Abstract
We have previously reported that angiotensin type 1 receptor (AT1R) blockade with candesartan exerts neurovascular protection after experimental cerebral ischemia. Here, we tested the hypothesis that a low, subhypotensive dose of candesartan enhances neuroplasticity and subsequent functional recovery through enhanced neurotrophic factor expression in rats subjected to ischemia reperfusion injury. Male Wistar rats (290–300 g) underwent 90 min of middle cerebral artery occlusion (MCAO) and received candesartan (0.3 mg/kg) or saline at reperfusion and then once every 24 h for 7 days. Functional deficits were assessed in a blinded manner at 1, 3, 7, and 14 days after MCAO. Animals were sacrificed 14-day post-stroke and the brains perfused for infarct size by cresyl violet. Western blot and immunohisto-chemistry were used to assess the expression of growth factors and synaptic proteins. Candesartan-treated animals showed a significant reduction in the infarct size [t (13)=−5.5, P= 0.0001] accompanied by functional recovery in Bederson [F (1, 13)=7.9, P=0.015], beam walk [F (1, 13)=6.7, P=0.023], grip strength [F (1, 13)=15.2, P=0.0031], and rotarod performance [F (1, 14)=29.8, P<0.0001]. In addition, candesartan-treated animals showed significantly higher expression of active metalloproteinase-3 (MMP-3), laminin, and angiopoietin-1 (Ang-1). The expression of vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) and its receptor was significantly increased in the animals treated with candesartan. Also, we observed significant increases in neuroplasticity markers, synaptophysin, and PSD-95. These results indicate that low-dose candesartan had a large and enduring effect on measures of plasticity, and this accompanied the functional recovery after ischemic stroke.
Keywords: Ischemic stroke, Candesartan, Angiotensin type 1 receptor, Neurotropic factor, Neuroplasticity, Functional recovery
Introduction
Ischemic stroke is one of the most common causes of death and disability in the USA. Although much progress has been made toward understanding the mechanistic basis of stroke, the effectiveness of drugs available for stroke patients is limited. Tissue plasminogen activator (tPA), the only FDA-approved treatment for stroke, has various limitations due to neurovascular toxicity [1, 2]. Thus, finding an effective therapeutic strategy for stroke remains a high priority.
In stroke patients, acutely elevated blood pressure (BP) is associated with unfavorable stroke outcomes [3], but lowering BP acutely is not recommended, due to the potential to worsen stroke injury [4]. In fact, both the Scandinavian Candesartan Acute Stroke Trial (SCAST) and the China Antihypertensive Trial in Acute Stroke (CATIS) demonstrated no benefit of BP lowering in the acute stroke period [5, 6]. There is preclinical evidence, however, that supports a neurovascular benefit of angiotensin receptor blockers (ARBs) beyond lowering BP [7, 8], compared with other BP lowering agents [9]. It remains possible that low doses of the ARB, candesartan, may have beneficial effects on long-term recovery after ischemic stroke.
Recent studies showed that activation of endogenous restorative mechanisms, rather than just simply reducing the area of infarction, may provide better functional recovery after stroke [10–12]. Several growth factors, including the two most important neurotrophic factors, vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF), have pleiotropic effects on brain function, including neuroprotection, vascular remodeling, neurogenesis, neuronal survival, and neuroplasticity [13, 14]. VEGF has been shown to stimulate angiogenesis, and this can be beneficial after ischemic stroke [13]. Additionally, BDNF regulates neuronal survival, cell migration, and synaptic function [15, 16]. VEGF and BDNF levels have both been shown to increase in ischemic brain after treatment with candesartan and other neurorestorative drugs [17–19, 10, 20]. Molecular mediators underlying the effect of candesartan on the induction of neuroplasticity and subsequent improvement of functional outcome after treatment of stroke have not been adequately determined.
In this study, we examined the effects of low-dose candesartan treatment on neurotrophic factors, neuronal plasticity, and functional outcome in a rat model of middle cerebral artery occlusion (MCAO). Although this low dose of candesartan did not act as an acute neurovascular protectant in our model, repeated administration increased the production of neurotrophic factors, which we believe increased vascular remodeling, neuroplasticity, and subsequent functional recovery after stroke.
Materials and Methods
Animals and Treatment Regimen
Male Wistar rats (290–300 g; Charles River Laboratories, Wilmington, MA) were used according to procedures approved by the Institutional Animal Care and Use Committee (IACUC) of the Charlie Norwood VA Medical Center. The rats were quarantined for at least 5 days before the experiment. The animals were housed in individual cages in a room maintained at 21–25 °C, 45–50 % humidity, and 12-h light/dark cycle with free access to pellet chow and water.
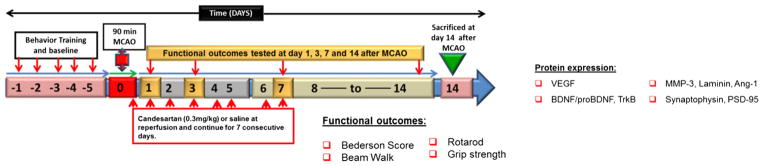
The animals were separated into three groups: group I, sham-operated saline-treated control (S); group II, MCAO and saline-treated stroke (MCAO); and group III, MCAO and candesartan (0.3 mg/kg) (MCAO + cand). Consistent with previous research, candesartan (Astra-Zeneca) was dissolved in saline and given in a dose of 0.3 mg/kg by intravenous injection 90-min postocclusion to ensure rapid delivery following injury. Additional injections of 0.3 mg/kg were administered intraperitoneally every 24 h continuing daily for up to 7 days after MCAO. In this 14-day survival study, we used 90-min MCAO to reduce the mortality. A diagram of the experimental design is shown in Fig. 1. A total of 38 rats were used in the present study; one rat each in the saline and candesartan groups died at 24 h and 3-day post-MCAO, respectively.
Fig. 1.
Schematic representation of study design. MCAO middle cerebral artery occlusion, VEGF vascular endothelial growth factor, BDNF brain-derived neurotrophic factor, TrkB tropomyosin-related kinase-B, MMP-3 matrix metalloproteinase-3, Ang-1 angiopoietin-1, PSD-95 postsynaptic density protein 95. Day 0 refers to the day of MCAO surgery
Candesartan Administration and BP Monitoring
Consistent with our previous published studies on 1-mg/kg dose of candesartan on acute BP after 3-h MCAO and 24-h survival [9, 21], here in this separate set of experiment, we wanted to demonstrate the subhypotensive effect of low-dose candesartan (0.3 mg/kg) under the same conditions, and rats were sacrificed at 24 h. To examine the effect of 0.3 mg/kg candesartan on acute BP, we monitored and recorded mean arterial blood pressure (MABP) every 10 min using telemetry transmitters (Data Sciences, Inc.) for the 24 h after the onset of stroke. After 3 h of occlusion, the animals received either candesartan (Astra-Zeneca) of 0.3 mg/kg or an equal volume of saline (1 ml/kg) intravenously. Animals were sacrificed at 24 h after stroke (detailed experimental design in Supplement Fig. 1). In all animals, baseline MAP was between 95 and 97 mmHg and was elevated by 30–35 mmHg upon MCAO. When compared to our previous data in the dose of 1 mg/kg candesartan, acute low-dose candesartan (0.3 mg/kg) did not lower BP within a few hours of administration (Supplement Fig. 2). In fact, we observed a mild effect on early (24 h) BP after candesartan treatment at 3-h post-MCAO, a critical time point, after which the BP starts to decline in MCAO. Mild BP lowering after a single candesartan dose was found to have no protective effect on early stroke outcomes. Therefore, we used 0.3-mg/kg dose of candesartan for 7 consecutive days to determine the effects on neuroplasticity and long-term functional recovery after MCAO.
Transient Middle Cerebral Artery Occlusion
Prior to MCAO surgery, isoflurane anesthesia was induced at 5 % and then maintained at 1.5–2 % during surgery. Focal cerebral ischemia was induced by occlusion of the right middle cerebral artery as previously described [22]. A midline incision was made on the ventral surface of the neck, and the right common carotid arteries were isolated and ligated with 6.0 silk suture. The internal carotid artery and the pterygopalatine artery were temporarily occluded with a microvascular clip. A 4-0 Doccol filament (Doccol Corporation, Redlands, CA) was introduced into the internal carotid artery through the incision in the external carotid artery. The filament was advanced approximately 20 mm distal to the carotid bifurcation. The animals were kept under anesthesia for only 15 min for the surgical procedure. Temperature was maintained at 36.5–37.5 °C all the time using a controlled heating system. After 90 min of MCAO, the occluding filament was withdrawn back into the common carotid artery to allow for reperfusion. The rats were allowed to recover from anesthesia on the heating pad and then returned to their home cages after full recovery from anesthesia. Sham-operated rats were subjected only to exposure of the MCA without occlusion. Anesthesia duration was similar in all groups.
Cerebral Blood Flow Imaging Using Laser Doppler Imaging System
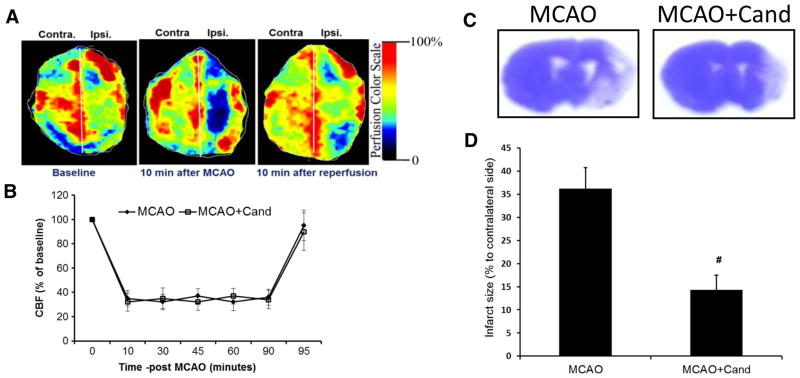
To ensure relative uniformity of the ischemic insult, in a separate subset of animals (N=5), cerebral blood flow was monitored using the Periscan PIM 3 System (Stockholm, Sweden) for repeated time interval during 90 min of occlusion. The animals were anesthetized and placed on a stereotaxic frame, and the skull was exposed. A whole brain scan was performed using the PIM3 to measure blood flow in both hemispheres. A built-in photo detector assisted with LDPI win software (PerimedInc) detected the reflected light from moving blood cells within 0.5 cm below of the cortical surface. Color-coded images were acquired three times consecutively, and the average blood flow was calculated based on the concentration and mean velocity of the blood cells using the LDPI win software. On induction of ischemia, cerebral blood flow decreased to 30 to 35 %, remaining stable throughout the 90 min of MCA occlusion. Reperfusion was associated with a restoration of blood flow to 80–90 % of baseline values in saline-treated control animals (Fig. 2b).
Fig. 2.
Low-dose candesartan treatment reduces infarct size 14 days after MCAO. a Representative images of cerebral blood flow (CBF) detected with laser doppler imaging system (Periscan PIM 3, laser scanner). The image illustrates the percentage of CBF change in ischemic (ipsilateral) versus contralateral hemisphere at baseline, 10 min after occlusion, and 10 min after reperfusion. b Measurement of CBF in the ipsilateral parietal cortex using laser Doppler flowmetry during the 90-min MCAO and 5-min postreperfusion. CBF was expressed as percentage of baseline. c Representative images of cresyl violet-stained coronal sections collected from saline- and candesartan-treated animals 14-day post-MCAO. Infarcts are shown as unstained regions. d Quantification of infarct size in saline- and candesartan-treated animals 14 days after MCAO. Results are expressed as % of contralateral side (mean ± SEM, #P=0.0001= MCAO+saline vs MCAO + cand, n=6–8/group)
Assessment of Functional Outcome
The experiment was performed between 9:00 a.m. and 4:00 p.m. All animals underwent neurobehavioral testing before MCAO and at days 1, 3, 7, and 14 after MCAO. Tests that were used included Bederson score, beam walk, grip strength, and rotarod performed in a blinded fashion.
Bederson Score
Neurological function was measured using the Bederson score [21]. An animal with no apparent deficits obtained a 0; the presence of forelimb flexion, 1; decreased resistance to push, 2; and circling, 3. A score of 3 is consistent with eMCAO. Only animals with a score of 3 at the time of reperfusion were included in the analysis of infarct size, hemoglobin, and neurological function.
Beam Walk
Beam walk in rats was determined as previously described [23]. Animals were placed on a beam (60 cm long and 4.5 cm wide) for 1 min and given a score from 0 to 6 as follows: balances on the beam with a steady posture=0, grasps side of the beam=1, hugs the beam with 1 limb falling=2, hugs the beam with 2 limbs falling=3, falls off the beam within 40–60 s=4, falls off the beam within 20–40 s=5; and falls off the beam within 20 s=6.
Grip Strength
Forelimb grip strength in rats was determined using a grip strength meter (Columbus Instruments, OH, USA) as previously described [24]. We used an electronic digital force gauge that measured the peak force exerted by the action of the animal while gripping the sensor bar. While being drawn back along a straight line leading away from the sensor, the animal released its grip at some point, and the gauge then recorded the maximum force attained at the time of release. The digital reading (in Newtons) of three successive trials was obtained for each rat, averaged, and used for data analysis. The MCAO rats were tested simultaneously with the shams.
Rotarod
Motor impairment was assessed using an accelerating rotarod (Columbus Instruments Rotamex 4/8 system, OH, USA). Rotameric tests were performed according to our previous published study [24]. All rats were given three training sessions, 10 min apart, before surgery to establish baseline performance. Rats were first habituated to the stationary rod, and then exposed to the rotating rod. The rod was started at 4 rpm and accelerated linearly to 40 rpm within 300 s. Latency to fall off the rotarod was then determined before ischemia (presurgery) and at days 1, 3, 7, and 14 after surgery in all rats. The rats were required to stay on the accelerating rod up to a minimum of 30 s. If they were unable to reach this criterion, the trial was repeated for a maximum of five times. The two best (long duration) fall latency values achieved by each rat were then averaged and used for data analysis. Rats not falling off within 5 min were given a maximum score of 300 s.
Histologic and Immunohistochemical Assessment
Cerebral infarct size was evaluated according to previously applied methods [25]. After completing the behavior tests, rats were given an overdose of ketamine/xylazine and then transcardially perfused with cold saline followed by 10 % buffer-formalin via the ascending aorta. The brains were removed and postfixed in 10 % buffer-formalin (Fischer Scientific) for 48 h and then stored at 4 °C in a solution of 30 % sucrose–saline for 2 days. The brains were embedded in OCT and sectioned coronally in 12-μm-thick slices starting from the frontal pole at an interval of 2 mm. The sections were stained with 1 % cresyl violet (Nissl staining). The infarct areas, defined as areas showing reduced Nissl staining under light microscopy, were traced and quantified with an image analysis system. Infarct volumes are expressed as a percentage of the contralateral side ± SEM.
Immunofluorescence was performed to identify the distribution and cell types. Colocalization of VEGF and TrkB with the different cellular markers [endothelial (CD31, 1:100; BD Pharmingen, San Jose, CA), astrocytes (GFAP, 1:200; Sigma, St. Louis, MO), and neurons (NeuN, 1:100; Millipore, Billerica, MA)] was processed simultaneously in 10-μm-thick sections from different animals as described previously [26]. Primary antibodies were incubated overnight at 4 °C at the following dilutions: rabbit anti-laminin (1:50; Dako Cytomation, Carpinteria, CA), rabbit anti-VEGF (1:100; Calbiochem Gibbstown, NJ), and rabbit anti-TrkB (1:100; Santa Cruz biotech, Santa Cruz, CA). After washing, slides were incubated with fluorescent secondary antibodies and cover slipped with Vectashield mounting medium (Vector Laboratories). Negative controls were prepared by omitting the primary antibodies. The number of laminin-stained vessels was counted using ImageJ software (NIH) in five different fields per section digitized from the ischemic border zone using a ×20 objective lens (Axio Observer fluorescent microscope, Zeiss). To examine the effect of candesartan on synaptophysin and PSD-95, after washing with PBS, 12-μm coronal sections were incubated with 1 % BSA containing 0.3 % Triton-X 100 for blocking for 1 h at room temperature and incubated with mouse anti-synaptophysin (1:200; Abcam, Cambridge, MA) and PSD-95 (1:200; Abcam, Cambridge, MA) at 4 °C overnight. After washing, slides were incubated with fluorescent secondary antibodies, cover slipped with Vectashield mounting medium (Vector Laboratories). All the sections were viewed using Zeiss Axio Observer.Z1 fluorescent microscope.
Western Blot
For WB analysis, we used peri-infarct (penumbra) cortical regions. By using a brain matrix, the brains were rapidly dissected into 4.0-mm coronal sections (approximately 0.5 and −3.5 mm from bregma). Brain tissue was homogenized in lysis buffer (RIPA); after centrifugation, protein concentration was determined. Fifty micrograms of the protein was subjected to electrophoresis in 4–20 % SDS-PAGE gels (BioRad) and transferred to nitrocellulose membranes. Membranes were then blocked at room temperature for 1 h in 5 % bovine serum albumin (BSA) and incubated with anti-VEGF (1:2,000; Calbiochem Gibbstown, NJ), BDNF (1:1,000; Santa Cruz biotech, Santa Cruz, CA), TrkB (1:500, Santa Cruz biotech, Santa Cruz, CA), MMP-3 (1:2,000; Abcam Cambridge, MA), angiopoietin-1 [Ang-1 (1:500; Santa Cruz biotech, Santa Cruz, CA)], synaptophysin (1:2,000; Abcam, Cambridge, MA), and density protein-95 [PSD-95 (1:2,000; Abcam, Cambridge, MA)]. All blots were stripped and reincubated with loading control antibodies. Intensity of the bands was measured by densitometry and quantified using ImageJ analysis software (ImageJ, NIH).
Statistical Analysis
All results were expressed as mean ± SEM, and calculations were obtained using GraphPad Prism and SAS® 9.3 (SAS Institute, Inc., Cary, NC). Infarct size and laminin density were analyzed using the Student’s t test (MCAO vs MCAO + cand). Data obtained from Western blot were log transformed prior to analysis by one-way analysis of variance (ANOVA) with three groups (Sham, MCAO, and MCAO + cand), and contrasts were obtained for planned comparisons (Sham vs MCAO and MCAO vs MCAO + cand). Bederson score, beam walk, grip strength, and rotarod performance were analyzed using multivariate repeated measure analysis of variance (RMANOVA) to assess group (MCAO or MCAO + cand) and day (1, 3, 7, and 14) effects along with the interaction between group and day. These tests were followed by a Bonferroni correction for the within-day pairwise comparisons of interest. Percent difference between presurgical and postsurgical scores for each animal was used to control for individual differences in grip strength and rotarod performance. Statistical significance was determined at alpha=0.05.
Results
Low-Dose Candesartan Reduces Infarct Size and Promotes Functional Recovery After MCAO
The infarct size of saline-treated and candesartan-treated animals 14 days after MCAO are presented as a percent of the contralateral hemisphere in Fig. 2d. Low-dose candesartan significantly [t (13)=−5.5, P=0.0001] reduced infarct size by 61 % compared to saline-treated animals.
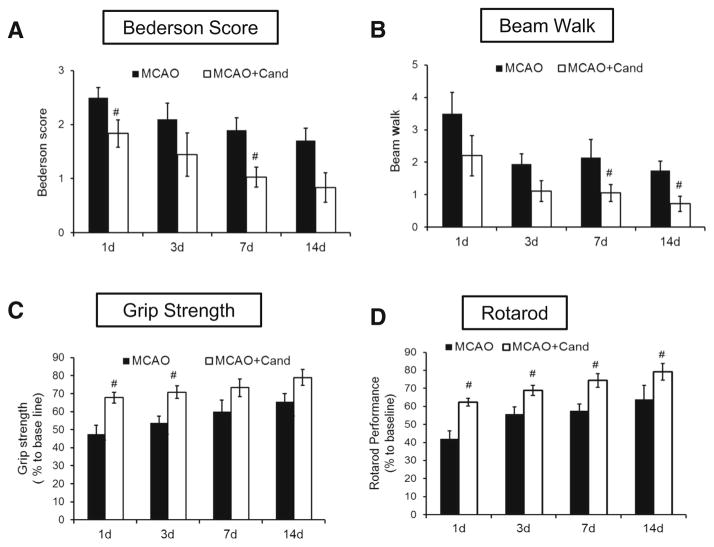
To examine whether the low-dose candesartan attenuates neurological deficits, we used Bederson score and beam walk tests at 24 h and days 3, 7, and 14 after MCAO (Fig. 3a, b). All rats improved over time in both Bederson [(F (3, 11)=5.8, P= 0.013)] and beam walk score (F (3, 11)=7.3, P=0.0059). Rats treated with candesartan showed significant improvement in both Bederson [F (1, 13)=7.9, P=0.015] and beam walk scores [F (1, 13)=6.7, P=0.023] after MCAO compared with saline-treated animals.
Fig. 3.
Low-dose candesartan treatment improves functional outcome after MCAO. Assessment of neurological deficit 1, 3, 7, and 14 days after MCAO using Bederson score (a), beam walk score (b), grip strength (c), and rotarod performance (d). Values are expressed as mean ± SEM [n=6–8/group, Bederson (#P=0.015), beam walk (#P= 0.023), grip strength (#P= 0.0031), and rotarod performance (#P<0.0001)=MCAO + saline vs MCAO + cand]
We also examined the effect of candesartan on grip strength, using grip strength meter. The maximum digital reading (in Newtons) of three successive trials obtained for each animal was used as the dependent variable. Grip strength following MCAO in Fig. 3c is presented as percentage of baseline. All rats improved over time [F (3, 10)=7.1, P= 0.0024]. Animals treated with candesartan showed significant improvement in the grip strength when compared with the saline group [F (1, 13)=15.2, P=0.0031].
Time spent on the rotarod is expressed as a percentage of presurgery control value. As with the other measures of behavior, all rats improved with time [F (3, 12)=12.1, P= 0.0006]. Repeated treatments with candesartan resulted in significant improvement compared with the saline group across time on latency to remain on the rotarod [F (1, 14)= 29.8, P<0.0001].
Low-Dose Candesartan Preserves Neurovasculature After MCAO
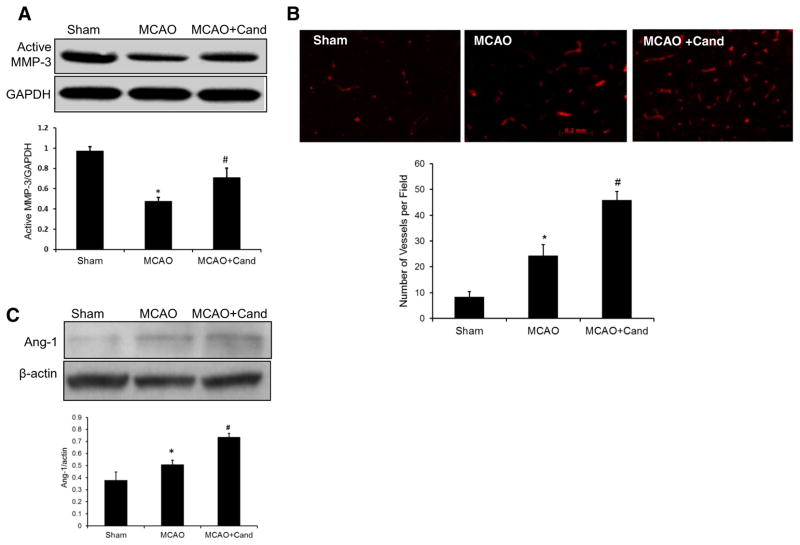
We considered the potential role of MMP-3 after ischemic injury, since MMP-3 is associated with neuronal migration and neurite outgrowth and contributes to neuroplasticity in the late phase of stroke [27, 28]. To examine the influence of candesartan on active MMP-3, Western blot analysis was done 14 days after MCAO and showed a significant [F (2, 13)= 13.5, P=0.0011] group difference in active MMP-3 (Fig. 4a). The saline-treated MCAO group showed significantly decreased active MMP-3 compared to shams while candesartan administration significantly upregulated MMP-3 compared to the saline MCAO.
Fig. 4.
Low-dose candesartan treatment attenuates ischemia/reperfusion-induced neurovascular injury. a Representative and quantitative analysis of Western blots showing that expression of active MMP-3 was significantly decreased in the ipsilateral hemisphere 14 days after MCAO and salvaged by low-dose candesartan treatment. b Representative micrographs of laminin-stained brain sections collected 14 days after MCAO from saline- and candesartan-treated groups. Quantification of laminin-positive vessels shows higher vascular density in low-dose candesartan treatment as compared to saline-treated animals. c Representative and quantitative analysis of Western blots showing that low-dose candesartan treatment significantly increased Ang-1 expression after MCAO. Values are expressed as mean ± SEM [n=4–5/group, *P<0.05=MCAO + saline vs sham; MMP-3 (#P=0.0011), laminin (#P=0.0032), and Ang-1 (#P<0.0001)=MCAO + saline vs MCAO + cand]
Importantly, MMP-3 levels correlated with increased expression of its target extracellular matrix substrates, molecules necessary to prepare the local environment for reactive synaptogenesis [29, 30]. Our previous work using a hypotensive dosage 1-mg/kg candesartan treatment demonstrated significant upregulation of laminin staining, a marker of components of the extracellular matrix at 7 days after MCAO [21]. Here, we examine the effect of low-dose candesartan on laminin density after 14 days of MCAO. Immunoflourescent staining of brain sections showed significantly higher vascular density in the ischemic border zone with candesartan treatment [t (8)= 4.15, P=0.0032] compared to saline treatment for number of vessels per field (Fig. 4b).
We further investigated the effect of candesartan treatment on angiopoietin 1 (Ang-1) expression after stroke. Ang-1 exerts a barrier protective function as well as a synergistic angiogenic effect after stroke and contributes to nascent vessel maturation [31]. There was a significant effect of group [F (2, 13)=27.5, P<0.0001] where Ang-1 expression in the saline-treated MCAO group was significantly higher than the shams and candesartan treatment significantly increased the Ang-1 compared to the saline-treated MCAO group (Fig. 4c).
Low-Dose Candesartan Enhances Endogenous Mediators of Neuroplasticity After MCAO
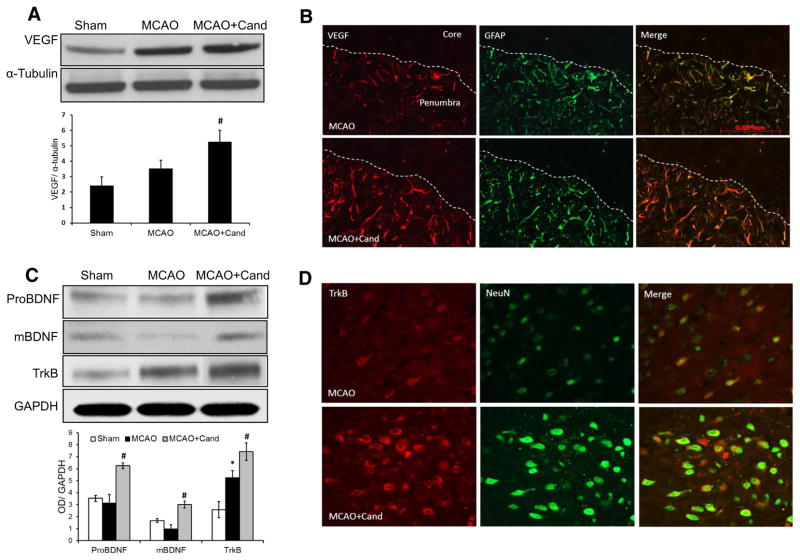
VEGF and BDNF are two important endogenous factors for vascular remodeling and neuroplasticity. Our previous work demonstrated a robust increase in VEGF and BDNF in the ischemic hemisphere at 24 h after MCAO, in animals treated with candesartan at a dose of 1 mg/kg IV administered at reperfusion [18, 17]. To determine the influence of low-dose candesartan (0.3 mg/kg) on VEGF and BDNF expression, Western blot analysis was done 14 days after MCAO. There were significant group differences for VEGF [F (2, 13)=7.4, P=0.0094], ProBDNF [F (2, 13)=16.9, P=0.0004], mature BDNF [F (2, 13)=15.6], and TrkB [F (2, 13)=23.7, P= 0.0001]. We found a significant increase in VEGF (Fig. 5a), ProBDNF, and mature BDNF (mBDNF) and its receptor, TrkB (Fig. 5c), in the ischemic border zone (penumbra) of animals treated with the low-dose candesartan compared to the saline-treated MCAO group.
Fig. 5.
Low-dose candesartan treatment upregulates neurotrophic growth factor expression at 14-day post-MCAO. a Representative and quantitative analysis of Western blots showing that candesartan significantly increased VEGF expression as compared to the saline-treated or sham-operated animals. b Immunolocalization of VEGF in astrocytes around the penumbra 14 days after MCAO. Overlay of double immunohistofluorescence for GFAP (astrocytic marker, green) and VEGF (red) increased in candesartan-treated group as compared to their saline-treated counterparts. c Representative and quantitative analysis of Western blots showing that candesartan significantly increased BDNF and TrkB expression as compared to saline-treated and sham-operated animals. d Immunolocalization of TrkB in neurons around the penumbra 14 days after MCAO. Overlay of double immunohistofluorescence for NeuN (neuronal marker, green) and TrkB (red) increased in the candesartan-treated group as compared to saline treatment. Values are expressed as mean ± SEM [n=4–5, *P<0.05=saline vs sham; VEGF (#P=0.0094), ProBDNF (#P=0.0004), and TrkB (#P=0.0001)=saline vs candesartan]
In order to characterize the role of the different brain cells in VEGF expression, immunohistofluorescence for VEGF coupled to NeuN (neurons) and GFAP (astrocytes) was performed at 14-day post-MCAO. Following MCAO, repeated treatments with candesartan increased the immunopositive signals of VEGF compared to the saline-treated MCAO group (Fig. 5b). The immunohistochemical expression of VEGF colocalized with the astrocyte marker, GFAP, in the peri-infarct area of brain sections was markedly increased at 14 days following MCAO in the candesartan-treated compared to the saline-treated group. We further explored the colocalization of TrkB using markers for neurons, astrocytes, and endothelial cells. TrkB showed strong colocalization with the neuronal marker, NeuN, in the ischemic penumbra. Compared to saline-treated animals, brain sections derived from candesartan-treated animals exhibited significantly increased TrkB expression (Fig. 5d).
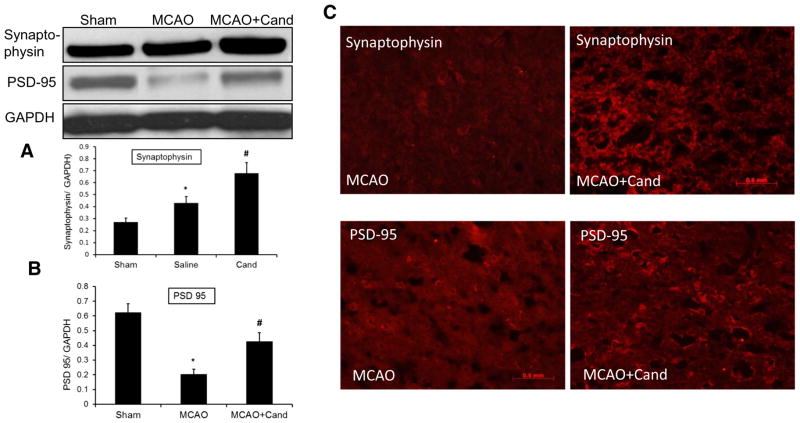
To confirm that the observed molecular mediators promoted neuroplasticity, we also examined the expression of synaptophysin and PSD-95, a key scaffolding protein implicated in excitatory synaptic signaling. There was a significant group effect for both synaptophysin [F (2, 13)=15.7, P= 0.0006] and PSD-95 [F (2, 13) = 35.3, P < 0.0001]. Synaptophysin expression was increased while PSD-95 was reduced significantly in the saline-treated MCAO group compared to shams. Candesartan administration significantly up-regulated the expression of both proteins compared to the saline-treated MCAO group (Fig. 6a, b). These findings were further confirmed by immunohistochemistry, with increased expression of synaptophysin and PSD-95 in the candesartan-treated group (Fig. 6c).
Fig. 6.
Low-dose candesartan treatment upregulates neuroplasticity markers 14-day post-MCAO. a Representative and quantitative analysis of Western blots showing that synaptophysin significantly increased after MCAO and further increased by candesartan treatment compared to saline-treated and sham-operated animals. b Representative and quantitative analysis of Western blots showing a significant reduction of PSD-95 expression that was ameliorated by low-dose candesartan treatment. c Immunohistochemical representation showing that candesartan increased the immunopositive signals of synaptophysin and PSD-95 in candesartan-treated animals as compared to their saline-treated counterparts. Values are expressed as mean ± SEM [n=4–5, *P<0.05=saline vs sham; synaptophysin (#P=0.0006) and PSD-95 (#P<0.0001)=saline vs candesartan]
Discussion
Our study demonstrated that a low-dose candesartan enhances neuroplasticity and subsequent functional neurologic recovery after ischemic reperfusion injury in rats. Further, we report here that the beneficial effects of candesartan are mediated through enhanced expression of VEGF and BDNF and the subsequent increase in neuroplasticity markers (synaptophysin and PDS95). We are encouraged that even the low-dose candesartan had a large and enduring effect on measures of plasticity, and this accompanied the sensorimotor recovery at 14 days. These findings are consistent with our previous studies with candesartan [17–19].
In the present study, the 0.3-mg/kg dose was selected based on previous findings showing the beneficial effects of candesartan on BP [32, 33]. Brdon et al. [32] found functional improvement during treatment with low-dose candesartan (0.3 mg/kg) consecutive for 7 days after MCAO. We now document a sustained motor recovery for 14 days even after the treatment is stopped, and we further elucidate the possible molecular mechanisms involved. We also reported that an acute single dose of candesartan (0.3 mg/kg) at 3 h after MCAO only mildly reduced BP and did not improve early (24 h) stroke outcomes (Supplement Figs. 1–3). While we can not rule out an acute neuroprotective effect when the drug was administered at 90 min, versus 3 h, as in the BP substudy (Supplement data), the behavior assessment and molecular data are consistent with a neurorestorative response. Recent clinical trials suggest that the BP lowering effects of AT1 receptor blockade in the acute stroke period could outweigh any benefit [5]. Subsequently, we considered it more relevant to administer candesartan in a low dose (0.3 mg/kg) because it only mildly affected BP.
Most studies report candesartan to be effective on acute reduction in lesion size and early behavioral impairments, but less is known about its long-term neurorestorative effects following stroke. To address this, we examined candesartan’s effects on a panel of behavioral tests (Bederson score, beam walk, grip strength, and accelerating rotarod) sensitive to unilateral ischemic insult. Consistent with the earlier observations [32, 33], we found that behavioral deficits caused by MCAO were significantly improved by candesartan treatment when measured at repeated intervals (1, 3, 7, and 14 days) after the MCAO. The degree of improvement was substantial (almost return to normal functioning) at days 7 and 14 post-MCAO. Further, treatment with candesartan significantly reduced the infarct size following MCAO in rats.
The matrix metalloproteinases (MMPs) are a gene family of extracellular matrix enzymes which damage the blood brain barrier (BBB) by degrading extracellular matrix proteins (e.g., laminin) and cause neuronal death in the acute injury phase [34]. However, at a later stage of injury, these same proteases have a beneficial role in accelerating neurovascular remodeling [35]. A recent study has shown that MMPs may promote vascular remodeling by accelerating angiogenesis [36] and are required for improving long-term recovery [37]. Emerging evidence suggests that inhibition of MMPs during the late phase is associated with more severe brain injury and worsened functional outcome [38]. Although there are many MMPs, an important role for MMP-3 (stromelysin-1) in particular has been identified, leading to increased expression of its target extracellular matrix proteins and plasticity [29, 30, 39]. Our results demonstrate that low-dose candesartan increased the expression of MMP-3 and prevented the degradation of laminin.
Ang-1 plays a prominent role in angiogenesis as well as vascular stability [40–42]. Ang-1 exerts a barrier protective function as well as anangiogenic effect after stroke [31, 43]. The expression of Ang-1 is acutely downregulated after focal cerebral ischemia [44] and linked to increased BBB permeability and edema [40]. On the other hand, treatment with recombinant adenoviruses expressing Ang-1 reduces BBB leakage in ischemic brain and decreases infarction in mice [45], and transgenic overexpression of Ang-1 increases vascular stabilization [46]. In the present study, Ang-1 expression was significantly increased after low-dose candesartan administration. Our data is consistent with our previous findings of candesartan’s vascular protective effects after ischemic stroke [19, 18]. This sheds light on the possible molecular mediators involved in such a response.
Neurotrophic factors are crucial in brain development and cell survival in adults. They participate in protection and proliferation of neuronal, glial, and endothelial cells. Among others, VEGF and BDNF are two important neurotrophic factors with pleiotropic effects on brain function, including neuroprotection, vascular remodeling, neurogenesis, neuronal survival, and plasticity [13, 14, 10]. As one of the most potentangiogenic factors, VEGF is upregulated by focal cerebral ischemia not only in animal models but also in human patients [47, 48] as an angiogenic, neurotrophic, and neuroprotective factor [49, 50]. VEGF also plays a vital role during neural [50] and vascular remodeling [51] after stroke. BDNF regulates neuronal survival, cell migration, and neuroplasticity [15, 16]. Increased production of neurotrophic factors has been proposed as a mechanism of functional recovery and neuroplasticity after cerebral ischemia [10, 52]. Neuroplasticity enhances functional recovery after brain injury [53] and is partially regulated by synaptic proteins (synaptophysin and PSD-95). Synaptophysin, also known as the major synaptic vesicle protein p38, is a transmembrane glycoprotein of neuroendocrine vesicles [54]. Synaptophysin plays an important role in presynaptic plasticity and synaptogenesis [55]. Additionally, PSD-95 plays an important function in the maturation of presynaptic and postsynaptic components [56–58]. The expression of PSD-95 is markedly decreased in the hippocampus after brain injury including TBI and stroke [23, 59]. Our results showed that ischemia-induced VEGF expression but candesartan treatment further increased VEGF expression, resulting in increased microvessel formation. We also found that candesartan treatment significantly increased the expression of BDNF and TrkB. Increased expression of BDNF and its receptors after ischemic injury to the brain can improve synaptic functional processes. Moreover, candesartan treatment increased the expression of synaptophysin and PSD-95. Our study, which is the first to document the long-term effect of candesartan on VEGF, BDNF, and synaptic protein expression after cerebral ischemia, suggests that candesartan’s induction of neurotrophic factors results in improved post-stroke plasticity.
This study focused on the efficacy of low-dose candesartan to promote neurotrophic factor involvement in plasticity and long-term recovery after ischemic injury and did not explore in detail the underlying mechanisms of this therapy. Further studies are needed to determine the specific role of VEGF or BDNF in neurogenesis and functional recovery by using VEGF or BDNF-targeting methods (such as gene transfer methods) locally in the brain.
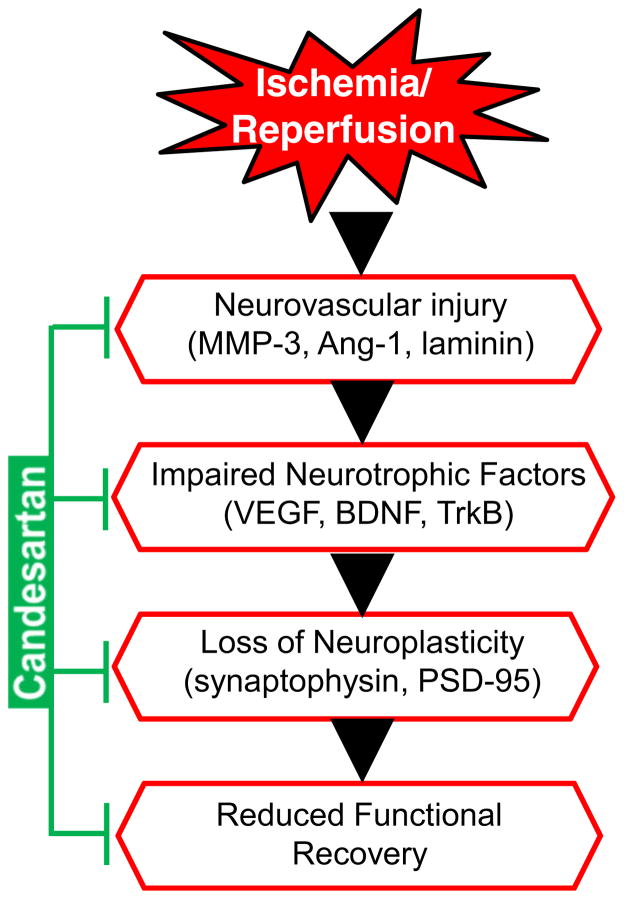
In conclusion, our data suggest that repeated treatments with a low, subhypotensive dose of candesartan enhanced neuroplasticity through increased VEGF and BDNF after stroke, which may be the mechanism by which candesartan reduced ischemic neuronal damage and enhanced functional recovery (Fig. 7). Taken together, these data suggest that blockade of AT1R after stroke promotes stroke recovery when excessive BP lowering is avoided.
Fig. 7.
Schematic representation of the ischemia reperfusion injury cascade and the steps influenced by candesartan treatment. MMP-3 matrix metalloproteinases-3, Ang-1 angiopoietin-1, BDNF brain-derived neuro-trophic factor, VEGF vascular endothelial growth factor, TrkB tropomyosin-related kinase B, PSD-95 postsynaptic density protein-95
Supplementary Material
Acknowledgments
This study was supported in part by the Veterans Affairs Merit Review (SCF, BX000891 and AE, BX000347) and NIH–NINDS (SCF, NS063965 and AE, NS054688). Adviye Ergul is a research career scientist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia.
Abbreviations
- ANOVA
Analysis of variance
- Ang-1
Angiopoietin-1
- AT1R
Angiotensin II type 1 receptor
- BP
Blood pressure
- BDNF
Brain-derived neurotrophic factor
- MMP-3
Matrix metalloproteinase-3
- MCAO
Middle cerebral artery occlusion
- PSD-95
Postsynaptic density protein-95
- TrkB
Tropomyosin-related kinase-B
- VEGF
Vascular endothelial growth factor
- ARBs
Angiotensin receptor blockers
- MABP
Mean arterial blood pressure
- tPA
Tissue plasminogen activator
Footnotes
Conflict of Interest SCF is a consultant for and has received funding from Pfizer. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Electronic supplementary material The online version of this article (doi:10.1007/s12035-014-8830-6) contains supplementary material, which is available to authorized users.
Contributor Information
Tauheed Ishrat, Email: tauheedarshi@gmail.com, Charlie Norwood VA Medical Center, Augusta, GA, USA. Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, HM 1212, 1120 15th St., Augusta, GA 30912, USA.
Bindu Pillai, Charlie Norwood VA Medical Center, Augusta, GA, USA. Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, HM 1212, 1120 15th St., Augusta, GA 30912, USA.
Sahar Soliman, Charlie Norwood VA Medical Center, Augusta, GA, USA. Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, HM 1212, 1120 15th St., Augusta, GA 30912, USA.
Abdelrahman Y. Fouda, Charlie Norwood VA Medical Center, Augusta, GA, USA. Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, HM 1212, 1120 15th St., Augusta, GA 30912, USA
Anna Kozak, Charlie Norwood VA Medical Center, Augusta, GA, USA. Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, HM 1212, 1120 15th St., Augusta, GA 30912, USA.
Maribeth H. Johnson, Department of Biostatistics, Georgia Regents University, Augusta, GA, USA
Adviye Ergul, Charlie Norwood VA Medical Center, Augusta, GA, USA. Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, HM 1212, 1120 15th St., Augusta, GA 30912, USA. Department of Physiology, Georgia Regents University, Augusta, GA, USA.
Susan C. Fagan, Email: sfagan@gru.edu, Charlie Norwood VA Medical Center, Augusta, GA, USA. Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, HM 1212, 1120 15th St., Augusta, GA 30912, USA. Department of Neurology, Georgia Regents University, Augusta, GA, USA
References
- 1.Lansberg MG, Albers GW, Wijman CA. Symptomatic intra-cerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24(1):1–10. doi: 10.1159/000103110. [DOI] [PubMed] [Google Scholar]
- 2.Marder VJ, Jahan R, Gruber T, Goyal A, Arora V. Thrombolysis with plasmin: implications for stroke treatment. Stroke J Cereb Circ. 2010;41(10 Suppl):S45–S49. doi: 10.1161/STROKEAHA.110.595157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishitsuka K, Kamouchi M, Hata J, Fukuda K, Matsuo R, Kuroda J, Ago T, Kuwashiro T, Sugimori H, Nakane H, Kitazono T. High blood pressure after acute ischemic stroke is associated with poor clinical outcomes: Fukuoka Stroke Registry. Hypertension. 2014;63(1):54–60. doi: 10.1161/HYPERTENSIONAHA.113.02189. [DOI] [PubMed] [Google Scholar]
- 4.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke J Cereb Circ. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 5.Sandset EC, Bath PM, Boysen G, Jatuzis D, Korv J, Luders S, Murray GD, Richter PS, Roine RO, Terent A, Thijs V, Berge E. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377(9767):741–750. doi: 10.1016/S0140-6736(11)60104-9. [DOI] [PubMed] [Google Scholar]
- 6.He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen CS, Tong W, Liu C, Ju Z, Peng Y, Peng H, Li Q, Geng D, Zhang J, Li D, Zhang F, Guo L, Sun Y, Wang X, Cui Y, Li Y, Ma D, Yang G, Gao Y, Yuan X, Bazzano LA, Chen J. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA J Am Med Assoc. 2014;311(5):479–489. doi: 10.1001/jama.2013.282543. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura Y, Ito T, Saavedra JM. Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke J Cereb Circ. 2000;31(10):2478–2486. doi: 10.1161/01.str.31.10.2478. [DOI] [PubMed] [Google Scholar]
- 8.Wilms H, Rosenstiel P, Unger T, Deuschl G, Lucius R. Neuroprotection with angiotensin receptor antagonists: a review of the evidence and potential mechanisms. Am J Cardiovasc Drugs Drugs Devices Other Interv. 2005;5(4):245–253. doi: 10.2165/00129784-200505040-00004. [DOI] [PubMed] [Google Scholar]
- 9.Elewa HF, Kozak A, Johnson MH, Ergul A, Fagan SC. Blood pressure lowering after experimental cerebral ischemia provides neurovascular protection. J Hypertens. 2007;25(4):855–859. doi: 10.1097/HJH.0b013e3280149708. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2005;25(2):281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris DC, Chopp M, Zhang L, Zhang ZG. Thymosin beta4: a candidate for treatment of stroke? Ann N Y Acad Sci. 2010;1194:112–117. doi: 10.1111/j.1749-6632.2010.05469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui X, Chopp M, Zacharek A, Cui Y, Roberts C, Chen J. The neurorestorative benefit of GW3965 treatment of stroke in mice. Stroke J Cereb Circ. 2013;44(1):153–161. doi: 10.1161/STROKEAHA.112.677682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111(12):1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci Off J Soc Neurosci. 2009;29(41):12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl-co-transporter KCC2. Development. 2003;130(7):1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- 16.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci Off J Soc Neurosci. 2003;23(17):6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhusban A, Kozak A, Ergul A, Fagan SC. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J Pharmacol Exp Ther. 2013;344(2):348–359. doi: 10.1124/jpet.112.197483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan W, Somanath PR, Kozak A, Goc A, El-Remessy AB, Ergul A, Johnson MH, Alhusban A, Soliman S, Fagan SC. Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke. PLoS One. 2011;6(9):e24551. doi: 10.1371/journal.pone.0024551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soliman S, Ishrat T, Pillai A, Somanath PR, Ergul A, El-Remessy AB, Fagan SC. Candesartan induces a prolonged proangiogenic effect and augments endothelium-mediated neuroprotection after oxygen and glucose deprivation: role of vascular endothelial growth factors a and B. J Pharmacol Exp Ther. 2014;349(3):444–457. doi: 10.1124/jpet.113.212613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone is neuroprotective against ischemic brain injury through its effects on the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neuroscience. 2012;210:442–450. doi: 10.1016/j.neuroscience.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozak A, Ergul A, El-Remessy AB, Johnson MH, Machado LS, Elewa HF, Abdelsaid M, Wiley DC, Fagan SC. Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke J Cereb Circ. 2009;40(5):1870–1876. doi: 10.1161/STROKEAHA.108.537225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke J Cereb Circ. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Okuda Y, Nonoguchi N, Zhao MZ, Kajimoto Y, Furutama D, Yukawa H, Shibata MA, Otsuki Y, Kuroiwa T, Miyatake S. Postischemic intraventricular administration of FGF-2 expressing adenoviral vectors improves neurologic outcome and reduces infarct volume after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2004;24(11):1205–1213. doi: 10.1097/01.WCB.0000136525.75839.41. [DOI] [PubMed] [Google Scholar]
- 24.Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol. 2010;226(1):183–190. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fouda A, Kozak A, Alhusban A, Switzer J, Fagan S. Anti-inflammatory IL-10 is upregulated in both hemispheres after experimental ischemic stroke: hypertension blunts the response. Exp Transl Stroke Med. 2013;5(1):12. doi: 10.1186/2040-7378-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyata S, Nakatani Y, Hayashi N, Nakashima T. Matrix-degrading enzymes tissue plasminogen activator and matrix metalloprotease-3 in the hypothalamoneurohypophysial system. Brain Res. 2005;1058(1–2):1–9. doi: 10.1016/j.brainres.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Wright JW, Meighan SE, Murphy ES, Holtfreter KL, Davis CJ, Olson ML, Benoist CC, Muhunthan K, Harding JW. Habituation of the head-shake response induces changes in brain matrix metalloproteinases-3 (MMP-3) and -9. Behav Brain Res. 2006;174(1):78–85. doi: 10.1016/j.bbr.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4(6):456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- 30.Falo MC, Fillmore HL, Reeves TM, Phillips LL. Matrix metalloproteinase-3 expression profile differentiates adaptive and maladaptive synaptic plasticity induced by traumatic brain injury. J Neurosci Res. 2006;84(4):768–781. doi: 10.1002/jnr.20986. [DOI] [PubMed] [Google Scholar]
- 31.Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET, Bernaudin M, Roussel S, Petit E. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25(11):1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- 32.Brdon J, Kaiser S, Hagemann F, Zhao Y, Culman J, Gohlke P. Comparison between early and delayed systemic treatment with candesartan of rats after ischaemic stroke. J Hypertens. 2007;25(1):187–196. doi: 10.1097/01.hjh.0000254376.80864.d3. [DOI] [PubMed] [Google Scholar]
- 33.Kozak W, Kozak A, Johnson MH, Elewa HF, Fagan SC. Vascular protection with candesartan after experimental acute stroke in hypertensive rats: a dose-response study. J Pharmacol Exp Ther. 2008;326(3):773–782. doi: 10.1124/jpet.108.139618. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg GA, Mun-Bryce S. Matrix metalloproteinases in neuroinflammation and cerebral ischemia. Ernst Schering Res Found Work. 2004;47:1–16. doi: 10.1007/978-3-662-05426-0_1. [DOI] [PubMed] [Google Scholar]
- 35.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14(5):497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 36.Pham LD, Hayakawa K, Seo JH, Nguyen MN, Som AT, Lee BJ, Guo S, Kim KW, Lo EH, Arai K. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60(6):875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12(4):441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 38.Rivera S, Ogier C, Jourquin J, Timsit S, Szklarczyk AW, Miller K, Gearing AJ, Kaczmarek L, Khrestchatisky M. Gelatinase B and TIMP-1 are regulated in a cell- and time-dependent manner in association with neuronal death and glial reactivity after global fore-brain ischemia. Eur J Neurosci. 2002;15(1):19–32. doi: 10.1046/j.0953-816x.2001.01838.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Hove I, Lemmens K, Van de Velde S, Verslegers M, Moons L. Matrix metalloproteinase-3 in the central nervous system: a look on the bright side. J Neurochem. 2012;123(2):203–216. doi: 10.1111/j.1471-4159.2012.07900.x. [DOI] [PubMed] [Google Scholar]
- 40.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286(5449):2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 41.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6(4):460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 42.Davis S, Yancopoulos GD. The angiopoietins: Yin and Yang in angiogenesis. Curr Top Microbiol Immunol. 1999;237:173–185. doi: 10.1007/978-3-642-59953-8_9. [DOI] [PubMed] [Google Scholar]
- 43.Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2007;27(10):1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2002;22(4):379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113(3):683–687. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 46.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282(5388):468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 47.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke J Cereb Circ. 1994;25(9):1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 48.Krupinski J, Stroemer P, Slevin M, Marti E, Kumar P, Rubio F. Three-dimensional structure and survival of newly formed blood vessels after focal cerebral ischemia. Neuroreport. 2003;14(8):1171–1176. doi: 10.1097/01.wnr.0000075304.76650.29. [DOI] [PubMed] [Google Scholar]
- 49.Chen YH, Wu HL, Chen CK, Huang YH, Yang BC, Wu LW. Angiostatin antagonizes the action of VEGF-A in human endothelial cells via two distinct pathways. Biochem Biophys Res Commun. 2003;310(3):804–810. doi: 10.1016/j.bbrc.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 50.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85(4):740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- 52.Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke J Cereb Circ. 2012;43(8):2270–2274. doi: 10.1161/STROKEAHA.111.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8(5):491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jahn R, Schiebler W, Ouimet C, Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ujike H, Takaki M, Kodama M, Kuroda S. Gene expression related to synaptogenesis, neuritogenesis, and MAP kinase in behavioral sensitization to psychostimulants. Ann N Y Acad Sci. 2002;965:55–67. doi: 10.1111/j.1749-6632.2002.tb04151.x. [DOI] [PubMed] [Google Scholar]
- 56.Lin Y, Jover-Mengual T, Wong J, Bennett MV, Zukin RS. PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proc Natl Acad Sci U S A. 2006;103(52):19902–19907. doi: 10.1073/pnas.0609924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci Off J Soc Neurosci. 2004;24(45):10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci Off J Soc Neurosci. 2000;20(3):1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan BC, Park JH, Ahn JH, Lee JC, Won MH, Kang IJ. Postsynaptic density protein (PSD)-95 expression is markedly decreased in the hippocampal CA1 region after experimental ischemia-reperfusion injury. J Neurol Sci. 2013;330(1–2):111–116. doi: 10.1016/j.jns.2013.04.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.