Abstract
Aim
Assessment of right ventricular (RV) function is a challenge, especially in patients with congenital heart disease (CHD). The aim of the present study is to assess whether knowledge-based RV reconstruction, used in the everyday practice of an echo-lab for adult CHD in a tertiary referral center, is accurate when compared to cardiac magnetic resonance (CMR) examination.
Subjects and methods
Adult patients who would undergo CMR for assessment of the RV were asked to undergo an echo of the heart for further knowledge-based reconstruction (KBR). Echocardiographic images were acquired in standard views using a predefined imaging protocol. RV volumes and ejection fraction (EF) calculated using knowledge-based technology were compared with the CMR data of the same patient.
Results
Nineteen consecutive patients with congenital right heart disease were studied. Median age of the patients was 28 years (range 46 years). Reconstruction was possible in 16 out of 19 patients (85%). RV volumes assessed with this new method were smaller than with CMR. Indexed end diastolic volumes were 114±17 ml vs 121±19 ml, P<0.05 and EFs were 45±8% vs 47±9%, P<0.05 respectively. The correlation between the methods was good with an intraclass correlation of 0.84 for EDV and 0.89 for EF, P value <0.001 in both cases.
Conclusion
KBR enables reliable measurement of RVs in patients with CHDs and can be used in clinical practice for analysis of volumes and EFs.
Keywords: ventripoint system, knowledge-based reconstruction, congenital heart disease, right ventricle volume
Introduction
Assessment of right ventricular (RV) function is a challenge due to its complex and alternated morphology, especially in patients with congenital heart disease (CHD). Regular and accurate assessment of RV function is an important part of the follow-up in such patients, since deterioration of RV function is closely related to mortality, and therefore, it guides therapy. As a rule of thumb, intervention, if possible, is considered, even in asymptomatic patients, when there are signs of worsening of RV function (1, 2).
RV ejection fraction (EF) is a crude measurement of global RV systolic function, but as it correlates well with clinical endpoints (3), it is very often used in clinical decision-making. Cardiac magnetic resonance (CMR) imaging is well validated and has excellent correlation with true RV volumes (4). It is considered the reference standard for assessment of the RV volumes and EFs (5) but is expensive and not widely available. Since echocardiography is more accessible than CMR, more patient-friendly, and less expensive, an echocardiographic alternative for assessment of RVEF has been long sought for. The correlation between 2D echocardiographic measurements of RV function, current standard in clinical practice, and CMR-derived values of RVEF is poor. Real-time 3D echocardiographic assessment of RVEF has proven to be reliable and feasible (6), but its use in clinical practice is still limited. This is mostly due to the limited image quality, with inferior spatial and temporal resolution compared to 2D echocardiography, and also due to the substantial learning curve. A new alternative technique enabling 3D reconstruction of the RV is ‘knowledge-based’ 3D reconstruction (3D-KBR). KBR uses standardized 2D cross-sections of the RV combined with information about their localization in the 3D space to produced 3D reconstruction of RV volume (7). KBR has shown to be accurate compared to CMR in children with various congenital heart anomalies (8) and in adults with the RV in systemic position and in pulmonary arterial hypertension (9, 10).
The aim of the present study is to assess whether this KBR of RV is accurate and clinically feasible in the everyday practice of an echo-lab for adult CHD (ACHD) of a tertiary referral centre.
Methods
Patients
Adult patients scheduled for routine CMR including assessment of RV volumes and EF, were asked to undergo an echo for KBR of the RV. CMR and echo were performed within 2 h from each other, to ensure comparable loading conditions.
Exclusion criteria were presence of cardiac devices, arrhythmia interfering with image acquisition, and inability to cooperate. Patients were not screened for the quality of acoustic window. The study was approved by the Institutional Research Ethics Board. Informed consent was obtained from all participants prior to the start of study procedures.
Image acquisition
Patients were studied in the left lateral decubitus position. They were asked to lie absolutely still during the entire study. As KBR uses diagnosis-specific algorithms for volume calculation, all patient's data, including diagnosis, gender, weight, and height were put into the examination protocol before image storage. Images for further KBR were acquired during end-expiratory breath holds in each of the various, predefined 2D cross-sections. The standard views and points of interest in all cross-section planes are shown in Table 1. Every clip included at least three cardiac cycles.
Table 1.
Knowledge-based reconstruction (KBR) image acquisition protocol.
| KBR image acquisition protocol | |
|---|---|
| View | Region of interest |
| Parasternal long axis | RV anterior free wall, septum, mitral valve, and aortic valve |
| RV inflow | Anterior/posterior tricuspid valve annular insertions and RV endocardium |
| RV outflow tract/pulmonary annulus | Pulmonary valve annular insertions |
| Parasternal short axis | |
| RVOT/PA | PV annular insertions, RV outflow tract, and conal septum |
| Mitral valve annular level | RV endocardium, anterior/septal tricuspid valve annular insertion |
| Papillary muscle level | RV endocardium, RV septum, RV inf/ant free wall and septum |
| Apex | RV Endocardium, RV septum, crux of RV free wall and septum |
| Apical four-chamber – focusing on RV anatomical structures | Anterior and septal tricuspid valve annular insertion |
| True RV apex – oblique apical | True RV apex, RV septum, and RV endocardium |
| Apical RV two-chamber | RV endocardium and RV septum |
| Foreshortened apical – RV inflow/outflow | RV lateral, anterior, free wall, RV inflow tract, and RV outflow tract |
RV, right ventricle; RVOT, RV outflow tract; PA, pulmonary artery; PV, pulmonary valve.
Echo examination was performed using the Toshiba Artida SSH-880CV Diagnostic Ultrasound System with a 5-MHz transducer. The ultrasound scanner was linked to a computer (Ventripoint Medical Systems; Ventripoint, Inc., Seattle, WA, USA) and a magnetic field receiver was attached to the ultrasound probe. The magnetic field transmitter generating the orthogonal magnetic field was located above the exam bed. The magnetic field data were recorded by the receiver at the time of image acquisition. Using this data, the plane of the acquired 2D image was placed in the 3D volume created by the magnetic field transmitter. Data were digitally stored and analyzed offline. Details of this system, technical specification, data acquisition and data analysis, are described elsewhere (7, 11).
Image analysis
The acquired 2D RV-images and their spatial information were stored on the Ventripoint Medical Systems computer attached to the ultrasound machine. To perform the image analysis, first end-diastole and end-systole were determined on each of the acquired clips. The definition of the end diastole was based on the ECG and visual assessment taking into account the largest RV cavity size and the opening and closure of tricuspid and pulmonary valves (if possible). The same procedure was repeated to define the end systole, tracking the smallest RV cavity sizes.
In the next step, 15–35 points were placed in predefined crucial anatomical structures, as illustrated in Table 1. The procedure was done in both end-diastole and end-systole.
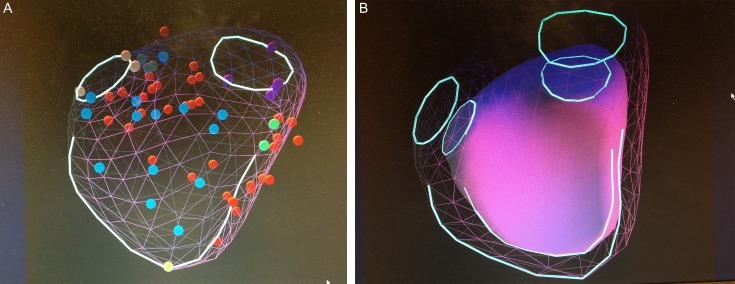
Combining data from these 2D cross-sections and their localization in the 3D space, the KBR system creates a 3D model of the RV using piecewise smooth subdivision surface reconstruction technology. The software compares acquired echo data with a (CMR-derived) reference database of patients with corresponding pathology (patients with Tetralogy of Fallot with the CMR-derived data base for patients with Tetralogy of Fallot, etc.), allowing reconstruction and measurements of RV volumes (Fig. 1A).
Figure 1.
Visualization of the right ventricle (RV) with knowledge-based reconstruction system. The two circles represent the position of the tricuspid and pulmonary valves. The open line represents the contour of the septal wall. (A) The mesh with the colored dots represent RV end diastolic volume (RVEDV). Demonstration of the position of the points on the mesh surface in relation to the calculated volume. Different colors represent different anatomic landmarks. Red, RV endocardium; light blue, RV septum; pink, pulmonary annulus; dark green, sub pulmonary; violet, tricuspid annulus; brown, basal bulge; yellow, apex; light green, RV septal edge. (B) Combined EDV and end systolic volume (ESV) rendering, visualize the inward movement of different segments of the RV.
After the first reconstruction, a quality check was performed using the incorporated features for quality assessment (border delineation, scan plane, intersections, and adjustment of the placement of points). The corrected, reconstructed RV shape was further reviewed in each of the 2D images in both end diastole and end systole and optimized by deletion or addition of points. After performed control and carrying out modifications, the final reconstruction with calculated volumes was accepted (Fig. 1B). These values, both volumes and EF, calculated with KBR technology were compared with the CMR data of the same patient.
CMR investigation
Subjects were scanned according to a pre-defined imaging protocol without anesthesia or sedation. A 1.5-T system (Ingenia R4.1.2, Philips Healthcare, Best, The Netherlands) was used with a dedicated chest phased-array parallel-imaging capable surface coil with a maximum of 36 active elements. Steady state free precession cine images were acquired in various orientations (short axis, four-chamber and two-chamber long axis, right and left ventricular outflow tract views in two planes) during repeated end-expiratory breath holds. Multi-slice cine short-axis acquisition was planned from the apex to well above the tricuspid and mitral valve: repetition time (TR)/echo time (TE) 3.4/1.69 ms, voxel size 1.25×1.25×8 mm, flip angle 90o, matrix 192×171 mm, and 30 frames/cycle.
RV volumetric analysis was performed by manual tracing of endocardial contours in end diastolic and end systolic phase in all slices, using Philips Cardiac Explorer (Philips EWS (release 2.6), Philips Medical Systems, Best, The Netherlands). The end diastolic and end systolic phase was selected by visual assessment as the phase with the largest and smallest RV cavity sizes respectively, taking into account the longitudinal four-chamber, vertical two-chamber, and RV outflow tract as reference views. If visual assessment was difficult, multiple frames were contoured to determine the correct end diastolic or end systolic phase. The RV epicardial and endocardial contours were manually traced from the most apical to the most basal short-axis slice. Only the portion of the outflow tract below the pulmonary valve was included in the blood volume in the slice where the valve was visible. If more than 50% of the tricuspid annulus or atrium was visible in a basal slice, the valve area was excluded from the blood volume. Trabeculae and papillary muscles were included in the blood volume.
Statistical analysis
Different statistical analyses were used to test the inter-technique correlation between parameters (end diastolic volume (EDV), indexed EDV (EDVi), end systolic volume (ESV), indexed ESV (ESVi), and EF) measured using two different methods. The Bland–Altman plot was constructed with the difference between measured values on y-axis and average of measured values on x-axis. Ninety-five percent limits of agreements were computed to see how far apart the measurements by the two methods were likely to be. Finally, an intraclass correlation (ICC) coefficient was performed for all parameters. Statistical analyses were performed using the IBM SPSS Statistics 23 System.
Results
Nineteen consecutive, unselected patients (13 men and six women) with CHD were studied. The diagnosis was Tetralogy of Fallot with stenosis and/or insufficiency in pulmonary homograft (ten patients), transposition of the great arteries in eight patients: three after atrial switch repair, four after arterial switch surgery, and one after Rastelli correction. One patient suffered from chronic pulmonary hypertension unrelated to CHD. Median age of the enrolled patients was 28 years (range 46 years). Mean body surface area was 1.8±0.25 m2 and mean body weight 68±14 kg.
Feasibility
The 2D-KBR was possible in 16 out of 19 patients (85%). In two out of 19 patients, reconstruction was not possible due to difficulty in identifying the anatomical landmarks (pulmonary valve and tricuspid annulus). In one patient, the KBR was not possible because the tracing did not lead to a sufficiently accurate reconstruction despite adjustments made during the quality check.
Accuracy
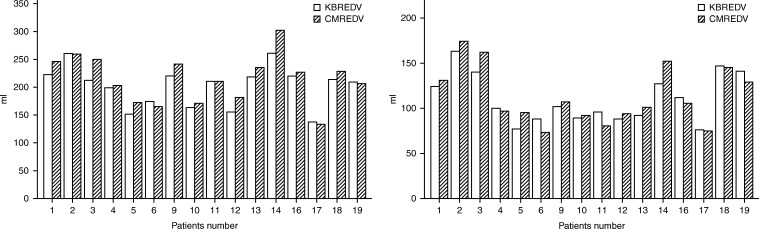
Results of the CMR and KBR analysis of the 16 individual patients were compared (Fig. 2). When grouped, RV volumes assessed with KBR were smaller than CMR.
Figure 2.
Comparison between knowledge-based reconstruction (KBR) and cardiac magnetic resonance (CMR) for right ventricle end systolic volumes (RVESV) and right ventricle end diastolic volumes (RVEDV).
KBR EDVi 114±17 ml vs CMR EDVi 121±19 ml (P<0.05), ESVi 63±14 ml vs 64±16 ml (P value 0.5) and RVEF 45±8% vs 47±9% respectively (P<0.05). The agreement between both methods assessed with ICC was of 0.84 for RV EDVi, 0.90 for RV ESVi and 0.89 for RVEF, P value <0.001.
Bland–Altman analysis revealed underestimation of EDV and ESV by KBR. The difference in indexed RV EDVi was −7 ml, with the limits of agreement (−23.0; 8.7), P=0.003. Difference for RVEF was −2% with the limits of agreement (−9.0; 5.0), P=0.04. A paired sample t-test was conducted to evaluate whether a statistically significant difference existed between the mean indexed RV volumes measured with the two modalities. The results of the paired sample t-test indicated no significant difference between indexed EDV and EF calculated with both modalities, on the P level 0.05 (Table 2).
Table 2.
Comparison between methods for different right ventricular volume parameters.
| Mean | s.d. | CI | df | Sig. | |
|---|---|---|---|---|---|
| EDV | −12.63 | 12.93 | −20.58; −4.67 | 15.00 | 0.004 |
| EDVi | −7.13 | 8.09 | −11.44; −2.81 | 15.00 | 0.003 |
| ESVi | −1.25 | 6.97 | −4.96; 2.46 | 15.00 | 0.48 |
| SV | −9.94 | 8.09 | −14.63; −5.25 | 15.00 | 0.000 |
| EF | −2.0 | 3.56 | −3.90; −1.04 | 15.00 | 0.04 |
EDV (ml), end diastolic volume; EDVi (ml/m2), EDV indexed; ESVi (ml/m2), end systolic volume indexed; SV (ml), stroke volume; EF%, ejection fraction measured with knowledge-based reconstruction (KBR) and cardiac magnetic resonance (CMR) respectively.
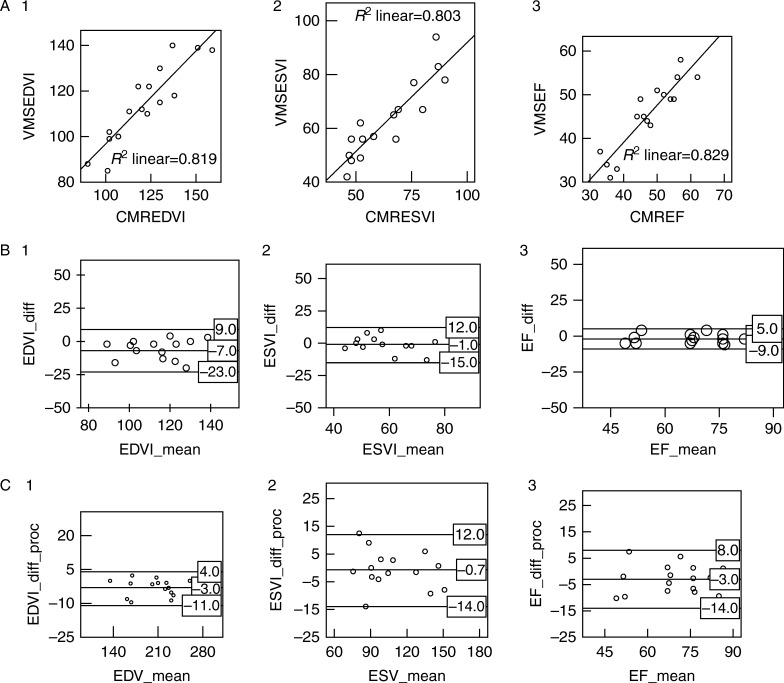
The linear regression analysis of RVEDVi, RVESVi, and EF, the Bland–Altman analysis and the percentage difference between both techniques (the difference between paired measurements divided by the average of the two measurements times 100) are shown in Fig. 3. Overall, the correlation between tested modalities was good.
Figure 3.
(A) Intertechnique correlation between knowledge-based reconstruction (KBR) and cardiac magnetic resonance (CMR) for end diastolic volume indexed (EDVi) (1), end systolic volume indexed (ESVi) (2), and ejection fraction (EF) (3). (B) Intertechnique agreement represented by Bland–Altman plots between KBR and CMR for EDVi (1), ESVi (2), and EF (3). EDVi_diff (KBREDVi−CMREDVi); EDVi_mean (KBREDVi+CMREDVi)/2. ESVi_diff (KBRSEVi−CMRESVi); ESVi_mean (KBRESVi+CMRESVi)/2. EF_diff (KBREF−CMREF); EF_mean (KBREF+CMREF)/2. (C) Percentage difference of intertechnique agreement represented by Bland–Altman plots between KBR and CMR for EDV (1), ESV (2), and EF (3). EDVi_diff_proc ((KBREDVi−CMREDVi)/EDV_mean×100). ESVi_diff_proc ((KBRESVi−CMRESVi)/ESV_mean×100). EF_diff_proc ((KBREF−CMREF)/EF_mean×100).
Discussion
This study shows that also in adult patients with congenital heart defects, KBR produces RV volumes and EF that are sufficiently parallel to those derived from CMR to be clinically useful in a ‘real-world’ setting. Despite a 2D image quality that is generally inferior to that in children, it is good enough to allow KBR to produce reliable 3D reconstructions of RV volumes.
Although the Bland–Altman test showed a systematic underestimation of echo derived RV volumes when compared with CMR, the differences were small. Apart from one study in which there is an over-estimation of RV volumes measured with echo, compared with CMR (9), our data are consistent with that of virtually all other studies on correlation between 3D echo and CMR for either left ventricular or RVEF (4, 12, 13). The underestimation of volumes with KBR is systematic and quantitatively limited and this should not hamper the use of KBR in clinical practice, provided echo-derived data are only compared to previously obtained echo-derived data. In contrast to conventional 2D echo, which is hardly of use for RV volume assessment in the individual patient in clinical practice (13, 14), the observed ICC of around 0.9 between KBR- and CMR-derived RVEDVi and RVESVi means that KBR can be used in clinical practice for assessment of RV volumes and function (Table 3). It is a very important aspect in the follow-up of adults with CHD, e.g., in timing of pulmonary valve replacement in patients with Tetralogy of Fallot (15, 16). The fact that these volumes may now be assessed reliably with KBR with limited investment of time and money (validated in the study by Laser et al. (12)) is, in our opinion, a step forward. CMR will still provide an additional information, prior to surgery, such as anatomic details, but its exploitation could probably be limited. In that way CMR resources would be more efficiently used in situations when we do not have other diagnostic tools.
Table 3.
ICC between methods for different RV volume parameters.
| Mean Δ/(limits of agreement) | P value* | ICC | P value † | |
|---|---|---|---|---|
| RV EDV | −12.63 (−41.9; 16.63) | 0.004 | 0.88 | <0.001 |
| RV EDVi (ml/m2) | −7.13 (−23.0; 8.7) | <0.001 | 0.84 | <0.001 |
| RV ESVi (ml/m2) | −1.25 (−14.9; 12.4) | NS | 0.90 | <0.001 |
| RVEF (%) | −2.0 (−9.0; 4.5) | <0.05 | 0.89 | <0.001 |
| RV SV (ml/m2) | −9.94 (−27.2; 7.3) | <0.001 | 0.87 | <0.001 |
RV, right ventricle; EDV, end diastolic volume; EDVi, EDV indexed; ESVi, end systolic volume indexed; EF, ejection fraction; SV, stroke volume; ICC, intraclass correlation. *P value of paired Student's t-test and † P value of ICC.
The limits of agreement for EF calculated with KBR compared with that calculated with CMR are quite wide: −2.4±11.4%. This should be taken into account when using KBR-derived EF in clinical practice. However, it should be realized that this is also true for CMR: when CMR is compared with itself, in repeated measurements of EF by test–retest (17) or inter- or intra-observer variability (18, 19, 20, 21) the bias±s.d. are of a comparable degree. The awareness about those limitations causes the decision about surgery to be based on repeated examinations in combination with patients' exercise capacities and symptoms.
The KBR system has the advantage of using 2D cross-sections as a basis for the 3D reconstruction. 2D echo has substantially higher spatial and temporal resolution than the RT3D echo images, acquired with the currently available 3D echo probes. The higher resolution makes identification of the endocardial border more straightforward and the anatomical structures that have to be defined are easier to recognize in the sharper 2D images than in the fuzzier RT3D images. A disadvantage is that KBR can only be used for volumetry of the RV (currently), not for the LV, and no analysis of anatomic structures like valves or abnormal anatomy is possible. In contrast to CMR, the KBR system is a bedside technique, which can be used in various clinical settings, including ICU. It is less expensive than CMR, faster in terms of data acquisition, with that more patient friendly, and data analysis (12). The learning curve for both CMR and KBR is comparable and quite long but time consumption per patient is relatively short for KBR. In experienced hands, time required for examination, image analysis, and post processing with KBR is about 20 min – given good internet connection with the central data base. This corresponds to time needed for performing a CMR examination, but then time for data post processing and analysis must be added. This statement is based on the authors' own experiences with KBR and CMR and on the previously cited paper by Laser et al. Because time consumption per examination is not systematically evaluated in the current study, the data are not reported in the part concerning results.
Cutting cost and increasing effectiveness, regarding both staff and equipment, is an important issue in the healthcare all around Europe and in the whole world.
Study limitations
The studied population is rather small and further investigation in larger and varying population, regarding both pathology and age, is recommended before the method can be the part of routine follow-up in ACHD population.
We excluded one patient with Rastelli corrected transposition due to poor image quality and inaccurate reconstructions. This reveals one of the limitations of the KBR. Very complicated morphologies and rare corrections are difficult to assess, as it is not possible to visualize the standardized cross-sections for detection of points of interest for further reconstruction. However, this not only the KBR problem, assessment of the RV function in those patients is very challenging with all modalities. KBR system requires a specific equipment, such as magnetic field generator and the specialized bed compatible with the magnetic field. This issue can currently be seen as a limitation. Modification of the system in order to make it more customer friendly and to decrease time necessary for preparation, will probably be done parallel with the method's further development.
Another limitation of presented study is the lack of data about inter and intra observer variability. In that aspect we will refer to the study by Dragulescu et al. (11) and Laser et al. (12). Both studies show a good reproducibility of the RV volume calculations with the limits of agreements being marginally wider than for CMR measurements, both for intra- and inter-observer variability.
Test–retest and intra- and inter-observer variability should be tested more profoundly, in large patient populations with various malformations before this technique can really be used with confidence in clinical practice. The experience in KBR is still relatively limited; future studies in larger patients' population with a greater variety of RV pathologies and an increased number of experienced investigators will probably give more information and more implications on how this new technology can be used in clinics.
Conclusion
In our experience, KBR enables reliable measurement of RV volumes in an ACHD population with sufficiently high feasibility and accuracy even when used in an unselected patient population. RV volumes and EF are comparable to that of CMR, acknowledging a small systemic underestimation. In contrast to 2D echo, KBR of the RV can be used in daily clinical practice for analysis of RV volumes and EF.
Acknowledgements
Acknowledgments to the staff at the echo lab at the Department of Cardiology, University Medical Center Utrecht, The Netherlands for their help and cooperation and to all co-authors for their critical comments during the work with this manuscript.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1. Baumgartner H Bonhoeffer P De Groot NM de Haan F Deanfield JE Galie N Gatzoulis MA Gohlke-Baerwolf C Kaemmerer H Kilner P et al. ESC guidelines for the management of grown-up congenital heart disease (new version 2010) European Heart Journal 31 2010. 2915–2957. 10.1093/eurheartj/ehq249 [DOI] [PubMed] [Google Scholar]
- 2. Rudski LG Lai WW Afilalo J Hua L Handschumacher MD Chandrasekaran K Solomon SD Louie EK Schiller NB Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography Journal of the American Society of Echocardiography 23 2010. 685–713.(quiz 86–88) 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 3. Haddad F Doyle R Murphy DJ Hunt SA Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure Circulation 117 2008. 1717–1731. 10.1161/CIRCULATIONAHA.107.653584 [DOI] [PubMed] [Google Scholar]
- 4. Sugeng L Mor-Avi V Weinert L Niel J Ebner C Steringer-Mascherbauer R Bartolles R Baumann R Schummers G Lang RM et al. Multimodality comparison of quantitative volumetric analysis of the right ventricle JACC. Cardiovascular Imaging 3 2010. 10–18. 10.1016/j.jcmg.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 5. Knauth AL Gauvreau K Powell AJ Landzberg MJ Walsh EP Lock JE del Nido PJ Geva T Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after Tetralogy of Fallot repair Heart 94 2008. 211–216. 10.1136/hrt.2006.104745 [DOI] [PubMed] [Google Scholar]
- 6. van der Zwaan HB Helbing WA McGhie JS Geleijnse ML Luijnenburg SE Roos-Hesselink JW Meijboom FJ Clinical value of real-time three-dimensional echocardiography for right ventricular quantification in congenital heart disease: validation with cardiac magnetic resonance imaging Journal of the American Society of Echocardiography 23 2010. 134–140. 10.1016/j.echo.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 7. Sheehan FH Kilner PJ Sahn DJ Vick GW III Stout KK Ge S Helbing WA Lewin M Shurman AJ Buechel EV et al. Accuracy of knowledge-based reconstruction for measurement of right ventricular volume and function in patients with Tetralogy of Fallot American Journal of Cardiology 105 2010. 993–999. 10.1016/j.amjcard.2009.11.032 [DOI] [PubMed] [Google Scholar]
- 8. Dragulescu A Grosse-Wortmann L Fackoury C Mertens L Echocardiographic assessment of right ventricular volumes: a comparison of different techniques in children after surgical repair of Tetralogy of Fallot European Heart Journal Cardiovascular Imaging 13 2012. 596–604. 10.1093/ejechocard/jer278 [DOI] [PubMed] [Google Scholar]
- 9. Bhave NM Patel AR Weinert L Yamat M Freed BH Mor-Avi V Gomberg-Maitland M Lang RM Three-dimensional modeling of the right ventricle from two-dimensional transthoracic echocardiographic images: utility of knowledge-based reconstruction in pulmonary arterial hypertension Journal of the American Society of Echocardiography 26 2013. 860–867. 10.1016/j.echo.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 10. Kutty S Li L Polak A Gribben P Danford DA Echocardiographic knowledge-based reconstruction for quantification of the systemic right ventricle in young adults with repaired D-transposition of great arteries American Journal of Cardiology 109 2012. 881–888. 10.1016/j.amjcard.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 11. Dragulescu A Grosse-Wortmann L Fackoury C Riffle S Waiss M Jaeggi E Yoo SJ Friedberg MK Mertens L Echocardiographic assessment of right ventricular volumes after surgical repair of Tetralogy of Fallot: clinical validation of a new echocardiographic method Journal of the American Society of Echocardiography 24 2011. 1191–1198. 10.1016/j.echo.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 12. Laser KT Horst JP Barth P Kelter-Klopping A Haas NA Burchert W Kececioglu D Körperich H Knowledge-based reconstruction of right ventricular volumes using real-time three-dimensional echocardiographic as well as cardiac magnetic resonance images: comparison with a cardiac magnetic resonance standard Journal of the American Society of Echocardiography 27 2014. 1087–1097. 10.1016/j.echo.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 13. Gopal AS Chukwu EO Iwuchukwu CJ Katz AS Toole RS Schapiro W Reichek N Normal values of right ventricular size and function by real-time 3-dimensional echocardiography: comparison with cardiac magnetic resonance imaging Journal of the American Society of Echocardiography 20 2007. 445–455. 10.1016/j.echo.2006.10.027 [DOI] [PubMed] [Google Scholar]
- 14. Bonnemains L Stos B Vaugrenard T Marie PY Odille F Boudjemline Y Echocardiographic right ventricle longitudinal contraction indices cannot predict ejection fraction in post-operative Fallot children European Heart Journal Cardiovascular Imaging 13 2012. 235–242. 10.1093/ejechocard/jer263 [DOI] [PubMed] [Google Scholar]
- 15. Bove EL Byrum CJ Thomas FD Kavey RE Sondheimer HM Blackman MS Parker FB Jr The influence of pulmonary insufficiency on ventricular function following repair of Tetralogy of Fallot. Evaluation using radionuclide ventriculography Journal of Thoracic and Cardiovascular Surgery 85 1983. 691–696. [PubMed] [Google Scholar]
- 16. Geva T Indications and timing of pulmonary valve replacement after Tetralogy of Fallot repair Seminars in Thoracic and Cardiovascular Surgery 9 2006. 11–22. 10.1053/j.pcsu.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 17. Pattynama PM Lamb HJ Van der Velde EA Van der Geest RJ Van der Wall EE De Roos A Reproducibility of MRI-derived measurements of right ventricular volumes and myocardial mass Magnetic Resonance Imaging 13 1995. 53–63. 10.1016/0730-725X(94)00076-F [DOI] [PubMed] [Google Scholar]
- 18. Winter MM Romeih S Bouma BJ Groenink M Blom NA Spijkerboer AM Mulder BJ Is cardiac CT a reproducible alternative for cardiac MR in adult patients with a systemic right ventricle? Netherlands Heart Journal 20 2012. 456–462. 10.1007/s12471-012-0310-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Catalano O Antonaci S Opasich C Moro G Mussida M Perotti M Calsamiglia G Frascaroli M Baldi M Cobelli F Intra-observer and interobserver reproducibility of right ventricle volumes, function and mass by cardiac magnetic resonance Journal of Cardiovascular Medicine 8 2007. 807–814. 10.2459/JCM.0b013e32801105ef [DOI] [PubMed] [Google Scholar]
- 20. Grothues F Moon JC Bellenger NG Smith GS Klein HU Pennell DJ Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance American Heart Journal 147 2004. 218–223. 10.1016/j.ahj.2003.10.005 [DOI] [PubMed] [Google Scholar]
- 21. Luijnenburg SE Robbers-Visser D Moelker A Vliegen HW Mulder BJ Helbing WA Intra-observer and interobserver variability of biventricular function, volumes and mass in patients with congenital heart disease measured by CMR imaging International Journal of Cardiovascular Imaging 26 2010. 57–64. 10.1007/s10554-009-9501-y [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a