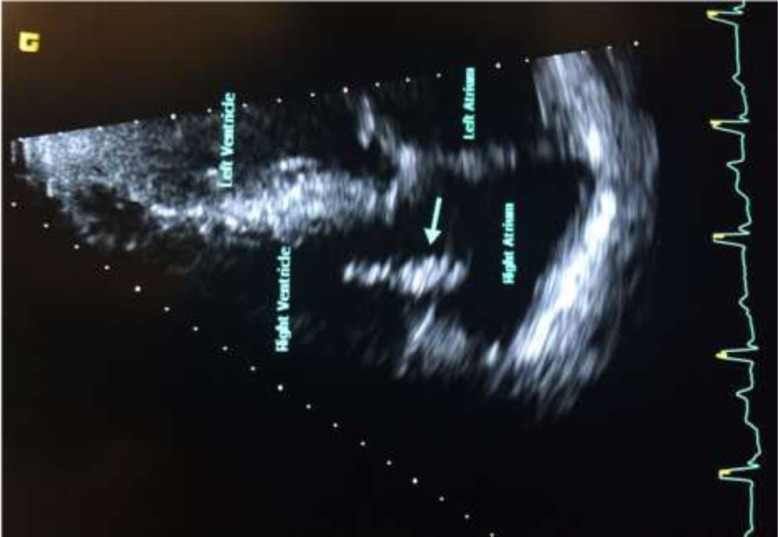
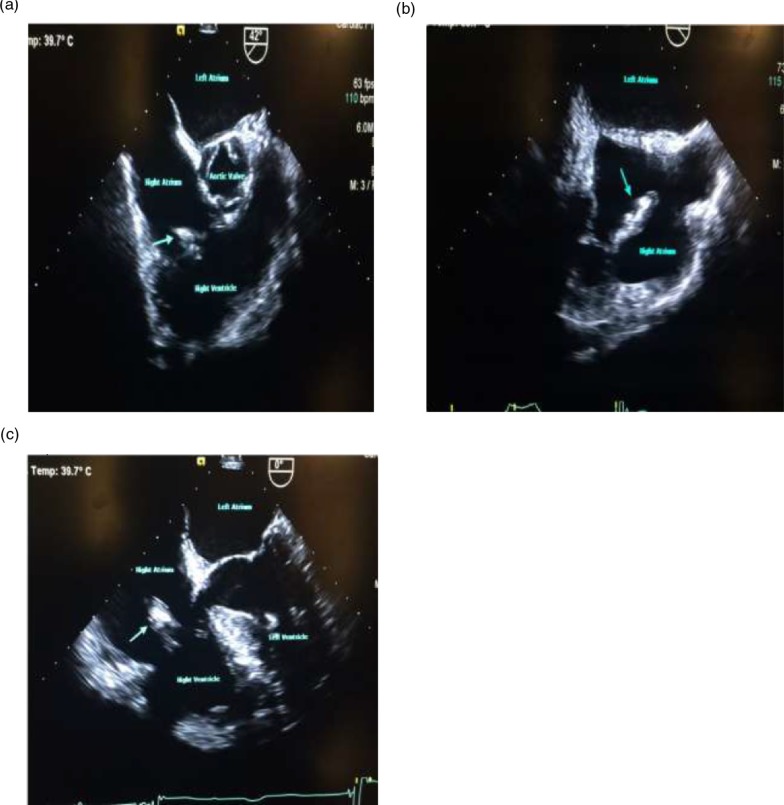
He also denied any history of IV drug use. Colonoscopy performed 5 years ago was unremarkable. Vitals signs were as follows: temperature 100.6°F; pulse 72 bpm; 20 breaths per minute; BP 148/75 mmHg, and SpO2 of 96% on room air. Physical examination was remarkable for a new systolic murmur (3/6) at the left sternal border with no radiation and tenderness to palpation and percussion of the lower lumbar vertebrae. Otherwise, the physical examination was unremarkable. Laboratory testing was unremarkable except for an elevated erythrocyte sedimentation rate of 99 mm/h (0–15 mm/h) and a C-reactive protein of 56.1 mg/dl (0–4.9 mg/dl). Urinalysis and urine toxicology were negative. HIV serology was also negative. EKG initially showed an old incomplete left bundle branch block (Fig. 1a); chest X-ray was normal and lumbar spine X-ray revealed degenerative disc disease with suspected discitis and spinal stenosis noted significantly at L3–L4, L4–L5, and L5–S1. MRI of the spine revealed L3–L4 and L5–S1 disc endplate changes with significant marrow edema suggestive of discitis and vertebral osteomyelitis (Fig. 2a and b). Three-phase bone scan also showed increased uptake at L3–L4 and L5–S1 (Fig. 3). Because of the presence of low-grade fever and a new murmur, transthoracic echocardiogram was done which showed tricuspid vegetation with moderate tricuspid regurgitation (Fig. 4). Three sets of blood cultures were positive for Enterococcus faecalis sensitive to ampicillin and streptomycin. There was no evidence of pulmonary septic emboli on chest imaging. Vertebral (bone) biopsy was suggested to the patient but he refused the procedure. Antibiotic therapy was instituted and planned for 6 weeks. Early during the course of antibiotic therapy, he developed light-headedness with persistent bradycardia but no persistent hypotension. A repeat EKG revealed Mobitz type II atrioventricular block (Fig. 1b). With the occurrence of conduction abnormalities, a transesophageal echocardiogram (TEE) was performed to identify any perivalvular extension of the infection. TEE revealed large fixed vegetation measuring 27×16 mm2 on the right atrial aspect of the tricuspid valve but no valve ring or septal abscess (Fig. 5a–c). Antibiotic therapy was continued with continuous cardiac monitoring; however, due to persistent symptomatic bradycardia, epicardial pacing was done (see Fig. 1c). Transvenous pacing route was not chosen due to the presence of significant tricuspid regurgitation and the risk of dislodgment of the large tricuspid vegetation. The procedure was well tolerated and the patient was later discharged home to continue long-term antibiotic therapy. At 2-month follow-up, the patient felt much better and his back pain was significantly improved.
Fig. 1.
(a) EKG on admission with an old incomplete left bundle branch block, (b) EKG showing second-degree AV block (2:1) – Mobitz type II and (c) EKG showing ventricular pacing.
Fig. 2.
(a, b) MRI of the lumbosacral spine showing a prominent marrow edema pattern adjacent to the L3–L4 and L5–S1 suggestive of osteomyelitis/discitis (see arrows): (a) coronal T1-weighted image and (b) sagittal T1-weighted image.
Fig. 3.
Three-phase bone scan showing focal abnormal radiotracer accumulation at the L3–L4 and L5–S1 intervertebral disc level suspicious for osteomyelitis/discitis.
Fig. 4.
Transthoracic echocardiogram (four-chamber view) showing vegetation on tricuspid valve (see arrow).
Fig. 5.
(a, b, and c) Transesophageal echocardiogram showing large vegetation on tricuspid valve (see arrows). No paravalvular complications visualized.
Discussion
We present a case of TVE complicated by persistent high-degree AV block. TVE in our case was not associated with traditional risk factors such as IV drug use, pacemakers, central venous catheters, or congenital heart disease. New conduction abnormalities are generally an uncommon manifestation of native valve endocarditis (2). They tend to occur in only about 9% of cases and are associated with worse prognosis and higher mortality (2, 4). Patients with persistent conduction abnormalities despite antibiotic therapy have even poorer prognosis than those with transient conduction abnormalities (4). They are commonly believed to be caused by spread of infection beyond the free valve area, involving the conduction pathways and thus are generally associated with perivalvular complications (2, 5). High-grade AV blocks are most commonly associated with left-sided endocarditis (aortic, 36%; mitral, 33%) and very rarely with tricuspid endocarditis (2, 4). A study by Weisse and Khan (5) has shown that patients who developed new second- or third-degree heart block or fascicular blocks had complications like valve ring abscesses, interatrial, interventricular septal abscesses, or mycotic aneurysms of the sinuses of Valsalva. Only a few cases of new conduction defects associated with TVE have been reported in the medical literature and in most cases they are transient and resolve with antibiotic therapy without the need for permanent pacemaker placement. Martínez-Urueña et al. (2) reported a case of tricuspid endocarditis with complete AVB and left bundle-branch block that was reversed upon treatment with antibiotics. The rare association of TVE with conduction defects may be best explained by the anatomic relationship of these respective valves with the triangle of Koch. The triangle of Koch is a triangular area located on the septal wall of the right atrium and roughly bounded by the septal leaflet of the tricuspid valve, the coronary sinus, and the membranous part of the interatrial septum (6). The atrioventricular node lies at the apex of the triangle (at the interatrial septum) and is anatomically more closely related to the non-coronary aortic leaflet and the anterior mitral leaflet than to the more distant tricuspid valve which lies at the base of the triangle (2, 6). Therefore, perivalvular infections resulting from aortic or mitral valve endocarditis are more likely to cause severe conduction defects than with TVE. Conduction abnormalities in our patient may be due to perivalvular inflammation affecting the conducting pathways, as no abscesses were identified on TEE. We therefore recommend that patients diagnosed with tricuspid endocarditis be monitored for conduction abnormalities. We also highlight the co-occurrence of vertebral osteomyelitis/discitis. It is relatively common for infectious endocarditis and osteomyelitis to coexist likely secondary to hematogenous spread of infection to the vertebrae (3) and vice versa. However, hematogenous enterococcus vertebral osteomyelitis/discitis is uncommon. Known risk factors for enterococcus bacteremia include intravascular devices; intra-abdominal, urinary tract, or wound Infections; and gastrointestinal or genitourinary procedures (3). As in many cases, in our patient the primary site of infection could not be determined. The most common pathogen implicated in hematogenous vertebral osteomyelitis is Staphylococcus aureus. Other commonly isolated organisms include enteric Gram-negative bacilli, Pseudomonas aeruginosa, Candida spp., and groups B and G hemolytic streptococci (7). We recommend that all patients with bacteremia and new or worsening back pain be worked up for vertebral osteomyelitis.
Conflict of interest and funding
There are no conflicts of interest to declare.
References
- 1.Heydari AA, Safari H, Sarvghad MR. Isolated tricuspid valve endocarditis. Int J Infect Dis. 2008;13(3):e109–11. doi: 10.1016/j.ijid.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Martínez-Urueña N, Hernández C, Duro IC, Sandín MG, Zatarain E, San Román A. Transient trifascicular block secondary to tricuspid valve endocarditis. Rev Esp Cardiol. 2012;65:767–8. doi: 10.1016/j.recesp.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Marella PC, Hasan S, Habte-Gabr E. Report of 2 cases of vertebral osteomyelitis/discitis caused by Enterococcus faecalis in dialysis patients. Infect Dis Clin Pract. 2007;15(3):199–200. [Google Scholar]
- 4.DiNubile MJ, Calderwood SB, Steinhaus DM, Karchmer AW. Cardiac conduction abnormalities complicating native valve active infective endocarditis. Am J Cardiol. 1986;58(13):1213–17. doi: 10.1016/0002-9149(86)90384-x. [DOI] [PubMed] [Google Scholar]
- 5.Weisse AB, Khan MY. The relationship between new cardiac conduction defects and extension of valve infection in native valve endocarditis. Clin Cardiol. 1990;13(5):337–45. doi: 10.1002/clc.4960130507. [DOI] [PubMed] [Google Scholar]
- 6.Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, et al. Harrison's principles of internal medicine. 17th ed. New York: McGraw-Hill; 2008. [Google Scholar]
- 7.Ülçay A, Karagöz E, Aribal S, Turhan V, Sari S, Akarsu S. A rare case of vertebral osteomyelitis developing after enterococcal bacteremia in a geriatric patient. Türk Fiz TIp Rehab Derg. 2014;60:348–52. [Google Scholar]