Abstract
This study reports antimicrobial susceptibility of Staphylococcus pseudintermedius carried by healthy dogs in Saskatoon, and describes changes in antimicrobial resistance since a 2008 study. One hundred healthy dogs presenting to the wellness service at the Western College of Veterinary Medicine were screened for S. pseudintermedius by culturing rectal and pharyngeal swabs. Staphylococcus pseudintermedius was identified biochemically and antimicrobial minimum inhibitory concentrations were determined by broth microdilution. Methicillin resistance was confirmed by polymerase chain reaction (PCR) and sequencing of the mecA gene. Of 221 S. pseudintermedius isolates from 78 dogs, 7 were methicillin resistant. No resistance to the fluoroquinolones, nitrofurantoin, tigecycline, vancomycin, quinupristin-dalfopristin, linezolid, or daptomycin was identified. Of the 78 positive dogs, isolates resistant to penicillin were found in 78%, to ampicillin in 61% and to tetracycline in 26%; resistance to oxacillin, erythromycin, clindamycin, trimethoprim + sulfamethoxazole, chloramphenicol, and gentamicin was found in < 10% of dogs. Compared to the 2008 study, the frequency of resistance to all drugs increased, and the frequency of colonization with pan-susceptible isolates decreased from 46% to 30%.
Résumé
Susceptibilité antimicrobienne de Staphylococcus pseudintermedius colonisant des chiens en santé à Saskatoon, au Canada. Cette étude présente un rapport sur la susceptibilité de Staphylococcus pseudintermedius chez des chiens porteurs en santé à Saskatoon et décrit les changements de la résistance antimicrobienne depuis une étude réalisée en 2008. On a réalisé un dépistage auprès de 100 chiens en santé présentés au service de bien-être du Western College of Veterinary Medicine pour S. pseudintermedius en réalisant une culture d’écouvillons rectaux et pharyngés. Staphylococcus pseudintermedius a été identifié par des tests biochimiques et les concentrations minimales inhibitrices d’antimicrobiens ont été déterminées par microdilution en bouillon. La résistance à la méthicilline a été confirmée par ACP et le séquençage du gène mecA. Parmi les 221 isolats de S. pseudintermedius provenant de 78 chiens, 7 étaient résistants à la méthicilline. Aucune résistance aux fluoroquinolones, à la nitrofurantoine, à la tigecycline, à la vancomycine, à la quinupristine-dalfopristine, au linézolide ou à la daptomycine n’a été identifiée. Parmi les 78 chiens positifs, des isolats résistants à la pénicilline ont été trouvés chez 78 %, à l’ampicilline chez 61 % et à la tétracycline chez 26 %; la résistance à l’oxacilline, à l’érythromycine, à la clindamycine, au triméthoprime + sulfaméthoxazole, au chloramphenicol et à la gentamicine a été trouvée chez < 10 % des chiens. Comparativement à l’étude de 2008, la fréquence de la résistance à tous les médicaments a augmenté et la fréquence de la colonisation par des isolats sensibles a chuté de 46 % à 30 %.
(Traduit par Isabelle Vallières)
Introduction
Staphylococcus pseudintermedius (recognized as distinct from S. intermedius in 2005) colonizes the skin and mucosal surfaces of up to 90% of healthy dogs (1–3). Clinically, S. pseudintermedius is the most common cause of pyoderma and otitis externa, the second most common cause of urinary tract infections, and is frequently implicated in nosocomial infections in dogs (4,5). The ubiquity of canine S. pseudintermedius infections in the community and the frequency of empiric treatment by veterinarians highlight the importance of antimicrobial resistance surveillance to inform evidence-based empiric therapeutic selection.
The emergence of antimicrobial resistance is a great challenge to antimicrobial therapy for animals and humans. The propensity of staphylococci to adapt to the selection pressure of antimicrobial use has been recognized since the first description of penicillin resistant S. aureus in the 1940s (6). Resistance to penicillin among staphylococci, including companion animal S. pseudintermedius isolates, is most commonly due to the production of staphylococcal beta-lactamase, conferred by the blaZ gene (7,8). Staphylococcus pseudintermedius has historically remained remarkably susceptible to antimicrobials, but since 2006 there has been a dramatic worldwide increase in the frequency of methicillin resistance (4,9). Methicillin resistance, which is rapidly emerging among S. pseudintermedius in dogs and common among S. aureus in humans is a serious threat to the efficacy of the most frequently used antibiotics, the beta-lactams (10–12). Methicillin resistance conferred by the mecA and mecC genes results in the production of altered cell wall proteins with a low affinity for beta-lactam drugs; leading to resistance to all beta-lactam antimicrobials currently licensed for use in veterinary medicine including the penicillins, cephalosporins, and carbapenems (13). Because methicillin resistance is not the product of beta-lactamase production, addition of beta-lactamase inhibitors such as clavulanic acid does not restore susceptibility. Furthermore, methicillin resistance in S. pseudintermedius is often associated with multidrug resistance, further limiting the treatment options available to veterinarians (4,9).
In the late 2000’s there was an explosive increase in the incidence of MRSP associated with 2 lineages of S. pseudintermedius, sequence type (ST) 71 in Europe and ST68 in North America (11,14). Among healthy dogs in North America and Europe 0 to 4.5% have been found to carry MRSP, while up to 66% of clinical S. pseudintermedius isolates have been reported to be methicillin resistant (4,15–19). In Saskatoon, S. pseudintermedius carried by healthy dogs and those causing infections have historically been remarkably susceptible; a 2008 study failed to identify any animals carrying MRSP (2,5). Since 2009, reports of canine infections with MRSP in Saskatoon including urinary tract infections and necrotizing fasciitis suggest the emergence of resistance in this region (20,21). The objective of this study was to determine the antimicrobial susceptibility profiles of S. pseudintermedius colonizing healthy dogs in Saskatoon, and identify changes in the frequency of resistance since the 2008 investigation.
Materials and methods
Sample collection
Between June and September 2014, 100 clinically healthy dogs presenting to the wellness service of the Veterinary Medical Centre at the Western College of Veterinary Medicine were investigated (Table 1). Pharyngeal and rectal samples were collected using sterile swabs with Stuart transport media (Becton Dickinson, Sparks, Maryland, USA) as previously described (2). Pharyngeal samples were collected by gently rolling a sterile swab across the pharynx for 1 to 3 s, and rectal swabs were collected by gently inserting a second swab 3 cm into the dog’s rectum and rotating for 1 to 3 s. All samples were processed within 4 h of collection. This study was approved by the University of Saskatchewan animal research ethics board (protocol #20130135).
Table 1.
Characteristics of sampled dogs (n = 100)
| Age | 2.5 mo to 12 y (median = 3 y) |
|---|---|
| Gendera | |
| Intact male | 18 |
| Neutered male | 33 |
| Intact female | 14 |
| Neutered female | 32 |
| History of antimicrobial use, past 6 months | |
| Yes | 9 |
| No | 91 |
Information on gender was not recorded for 1 dog.
Culture and susceptibility testing
All swabs were plated on CHROMagar Staph aureus (CHROMagar, Paris, France), and Mueller-Hinton agar + 4 μg/mL oxacillin. Plates were then incubated overnight at 35°C and up to 5 S. pseudintermedius-like colonies (mauve color) were sub-cultured to Columbia agar with 5% sheep blood (Becton, Dickinson). Isolates were identified based on colony morphology (small, creamy grey to white, round colonies with a smooth margin and double zone of hemolysis on blood agar) and biochemically using the catalase test, and tube coagulase test with rabbit plasma, the production of acetoin and hyaluronidase, and the fermentation of mannitol, maltose, and trehalose (2,22). Since the carriage of genetically diverse S. pseudintermedius strains by individual dogs has been recognized, 3 isolates per animal were saved for future testing to increase the likelihood of detecting resistant organisms (23). Bacteria were stored at −80°C in trypticase soy broth + 15% glycerol. For dogs carrying S. pseudintermedius at both sites, 2 pharyngeal and 1 rectal isolate were saved.
Antimicrobial minimum inhibitory concentrations were determined by broth microdilution using the GAPLL1F Sensititre panel (Thermo Fisher Scientific, Oakwood Village, Ohio, USA). Tests were conducted according to the Clinical and Laboratory Standards Institute (CLSI) and manufacturer’s guidelines (24). A panel of drugs including: penicillin (PEN), ampicillin (AMP), oxacillin (OXA) with 2% NaCl, erythromycin (ERY), clindamycin (CLI), tetracycline (TET), tigecycline (TGC), trimethoprim + sulfamethoxazole (SXT), ciprofloxacin (CIP), levofloxacin (LEV), moxifloxacin (MOX), gentamicin (GEN), chloramphenicol (CHL), rifampin (RIF), nitrofurantoin (NIT), vancomycin (VAN), linezolid (LZD), daptomycin (DAP) and quinupristin + dalfopristin (QDA) was used. For quality control S. aureus ACTCC 29213 and Enterococcus faecalis ACTCC 29212 were used (25). Antimicrobial MICs were used to categorized isolates as susceptible or resistant using CLSI breakpoints for all drugs except tigecycline and daptomycin for which the EUCAST interpretive criteria were used (25–27). Isolates were considered to be MRSP when resistant to oxacillin (MIC ≥ 0.5 μg/mL); genotypic resistance was confirmed by PCR and sequencing of the mecA and mecC genes using previously described primers (28). Isolates resistant to erythromycin and susceptible to clindamycin were tested for inducible clindamycin resistance using the D-test as described by the CLSI (26).
Results
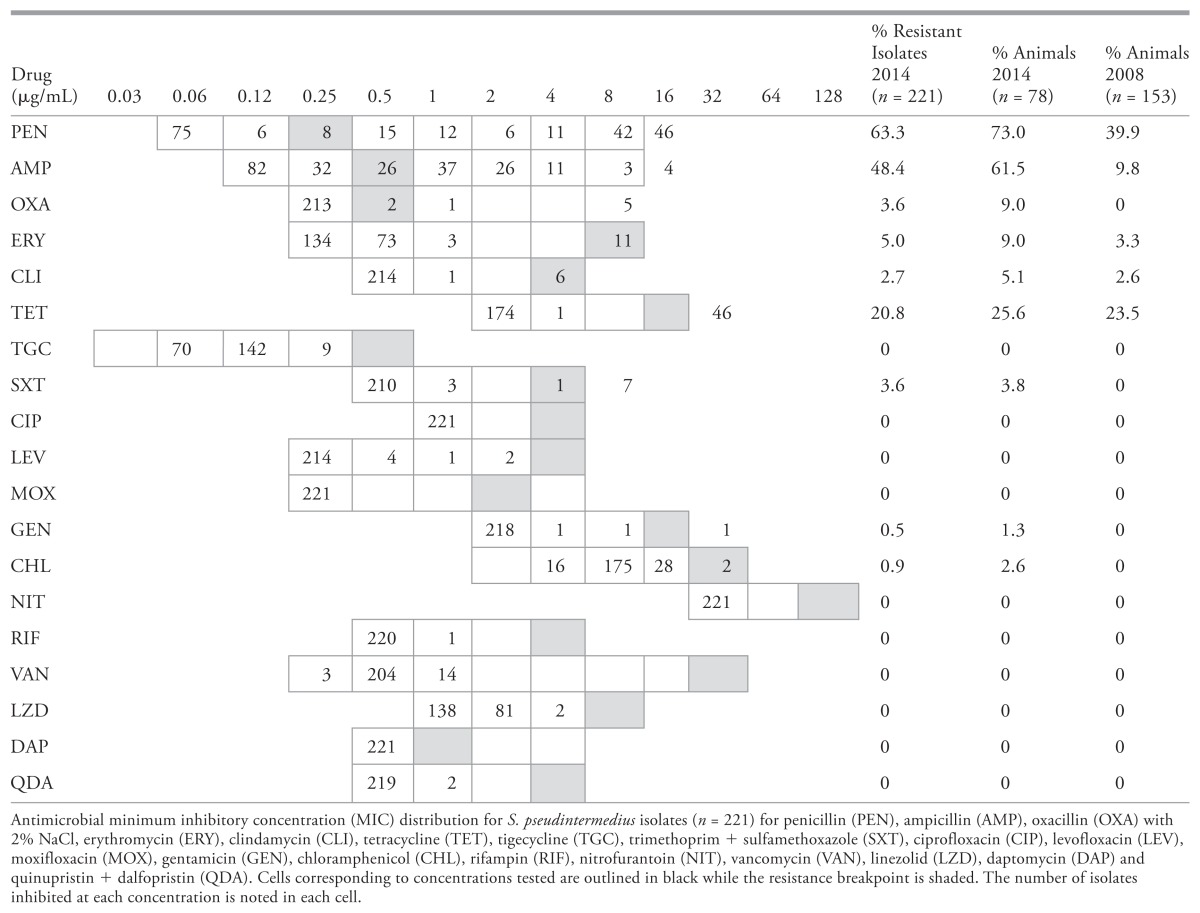
Of the 100 dogs tested, S. pseudintermedius was isolated from 78. A total of 221 isolates were collected, including single isolates from 5 dogs, 2 isolates from 3 dogs, and 3 isolates from 70 dogs. For dogs in which < 3 isolates were initially identified, all isolates were saved. No S. pseudintermedius was isolated from Müeller-Hinton agar with 4 μg/mL oxacillin; all isolates were recovered from CHROMagar Staph aureus. Antimicrobial susceptibility testing revealed phenotypic diversity among multiple isolates from individual dogs. Of the 78 positive animals, isolates with varying susceptibility profiles were grown from 30, while phenotypically homogeneous isolates were grown from 48. Consequently, the frequency of resistance among the overall isolate collection was lower than the percentage of animals carrying isolates expressing any particular resistance phenotype; for example, if 1 of 3 isolates carried by a dog was resistant to tetracycline, that dog was considered to carry tetracycline-resistant isolates (Table 2). No resistance to ciprofloxacin, levofloxacin, moxifloxacin, nitrofurantoin, rifampin, tigecycline, vancomycin, quinupristin + dalfopristin, linezolid or daptomycin was identified. The most common resistance profile was penicillin + ampicillin resistance (n = 70; 31.7%) followed by pan-susceptibility (n = 67; 30.3%) (Table 3). Methicillin resistant isolates (n = 8) were identified in 7 dogs carrying S. pseudintermedius. Resistance to trimethoprim + sulfamethoxazole, chloramphenicol, and gentamicin was less common (Table 3). All oxacillin resistant isolates posessed the mecA gene, while mecC was not identified. None of the 5 erythromycin resistant, clindamycin susceptible isolates were inducibly clindamycin resistant.
Table 2.
MIC distribution of isolates and the percentage of animals colonized with resistant isolates in 2008 and 2014
Table 3.
Summary of resistance profiles of S. pseudintermedius isolates (n = 221)
| Resistance profile | Number of isolates |
|---|---|
| Pan-susceptible | 67 |
| PEN + AMP | 70 |
| PEN | 27 |
| TET | 13 |
| PEN + AMP + TET | 13 |
| PEN + AMP + OXA | 6 |
| PEN + AMP + SXT + TET | 6 |
| PEN + TET | 4 |
| PEN + AMP + TET + ERY + CLI | 4 |
| PEN + AMP + TET + ERY | 3 |
| PEN + AMP + SXT | 2 |
| PEN + AMP + OXA + TET | 1 |
| PEN + AMP + OXA + ERY | 1 |
| TET + ERY | 1 |
| PEN + ERY + CLI | 1 |
| PEN + AMP + TET + CHL | 1 |
| PEN + AMP + ERY + CLI + CHL + GEN | 1 |
Resistance profiles of S. pseudintermedius isolates (left column), and number of isolates with each profile (right column). Penicillin (PEN), ampicillin (AMP), oxacillin (OXA) with 2% NaCl, erythromycin (ERY), clindamycin (CLI), tetracycline (TET), trimethoprim + sulfamethoxazole (SXT), gentamicin (GEN), chloramphenicol (CHL).
Fifteen multidrug resistant isolates (MDR; resistance to 3 or more drugs classes) were identified, all were methicillin susceptible S. pseudintermedius (MSSP) (Table 3). Notably, 1 isolate was resistant to PEN, AMP, TET, ERY, CLI, CHL and GEN.
Discussion
Compared to the previous resistance surveillance study targeting S. pseudintermedius from healthy dogs presenting to the wellness service at our institution in Saskatoon in 2008, a higher frequency of resistance to specific antimicrobials, and resistance to more drugs including methicillin was identified. Furthermore, only 30% of the dogs carried pan-susceptible isolates compared to 46% in 2008 (2). Differences in sample collection between the present investigation and that done in 2008 (the inclusion of a single isolate per dog in 2008 versus 3 presently, and the inclusion of nasal swabs in the 2008 investigation) preclude statistical comparisons between studies. However, the higher frequency of resistance including MRSP in 2014 is consistent with local clinical observations suggesting the emergence of resistance and with global MRSP trends. The frequency of carriage of healthy dogs with MRSP (7%) was higher than previously described elsewhere in North America or Europe (≤ 4.5%) perhaps reflecting the continued emergence of MRSP following previous studies (4,18). This frequency was lower than that reported in Asia, where up to 45% colonization has been reported in Thailand, Japan and Hong Kong (29–31). The inclusion of Müeller-Hinton agar with 4 μg/mL oxacillin did not improve our ability to recover MRSP despite the identification of 5 isolates with oxacillin MICs of > 4 μg/mL.
Risk factors for dogs to be infected with or carry MRSP have not been adequately characterized. There is conflicting evidence describing an association between infection with MRSP versus MSSP and previous antimicrobial administration (32,33). Hospitalization and surgical procedures have also been positively associated with MRSP colonization (33,34). In the present investigation, none of the 7 MRSP positive animals had been treated with antimicrobials in the previous 6 mo, and all were clinically healthy suggesting community acquisition of the MRSP. Further study is required to define risk factors associated with MRSP in dogs.
A total of 15 (6.8%) isolates from 7 dogs (9% of colonized dogs) were MDR, higher than in 2008 where only 1 dog carried MDR S. pseudintermedius (2). In contrast to the literature, MDR was more frequently identified among MSSP than MRSP (35). The most common resistance profile among MRSP, including 6 of 8 isolates, was simply beta-lactam resistance. MDR among MRSP is a serious threat to the ability of veterinarians to treat their patients. In 2009, a community associated urinary tract infection caused by MRSP resistant to the beta-lactams, macrolides, fluoroquinolones, aminoglycosides, trimethoprim-sulfamethoxazole, chloramphenicol and rifampin was reported in an otherwise healthy, neutered male Pug dog (20). Elsewhere, MRSP resistant to all antimicrobials licensed for use in companion animals have been described, highlighting the critical role of culture and susceptibility testing to guide therapy (4,11,35). Differences in clinical outcome for human patients infected with methicillin resistant versus susceptible staphylococci have not been observed, although the typically superficial nature of staphylococcal infections (pyoderma and otitis) may mask differences which have been seen in invasive MRSA vs. MSSA infections in people (33,36). More studies are needed to define risk factors associated with MRSP infection so that appropriate empiric treatments can be applied pending laboratory guided therapy.
Although ill-defined, antimicrobial resistant S. pseudintermedius is also a public health risk; human infections have been reported (37). Because S. pseudintermedius is not part of the normal microbiota of humans, carriage has been reported to be sporadic, colonization or infection with this organism is likely zoonotic (38,39). Presumptive transmission of S. pseudintermedius from dogs to humans working closely with them (veterinary staff) and pet owners has been reported; 3.9% to 13% of those humans have been found to carry this organism (31,38,40). The frequency of human S. pseudintermedius infections may be under-appreciated due to its morphological and biochemical similarity to S. aureus leading to misidentification in diagnostic labs. The introduction of highly discriminatory identification methods such as MALDI-TOF which readily differentiate S. pseudintermedius and the closely related S. intermedius and S. delphini from S. aureus is helping to identify this previously under-recognized zoonosis (41).
Antimicrobial resistance appears to be emerging among S. pseudintermedius colonizing healthy dogs in Saskatoon. Although we presume that infections with MRSP are encountered with increasing frequency in our region, these data are not available. Culture and susceptibility testing should be encouraged to aid in the identification of MRSP in veterinary patients and to guide antimicrobial therapy. Methicillin resistance should be suspected when empiric beta-lactam therapy fails to cure S. pseudintermedius infections or for isolates demonstrated to be resistant to potentiated penicillins such as amoxicillin + clavulanic acid. Further studies to describe the susceptibility of clinical isolates in this region would be complementary to this investigation.
Acknowledgments
The authors acknowledge Dr. Jordan Woodsworth for sample collection and Champika Fernando for technical support. We also thank the Companion Animal Health Fund for funding this project and providing a fellowship to Roshan Priyantha. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Devriese LA, Vancanneyt M, Baele M, et al. Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int J Syst Evol Microbiol. 2005;55:1569–1573. doi: 10.1099/ijs.0.63413-0. [DOI] [PubMed] [Google Scholar]
- 2.Rubin JE, Chirino-Trejo M. Prevalence, sites of colonization, and antimicrobial resistance among Staphylococcus pseudintermedius isolated from healthy dogs in Saskatoon, Canada. J Vet Diag Invest. 2011;23:351–354. doi: 10.1177/104063871102300227. [DOI] [PubMed] [Google Scholar]
- 3.Bannoehr J, Guardabassi L. Staphylococcus pseudintermedius in the dog: Taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet Dermatol. 2012;23:253–266. e251–252. doi: 10.1111/j.1365-3164.2012.01046.x. [DOI] [PubMed] [Google Scholar]
- 4.van Duijkeren E, Catry B, Greko C, et al. Review on methicillin-resistant Staphylococcus pseudintermedius. J Antimicrob Chemother. 2011;66:2705–2714. doi: 10.1093/jac/dkr367. [DOI] [PubMed] [Google Scholar]
- 5.Ball KR, Rubin JE, Chirino-Trejo M, Dowling PM. Antimicrobial resistance and prevalence of canine uropathogens at the Western College of Veterinary Medicine Veterinary Teaching Hospital, 2002–2007. Can Vet J. 2008;49:985–990. [PMC free article] [PubMed] [Google Scholar]
- 6.Barber M. Staphylococcal infection due to penicillin-resistant strains. Br Med J. 1947;2(4534):863–865. doi: 10.1136/bmj.2.4534.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malik S, Christensen H, Peng H, Barton MD. Presence and diversity of the beta-lactamase gene in cat and dog staphylococci. Vet Microbiol. 2007;123:162–168. doi: 10.1016/j.vetmic.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Fuda CC, Fisher JF, Mobashery S. Beta-lactam resistance in Staphylococcus aureus: The adaptive resistance of a plastic genome. Cell Mol Life Sci. 2005;62:2617–2633. doi: 10.1007/s00018-005-5148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moodley A, Damborg P, Nielsen SS. Antimicrobial resistance in methicillin susceptible and methicillin resistant Staphylococcus pseudintermedius of canine origin: Literature review from 1980 to 2013. Vet Microbiol. 2014;171:337–341. doi: 10.1016/j.vetmic.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Prescott JF, Hanna WJ, Reid-Smith R, Drost K. Antimicrobial drug use and resistance in dogs. Can Vet J. 2002;43:107–116. [PMC free article] [PubMed] [Google Scholar]
- 11.Perreten V, Kadlec K, Schwarz S, et al. Clonal spread of methicillin-resistant Staphylococcus pseudintermedius in Europe and North America: An international multicentre study. J Antimicrob Chemother. 2010;65:1145–1154. doi: 10.1093/jac/dkq078. [DOI] [PubMed] [Google Scholar]
- 12.Weese JS, van Duijkeren E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet Microbiol. 2010;140:418–429. doi: 10.1016/j.vetmic.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 13.Becker K, Ballhausen B, Kock R, Kriegeskorte A. Methicillin resistance in Staphylococcus isolates: The “mec alphabet” with specific consideration of mecC, a mec homolog associated with zoonotic S. aureus lineages. Int J Med Microbiol. 2014;304:794–804. doi: 10.1016/j.ijmm.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Bardiau M, Yamazaki K, Ote I, Misawa N, Mainil JG. Characterization of methicillin-resistant Staphylococcus pseudintermedius isolated from dogs and cats. Microbiol Immunol. 2013;57:496–501. doi: 10.1111/1348-0421.12059. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami T, Shibata S, Murayama N, et al. Antimicrobial susceptibility and methicillin resistance in Staphylococcus pseudintermedius and Staphylococcus schleiferi subsp. coagulans isolated from dogs with pyoderma in Japan. J Vet Med Sci. 2010;72:1615–1619. doi: 10.1292/jvms.10-0172. [DOI] [PubMed] [Google Scholar]
- 16.Gingrich EN, Kurt T, Hyatt DR, Lappin MR, Ruch-Gallie R. Prevalence of methicillin-resistant staphylococci in northern Colorado shelter animals. J Vet Diag Invest. 2011;23:947–950. doi: 10.1177/1040638711407301. [DOI] [PubMed] [Google Scholar]
- 17.Griffeth GC, Morris DO, Abraham JL, Shofer FS, Rankin SC. Screening for skin carriage of methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Vet Dermatol. 2008;19:142–149. doi: 10.1111/j.1365-3164.2008.00663.x. [DOI] [PubMed] [Google Scholar]
- 18.Hanselman BA, Kruth SA, Rousseau J, Weese JS. Coagulase positive staphylococcal colonization of humans and their household pets. Can Vet J. 2009;50:954–958. [PMC free article] [PubMed] [Google Scholar]
- 19.Hanselman BA, Kruth S, Weese JS. Methicillin-resistant staphylococcal colonization in dogs entering a veterinary teaching hospital. Vet Microbiol. 2007;126:277–281. doi: 10.1016/j.vetmic.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Rubin JE, Gaunt MC. Urinary tract infection caused by methicillin-resistant Staphylococcus pseudintermedius in a dog. Can Vet J. 2011;52:162–164. [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer MN, Rubin JE. Necrotizing fasciitis caused by methicillin-resistant Staphylococcus pseudintermedius at a previously irradiated site in a dog. Can Vet J. 2012;53:1207–1210. [PMC free article] [PubMed] [Google Scholar]
- 22.Winn W, Allen S, Janda W, et al. Gram-positive cocci Part 1: Staphylococci and related Gram-positive cocci. In: Winn W, Allen S, Janda J, et al., editors. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. 6th ed. Baltimore, Maryland: Lippincott Williams & Wilkins; 2006. pp. 623–671. [Google Scholar]
- 23.Paul NC, Bargman SC, Moodley A, Nielsen SS, Guardabassi L. Staphylococcus pseudintermedius colonization patterns and strain diversity in healthy dogs: A cross-sectional and longitudinal study. Vet Microbiol. 2012;160:420–427. doi: 10.1016/j.vetmic.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 24.CLSI. M07-A9 Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 9th ed. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 25.CLSI. 2nd informational supplement. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2013. VET01-S2 Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. [Google Scholar]
- 26.CLSI. 24th informational supplement. Clinical and Laboratory Standards Institute; 2014. M100-S24 Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 27.EUCAST. European Committee on Antimicrobial Susceptibility Testing breakpoint tables for interpretation of MICs and zone diameters. 2014. [Google Scholar]
- 28.Stegger M, Andersen PS, Kearns A, et al. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA(LGA251) Clin Microbiol Infect. 2012;18:395–400. doi: 10.1111/j.1469-0691.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki T, Kikuchi K, Tanaka Y, Takahashi N, Kamata S, Hiramatsu K. Methicillin-resistant Staphylococcus pseudintermedius in a veterinary teaching hospital. J Clin Microbiol. 2007;45:1118–1125. doi: 10.1128/JCM.02193-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epstein CR, Yam WC, Peiris JS, Epstein RJ. Methicillin-resistant commensal staphylococci in healthy dogs as a potential zoonotic reservoir for community-acquired antibiotic resistance. Infect Genet Evol. 2009;9:283–285. doi: 10.1016/j.meegid.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Chanchaithong P, Perreten V, Schwendener S, et al. Strain typing and antimicrobial susceptibility of methicillin-resistant coagulase-positive staphylococcal species in dogs and people associated with dogs in Thailand. J Appl Microbiol. 2014;117:572–586. doi: 10.1111/jam.12545. [DOI] [PubMed] [Google Scholar]
- 32.Lehner G, Linek M, Bond R, et al. Case-control risk factor study of methicillin-resistant Staphylococcus pseudintermedius (MRSP) infection in dogs and cats in Germany. Vet Microbiol. 2014;168:154–160. doi: 10.1016/j.vetmic.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Weese JS, Faires MC, Frank LA, Reynolds LM, Battisti A. Factors associated with methicillin-resistant versus methicillin-susceptible Staphylococcus pseudintermedius infection in dogs. J Am Vet Med Assoc. 2012;240:1450–1455. doi: 10.2460/javma.240.12.1450. [DOI] [PubMed] [Google Scholar]
- 34.Nienhoff U, Kadlec K, Chaberny IF, et al. Methicillin-resistant Staphylococcus pseudintermedius among dogs admitted to a small animal hospital. Vet Microbiol. 2011;150:191–197. doi: 10.1016/j.vetmic.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Detwiler A, Bloom P, Petersen A, Rosser EJ., Jr Multi-drug and methicillin resistance of staphylococci from canine patients at a veterinary teaching hospital (2006–2011) Vet Q. 2013;33:60–67. doi: 10.1080/01652176.2013.799792. [DOI] [PubMed] [Google Scholar]
- 36.Ott E, Bange FC, Reichardt C, et al. Costs of nosocomial pneumonia caused by meticillin-resistant Staphylococcus aureus. J Hosp Infect. 2010;76:300–303. doi: 10.1016/j.jhin.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Talan DA, Staatz D, Staatz A, Goldstein EJ, Singer K, Overturf GD. Staphylococcus intermedius in canine gingiva and canine-inflicted human wound infections: Laboratory characterization of a newly recognized zoonotic pathogen. J Clin Microbiol. 1989;27:78–81. doi: 10.1128/jcm.27.1.78-81.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul NC, Moodley A, Ghibaudo G, Guardabassi L. Carriage of methicillin-resistant Staphylococcus pseudintermedius in small animal veterinarians: Indirect evidence of zoonotic transmission. Zoonoses Public Health. 2011;58:533–539. doi: 10.1111/j.1863-2378.2011.01398.x. [DOI] [PubMed] [Google Scholar]
- 39.Paul NC. MRSP: Prevalence in practice. Vet Rec. 2015;176:170–171. doi: 10.1136/vr.h443. [DOI] [PubMed] [Google Scholar]
- 40.Walther B, Hermes J, Cuny C, et al. Sharing more than friendship — Nasal colonization with coagulase-positive staphylococci (CPS) and co-habitation aspects of dogs and their owners. PLoS One. 2012;7:e35197. doi: 10.1371/journal.pone.0035197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva MB, Ferreira FA, Garcia LN, et al. An evaluation of matrix-assisted laser desorption ionization time-of-flight mass spectrometry for the identification of Staphylococcus pseudintermedius isolates from canine infections. J Vet Diag Invest. 2015;27:231–235. doi: 10.1177/1040638715573297. [DOI] [PubMed] [Google Scholar]