Background:
The incidence of enterovirus D68 (EV-D68) and the spectrum of clinical disease in children are not well known in European countries. We have designed a study with the objective of describing the clinical impact of EV-D68 detected in children with respiratory tract infections.
Methods:
As a part of a prospective study to identify the etiology and clinical characteristics of viral respiratory infections in children in Spain, we performed the analysis of the cases of EV infections in all children hospitalized in a secondary hospital in Madrid, during the epidemic respiratory season 2012–2013. A second group of samples was corresponded to infants of the same area, with ambulatory respiratory infection or asymptomatic. Phylogenetic EV-D68 analysis was made using the viral protein 1 gene (VP1). Clinical data of EV-D68 patients were compared with those infected by rhinovirus in the same period and population.
Results:
The study population consisted of 720 patients corresponding to 399 episodes of hospitalization for respiratory causes, 44 episodes of ambulatory respiratory infections and 277 children determined as a healthy control group. A total of 22 patients were positive for EVs (3.05%), and 12 of them were specifically typed as EV-D68 (11/443 respiratory infections, 2.5%). The most frequent diagnosis in the 10 hospitalized children with EV-D68 detection was recurrent wheezing. Hypoxia was present in 70% of cases, but admission in the intensive care unit was not required. No neurological signs or symptoms were observed. One patient had an ambulatory mild bronchiolitis and another was asymptomatic. No differences were found with rhinovirus infections except less duration of hypoxia and fever in EV-D68 group.
Conclusions:
EV-D68 infections were detected in 3.05% of respiratory studied samples (2.5% of admissions). The infection was associated with wheezing episodes with hypoxia. No admissions to intensive care unit or neurological symptoms were found.
Keywords: enterovirus, enterovirus D68, respiratory infections, hospitalized children, VP1 gene
The genus Enterovirus (belonging family Picornaviridae) comprises 10 species and 7 of them infect humans: enterovirus (EV) A–D and rhinovirus (RV) A–C. Human EV 68 (EV-D68) belongs to the EV species D together with 3 other EV types (EV-D70, EV-D94 and EV-D111).1 It was discovered in 1962 in respiratory samples2 and was earlier named human RV-87 on the basis of the optimal growth temperature, low pH sensitivity and antigenic characteristics being related to RV. It was later reclassified as EV-D68.3 During the following 40 years, human infection caused by EV-D68 was rarely reported.
EV-D68 can be detected by generic reverse transcription-polymerase chain reactions (RT-PCRs) designed in the 5′ noncoding region (5′NCR), but the specific detection of EV-D68 requires genotyping using the gene that codifies the viral protein 1 (VP1) or the VP2–VP4 genome region. The phylogenetic analysis of circulating EV-D68 using VP1 gene sequences allowed its classification in 3 major genetic groups: major groups 1, 2 and 3 as was published by Meijer et al4 or clades B, C and A, respectively, published by Tokarz et al.5 In recent months, PCR techniques are being incorporated into the routine diagnosis of this virus.6
The spectrum of clinical disease comprises asymptomatic to acute respiratory infections, upper and mild to severe lower respiratory disease requiring hospitalization and occasionally neurological symptoms and eventually death.7–9 In the last 10 years, several EV-D68 outbreaks have been reported,10,11 mainly in 2010. After the 2010 outbreak, EV-D68 continues circulating in several countries like Netherlands,12 and the most important outbreak has occurred in US and Canada.13 From August to September 2014, a total of 220 people had a confirmed EV-D68 respiratory illness in 32 different US states, and in Canada there was an increase of severe respiratory infections associated to this virus. In these countries, the patient’s age ranged between 6 weeks and 16 years, and an important percentage needed admission to the intensive care unit (ICU). Asthma has been the underlying disease most frequently detected in these patients. In European countries, the laboratory-confirmed cases of infections by EV-D68 are low because respiratory infections of RV and nonpolio EV are not of mandatory notification in the European Union. Until now, only a few specialized laboratories perform the specific EV-D68 identification, and it is unknown which countries have established surveillance schemes for respiratory specimens with screening for EV.
Based on the current information, the European Center for Disease Prevention and Control consider that European Union countries have a moderate risk of EV-D68 transmission because the circulation for this virus is low.14
As a part of a prospective study to identify the etiology and clinical characteristics of viral respiratory infections in children in Spain, we performed the analysis of the cases of EV infections in all children hospitalized in a secondary hospital in Madrid, during the epidemic respiratory season 2012–2013. Phylogenetic EV-D68 analysis was made using the VP1 gene. Clinical data of EV-D68 patients were compared with those infected by RV in the same period and population. The RV, belonged in the same genus Enterovirus, was chosen on the basis of similar clinical presentation.
PATIENTS AND METHODS
Clinical Assessment
The study population comprised all children younger than 14 years with a respiratory tract disease admitted to the secondary public hospital Severo Ochoa (Leganés, Madrid), from September 2012 through August 2013. At the reception, all patients were evaluated by an attending physician. Clinical characteristics of patients were analyzed. During the hospital stay, and as part of the study, a physician filled out a study questionnaire with the clinical data. A second group of samples corresponded to infants of the same area, belonging to a cohort recruited at birth to study the development of recurrent wheezing after viral infections. The samples in this second group were taken when an ambulatory respiratory infection was detected and also in the routine health visits for vaccination, and they are used as healthy controls. The recruitment period was also from September 2012 through August 2013. The study was approved by The Medical Ethics Committee. Informed consent was obtained from parents or legal guardians. The clinical diagnostic criteria were the same as used in other publications of our group.15
Virus Detection
Specimens consisted of nasopharyngeal aspirates (NPAs) were taken from each patient at admission (Monday to Friday). Each specimen (one for each patient) was sent for virological investigation to the Respiratory Virus and Influenza Unit at the National Center for Microbiology (CNM, ISCIII). NPAs were processed within 24 hours after collection. Upon reception, 3 aliquots were prepared and stored at −80°C. Both, the reception and the NPA sample processing areas were separate from those defined as working areas.
Polymerase Chain Reactions Methods for Detection of 16 Respiratory Viruses
Initially for diagnosis, 3 independent RT-PCR assays were performed to detect a total of 16 respiratory viruses.16–18 The specific identification of EV and RV was made by using degenerated primers designed in the polyprotein gene, between the 3′ end of the 5′ noncoding region (5′-NCR) and the 5′ end of VP2 gene and implemented in the third multiplex RT-PCR. The size of amplified products, 226 nt for EV and 110 nt for RV (both sizes on average), permitted the specific detection of both groups of virus.16
Enterovirus D68 Detection, Genotyping and Phylogenetic Analysis
Between September 2012 and August 2013, every EV detected was typed using degenerated primers designed for EV/RV typing in the 5′NCR-VP4/VP2 gene protein as previously described.19 Amplified products, ranging between 653 nt for EV-D68 Fermon reference strain and 630 nt for EV-D68 US/KY/14 (GenBank IDs: AY426531 and KM851231, respectively), were purified and sequenced in both directions using an automated ABI PRISM 377 model sequencer (Applied Biosystems, Foster City, CA) at the DNA Sequencing Facility, Genomics Unit (CNM, ISCIII).
VP1 gene was amplified by using two specific EV-D68 primers. Sense primer was located in the VP3 gene, EV68-VP3S-5′-GTTCYTTAATAGGRTTCRTAGCAGC-3′(2312–2336 of EV-D68 Fermon strain), and the antisense primer was located in the VP1 gene, EV68-VP1A-5′-CTCTATTRCCAATTATGGCATTRAG-3′ (3253–3277 of EV-D68 Fermon strain). Amplifications were performed at 52°C for the RT step and 52°C for the annealing temperature and 40 cycles. Amplified products of 965 nt were obtained and sequenced in both directions. The nucleotide consensus EV-D68 VP1 sequences were compared and aligned against other sequences retrieved from the GenBank database using the program CLUSTAL X (version 1.83; http://www.clustal.org/). The relationships between individual viruses were inferred from a phylogenetic tree based on the nucleotide sequence of a partial VP1 region (783 nt) that was constructed using the maximum-likelihood method based on the T92 model with γ-distribution (ModelTest best Fit Substitution Model) using MEGA6 software.20
Statistical Analysis
Values were expressed as percentages for discrete variables, or as mean and standard deviation for continuous variables. Clinical characteristics of patients with infections associated to EV-D68 were compared with those associated with single infection by RV in the same period. Clinical characteristics and laboratory variables were compared using the Student’s t test, the Mann–Whitney U test, the χ2 test and Fisher exact test. A 2-sided value of P < 0.05 was considered statistically significant. Results were adjusted for age. All analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 21.0 (IBM Corp, Armonk, NY).
RESULTS
The study population consisted of 720 patients corresponding to 399 episodes of hospitalization for respiratory causes in children younger than 14 years, 44 episodes of outpatient/ambulatory respiratory infections and 277 children determined as a healthy control group. A total of 22 patients were positive for EVs (3.05%) and 12 of them were specifically typed as EV-D68 (54.5%). Ten cases corresponded to hospitalized children, 1 case (SO-9749) was an outpatient who suffered mild bronchiolitis and finally, 1 case (SO-9770) belonged to the healthy control group, the sample being obtained during 2 months vaccination visit.
The clinical data of all 12 patients are shown in Table 1. The most frequent diagnosis in the 10 hospitalized children with EV-D68 detection (2.5% of hospitalized episodes) was recurrent wheezing. Hypoxia was present in 7 cases but none of them had radiological infiltrates, and admission in the ICU was not required. Additionally, no neurological signs or symptoms were observed. A first group of cases were detected in autumn between the end of October and the end of November 2012 and a second group in winter at the end of January and the beginning of February 2013.
TABLE 1.
Identification, Clinical Data and VP1 Sequence GenBank Accession Number of Patients with EVD68 Infections

Comparison Among EV-D68 and RV Infections
During the same period a total of 73 episodes of single RV infections in hospitalized children were analyzed and compared with EV-D68 episodes (Table 2). Clinical data for infections caused by EV-D68 were similar to infections associated with RV. Patients with EV-D68 have fever more frequently (P = 0.001). Nevertheless, infections caused by RV had more radiological infiltrate (P = 0.01) and needed oxygen during longer periods (P = 0.026). No clinical data of more severity were found in patients with EV-D68.
TABLE 2.
Clinical Characteristics Associated with EV-D68 Infections and Single RV Infections in Hospitalized Children

In spite of the small number of single EV-D68 infections (n = 7), we excluded the coinfections with other viruses and performed the comparison between EV-D68 and RV single infections, and the clinical differences were maintained with more frequent fever and less duration of hypoxia in children infected by EV-D68.
Phylogenetic Analysis of VP1
The EV-D68 sequences for VP1 gene studied (889 nt) were deposited in the GenBank database under the accession numbers KF254914 to KF254924. Phylogenetic analysis of Spanish EV-D68 VP1 genomic region indicated that 8 sequences clustered into 1 out of the 2 sublineages of the major group 1 (Tokarz clade B) and 3 of them were included in 2 sublineages of the major group 3 (Tokarz clade A) (Fig. 1).
FIGURE 1.
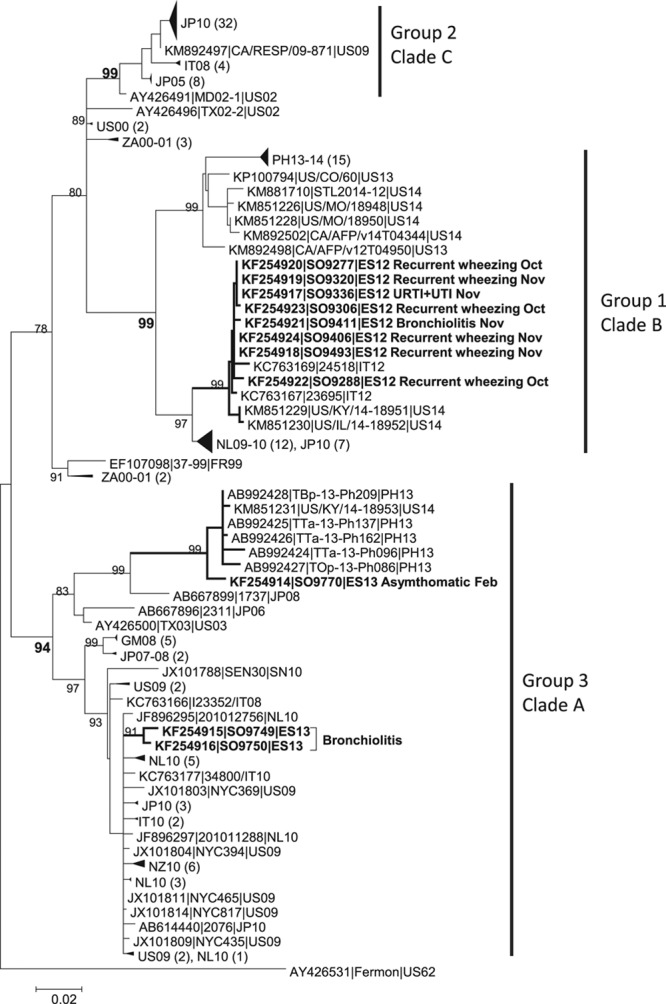
Phylogenetic tree based on the alignment of 161 VP1 genomic region sequences including Spanish EV-D68 virus and other circulating and no circulating virus. Positions were relative to the VP1 genomic region of the reference Fermon strain (AF081348), nucleotides 1–783 (1–261 amino acids). Two-letter International Organization for Standardization codes for countries and year of detection identified the sequences. The tree was constructed using the maximum-likelihood method using the T92 model with gamma distribution (ModelTest best Fit Substitution Model). Bootstrap values of >80 (1000 replications) are shown. Major phylogenetic groups/clades are indicated at right of the tree. Collapse branches or groups of braches were used to its graphical simplest form and the number of collapsed sequences is indicated in the tree in parenthesis. In the Spanish virus, in bold, the clinical diagnosis of patients and month of detection is detailed.
Group 1 Spanish EV-D68 sequences were very closely related with 2 Italian viruses that also circulated in 2012 (KC763169 and KC763167) and also with 2 viruses that circulated in US during 2014 (KM851229 and KM851230), which were fully sequenced as 2 of the 7 represented cocirculating strains identified by the Centers for Disease Control and Prevention in the US.21 This clade belonged to major group 1 in 1 of the 2 sublineages showed previously,10 and it was supported by a bootstrap value of 99. The sequences had highly similar amino acid signatures between them with changes previously described at the BC loop (amino acid positions 90–99) and the DE loop (amino acid positions 140–145, 148 and 152) regions.4 Patients from this clade presented recurrent wheezing, bronchiolitis in one case and upper respiratory tract infection.
From the major group 3, sequences KF254914 (SO-9770), KF254915 (SO-9749) and KF254916 (SO-9750) clustered into 2 different sublineages. These 3 viruses circulated in winter at the beginning of 2013. Notably, KF254914 (SO-9770) taken in February 2013 from an asymptomatic patient was highly related with sequences from the Philippines circulating in 2013, with the KM851231-US/KY/14-18953, which was fully sequenced.21 This separate clade was supported by a bootstrap value of 99. All sequences from this clade A group 3 presented a 3 nt deletion in the VP1 gene in position 140.
DISCUSSION
In recent years, the detection and circulation of EV-D68 has been reported worldwide. In Spain, the respiratory viral infections with laboratory confirmed that EV-D68 could be established approximately 2.5% of tested episodes in both hospitalized children and outpatients. The strength of our study is the inclusion during a complete epidemiological season of all inpatients and also a proportion of mild infections and asymptomatic children. EV-D68 VP1 genome region was compared with all other sequences available in the GenBank database (data not shown). With this approach, the entire spectrum of clinically respiratory infections and also a control group of asymptomatic children were covered. In addition, we have compared the clinical characteristics of EV-D68 infections with those where a RV was confirmed.
In this study, EV-D68 represented 54.5% of all the detected infections by EV. The main clinical characteristics associated with this virus are episodes of recurrent wheezing, with hypoxia requiring hospitalization in preschool children. These episodes are practically indistinguishable from those associated with RV, with the exception of less duration of oxygen treatment in the case of infection by EV-D68. Although children with EV-D68 infections seem slightly older than RV ones, age was not significantly different, possibly because of the sample size. We have found neither neurological data nor severe respiratory distress that might have required ICU admission in any EV-D68 patient.
The EV-D68 screening was not included as a routine test, and therefore, only sporadic cases have been reported in different European countries. In France (2009–2010), out of 16 EV strains, 10 (63%) were identified as EV-D68 and were taken from hospitalized children aged from 6 month to 10 years, with acute wheezing and bronchitis, and none of them required admission to ICU.22 In Italy from 2008 to 2009, 12 of 1500 samples tested were positive for EV-D68 among adults and children with respiratory infections.23 Two different studies in the Netherlands presented a more complete picture of EV-D68 infections where authors described the incidence of infections first between 1994 and 2010 and recently from 2010 to 2014.7,10 The Dutch cases occurred mainly in the under 20 and the 50–59 years age groups and notably in children younger than 10 years. With the recent addition of determining EV-D68 in diagnostic virology laboratories, more virological and epidemiological details are known. The circulation of EV-D68 in the Netherlands from 2011 to 2014 confirmed a relatively stable circulation in autumn–winter every year. Our series are consistent with these European studies, showing a moderate proportion of EV-D68 infections in children younger than 5 years, with asthma or recurrent wheezing, and of moderate severity, not requiring ICU admission. This is the case of the Norway series, also with 1 outpatient children, although they had 1 paralysis case.24 The percentage of circulation is low (approximately 2%–7%), and it has been detected in hospitalized and in outpatients.6,25 Some cases are also described in Spain.26
The majority of molecular assays for detecting EV and RV have been designed in the 5′NCR. Several years ago, we designed a molecular assay that was able to differentiate EV and RV by the relative amplified product size (approximately 226 and 110 nt, respectively) based on amplification of the partial 5′NCR and the spacer region.15 Between early 1960s and mid 1990s, the EV-D68 genome developed a rearrangement in the spacer region resulting in a 24-nt deletion and after that a second rearrangement resulting in an additional 11-nt deletion.27 We noticed that some EV were misidentified as parainfluenza virus type 4AB (174 nt) because of the similar size of amplified products visualized in agarose gels. By a retrospective study using the sequence of the corresponding amplified products and by a specific EV-D68 VP1 assay, we confirmed positive results and defined the circulation of 2 of the EV-D68 major groups in Spain. Probably, EV-D68 was circulating in Spain long time ago, and we have underestimated its prevalence until now.
In general, the length of EVs 5′ NCR spacer region is approximately 150–180 nt, but in the case of EV-D68, successive deletions generated a length of approximately 115 nt for virus clustered in major groups 1 and 2 and 130 nt for major group 3. These deletions may affect the virulence of these viruses, and EV-D68 could increase the respiratory tropism as it is the case with the RV, whose spacer region is even shorter (approximately 50 nt). A second factor is the change of the antigenicity in recent virus as it was showed by Imamura et al.28 The recent EV-D68 presented divergent antigenicity with the Fermon reference strain and also among the 3 genetic groups, which preferably bind to α-2-6–linked sialic acid mainly located in the upper respiratory tract. The phylogenetic analysis of the VP1 genomic region showed that the majority of Spanish EV-D68 from 2012 and 2013 clustered, with significant bootstrap values of 99, with contemporary Italians 2012 and more modern US 2014 EV-D68 virus in the major group 1 (Tokarz clade B). Patients from this group 1 were hospitalized with mainly episodes of recurrent wheezing with hypoxia. Only 3 of our virus were included in the major group 3 (Tokarz clade A): 1 clustered with sequences from Philippines 2013 and US 2014 in 1 of the 2 sublineages and 2, from patients with bronchiolitis, clustered in the other sublineage of the group 3 with a high number of sequences distributed worldwide. Our virus from only 1 epidemic respiratory infection season indicated the cocirculation of different phylogenetic groups and sublineages with an increase of variability of the BC and DE loops in the VP1 genomic region. As the US 2014, Spanish virus of 2012–2013 corresponded to groups 1 and 3 and a very high genetic homology was identified when comparison of sequences was made. More systematic studies covering the study of children with asthma and other severe cases are necessary to clarify the incidence and severity of these infections.
ACKNOWLEDGMENTS
This study has been partially supported by FIS (Fondo de Investigaciones Sanitarias – Spanish Health Research Fund) Grants No: PI12/01291 and a Research grant from the Spanish Association of Pediatrics.
Footnotes
This study has been partially supported by FIS (Fondo de Investigaciones Sanitarias—Spanish Health Research Fund) Grant No: PI12/01291 and I Research grant from the Spanish Association of Pediatrics. The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Oberste MS, Maher K, Kilpatrick DR, et al. Typing of human enteroviruses by partial sequencing of VP1. J Clin Microbiol. 1999;37:1288–1293. doi: 10.1128/jcm.37.5.1288-1293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol. 1967;85:297–310. doi: 10.1093/oxfordjournals.aje.a120693. [DOI] [PubMed] [Google Scholar]
- 3.Blomqvist S, Savolainen C, Råman L, et al. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J Clin Microbiol. 2002;40:4218–4223. doi: 10.1128/JCM.40.11.4218-4223.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meijer A, van der Sanden S, Snijders BE, et al. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in The Netherlands in 2010. Virology. 2012;423:49–57. doi: 10.1016/j.virol.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol. 2012;93(Pt 9):1952–1958. doi: 10.1099/vir.0.043935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poelman R, Schölvinck EH, Borger R, et al. The emergence of enterovirus D68 in a Dutch University Medical Center and the necessity for routinely screening for respiratory viruses. J Clin Virol. 2015;62:1–5. doi: 10.1016/j.jcv.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol. 2011;52:103–106. doi: 10.1016/j.jcv.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med. 2011;135:793–796. doi: 10.5858/2010-0174-CR.1. [DOI] [PubMed] [Google Scholar]
- 9.Imamura T, Fuji N, Suzuki A, et al. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerg Infect Dis. 2011;17:1430–1435. doi: 10.3201/eid1708.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson R. Outbreaks of enterovirus D68 continue across the USA. Lancet Respir Med. 2014;2:791. doi: 10.1016/s2213-2600(14)70190-0. [DOI] [PubMed] [Google Scholar]
- 11.Centers of Disease Control and Prevention. Clusters of acute respiratory illness associated with human enterovirus 68- Asia, Europe, and United States, 2008–2010. MMWR morbidity and mortality weekly report. 2011;60:1301–1304. [PubMed] [Google Scholar]
- 12.Meijer A, Benschop KS, Donker GA, et al. Continued seasonal circulation of enterovirus D68 in the Netherlands, 2011–2014. Euro Survell. 2014;19 doi: 10.2807/1560-7917.es2014.19.42.20935. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?Articleld=20935. Accessed March 8, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68 - Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:798–799. [PMC free article] [PubMed] [Google Scholar]
- 14.European Center for Disease Prevention and Control. Enterovirus 68 detections in the USA and Canada—26 September 2014. Stockholm, Sweden: ECDC; 2014. [Google Scholar]
- 15.Calvo C, García-García ML, Sanchez-Dehesa R, et al. Eight year prospective study of adenoviruses infections in hospitalized children. Comparison with other respiratory viruses. PLoS One. 2015;10:e0132162. doi: 10.1371/journal.pone.0132162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coiras MT, Pérez-Breña P, García ML, et al. Simultaneous detection of influenza A, B, and C viruses, respiratory syncytial virus, and adenoviruses in clinical samples by multiplex reverse transcription nested-PCR assay. J Med Virol. 2003;69:132–144. doi: 10.1002/jmv.10255. [DOI] [PubMed] [Google Scholar]
- 17.Calvo C, Pozo F, García-García ML, et al. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three-year prospective study. Acta Paediatr. 2010;99:883–887. doi: 10.1111/j.1651-2227.2010.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coiras MT, Aguilar JC, García ML, et al. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested-PCR assays. J Med Virol. 2004;72:484–495. doi: 10.1002/jmv.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvo C, Casas I, García-García ML, et al. Role of rhinovirus C respiratory infections in sick and healthy children in Spain. Pediatr Infect Dis J. 2010;29:717–720. doi: 10.1097/INF.0b013e3181d7a708. [DOI] [PubMed] [Google Scholar]
- 20.Tamura K, Stecher G, Peterson D, et al. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown BA, Nix WA, Sheth M, et al. Seven strains of enterovirus D68 detected in the United States during the 2014 severe respiratory disease outbreak. Genome Announc. 2014;2 doi: 10.1128/genomeA.01201-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renois F, Bouin A, Andreoletti L. Enterovirus 68 in pediatric patients hospitalized for acute airway diseases. J Clin Microbiol. 2013;51:640–643. doi: 10.1128/JCM.02640-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piralla A, Baldanti F, Gerna G. Phylogenetic patterns of human respiratory picornavirus species, including the newly identified group C rhinoviruses, during a 1-year surveillance of a hospitalized patient population in Italy. J Clin Microbiol. 2011;49:373–376. doi: 10.1128/JCM.01814-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bragstad K, Jakobsen K, Rojahn AE, et al. High frequency of enterovirus D68 in children hospitalised with respiratory illness in Norway, autumn 2014. Influenza Other Respir Viruses. 2015;9:59–63. doi: 10.1111/irv.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiche J, Böttcher S, Diedrich S, et al. Low-level circulation of enterovirus D68-associated acute respiratory infections, Germany, 2014. Emerg Infect Dis. 2015;21:837–841. doi: 10.3201/eid2105.141900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Launes C, Armero G, Anton A, et al. Molecular epidemiology of severe respiratory disease by human rhinoviruses and enteroviruses at a tertiary paediatric hospital in Barcelona, Spain. Clin Microbiol Infect. 2015;21:799.e5–799.e7. doi: 10.1016/j.cmi.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Kaida A, Kubo H, Sekiguchi J, et al. Enterovirus 68 in children with acute respiratory tract infections, Osaka, Japan. Emerg Infect Dis. 2011;17:1494–1497. doi: 10.3201/eid1708.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura T, Okamoto M, Nakakita S, et al. Antigenic and receptor binding properties of enterovirus 68. J Virol. 2014;88:2374–2384. doi: 10.1128/JVI.03070-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


