Abstract
Background. Echinocandins are first-line treatment for Candida glabrata candidemia. Echinocandin resistance is concerning due to limited remaining treatment options. We used data from a multisite, population-based surveillance program to describe the epidemiology and risk factors for echinocandin nonsusceptible (NS) C glabrata candidemia.
Methods. The Centers for Disease Control and Prevention's Emerging Infections Program conducts population-based laboratory surveillance for candidemia in 4 metropolitan areas (7.9 million persons; 80 hospitals). We identified C glabrata cases occurring during 2008–2014; medical records of cases were reviewed, and C glabrata isolates underwent broth microdilution antifungal susceptibility testing. We defined echinocandin-NS C glabrata (intermediate or resistant) based on 2012 Clinical and Laboratory Standards Institute minimum inhibitory concentration breakpoints. Independent risk factors for NS C glabrata were determined by stepwise logistic regression.
Results. Of 1385 C glabrata cases, 83 (6.0%) had NS isolates (19 intermediate and 64 resistant); the proportion of NS isolates rose from 4.2% in 2008 to 7.8% in 2014 (P < .001). The proportion of NS isolates at each hospital ranged from 0% to 25.8%; 3 large, academic hospitals accounted for almost half of all NS isolates. In multivariate analysis, prior echinocandin exposure (adjusted odds ratio [aOR], 5.3; 95% CI, 2.6–1.2), previous candidemia episode (aOR, 2.5; 95% CI, 1.2–5.1), hospitalization in the last 90 days (aOR, 1.9; 95% CI, 1.0–3.5, and fluconazole resistance [aOR, 3.6; 95% CI, 2.0–6.4]) were significantly associated with NS C glabrata. Fifty-nine percent of NS C glabrata cases had no known prior echinocandin exposure.
Conclusion. The proportion of NS C glabrata isolates rose significantly during 2008–2014, and NS C glabrata frequency differed across hospitals. In addition to acquired resistance resulting from prior drug exposure, occurrence of NS C glabrata without prior echinocandin exposure suggests possible transmission of resistant organisms.
Keywords: Candida glabrata, Candidemia, echinocandin resistance, epidemiology, risk factors, transmission of drug resistance
Candida species are the most common cause of healthcare-associated bloodstream infections [1], and Candida glabrata accounts for one third of all candidemia in the United States [2, 3]. Because C glabrata demonstrates increased resistance to the azole class of antifungal medications [4], echinocandins are the preferred treatment for this organism [5]. Echinocandins were first introduced in the early 2000s, and reports of resistance were rare until recently [6]. In the last few years, the Centers for Disease Control and Prevention (CDC) and others have reported increases in bloodstream infections caused by echinocandin and multidrug-resistant Candida species, the majority of which were C glabrata [2, 7–10].
Echinocandin resistance, thought to be acquired from prior exposure to echinocandins and mediated through mutations in the FKS genes [11], is problematic because there are few other options remaining for treatment of C glabrata. Alternative treatments such as amphotericin B are more toxic and often poorly tolerated [12]. Furthermore, testing for resistance to echinocandins is not routinely performed in most clinical laboratories, and therefore resistance may only be recognized when treatment failure occurs.
The objectives of this study were to describe the epidemiology of and risk factors for echinocandin nonsusceptible (NS) C glabrata bloodstream infections using data from a large, multisite, population-based surveillance program in the United States.
METHODS
The CDC's Emerging Infections Program conducts surveillance for Candida bloodstream infections in 4 metropolitan areas: Atlanta, Georgia (started in March 2008), Baltimore City and County, Maryland, (started in June 2008), the tri-county region of Portland Oregon (started in January 2011), and Knox County (Knoxville) and surrounding counties in Tennessee (started in January 2011); combined population is ∼7.9 million persons and includes 80 hospital sites. The methods of surveillance have been previously described [2]; in brief, a case of candidemia is defined as a blood culture positive for a Candida species collected from a resident of the surveillance area at least 30 days after any other blood culture positive for Candida species. Surveillance personnel use standardized case report forms to abstract demographic and clinical data from medical records. All available laboratory isolates are sent to the CDC for species confirmation and antifungal drug-susceptibility testing. FKS mutation analysis was performed on isolates that demonstrated elevated minimum inhibitory concentration (MIC) values during antifungal drug-susceptibility testing. Only cases with C glabrata infection that occurred during 2008–2014 and for which isolates were available for laboratory analysis are included in this report.
Laboratory Methods
Molecular identification of isolates at the CDC was conducted using a Luminex assay or DNA sequencing of the D1/D2 subunit of the 28S ribosomal DNA, as previously described [13]. Antifungal susceptibility testing was performed with broth microdilution with fluconazole, anidulafungin, caspofungin, and micafungin, according to the Clinical and Laboratory Standards Institute (CLSI) M27-A3 document guidelines [14], using frozen Roswell Park Memorial Institute (RPMI) microbroth trays custom manufactured by TREK Diagnostics (Cleveland, OH) with no indicator dye. Results were read after 24 hours, and the MIC was identified as the lowest concentration of drug that caused a significant decrease in growth compared with the control well. The mutations in hotspot-1 regions of FKS1 and FKS2 genes were detected by either the Luminex-based multiplex assay [15] or by DNA sequencing at the CDC as described previously [16].
Definitions
Isolates of C glabrata were categorized as susceptible, intermediate, or resistant to each echinocandin using the 2012 CLSI M27-S4 breakpoints [17]. Isolates with MICs in the intermediate or resistance range to 1 or more echinocandins were considered NS and are the focus of this analysis. For fluconazole, isolates with an MIC ≤ 32 μg/mL were considered susceptible dose-dependent, whereas those with an MIC of ≥64 μg/mL were considered resistant. Any isolate that was resistant to both fluconazole and 1 or more echinocandin was considered to be multidrug resistant. An isolate demonstrating mutations in the hotspot-1 regions of FKS1 and FKS2 genes was considered to have an FKS mutation.
Statistical Methods
We used the Cochrane-Armitage test to assess longitudinal trends in the proportion of NS isolates. Categorical variables were analyzed using χ2 or Fisher's exact tests, and medians of continuous variables were analyzed using the Wilcoxon rank-sum test. Logistic regression analysis was applied to identify demographic and clinical variables associated with echinocandin nonsusceptibility and with having an FKS mutation. FKS mutation testing was not available for echinocandin susceptible isolates. Variables with a P < .20 by bivariate analysis or were clinically relevant were included in multivariable model selection. Model selection was conducted using stepwise logistic regression and consideration of 2-way interaction terms. The level of significance was set at α = 0.05. All analyses were done using SAS software (version 9.3; SAS Institute, Inc., Cary, NC).
Human Subjects
The CDC ethics liaisons reviewed this surveillance project and deemed it a nonresearch activity. This activity was also evaluated individually at each surveillance location, and it was either deemed a public health assessment or human subjects research and then approved by local institutional review boards.
RESULTS
There were 1591 reported cases of C glabrata infections during 2008–2014 in the 4 surveillance sites; 1385 (87%) cases with isolates and clinical information were available and included in analysis. There were no significant differences in demographic characteristics of cases with and without available isolates. Of these 1385 cases, 6.0% (n = 83) had isolates that were NS to echinocandins (1.4% [n = 19] intermediate, 4.6% [n = 64] resistant to 1 or more echinocandin). Susceptibility varied by echinocandin: 4.8% (n = 66) of cases had isolates that were NS to micafungin (13 intermediate and 53 resistant), 4.0% (n = 55) were NS to caspofungin (17 intermediate and 38 resistant), and 3.7% (n = 51) were NS to anidulafungin (6 intermediate and 45 resistant); 2.9% (n = 40) were NS to all 3 echinocandins, and 2.1% (n = 29) were resistant to all 3 echinocandins. Isolates with fluconazole resistance were detected in 9.6% of cases (n = 132), and multidrug resistance was detected in 1.7% (n = 23).
Time and Location Trends
The proportion of cases with an echinocandin-NS C glabrata isolate increased over time: 4.2% (7 of 161) of isolates in 2008 were NS, compared with 7.8% (13 of 153) in 2014 (test for trend: P < .001) (Figure 1). The surveillance area in Georgia had the highest proportion of cases with NS isolates (6.9%; 43 of 620), followed by the Maryland surveillance area (5.9%; 35 of 598), the Oregon surveillance area (3.0%; 2 of 67), and the Tennessee surveillance area (3.0%; 3 of 100). All surveillance areas had an increase in proportion of NS cases with the Georgia surveillance area experiencing the highest increase from 3.4% (3 of 89) in 2008 to 14.5% (9 of 62) in 2014 (Figure 1).
Figure 1.
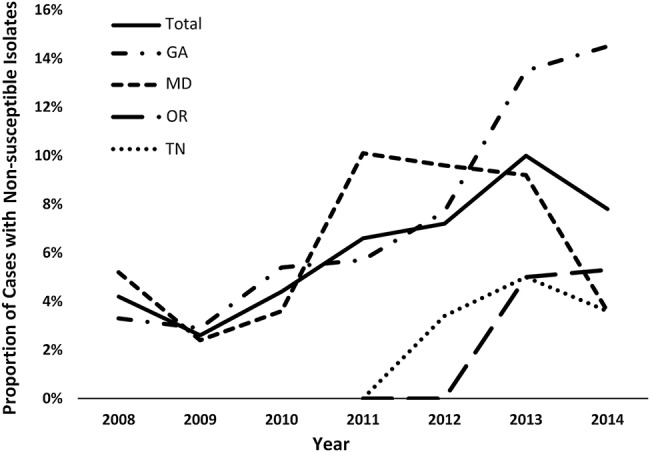
Proportion of cases with Candida glabrata isolates that were nonsusceptible to echinocandins, by surveillance site and year, 2008–2014. Surveillance areas: Atlanta metropolitan area, Georgia ([GA] started in March 2008), Baltimore City and County, Maryland ([MD] started in June 2008), Tri-county region of Portland Oregon ([OR] started in January 2011), and Knoxville, Tennessee ([TN] started in January 2011); combined population is 7.9 million persons and includes 80 hospital sites.
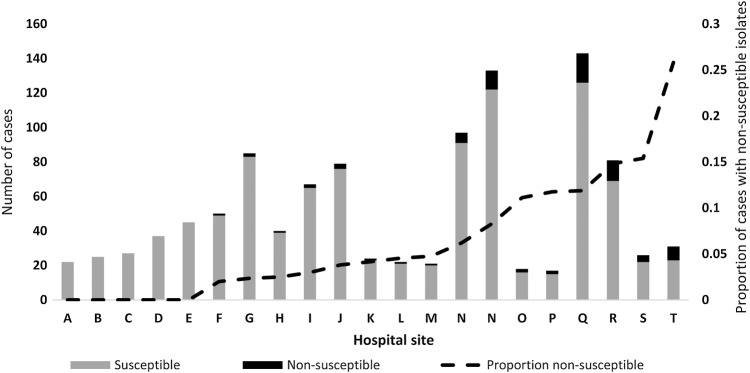
Among the 80 hospitals within all 4 surveillance areas, C glabrata isolates were identified at 57 (71.3%); the number ranged from 1 to 143 (median, 12). Twenty-three (40.3%) of these 57 hospitals had any cases with NS C glabrata. Among the 21 hospitals that submitted at least 20 C glabrata isolates during the study period, the proportion of NS isolates identified ranged from 0%–25.8% (Figure 2). Three large, academic hospitals accounted for nearly half (n = 40 of 83) of all cases with NS isolates.
Figure 2.
Proportion of Candida glabrata cases with echinocandin nonsusceptible (NS) isolates by hospital site (among hospitals with ≥20 isolates submitted during the study period), 2008–2014, sorted by proportion of NS isolates.
Factors Associated With Candidemia Caused by Echinocandin Nonsusceptible Candida glabrata
Univariable Analyses
The proportion of cases with NS isolates was significantly different by age category (P < .001) (Table 1). Cases with NS isolates were also more likely to have had a prior hospitalization in the 90 days before C glabrata-positive blood culture (echinocandin NS: 78.3% vs susceptible: 59.1%; P < .001), have received total parenteral nutrition in the 14 days before having C glabrata-positive blood culture (NS: 41.0% vs susceptible: 26.9%; P = .008), or have had a previous episode of candidemia (NS: 40.2% vs susceptible: 7.6%; P < .001). Cases with NS isolates were also more likely to have ever received an echinocandin (NS: 38.6% vs susceptible: 5.2%; P < .001) and have isolates with fluconazole resistance (NS: 32.9% vs susceptible: 8.1%; P < .001). Cases with NS isolates were in the hospital for a median duration of 7 days (interquartile range [IQR]: 0–20.5) before candidemia diagnosis compared with 5 days (IQR: 0–15) for cases with susceptible isolates (P = .30). There were no significant differences between those with echinocandin susceptible and NS isolates by sex, race (black vs non-black), presence of certain underlying conditions, including diabetes, underlying liver disease, cancer, renal disease, organ transplantation, or abdominal surgery in the 90 days before C glabrata-positive blood culture. Residence in a nursing home at the time of C glabrata-positive blood culture, intensive care unit stay, presence of a central venous catheter in the 2 days before C glabrata-positive blood culture, and 30-day mortality were also not significantly different between the 2 groups.
Table 1.
Characteristics of Patients With Candida glabrata Candidemia by Echinocandin Susceptibility
| Characteristic | Echinocandin Nonsusceptible, n (%) | Echinocandin Susceptible, n (%) | P Valuea |
|---|---|---|---|
| Total: n = 1334 | 83 (6.0) | 1302 (94.0) | <.001 |
| Age in years | |||
| 0–1 | 0 (0) | 15 (1.2) | |
| 2–17 | 2 (2.4) | 7 (0.5) | |
| 18–44 | 28 (33.7) | 187 (14.5) | |
| 45–64 | 25 (30.1) | 531 (41.1) | |
| ≥65 | 28 (33.7) | 552 (42.7) | |
| Female sex | 39 (47.6) | 675 (52.0) | .494 |
| Black race | 43 (54.4) | 688 (54.9) | >.999 |
| Underlying liver condition | 10 (12.1) | 191(14.7) | .630 |
| Diabetes | 35 (42.2) | 525 (40.3) | .731 |
| Abdominal surgery in the 90 days prior to candidemia | 18 (21.7) | 252 (19.4) | .668 |
| Previous hospitalization in the 90 days prior to candidemia | 65 (78.3) | 729 (59.1) | <.001 |
| Nursing home resident at the time of candidemia | 23 (30.3) | 293 (25.5) | .347 |
| Intensive care unit admission for candidemia | 51 (62.2) | 815 (64.1) | .723 |
| Central venous catheter present in the 2 days prior to candidemia | 70 (86.4) | 947 (76.8) | .054 |
| Total parenteral nutrition in the 14 days prior to candidemia | 34 (41.0) | 350 (26.9) | .008 |
| Previous candidemia episode | 33 (40.2) | 98 (7.6) | <.001 |
| Ever received echinocandin | 32 (38.6) | 68 (5.2) | <.001 |
| Fluconazole-resistant isolate | 27 (32.9) | 105 (8.1) | <.001 |
| Number of days in the hospital before candidemia, median (IQR) | 7 (0–15) | 5 (0–20.5) | .303b |
| Death within 30 days of candidemia | 18 (21.7) | 357 (27.5) | .308 |
The significant P Values are indicated in bold.
Abbreviation: IQR, interquartile range.
a Fishers exact test.
b Wilcoxon rank-sum test.
Multivariable Analyses
Table 2 shows the unadjusted and adjusted odds ratios for variables included in the multivariable model selection step. In the multivariable analysis, factors that remained independently associated with having a NS isolate were as follows: hospitalization in the 90 days before candidemia (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.0–3.4), previous episode of candidemia (aOR, 2.5; 95% CI, 1.5–5.1), prior exposure to echinocandins (aOR, 5.3; 95% CI, 2.6–10.8), and fluconazole resistance (aOR, 3.6; 95% CI, 2.0–6.4) (Table 2).
Table 2.
Risk Factors for Echinocandin nonsusceptible Candida glabrata Infection and Presence of FKS Mutations Using Multivariable Modeling
| Characteristic | Nonsusceptible |
FKS Mutations |
||
|---|---|---|---|---|
| Unadjusted Odds Ratio (95% CI) | Adjusted Odd Ratio (95% CI) | Unadjusted Odds Ratio (95% CI) | Adjusted Odd Ratio (95% CI) | |
| Age | ||||
| 0–17 | 1.7 (.4–8.0) | 1.7 (.4–8.6) | 3.6 (.4–31.3) | 3.9 (.4–37.9) |
| 18–44 | 3.0 (1.7–5.1) | 1.7 (.9–3.3) | 6.5 (2.9–14.7) | 4.4 (1.6–11.8) |
| 45–64 | 0.9 (.5–1.6) | 0.6 (.3–1.2) | 1.2 (.5–2.9) | 0.8 (.3–2.2) |
| ≥65 | Reference | Reference | Reference | Reference |
| Hospitalization in prior 90 days | 2.5 (1.5–4.3) | 1.9 (1.1–3.5) | 2.8 (1.3–6.0) | 5.9 (1.7–20.6) |
| Previous candidemia | 8.2 (5.0–13.3) | 2.5 (1.3–5.1) | 11.1 (5.8–21.5) | 1.6 (.6–4.4) |
| Prior echinocandin | 11.4 (6.8–18.9) | 5.3 (2.6–10.8) | 18.9 (9.7–37.2) | 11.6 (4.4–31.3) |
| Fluconazole resistance | 5.6 (3.4–9.2) | 3.6 (2.0–6.4) | 6.0 (3.1–11.8) | 3.2 (1.4–7.2) |
| Time between admission and Candida culture date (per week) | 1.1 (1.0–1.1) | Not selected to remain in the model | 1.1 (1.1–1.2) | 1.2 (1.1–1.3) |
| Received TPN | 1.9 (1.2–3.0) | Not selected to remain in the model | 3.0 (1.6–5.8) | Not selected to remain in the model |
| Central venous catheter | 2.0 (1.01–3.9) | Not selected to remain in the model | 4.8 (1.2–20.2) | Not selected to remain in the model |
Abbreviations: CI, confidence interval; TPN, total parenteral nutrition.
We also examined factors associated with nonsusceptibility to caspofungin and micafungin/anidulafungin separately. Results of this analysis are presented in a supplementary table (Supplementary Table S1). Risk factors for nonsusceptibility for each of the echinocandins were largely the same as risk factors for nonsusceptibility to any echinocandin. However, there was a stronger association between prior echinocandin exposure and caspofungin nonsusceptibility than to micafungin/anidulafungin nonsusceptibility (aOR 8.3 [95% CI, 3.7–18.8] for caspofungin vs aOR 4.9 [95% CI, 2.3–10.3] for micafungin/anidulafungin).
FKS Mutations
Testing for FKS mutation was performed on 859 of 1302 (66.0%) of susceptible isolates and 73 of 83 (88.0%) NS isolates. None of the isolates that were categorized as susceptible had FKS mutations, and 41 (56.2%) of isolates categorized as NS had FKS mutations present. Among resistant isolates, 70% (41 of 58) had an FKS mutation present, whereas none of the intermediate isolates (0 of 15) had FKS mutations. Among the 28 cases that had an isolate resistant to all 3 echinocandins by MIC values, 92.9% (n = 26) had an FKS mutation.
Risk factors for having FKS mutations were evaluated using the 932 cases with FKS mutation testing performed. Risk factors were similar to those for having an NS isolate (Table 2). An exception was that previous candidemia episode was not an independent risk factor for having an FKS mutation but remained in the multivariable model as a confounder. In addition, a new variable, time between admission and C glabrata-positive blood culture, was selected to remain in the model, and this was associated with a 16% higher odds of an isolate with a FKS mutation for every week spent in the hospital (aOR, 1.16; 95% CI, 1.06–1.26).
Resistance Without Any Prior Antifungal Drug Exposure
Of the 83 cases with NS isolates, more than half (59%; n = 51) did not have a known prior echinocandin exposure and 48.2% (n = 40) did not have known prior echinocandin exposure or a known previous episode of candidemia. Forty-four percent (18 of 41) of cases with FKS mutations did not have any known echinocandin exposure, and 37% (15 of 41) did not have any known echinocandin exposure or known previous episode of candidemia.
DISCUSSION
In this large population-based surveillance program, we found that isolates NS to echinocandins were recovered from 6% of cases of C glabrata candidemia. Furthermore, the proportion of cases with NS isolates has increased in all surveillance areas, despite demonstration that the total incidence of candidemia, including C glabrata candidemia, is declining in the United States [7]. An equally important finding is that nonsusceptibility was not uniformly distributed across hospitals. There were many hospitals with zero or only a few cases with echinocandin NS C glabrata isolates, whereas at some hospitals, 1 of every 6 C glabrata cases had an isolate with echinocandin MICs in the NS range.
The difference in the proportion of NS isolates by hospital site may be due to differences in echinocandin utilization by hospital [18]. Echinocandin utilization is likely to vary both by patient population and acuity. For instance, because echinocandins are the recommended first-line treatment for candidemia in cancer patients with neutropenia [5], hospitals with higher numbers of neutropenic cancer patients are likely to use more echinocandins than hospitals without such populations. Echinocandin use may also be influenced by hospital antimicrobial stewardship policies [19, 20]. Different strategies may be required to control the rise of echinocandin resistance, depending on the prevalence of NS C glabrata isolates in any given facility. All hospitals would likely benefit from a strong antimicrobial stewardship program that includes antifungal drugs [21]. In addition, hospitals that already have a substantial burden of echinocandin NS isolates should consider routine echinocandin susceptibility testing to identify patients who are at risk for antifungal treatment failure before clinical failure occurs.
Prior exposure to echinocandins was strongly associated with having infection with a NS isolate in this analysis, a finding that is consistent with prior studies [8, 10, 22]. Prior exposure likely results in selection pressure and facilitates generation and persistence of resistant isolates. We were surprised to find that more than half of cases with an NS isolate did not have known echinocandin exposure. In addition, 40% of isolates with FKS mutations did not come from patients with known prior echinocandin exposure. Lack of echinocandin exposure among those with echinocandin-resistant isolates has also been noted in at least 1 other study [10]. Although the data are limited by incomplete information on dose, duration, and sequence of antifungal therapy, the finding that patients without a prior history of echinocandin exposure had echinocandin NS isolates raises the possibility that resistance is not just acquired from prior exposure to echinocandins, but that NS organisms may also be transmitted within medical and community settings.
The clustering of cases with NS isolates at relatively few hospitals could also be explained by circulation of NS isolates either in the hospital or in the community that the hospital serves. The finding that each additional week in the hospital before the C glabrata-positive blood culture independently raised the odds of having an NS isolate and an FKS mutation suggests that resistant isolates may be transmitted in hospitals. The possibility of transmission could be further evaluated by studying relationships of NS isolates via whole genome sequencing in the same hospital over a short period of time.
Fluconazole resistance was associated with echinocandin nonsusceptibility in this study and has been reported in other studies [9, 10]. Resistance to azoles in isolates of C glabrata is a result of overexpression of Candida drug resistance efflux pumps [23], which are not involved in echinocandin resistance, although C glabrata is thought to be unique in its ability to sequentially acquire and express unlinked resistance-conferring mutations [8]. It is notable that we observed multidrug resistance in approximately 2% of C glabrata isolates. Previous candidemia was independently associated with having an NS isolate, even after controlling for prior exposure to echinocandins and fluconazole resistance. It is possible that fluconazole treatment of previous C glabrata candidemia exerts selection pressure, resulting in genomic changes in the pathogen even in the absence of overt fluconazole resistance. Such organisms may persist as colonizers in patients and, if subsequently exposed to antifungal drugs, emerge as NS to echinocandins [23].
Older age has been shown to be a risk factor for candidemia itself [2] and for colonization with C glabrata [24], but not necessarily for resistant infection. In this analysis, we found that in fact, younger age group of 18–44 years was associated with higher odds having an echinocandin NS isolate compared with the ≥65 years age group. Other investigators have also reported similar findings with fluconazole resistance; Pfaller et al [25], reported that although adults over the age of 60 were more likely to have candidemia with C glabrata, they were less likely to have an isolate resistant to fluconazole than adults aged 20–59. It is possible that differences in underlying conditions (eg, bone marrow transplantation is more common in younger adults and is associated with receipt of antifungal prophylaxis, which in turn may result in development of resistance) and mode of acquisition of C glabrata infection (colonization more likely in older adults) may account for differences in proportion of echinocandin NS isolates seen in the various age groups.
We conducted a separate analysis examining risk factors for FKS mutations because presence of these mutations is more closely correlated with treatment failure than elevated echinocandin MICs [26]. The associations we found were largely the same as those found for NS isolates, although the strength of the association was higher for those with FKS mutations. This suggests that the associations identified by this analysis can be used in the clinical setting to identify not only patients at high risk for echinocandin NS C glabrata infection, but also those at risk for potential treatment failure based on presence of FKS mutations.
There are several limitations to this study. First, although we did re-evaluate the medical records for a subset of patients with echinocandin-resistant infections and no known prior echinocandin exposure to confirm lack of echinocandin exposure, it is possible that presence of risk factors such as previous candidemia episodes and prior exposures to echinocandins were missed. We would be less likely to miss such information than a single-center study because the surveillance system includes all hospitals within the catchment area. This means that previous episodes of candidemia and associated treatment data would be captured even if infections were detected at another hospital in the surveillance system. Second, we did not collect dates, duration, dose, or sequence of antifungal therapy at all sites for all years of the study and were therefore unable to study treatment failure or duration of echinocandin therapy prior to resistance development; this limitation is somewhat allayed by previous studies demonstrating that echinocandin treatment failure is associated with presence of an FKS mutation [8]. Although this study is population-based surveillance, findings from these surveillance areas may not be generalizable to the entire United States. Hospitals are deidentified in the surveillance system. Therefore, we do not have details on patient populations treated and echinocandin usage patterns at the 3 hospitals with a high burden of NS isolates compared with other hospital with a lower proportion of NS isolates. This information would shed more light on hospital-level factors associated with echinocandin nonsusceptibility.
CONCLUSIONS
Increases in echinocandin NS C glabrata infections are concerning due to limited treatment options. Although currently NS isolates seem to be concentrated in a small number of hospitals, increased use of echinocandins may result in more widespread resistance. There is an urgent need for antifungal resistance control measures to prevent further increases in echinocandin NS C glabrata. A better understanding of how nonsusceptibility is acquired, including whether echinocandin resistance is transmitted between patients, is important to guide infection prevention measures.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We gratefully acknowledge the many individuals in the hospitals and laboratories in Baltimore, Atlanta, Knox County, and Portland for help in identifying cases and isolates, and we also thank the following people: Wendy Baughman, Betsy Stein, Sasha Harb, Jessica Reno, Lewis Perry, Chris Bower, Andrew Revis, Zirka Smith, Catherine Espinosa, Michelle Wiles (Georgia Emerging Infections Program); Rosemary Hollick, Kim Holmes, Kathleen Shutt, Vijitha Lahanda Wadu, Lindsay Bonner (Maryland Emerging Infections Program); Brenda Barnes, Terri McMinn, Caroline Graber (Tennessee Emerging Infections Program); Valerie Ocampo, Magdalena Scott, Heather Jamieson, Monika Samper, Ariane Mangune, Lydia Mcdonald (Oregon Emerging Infections Program); Matthew Westercamp, Shirley McClinton, Colleen Lysen, Randy Kuykendall, Joyce Peterson, Carol Bolden, Rajal Mody, Tom Chiller, and Mary Brandt (Mycotic Diseases Branch, Centers for Disease Control and Prevention).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Magill SS, Edwards JR, Bamberg W et al. . Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cleveland AA, Farley MM, Harrison LH et al. . Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis 2012; 55:1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaller MA, Andes DR, Diekema DJ et al. . Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the prospective antifungal therapy (PATH) registry 2004–2008. PLoS One 2014; 9:e101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanafani ZA, Perfect JR. Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis 2008; 46:120–8. [DOI] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes D et al. . Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfaller MA, Boyken L, Hollis RJ et al. . In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol 2008; 46:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleveland AA, Harrison LH, Farley MM et al. . Declining incidence of candidemia and the shifting epidemiology of candida resistance in two US metropolitan areas, 2008–2013: results from population-based surveillance. PloS One 2015; 10:e0120452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander BD, Johnson MD, Pfeiffer CD et al. . Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 2013; 56:1724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller MA, Castanheira M, Lockhart SR et al. . Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol 2012; 50:1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farmakiotis D, Tarrand JJ, Kontoyiannis DP. Drug-resistant Candida glabrata infection in cancer patients. Emerg Infect Dis 2014; 20:1833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyda ND, John J, Kilic A et al. . FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis 2014; 59:819–25. [DOI] [PubMed] [Google Scholar]
- 12.Kagan S, Ickowicz D, Shmuel M et al. . Toxicity mechanisms of Amphotericin B and Its neutralization by conjugation with arabinogalactan. Antimicrob Agents Chemother 2012; 56:5603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockhart SR, Iqbal N, Cleveland AA et al. . Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two United States cities from 2008 to 2011. J Clin Microbiol 2012; 50:3435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Third Edition. CLSI document MM27-A3 Wayne, PA; Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 15.Pham CD, Bolden CB, Kuykendall RJ, Lockhart SR. Development of a luminex-based multiplex assay for detection of mutations conferring resistance to echinocandins in Candida glabrata. J Clin Microbiol 2014; 52:790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimbeck AJ, Iqbal N, Ahlquist AM et al. . FKS mutations and elevated echinocandin MIC values among Candida glabrata isolates from United States population-based surveillance. Antimicrob Agents Chemother 2010; 54:5042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CLSI. M27-S4. Reference Method for Broth Dilution antifungal Susceptibility Testing of Yeasts; Fourth Informational Supplement. CLSI document MM18-A Wayne, PA; Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 18.Fekkar A, Dannaoui E, Meyer I et al. . Emergence of echinocandin-resistant Candida spp. in a hospital setting: a consequence of 10 years of increasing use of antifungal therapy? Eur J Clin Microbiol Infect Dis 2014; 33:1489–96. [DOI] [PubMed] [Google Scholar]
- 19.Ruttimann S, Keck B, Hartmeier C et al. . Long-term antibiotic cost savings from a comprehensive intervention program in a medical department of a university-affiliated teaching hospital. Clin Infect Dis 2004; 38:348–56. [DOI] [PubMed] [Google Scholar]
- 20.Ansari F, Gray K, Nathwani D et al. . Outcomes of an intervention to improve hospital antibiotic prescribing: interrupted time series with segmented regression analysis. J Antimicrob Chemother 2003; 52:842–8. [DOI] [PubMed] [Google Scholar]
- 21.Valerio M, Rodriguez-Gonzalez CG, Munoz P et al. . Evaluation of antifungal use in a tertiary care institution: antifungal stewardship urgently needed. J Antimicrob Chemother 2014; 69:1993–9. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard E, Lortholary O, Boukris-Sitbon K et al. . Prior caspofungin exposure in patients with hematological malignancies is a risk factor for subsequent fungemia due to decreased susceptibility in Candida spp.: a case-control study in Paris, France. Antimicrob Agents Chemother 2011; 55:5358–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanguinetti M, Posteraro B, Fiori B et al. . Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance. Antimicrob Agents Chemother 2005; 49:668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malani AN, Psarros G, Malani PN, Kauffman CA. Is age a risk factor for Candida glabrata colonization? Mycoses 2011; 54:531–7. [DOI] [PubMed] [Google Scholar]
- 25.Pfaller MA, Messer SA, Hollis RJ et al. . Variation in susceptibility of bloodstream isolates of Candida glabrata to fluconazole according to patient age and geographic location in the United States in 2001 to 2007. J Clin Microbiol 2009; 47:3185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shields RK, Nguyen MH, Press EG et al. . The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob Agents Chemother 2012; 56:4862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]