Abstract
Secondary aortoenteric fistula (SAEF) is a rare yet lethal cause of gastrointestinal bleeding and occurs as a complication of an abdominal aortic aneurysm repair. Clinical presentation may vary from herald bleeding to overt sepsis and requires high index of suspicion and clinical judgment to establish diagnosis. Initial diagnostic tests may include computerized tomography scan and esophagogastroduodenoscopy. Each test has variable sensitivity and specificity. Maintaining the hemodynamic status, control of bleeding, removal of the infected graft, and infection control may improve clinical outcomes. This review entails the updated literature on diagnosis and management of SAEF. A literature search was conducted for articles published in English, on PubMed and Scopus using the following search terms: secondary, aortoenteric, aorto-enteric, aortoduodenal, aorto-duodenal, aortoesophageal, and aorto-esophageal. A combination of MeSH terms and Boolean operators were used to device search strategy. In addition, a bibliography of clinically relevant articles was searched to find additional articles (Appendix A). The aim of this review is to provide a comprehensive update on the diagnosis, management, and prognosis of SAEF.
Keywords: aortoenteric fistula, aortoduodenal fistula, gastrointestinal bleeding, abdominal aortic aneurysm
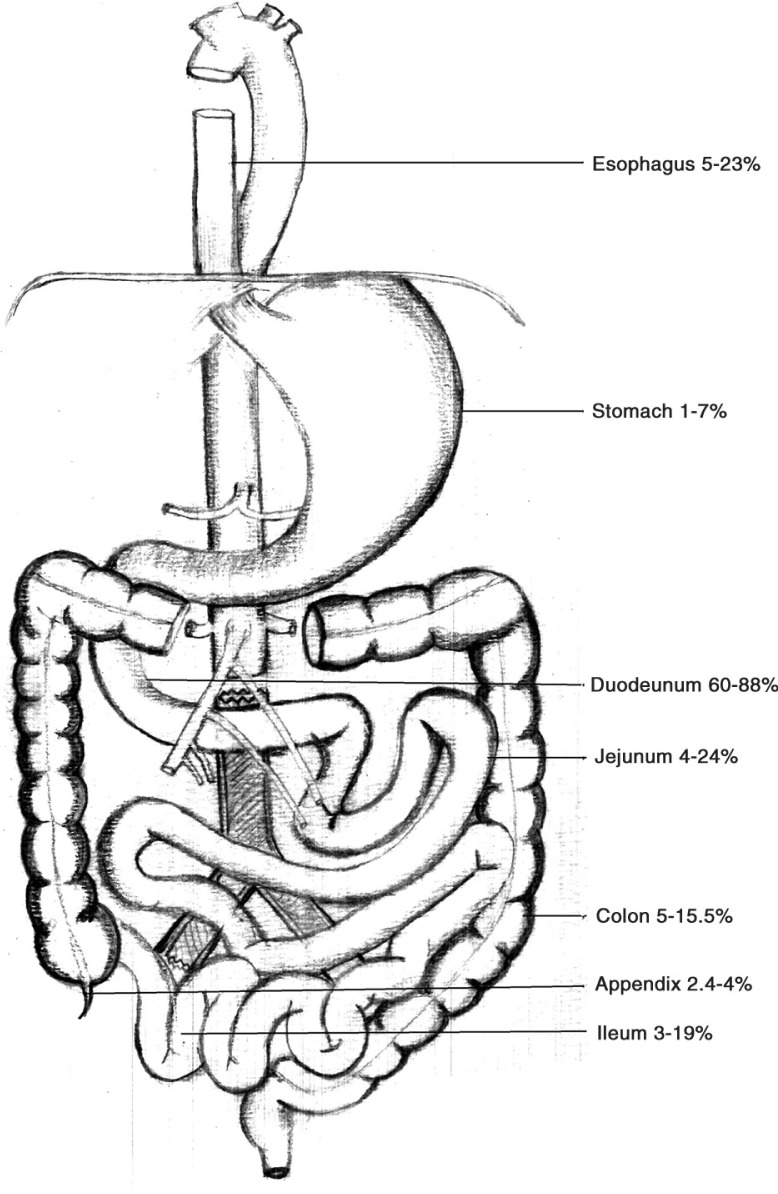
An aortoenteric fistula (AEF) is defined as a conduit between an aortic aneurysm and the gastrointestinal (GI) tract (1). Primary AEF (PAEF) exists in patients without a pre-existing abdominal aortic aneurysm (AAA) repair. On the other hand, secondary aortoenteric fistula (SAEF) occurs as a complication of an AAA repair (2–5). The abdominal aorta is more likely to be involved than the thoracic aorta (56 vs. 44%) (6). In one-third of the cases, the abdominal aorta forms a channel with the duodenum (7). This is secondary to the anatomical position of the third part of the duodenum, which lies between the superior mesenteric artery and the abdominal aorta (8–10). Rarely, conduit may involve ascending, transverse, sigmoid colon, or rectum (11) (Fig. 1). A PAEF occurs in the setting of an unrepaired aortic aneurysm sac that erodes into the GI lumen, in the presence of predisposing factors such as atherosclerosis (60–80% of cases), infections (e.g., syphilis and tuberculosis), or mechanical stress (e.g., biliary calculi, ulcers, or radiation) (2, 21, 22). Conversely, a SAEF occurs in patients with a previous AAA repair that may have a concurrent infection of the graft, hence leading to duodenal wall weakening and formation of a fistula or track. It is suggested that in the case of SAEF, the aortic pulsations, in some instances, may form a pseudoaneurysm. Each pulsation may produce shear stress on the GI mucosa, resulting in erosion (23). The close proximity of the graft with the duodenum may hasten the migration of bowel flora. In most cases, the portal of entry is the suture line (24). The infection may also be introduced at the time of AAA repair. The prosthetic graft may be colonized by Staphylococcus epidermidis – a skin commensal known to form biofilm on prosthetic material. It may gain entry into the blood stream at the time of surgical repair, what is known as primary contamination (3). Given the complicated surgical course and proximity of the bowel flora, it is not uncommon that blood and endovascular graft cultures may identify diverse microbes including Gram-positive organisms, Gram-negative organisms, and anaerobes (gut flora leading to secondary contamination). In some cases, no organism is identified on the culture (23). Other organisms that have been isolated via different techniques include Enterobacter, E. coli, Pseudomonas, Klebsiella, Streptococcus, and methicillin-resistant Staphylococcus aureus (MRSA), Listeria, Chlamydia, Clostridium, Propionibacterium, and Candida (12–14, 25–28) (Table 1).
Fig. 1.
The pooled incidence of secondary aortoenteric fistula at various anatomical locations (4, 5, 7, 8, 11–20).
Table 1.
Secondary aortoenteric fistula: causative organism and clinical features
| Causative organisms (2, 12, 13, 15, 17, 18, 23, 29–31) | Staphylococcus epidermidis | 12–21% | |
| Staphylococcus aureus coagulase negative | 9–41% | ||
| Methicillin-resistant Staphylococcus aureus (MRSA) | 4–19% | ||
| Enterobacter | 9–38% | ||
| E. coli | 6–52% | ||
| Salmonella | |||
| Pseudomonas | 9–11% | ||
| Clostridium | |||
| Bacteroides | |||
| Proteus | 14% | ||
| Klebsiella | 14–17% | ||
| Enterococcus | 12% | ||
| Streptococcus | 14–21% | ||
| Aspergillus | |||
| Polymicrobial | 40–56% | ||
| Candida species | 18–25% | ||
| Clinical features (2, 3, 7, 8, 12, 23, 15, 16, 18, 19, 29, 31–34) | Hemorrhagic shock |
|
18–100% |
| Bright red blood per rectum | |||
| Coffee ground emesis | |||
| Herald bleeding | |||
| Melena | |||
| Fever |
|
30.3–87% | |
| Sepsis | |||
| Abscess | 8–11% | ||
| Pulsating abdominal mass | 5–18.2% | ||
| Groin mass | 12% | ||
| Limb ischemia | 18.2–30% | ||
| Pseudo aneurysm | |||
| Abdominal pain | 10–30% | ||
| Back pain | 5–10% | ||
| Graft occlusion | 21.2% | ||
| Femoral pseudoaneurysm | 5% | ||
| Weight loss | 15.2% | ||
Clinical features
A patient with a SAEF may present with upper or lower GI bleeding, sepsis, and/or hemodynamic instability (Table 1). By far the commonest reported presentation is GI bleeding, which may vary in its presentation from herald bleeding to overt hemorrhagic shock (8, 23, 29). Herald bleeding is an episode of transient or self-limiting early hemorrhage that may precede the ominous clinical hemorrhage. It may be present in approximately 20–75% of cases (8, 23). The onset of bleeding to the time of initial presentation may range from a few hours to a year (mean=3 days) (23). The classic triad of GI bleeding, palpable abdominal mass, and abdominal pain is an infrequent clinical presentation (35). After GI bleeding, sepsis or fever is the commonest presentation in patients with SAEF. In one study, GI bleeding occurred in 39/48 patients, whereas sepsis was the initial presentation in 20/48 patients (29). When looking at the patients with sepsis; fever, leukocytosis, wound infection, septic embolism, and hypotension were the frequent presentations (13). Other rare clinical features may include, groin infection, pseudoaneurysms, limb ischemia, retroperitoneal abscess, anorexia, malaise, and back pain (23, 29, 36).
Establishing the diagnosis
The diagnosis of a SAEF is based on clinical grounds aided by readily available, non-invasive, and safer radiographic modalities such as computerized tomography (CT) scan (2) (Table 2). In addition to identifying the location of the conduit, CT scan may aid in identification of an infection or abscess if present (37). The reported sensitivity and specificity of CT scan is approximately 90% (sensitivity 94% and specificity 85%) (26). In one retrospective study, CT was useful in establishing the diagnosis of SAEF in 93% (26/28) of cases (23). In a different study, abnormal CT findings were suggestive of SAEF in 92% (22/24) of patients and confirmatory in 8% (8/24) of patients (13). CT scan findings that may be indicative of an AEF include: a gas shadow in or around the graft (sensitivity 40% and specificity 100%), focal wall inflammation and thickening, visible graft (sensitivity 22% and specificity 100%), soft tissue collection around the aorta >5 mm (specificity 92% and sensitivity 90%), swelling or hematoma around the graft, intravenous contrast within the GI lumen or around the aorta, loss of calcification or tear in the aortic wall (specificity 75% and sensitivity 89%), pseudoaneurysm, and duodenal hematoma (2, 9, 32).
Table 2.
Secondary aortoenteric fistula: diagnostic assessment
| Diagnosis (2–5, 7, 9, 12, 13, 15, 18, 20, 23, 25, 29–34) | Exploratory laparotomy | Sensitivity and specificity 91–100% |
| Computerized tomography scan | Sensitivity 40–100%; specificity 33.3–100% | |
| Computerized tomography angiography | Sensitivity 33–100% | |
| EGD | Sensitivity 10–80%, specificity 8.3–75%, accuracy 30% | |
| Radionuclide scanning with technetium-99-labeled leukocytes | Sensitivity 0%, specificity 0–80% | |
| Nuclear medicine RBC | Sensitivity 0% |
Esophagogastroduodenoscopy (EGD) is recommended in patients with a history of an AAA repair and/or upper GI bleeding (8). An EGD may offer advantages in these particular patients when excluding other causes of GI bleeding (30). Findings suggestive of SAEF on EGD include visible graft, bleeding, adherent clot, or ulcer or pulsatile mass (13, 15). Considerable variation may exist in diagnostic yield of EGD. This variation may partly be explained by operative technique or experience. In a case series of patients with AEF (SAEF=25), an EGD established the diagnosis in 6/15 patients (40%) (24). In comparison, a study by Champion et al. (8) revealed a better diagnostic yield with EGD – 8/11 patients (73%). Because most of the AEFs occur in the third or the fourth parts of the duodenum, enteroscope or pediatric colonoscope may be utilized instead of the regular gastroscope to aid better visualization of distal duodenum and proximal jejunum (38, 39). Other modalities that may be used with variable success rate include magnetic resonance imaging, intravascular ultrasound, arteriography nuclear scans (e.g., tagged leukocyte scintigraphy), digital subtraction angiography, multidetector CT scan, and the single-photon emission computed tomography (for stent graft infection) (2, 27, 40) (Table 2).
The repair of SAEF
Optimal outcomes of SAEF depend on maintaining hemodynamic stability by aggressive blood loss control, surgical repair of the underlying defect, infection control via empiric intravenous antibiotics, and revascularization and maintenance of perfusion to the lower limb (2, 6). Factors that may govern clinical outcomes depends on timeliness of the procedure; the revascularization approach (open vs. in situ); type of surgery (emergent vs. non-emergent); or the type of the graft utilized. Currently, there are no established guidelines for repair of the SAEF. The open approach entails graft excision and extra-anatomic bypass, whereas the endovascular approach consists of endovascular in situ graft replacement and partial or complete graft excision. The open approach may be advantageous in the setting of an underlying infection when an infected endograft can safely be excised thus eradicating the source of infection. In addition, simultaneous staged bowel repair can be performed in such cases. The endovascular repair offers shorter duration of surgery and hence requires minimal anesthesia. In addition, this surgical technique avoids complications associated with clamping of the aorta such as thrombosis, infection, and decreased perfusion of the colon and lower limbs (41). In a multicenter retrospective study that compared endovascular (n=8) and open repair (n=17) for AEF (PAEF=1, SAEF=24), the overall morbidity was lower in patients who underwent endovascular repair when compared with the group that underwent an open repair (25 vs. 77% p=0.028) (16). On the other hand, although the endovascular repair may have lower perioperative mortality and hospital stay, there may be a risk of infection relapse secondary to incomplete removal of the infected graft (12). In one study, the risk of reinfection approached 44% (persistent, new, or recurrent infection) during a follow-up duration of 13 months (6). In a different study, no difference in mortality or reinfection was observed between in-situ revascularization and extra-anatomic reconstruction (15). Other factors that may influence mortality include emergent procedure, where 30-day mortality may be as high as 60% when compared to a non-emergent procedure (38%). Similarly, better outcomes were reported for a silver impregnated graft via in situ implantation when compared to the cohort who received a Dacron graft via extra-anatomic bypass (34.7 vs. 46.1%). In a recent report, in situ prosthetic graft excision and replacement with cryopreserved allograft was shown to have 25% postoperative mortality over the median duration of 31 months (17).
Management of sepsis
Broad-spectrum antibiotics that cover Gram-positive and Gram-negative organisms and anaerobes should be administered early. In addition, the source of infection, that is, the in situ aortic graft, has to be surgically excised, repaired, or replaced with the end goal of achieving prompt revascularization. Failure to treat sepsis may result in mortality in 60% of the cases (6). The selection of antibiotics is determined by the results of blood, wound, intraoperative drainage, and tissue specimen culture. Intravenous vancomycin, piperacillin/tazobactam, and gentamicin may provide empiric coverage against the majority of identified organisms (25, 17). Antibiotics should be tailored after the culture and sensitivity results. Intravenous antibiotics should be continued for at least 4–6 weeks, followed by long-term oral antibiotics such as amoxicillin–clavulanate, levofloxacin, or trimethoprim–sulfamethoxazole (31, 33). The C-reactive protein may help guide the duration of antibiotic and monitor the response of therapy (28).
Perioperative complications
Complications may depend on type of revascularization and can occur during the intraoperative period, the perioperative period, or at several months follow-up. Sepsis remains a life-threatening complication following endovascular AEF repair (42). Other life-threatening complications include uncontrolled bleeding, multiorgan failure, acute coronary event, limb ischemia leading to gangrene or amputation, liver failure, and acute respiratory failure (4, 29). In one study, extra-anatomic bypass failure occurred in 18.2% (6/33 of cases), lower extremity amputation in 9.1% (3/33 of cases), and extra-anatomic bypass infection in 15% (5/33 of cases) (13). When comparing complication rate between open [(n=17) vs. endovascular repair (n=8)], multiple organ failure occurred in 29% (5/17 cases) in the open group and none in the endovascular group. Recurrent bleeding occurred in 12% (2/17 cases) in the open group and none in the endovascular group. Lower extremity ischemia occurred in the 29% (5/17 cases) in the open group and 13% (1/8 cases) in the endovascular group. Loss of the lower extremity occurred in 12% (2/17 cases) in the open group and 13% (1/8 cases) in the endovascular group. Last, mortality was found to be higher in the open group 35% (6/17) when compared to the endovascular group 0% (16). These results may favor the use of endovascular repair over open repair.
Predictors of recurrent sepsis and overall survival
Clinical variables may prognosticate better or worse outcomes, and can provide clinicians and surgeons with goal-directed management for high-risk cases. Kakkos et al. (16) reported that patients taking two antibiotics at the time of discharge had a lower incidence of sepsis at 2 years (0%) when compared to a single antibiotic (63%) or no antibiotic regimen (100%). In a different study, a lower survival was reported in patients who had fever at the time of presentation [fever vs. no fever (11% vs. 52%, p=0.04) or preoperative sepsis (24 vs. 50% p=0.32)]. In another study, intraoperative factors associated with early mortality after repair included hemodynamic instability, the need for blood transfusion, and aortic clamp placement above the renal arteries (23). In a systematic review, preoperative infection [OR 9.36 (2.24–39.12)] and any complication after endovascular procedure [OR 80.75 (8.20–794.944)] were identified as predictors of adverse outcomes in the univariate analysis. Furthermore, patients in the infection group (persistent or recurrent) had a shorter (1 month) survival when compared to patients without infection (p=0.042) (6).
The type of grafts utilized during the surgical procedure may also affect clinical outcomes. Cryopreserved grafts compared to fresh allografts were shown to have reduced risk of endoleak, rupture, and other graft-related complications (41). Successful outcomes have been reported for rifampin-soaked endografts in a few instances (43). Surgical complications may further contribute to morbidity.
The SAEF may be associated with post-implantation syndrome (PIS) – a frequent occurrence associated with aortic graft placement. The PIS is characterized by fever, leukocytosis, and/or hypercoagulability without a source of infection confirmed by negative culture. This may contribute to morbidity and, therefore, result in a prolonged hospital stay (44, 45). Thus, the presence of infection and hemodynamic instability portends poor prognosis and may necessitate aggressive management.
Prognosis of SAEF
The prognosis in patients with SAEF may depend on the hemodynamic status of the patient at presentation, the operative technique performed, and time to surgical exploration. A delay in surgical exploration may increase mortality, whereas an uncorrected SAEF is almost always fatal (12). In one report, the mortality rate at the first month reached 45.8%, 34% at 3 years, and 27.4% at 5 years (29). In a different study, there were six perioperative and three mortalities after 1 month with an overall mortality rate of 27.2% over the course of 4.4±3.7 years (13). Similarly, in a different report, the mortality rate approached 40% in the first month (24). Nevertheless, the reported survival seems to be improving over the years. In a recent report by Schoell et al. (17), 1-month, 1-year, and 5-year survival was 75, 70, and 66%, respectively. These results are promising and suggest that a SAEF is not as ominous as previously thought.
Conclusions
A SAEF should be ruled out in a patient presenting with GI bleeding with prior history of AAA repair. Early recognition of symptoms may avoid the untoward hemodynamic instability. The management of SAEF requires a multidisciplinary approach involving the intensivists, vascular surgeons, and gastroenterologists. The use of CT scan may assist in establishing an early diagnosis. Endovascular approach in selected cases may ameliorate complications associated with open revascularization. Future research direction exploring the benefit of various types of endograft and surgical techniques may hold promise for providing better care to patients with this debilitating clinical entity.
Acknowledgement
We acknowledge Stephanie Gonzalez, MSHS, for her editorial support in preparation of this manuscript.
Conflict of interest and funding
We do not have any disclosures or conflicts of interest to reveal.
Appendix A- The Search Strategy
Pubmed
(secondary, arotoenteric, aorto-enteric) OR secondary, aortoduodenal, aorto-duodenal) OR secondary, aorto-esophageal, aortoesophageal)
((((“secondary”[Subheading] OR “secondary”[All Fields] OR “neoplasm metastasis”[MeSH Terms] OR (“neoplasm”[All Fields] AND “metastasis”[All Fields]) OR “neoplasm metastasis”[All Fields]) AND aorto-enteric[All Fields]) OR ((“secondary”[Subheading] OR “secondary”[All Fields] OR “neoplasm metastasis”[MeSH Terms] OR (“neoplasm”[All Fields] AND “metastasis”[All Fields]) OR “neoplasm metastasis”[All Fields]) AND aortoduodenal[All Fields] AND aorto-duodenal[All Fields])) OR ((“secondary”[Subheading] OR “secondary”[All Fields] OR “neoplasm metastasis”[MeSH Terms] OR (“neoplasm”[All Fields] AND “metastasis”[All Fields]) OR “neoplasm metastasis”[All Fields]) AND aorto-esophageal[All Fields] AND aortoesophageal[All Fields])) AND English[lang]
Scopus
Aortoenteric fistula, secondary aortoenteric fistula OR secondary, aortoduodenal, aorto-duodenal.
References
- 1.Tyrell T. The lectures on the principles and practice of surgery with additional notes and cases. London: Thomas & George Underwood; 1824. [Google Scholar]
- 2.Xiromeritis K, Dalainas I, Stamatakos M, Filis K. Aortoenteric fistulae: Present-day management. Int Surg. 2011;96:266–73. doi: 10.9738/0020-8868-96.3.266. [DOI] [PubMed] [Google Scholar]
- 3.Tagowski M, Vieweg H, Wissgott C, Andresen R. Aortoenteric fistula as a complication of open reconstruction and endovascular repair of abdominal aorta. Radiol Res Pract. 2014;2014:383159. doi: 10.1155/2014/383159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergqvist D, Alm A, Claes G, Drott C, Forsberg O, Larsson M, et al. Secondary aortoenteric fistulas – An analysis of 42 cases. Eur J Vasc Surg. 1987;1:11–18. doi: 10.1016/s0950-821x(87)80018-x. [DOI] [PubMed] [Google Scholar]
- 5.Bergqvist D, Bjorck M. Secondary arterioenteric fistulation – A systematic literature analysis. Eur J Vasc Endovasc Surg. 2009;37:31–42. doi: 10.1016/j.ejvs.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Antoniou GA, Koutsias S, Antoniou SA, Georgiakakis A, Lazarides MK, Giannoukas AD. Outcome after endovascular stent graft repair of aortoenteric fistula: A systematic review. J Vasc Surg. 2009;49:782–9. doi: 10.1016/j.jvs.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 7.Cendan JC, Thomas JB, Seeger JM. Twenty-one cases of aortoenteric fistula: Lessons for the general surgeon. Am Surg. 2004;70:583–7. [PubMed] [Google Scholar]
- 8.Champion MC, Sullivan SN, Coles JC, Goldbach M, Watson WC. Aortoenteric fistula. Incidence, presentation recognition, and management. Ann Surg. 1982;195:314–17. doi: 10.1097/00000658-198203000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes FM, Kavanagh D, Barry M, Owens A, MacErlaine DP, Malone DE. Aortoenteric fistula: A diagnostic dilemma. Abdom Imaging. 2007;32:398–402. doi: 10.1007/s00261-006-9062-7. [DOI] [PubMed] [Google Scholar]
- 10.Schapiro RL, Hahn FJ. Primary aortoduodenal fistula. A rare cause of acute gastrointestinal hemorrhage. JAMA. 1976;236:2541–2. doi: 10.1001/jama.236.22.2541. [DOI] [PubMed] [Google Scholar]
- 11.Leon LR, Jr, Mills JL, Sr, Psalms SB, Kasher J, Kim J, Ihnat DM. Aortic paraprosthetic-colonic fistulae: A review of the literature. Eur J Vasc Endovasc Surg. 2007;34:682–92. doi: 10.1016/j.ejvs.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Baril DT, Carroccio A, Ellozy SH, Palchik E, Sachdev U, Jacobs TS, et al. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg. 2006;44:250–7. doi: 10.1016/j.jvs.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Kuestner LM, Reilly LM, Jicha DL, Ehrenfeld WK, Goldstone J, Stoney RJ. Secondary aortoenteric fistula: Contemporary outcome with use of extraanatomic bypass and infected graft excision. J Vasc Surg. 1995;21:184–95. doi: 10.1016/s0741-5214(95)70261-x. [DOI] [PubMed] [Google Scholar]
- 14.La GG, Barbagallo F, Gagliardo S, Latteri S, Scala V, Sofia M, et al. Recurrent aortoduodenal fistula. Ann Vasc Surg. 2011;25:386. doi: 10.1016/j.avsg.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Batt M, Jean-Baptiste E, O'Connor S, Saint-Lebes B, Feugier P, Patra P, et al. Early and late results of contemporary management of 37 secondary aortoenteric fistulae. Eur J Vasc Endovasc Surg. 2011;41:748–57. doi: 10.1016/j.ejvs.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Kakkos SK, Antoniadis PN, Klonaris CN, Papazoglou KO, Giannoukas AD, Matsagkas MI, et al. Open or endovascular repair of aortoenteric fistulas? A multicentre comparative study. Eur J Vasc Endovasc Surg. 2011;41:625–34. doi: 10.1016/j.ejvs.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Schoell T, Manceau G, Chiche L, Gaudric J, Gibert H, Tresallet C, et al. Surgery for secondary aorto-enteric fistula or erosion (SAEFE) complicating aortic graft replacement: A retrospective analysis of 32 patients with particular focus on digestive management. World J Surg. 2015;39:283–91. doi: 10.1007/s00268-014-2750-5. [DOI] [PubMed] [Google Scholar]
- 18.Dorigo W, Pulli R, Azas L, Pratesi G, Innocenti AA, Pratesi C. Early and long-term results of conventional surgical treatment of secondary aorto-enteric fistula. Eur J Vasc Endovasc Surg. 2003;26:512–18. doi: 10.1016/s1078-5884(03)00379-4. [DOI] [PubMed] [Google Scholar]
- 19.O'Mara CS, Williams GM, Ernst CB. Secondary aortoenteric fistula. A 20 year experience. Am J Surg. 1981;142:203–9. doi: 10.1016/0002-9610(81)90275-0. [DOI] [PubMed] [Google Scholar]
- 20.Burks JA, Jr, Faries PL, Gravereaux EC, Hollier LH, Marin ML. Endovascular repair of bleeding aortoenteric fistulas: A 5-year experience. J Vasc Surg. 2001;34:1055–9. doi: 10.1067/mva.2001.119752. [DOI] [PubMed] [Google Scholar]
- 21.Reckless JP, McColl I, Taylor GW. Aorto-enteric fistulae: An uncommon complication of abdominal aortic aneurysms. Br J Surg. 1972;59:458–60. doi: 10.1002/bjs.1800590613. [DOI] [PubMed] [Google Scholar]
- 22.Voorhoeve R, Moll FL, de Letter JA, Bast TJ, Wester JP, Slee PH. Primary aortoenteric fistula: Report of eight new cases and review of the literature. Ann Vasc Surg. 1996;10:40–8. doi: 10.1007/BF02002340. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong PA, Back MR, Wilson JS, Shames ML, Johnson BL, Bandyk DF. Improved outcomes in the recent management of secondary aortoenteric fistula. J Vasc Surg. 2005;42:660–6. doi: 10.1016/j.jvs.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Moulton S, Adams M, Johansen K. Aortoenteric fistula. A 7 year urban experience. Am J Surg. 1986;151:607–11. doi: 10.1016/0002-9610(86)90566-0. [DOI] [PubMed] [Google Scholar]
- 25.Herdrich BJ, Fairman RM. How to manage infected aortic endografts. J Cardiovasc Surg (Torino) 2013;54:595–604. [PubMed] [Google Scholar]
- 26.Low RN, Wall SD, Jeffrey RB, Jr, Sollitto RA, Reilly LM, Tierney LM., Jr Aortoenteric fistula and perigraft infection: Evaluation with CT. Radiology. 1990;175:157–62. doi: 10.1148/radiology.175.1.2315475. [DOI] [PubMed] [Google Scholar]
- 27.Sharif MA, Lee B, Lau LL, Ellis PK, Collins AJ, Blair PH, et al. Prosthetic stent graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2007;46:442–8. doi: 10.1016/j.jvs.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Sharif MA, McDowell DA, Badger SA. Chlamydia pneumoniae antibodies and C-reactive protein levels in patients with abdominal aortic aneurysms. Scientific World J. 2013;2013:212450. doi: 10.1155/2013/212450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biro G, Szabo G, Fehervari M, Munch Z, Szeberin Z, Acsady G. Late outcome following open surgical management of secondary aortoenteric fistula. Langenbecks Arch Surg. 2011;396:1221–9. doi: 10.1007/s00423-011-0807-6. [DOI] [PubMed] [Google Scholar]
- 30.Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg. 2007;45:834–6. doi: 10.1016/j.jvs.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 31.Murphy EH, Szeto WY, Herdrich BJ, Jackson BM, Wang GJ, Bavaria JE, et al. The management of endograft infections following endovascular thoracic and abdominal aneurysm repair. J Vasc Surg. 2013;58:1179–85. doi: 10.1016/j.jvs.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 32.Hagspiel KD, Turba UC, Bozlar U, Harthun NL, Cherry KJ, Ahmed H, et al. Diagnosis of aortoenteric fistulas with CT angiography. J Vasc Interv Radiol. 2007;18:497–504. doi: 10.1016/j.jvir.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Lonn L, Dias N, Veith ST, Resch T. Is EVAR the treatment of choice for aortoenteric fistula? J Cardiovasc Surg (Torino) 2010;51:319–27. [PubMed] [Google Scholar]
- 34.Sierra J, Kalangos A, Faidutti B, Christenson JT. Aorto-enteric fistula is a serious complication to aortic surgery. Modern trends in diagnosis and therapy. Cardiovasc Surg. 2003;11:185–8. doi: 10.1016/s0967-2109(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 35.Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg. 2005;92:143–52. doi: 10.1002/bjs.4928. [DOI] [PubMed] [Google Scholar]
- 36.Simon T, Feller E. Diverse presentation of secondary aortoenteric fistulae. Case Rep Med. 2011;2011:406730. doi: 10.1155/2011/406730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herdrich BJ, Fairman RM. How to manage infected aortic endografts. J Cardiovasc Surg (Torino) 2013;54:595–604. [PubMed] [Google Scholar]
- 38.Gerson LB, Fidler JL, Cave DR, Leighton JA. ACG clinical guideline: Diagnosis and management of small bowel bleeding. Am J Gastroenterol. 2015;110:1265–87. doi: 10.1038/ajg.2015.246. [DOI] [PubMed] [Google Scholar]
- 39.Singh A, Baptista V, Stoicov C, Cave DR. Evaluation of small bowel bleeding. Curr Opin Gastroenterol. 2013;29:119–24. doi: 10.1097/MOG.0b013e32835bdc1a. [DOI] [PubMed] [Google Scholar]
- 40.Maternini M, Tozzi P, Vuilleumier H, Von Segesser LK. Intravascular ultra sound: One more tool to diagnose aorto-duodenal fistula. Eur J Vasc Endovasc Surg. 2006;32:542–4. doi: 10.1016/j.ejvs.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Kieffer E, Gomes D, Chiche L, Fleron MH, Koskas F, Bahnini A. Allograft replacement for infrarenal aortic graft infection: Early and late results in 179 patients. J Vasc Surg. 2004;39:1009–17. doi: 10.1016/j.jvs.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 42.Jayarajan S, Napolitano LM, Rectenwald JE, Upchurch GR., Jr Primary aortoenteric fistula and endovascular repair. Vasc Endovascular Surg. 2009;43:592–6. doi: 10.1177/1538574409335275. [DOI] [PubMed] [Google Scholar]
- 43.Escobar GA, Eliason JL, Hurie J, Arya S, Rectenwald JE, Coleman DM. Rifampin soaking dacron-based endografts for implantation in infected aortic aneurysms – New application of a time-tested principle. Ann Vasc Surg. 2014;28:744–8. doi: 10.1016/j.avsg.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Arnaoutoglou E, Kouvelos G, Papa N, Kallinteri A, Milionis H, Koulouras V, et al. Prospective evaluation of post-implantation inflammatory response after EVAR for AAA: Influence on patients’ 30 day outcome. Eur J Vasc Endovasc Surg. 2015;49:175–83. doi: 10.1016/j.ejvs.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Arnaoutoglou E, Kouvelos G, Milionis H, Mavridis A, Kolaitis N, Papa N, et al. Post-implantation syndrome following endovascular abdominal aortic aneurysm repair: Preliminary data. Interact Cardiovasc Thorac Surg. 2011;12:609–14. doi: 10.1510/icvts.2010.256784. [DOI] [PubMed] [Google Scholar]