Abstract
Background
Research shows that type 2 diabetes mellitus (T2DM) affects the risk and prognosis of colorectal cancer (CRC). Here, we conducted a retrospective study to investigate whether the clinicopathological features of CRC patients correlate with their blood glucose levels.
Material/Methods
We enrolled 391 CRC patients hospitalized in our center between 2008 and 2013. Data of their first fasting plasma glucose (FPG) and 2-h postprandial glucose (2hPPG) level after admission, their clinicopathological features, and survival were collected. The correlations between blood glucose level and clinicopathological features were analyzed by Pearson chi-square analysis. Patient survival was analyzed by Kaplan-Meier and Cox-regression analysis.
Results
There were 116 out of the 391 CRC patients who had high blood glucose level (H-G group, 29.67%), among which 58 (14.83%), 18 (4.60%), and 40 (10.23%) were diabetes mellitus (DM), impaired glucose tolerance (IGT), and impaired fasting glucose (IFG), respectively, while 275 (70.33%) patients had normal glucose level (N-G group). Compared with the N-G group, patients in the H-G group had larger tumor diameters and lower tumor differentiation (p<0.05). A higher ratio of patients in the H-G group also had more advanced TNM staging and more ulcerative CRC gross type (p<0.05). No significant difference was observed in patient overall survival among different glucose groups. No effect of insulin therapy on CRC development and patient survival was observed.
Conclusions
Blood glucose level in CRC patients correlates significantly with local tumor malignancy, but no significant effect on distant metastasis and patient overall survival was observed.
MeSH Keywords: Blood Glucose; Colorectal Neoplasms; Diabetes Mellitus, Type 2
Background
The prevalence of type 2 diabetes mellitus (T2DM), a group of metabolic disorders with increased blood glucose level, is increasing dramatically worldwide. Currently, it is estimated that 387 million people around the world have diabetes and the number will reach 587 million by 2035 [1]. Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer death in the United States [2]. Internationally, the risks of both T2DM and CRC increase in parallel with economic development. It is suggested that the prevalence of a Western diet and sedentary lifestyle serves as strong contributors.
The positive association between T2DM and an increased risk of several cancer types, including CRC, has been reported. T2DM correlates tightly with the incidence and mortality of CRC [3–5]. Compared to CRC patients with normal glucose level, the coexisting of T2DM increases the overall and cancer-specific mortality and decreases disease-free survival [3]. In addition to increasing CRC incidence, T2DM is also an independent prognosis factor for CRC reoccurrence and metastasis [6]. Patients with T2DM had a lower survival rate even after curative surgery for colon cancer [7]. T2DM with high serum HbA1c levels is an independent prognosis factor for high incidence of colonic adenomatous polyps (APs) and CRC [8]. A large retrospective study of Korean CRC patients revealed that incidence of CRC was positively correlated with the fasting serum glucose level [9]. However, there are also studies showing no correlation between T2DM and CRC. For example, T2DM was found to have no effect on the short-term survival and cancer-specific survival of CRC patients [10]. It is suggested that the discrepancy might be related to ethnicity and sex [11,12].
Therapeutic options for T2DM include insulin injection, and/or oral anti-hyperglycemic drug administration, such as metformin. A study showed that long-term administration of insulin increased CRC incidence in T2DM patients [13]. High insulin and high glucose both increase the risk of recurrent colorectal cancer [14]. Zhang et al. showed that metformin treatment can significantly lower the risk of CRC in T2DM patients [15]. However, few studies have closely examined the correlation between antidiabetic medication and the clinicopathological features of CRC.
Although the association between T2DM and CRC has been reported, the effect of blood glucose level on the clinicopathological features of CRC has not been explored, especially in non-European populations. Our current retrospective study analyzed the correlation between CRC patients’ blood glucose levels and their disease severity, prognosis, and survival in a Chinese population. We also analyzed whether diabetes treatment affected CRC development and patient survival.
Material and Methods
Patients and their clinicopathological features
This study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Huzhou University, China. All study participants provided written informed consent.
We enrolled 391 CRC patients hospitalized in our center between 2008 and 2013 into our retrospective study. Clinicopathological data were collected and analyzed, including sex, age, tumor location, tumor type, tumor diameter, vascular/perineural invasion, histological stage, primary tumor invasion depth, lymph node metastasis, distant metastasis, and TNM classification. CRC patients were classified according to TNM system based on the America Joint Committee on cancer (AJCC)/Union for International Cancer Control (UICC) 2009 staging system for colorectal cancer (7th edition) [16].
All diagnosis of CRC was based on ultrasonic or CT scan results, and endoscopic biopsy or surgical resection. Exclusion includes familial adenomatous polyposis (FAP); hereditary nonpolyposis colorectal cancer (HNCRC) or anal canal cancer; acute or chronic inflammation; serious cardiovascular or cerebrovascular disease (such as acute coronary syndrome, chronic cardiac dysfunction, cerebral vascular accident); liver and kidney dysfunction or other stress conditions.
Glucose level
Fasting plasma glucose (FPG) and 2-h postprandial glucose (2hPPG) upon the initial hospitalization (before surgery, radio-, or chemotherapy) were obtained. Glucose level (mmol/L) was measured from the venous blood of the patients in the morning by the glucose oxidase method using glutamate assay kit (CAT GL7210, Beijing Leadman Biochemistry, China). Samples were then analyzed by a Hitachi 7600 series auto biochemistry analyzer (Hitachi, Tokyo, Japan). Two measurements were performed for each patient and the average was used as the final value. The FPG, 2hPPG standards were in accordance with the diagnosis standard of the World Health Organization (WHO) (1999): normal reference for FPG, 3.9~6.1 mmol/L; impaired fasting glucose (IFG): FPG=6.1~7.0 mmol/L, 2hPPG <7.8 mmol/L; impaired glucose tolerance (IGT): FPG <7.0 mmol/L, 2hPPG ≥7.8~<11.1 mmol/L; DM, FPG ≥7.0 mmol/L, 2hPPG ≥11.1 mmol/L, or with diabetes symptoms, or random venous blood glucose level ≥11.1 mmol/L.
The 391 CRC patients were divided into 4 groups according to their glucose level: diabetes mellitus (DM) group, impaired glucose tolerance (IGT) group, impaired fasting glucose (IFG) group, and normal glucose (N-G) group. The DM, IGT, and IFG groups were also collectively called the high-glucose (H-G) group.
Diabetes-controlling treatment
Data on diabetes-controlling treatment of all CRC patients with hyperglycosemia (including DM, IGT and IFG) were collected (Table 1). These patients were further categorized into patients with insulin treatment (plus oral hypoglycemic) (INS group), patients with oral hypoglycemic (without insulin) (OH group), patients without any diabetes-controlling treatment (No Treatment, N-T group), and patients with normal blood glucose level (Normal blood Glucose, N-G group).
Table 1.
Diabetes-controlling treatment/medication of DM patients.
| Group | Diabetes-controlling treatment | Patients number (58) | |
|---|---|---|---|
| n | % | ||
| INS | Insulin injection and oral administration of hypoglycemic drugs | 15 | 25.86% |
| OH | Biguanide (metformin) + non-sulfonylureans (repaglinide) | 9 | 15.52% |
| Biguanide (metformin) + sulfonylureans (glidazide, glimepiride) | 8 | 13.79% | |
| Biguanide (metformin) + glycosidase inhibitor (acarbose) | 6 | 10.34% | |
| Biguanide (metformin) + insulin sensitizer (pioglitazone, rosiglitazone) | 5 | 8.62% | |
| Biguanide (metformin) | 4 | 6.90% | |
| Glycosidase inhibitor (acarbose) | 2 | 3.45% | |
| Sulfonylureans (glidazide, glimepiride) | 2 | 3.45% | |
| Non-sulfonylureans (repaglinide) | 1 | 1.72% | |
| N-T | No hypoglycemic treatment | 6 | 10.34% |
DM – diabetes mellitus; INS – diabetes patients with insulin plus oral hypoglycemic treatment; OH – diabetes patients with oral hypoglycemic only (without insulin); N-T – diabetes patients without any diabetes-controlling treatment.
Patient follow-up
Patient survival was followed up through regular phone contact. Records were taken on patient status as “death”, “alive” or “lost contact”. The time between initial hospitalization and follow-up was recorded (months) until 80 months or death/lost contact, whichever came first.
Statistic analysis
Statistics were calculated using SPSS19.0 (IBM, USA). Correlation between glucose levels and clinicopathological parameters was analyzed using Pearson chi-square (c2) test. The correlation between glucose level and that of patient survival was analyzed using Kaplan-Meier survival analysis and log-rank test. Kaplan-Meier survival curve and Cox-regression were applied to analyze patient survival in different glucose groups. p<0.05 was considered statistically significant and p<0.01 was considered substantially different.
Result
Blood glucose level in CRC patients
The 391 CRC patients were all Asian, male (n=222), female (n=169). The number of patients in each glucose group and their percentage was listed in Table 2. The average age of DM patients was 65.483±11.776 (range, 31–97yrs); average age for IGT was 70.167±2.676 (range, 53–86yrs); and average age for IFG was 66.075±1.753 (range, 31–97 yrs). The history of high blood glucose in the 116 H-G patients was 1 month to 21 years, average, 5.2±3.6 years. The blood glucose level in the DM patients was 9.002±1.750mmol/L, the average glucose level in IGT patients were 6.829±0.023mmol/L, IFG, 6.293±0.021mmol/L, blood glucose level in N-G group, 5.042±0.042 mmol/L.
Table 2.
Colorectal cancer patients and their blood glucose level.
| CRC n=391 |
H-G | DM | IGT | IFG | N-G | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Total Number | 116 | 29.67% | 58 | 14.83% | 18 | 4.60% | 40 | 10.23% | 275 | 70.33% |
| Male | 63 | 16.11% | 30 | 7.67% | 11 | 2.81% | 22 | 5.63% | 159 | 40.66% |
| Female | 53 | 13.55% | 28 | 7.16% | 7 | 1.79% | 18 | 4.60% | 116 | 29.67% |
CRC – colorectal cancer; H-G – high glucose groups including DM (diabetes mellitus), IGT (impaired glucose tolerance), and IFG (impaired fasting glucose); N-G – normal blood glucose group.
Clinicopathological features of CRC patients and their blood glucose level
The clinicopathological features of the CRC patients were compared between the 3 high-glucose patients groups and the normal glucose group (Table 3). A significant difference was observed between the H-G vs the N-G group and the DM vs. N-G group in gross tumor type, tumor diameter, tumor differentiation, and TNM classification (Table 3, marked by asterisks). Specifically, high glucose level was significantly correlated with more frequent ulcerative tumor type, larger tumor diameter, lower tumor differentiation, and higher TNM classification (p<0.05). No significant differences were found between the groups in sex, age, tumor location, vascular/perineural invasion, lymphoma metastasis, distant metastasis and patient prognosis (p>0.05).
Table 3.
Clinopathological features of CRC patients and their blood glucose level by Pearson Chi-Square analysis.
| Clinicopathological features | n=391 | Fasting serum glucose | H-G vs. N-G | DM vs. N-G | IGT vs. N-G | IFG vs. N-G | |||
|---|---|---|---|---|---|---|---|---|---|
| DM=58 | IGT=18 | IFG=40 | N-G =275 | χ2/sig. | χ2/sig. | χ2/sig. | χ2/sig. | ||
| Sex | |||||||||
| Male | 222 | 30 | 11 | 22 | 159 | 0.409 | 0.725 | 0.075 | 0.113 |
| Female | 169 | 28 | 7 | 18 | 116 | 0.522 | 0.395 | 0.784 | 0.736 |
| Age (Years) | |||||||||
| <60 | 127 | 17 | 4 | 12 | 94 | 1.223 | 0.512 | 1.086 | 0.274 |
| ≥60 | 264 | 41 | 14 | 28 | 181 | 0.269 | 0.474 | 0.297 | 0.601 |
| Tumor location | |||||||||
| Cecum | 23 | 5 | 0 | 1 | 17 | 3.302 | 5.130 | 2.955 | 5.718 |
| Ascending colon | 117 | 22 | 3 | 17 | 75 | 0.654 | 0.400 | 0.707 | 0.335 |
| Transverse colon | 20 | 4 | 1 | 1 | 14 | ||||
| Descending colon | 19 | 1 | 1 | 3 | 14 | ||||
| Sigmoid colon | 63 | 9 | 3 | 4 | 47 | ||||
| Rectum | 149 | 17 | 10 | 14 | 108 | ||||
| Gross type | |||||||||
| Ulcerative | 241 | 43 | 8 | 30 | 160 | 8.003 | 11.633 | 1.558 | 4.152 |
| Invasive | 56 | 11 | 4 | 3 | 38 | 0.018* | 0.003** | 0.459 | 0.125 |
| Polyp | 94 | 4 | 6 | 7 | 77 | ||||
| Tumor diameter (cm) | |||||||||
| <3 | 84 | 7 | 1 | 5 | 71 | 10.326 | 5.048 | 3.742 | 3.384 |
| ≥3 | 307 | 51 | 17 | 35 | 204 | 0.001** | 0.025* | 0.053 | 0.066 |
| Neurovascular invasion | |||||||||
| No invasion | 145 | 18 | 6 | 13 | 108 | 1.903 | 1.382 | 0.251 | 0.677 |
| Neuro/vascular invasion | 246 | 40 | 12 | 27 | 167 | 0.168 | 0.240 | 0.617 | 0.411 |
| Differentiation | |||||||||
| High | 52 | 1 | 0 | 0 | 51 | 25.268 | 13.721 | 4.186 | 9.859 |
| Medium | 210 | 30 | 11 | 23 | 146 | 0.000** | 0.001** | 0.123 | 0.007** |
| Low | 129 | 27 | 7 | 17 | 78 | ||||
| Primary tumor invasion | |||||||||
| T1, T2 | 65 | 6 | 4 | 9 | 46 | 0.007 | 1.481 | 0.360 | 0.807 |
| T3, T4 | 326 | 52 | 14 | 31 | 229 | 0.933 | 0.224 | 0.548 | 0.369 |
| Lymph metastasis | |||||||||
| N0 | 228 | 27 | 8 | 25 | 168 | 4.352 | 4.223 | 6.563 | 0.590 |
| N1 | 86 | 16 | 8 | 9 | 53 | 0.113 | 0.121 | 0.038* | 0.744 |
| N2 | 77 | 15 | 2 | 6 | 54 | ||||
| Distant metastasis | |||||||||
| M0 | 379 | 55 | 18 | 40 | 266 | 0.129 | 0.498 | 0.608 | 1.348 |
| M1 | 12 | 3 | 0 | 0 | 9 | 0.719 | 0.481 | 0.436 | 0.246 |
| TNM classification | |||||||||
| I, II | 355 | 30 | 18 | 38 | 269 | 54.728 | 111.005 | 0.401 | 1.121 |
| III, IV | 36 | 28 | 0 | 2 | 6 | 0.000** | 0.000** | 0.527 | 0.290 |
| Prognosis and follow-up | |||||||||
| Death | 98 | 14 | 5 | 7 | 72 | 0.618 | 0.182 | 0.034 | 1.521 |
| Alive | 251 | 37 | 11 | 29 | 174 | 0.734 | 0.913 | 0.983 | 0.467 |
| Lost contact | 42 | 7 | 2 | 4 | 29 | ||||
P<0.01,
P<0.05.
DM – diabetes mellitus, IGT (impaired glucose tolerance), IFG (impaired fasting glucose), H-G (high glucose groups including DM, IGT and IFG). N-G (normal blood glucose group). TNM system based on the America joint committee on cancer (AJCC)/union for international cancer control (UICC) 2009 staging system for colorectal cancer (7th edition) [16]. Primary tumor (T): Tx – primary tumor cannot be evaluated; T0 – no signs of tumor; Tis – carcinoma in situ; T1,2,3,4 – size and/or extension of primary tumor. N – degree of spread to regional lymph nodes; Nx – lymph nodes cannot be evaluated; N0 – tumor cells absent from regional lymph nodes; N1 – 1–3 lymph nodes metastasis; N2 – more than 4 lymph nodes metastasis. Distant metastasis (M): M0 – no distant metastasis; M1 – distant metastasis. TNM classification standard: I – T1/T2, N0, M0; II – T3/ T4, N0, M0; III – any T, N1~N2, M0; IV – any T, any N, M1.
Diabetes-controlling treatment in CRC patients with D2M
Among the 58 D2M patients with CRC, 15 received insulin (INS) treatment and oral administration of hypoglycemic drugs. Their blood glucose level was 9.71–14.57 mmol/L (average 11.387±1.445 mmol/L). INS dosage was 20–30 U/d (average, 24.98±5.7 U/d). Patients in the IGT and IFG groups in our study did not receive any hypoglycemic/insulin treatment.
We divided the 58 D2M patients into sub-groups based on their diabetes-controlling medications (Table 1). Based on Pearson chi-square analysis, compared to CRC patients in the N-G group, DM patients who received INS plus hypoglycemic treatment (INS group) had larger tumor diameter (χ2=14.225, p=0.000) (Table 4). A higher proportion of D2M patients in the INS group were in TNM stage III and IV than stage I and II compared with CRC patients in the N-G group (χ2=81.003, p=0.000) (Table 4). Significant differences were also observed in distant metastasis (χ2=21.000, p=0.000) and TNM classification (χ2=81.003, p=0.000) between the INS group and the N-G group (Table 4). A significant difference was also found between DM patients who received no diabetes treatment (N-T group) vs. N-G group, indicating that high blood glucose level, not than INS treatment, was a significant factor contributing to CRC development. Consistently, no significant differences were found when comparing the clinicopathological features of CRC patients in the INS group versus the OH or N-T groups (Table 4).
Table 4.
The effect of diabetes-controlling treatment on the clinicopathological features of CRC patients with D2M by Pearson Chi-Square analysis.
| Clinicopathological features | DM=58 | N-G=275 | INS vs. OH | INS vs. N-T | INS vs. N-G | N-T vs. N-G | ||
|---|---|---|---|---|---|---|---|---|
| INS=15 | OH=37 | N-T=6 | χ2/P | χ2/P | χ2/P | χ2/P | ||
| Sex | ||||||||
| Male | 7 | 19 | 4 | 159 | 0.094 | 0.687 | 0.723 | 0.189 |
| Female | 8 | 18 | 2 | 116 | 0.760 | 0.407 | 0.395 | 0.664 |
| Age (yrs) | ||||||||
| <60 | 4 | 11 | 2 | 94 | 0.049 | 0.093 | 0.359 | 0.002 |
| ≥60 | 11 | 26 | 4 | 181 | 0.825 | 0.760 | 0.549 | 0.965 |
| Tumor diameter (cm) | ||||||||
| <3 | 1 | 6 | 0 | 71 | 0.836 | 0.420 | 14.225 | 7.841 |
| ≥3 | 14 | 31 | 6 | 51 | 0.361 | 0.517 | 0.000** | 0.005** |
| Neurovascular invasion | ||||||||
| No invasion | 5 | 11 | 2 | 108 | 0.650 | 0.000 | 0.211 | 0.087 |
| Neuro/vascular invasion | 10 | 26 | 4 | 167 | 0.799 | 1.000 | 0.646 | 0.768 |
| Differentiation | ||||||||
| High | 0 | 0 | 1 | 51 | 0.002 | 2.870 | 4.408 | 1.400 |
| Medium | 8 | 20 | 2 | 146 | 0.962 | 0.238 | 0.110 | 0.497 |
| Low | 7 | 17 | 3 | 78 | ||||
| Primary tumor invasion | ||||||||
| T1, T2 | 2 | 2 | 2 | 46 | 0.945 | 1.112 | 0.119 | 1.143 |
| T3, T4 | 13 | 35 | 4 | 229 | 0.331 | 0.292 | 0.731 | 0.285 |
| Lymph metastasis | ||||||||
| N0 | 6 | 19 | 2 | 168 | 0.631 | 1.167 | 2.762 | 3.556 |
| N1 | 4 | 9 | 3 | 53 | 0.729 | 0.558 | 0.251 | 0.169 |
| N2 | 5 | 9 | 1 | 54 | ||||
| Distant metastasis | ||||||||
| M0 | 15 | 36 | 6 | 0 | 0.413 | —— | 21.000 | 12.000 |
| M1 | 0 | 1 | 0 | 6 | 0.520 | —— | 0.000** | 0.001** |
| TNM Classification | ||||||||
| I, II | 7 | 21 | 2 | 269 | 0.437 | 0.311 | 81.003 | 71.144 |
| III, IV | 8 | 16 | 4 | 6 | 0.508 | 0.577 | 0.000** | 0.000** |
| Prognosis and follow-up | ||||||||
| Death | 4 | 7 | 3 | 72 | 0.397 | 1.575 | 0.129 | 2.045 |
| Alive | 9 | 25 | 3 | 174 | 0.820 | 0.455 | 0.938 | 0.360 |
| Lost contact | 2 | 5 | 0 | 29 | ||||
P<0.01,
P<0.05
DM – diabetes mellitus; INS – diabetes patients with insulin plus oral hypoglycemic treatment; OH – diabetes patients with oral hypoglycemic (without insulin); N-T – diabetes patients without any diabetes-controlling treatment; N-G – patients with normal blood glucose level.
We also compared the overall survival of DM patients who received insulin plus hypoglycemic treatment, DM patients with oral hypoglycemic (without insulin), and patients without any diabetes-controlling treatment, and no significant difference was found (Table 4).
Patients’ survival and their blood glucose levels
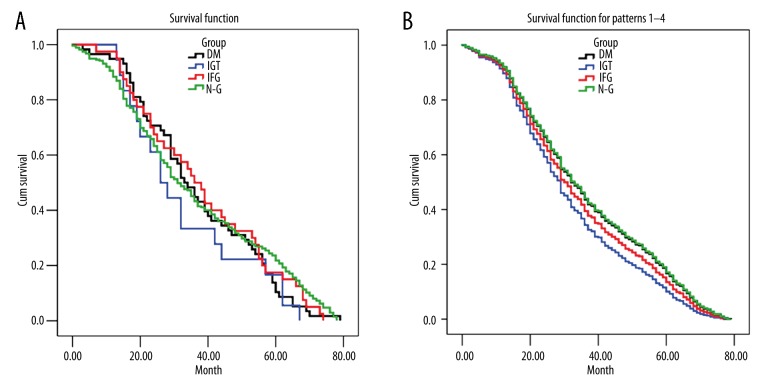
The correlation between glucose level and that of patient survival was analyzed using Kaplan-Meier survival analysis and log-rank test (Figure 1A, 1B and Table 5). Survival curve of CRC patients in the 4 groups was constructed by both the Kaplan-Meier method (Figure 1A) and Cox-Regression method (Figure 1B) with follow-up time of 0–80 months. No statistically significant differences were found between N-G and H-G groups (p>0.05) by either the Kaplan-Meier or the Cox-Regression method. However, patients in the H-G group (DM, IGT, IFG) exhibited a shorter survival trend as the follow-up time increased (Figure 1A). No significant difference was found between the H-G group and N-G group in mean or median survival (P>0.05) (Table 5).
Figure 1.
CRC survival curve and blood glucose level by Cox-Regression (A) and Kaplan-Meier analysis (B). No significant difference was found in CRC patients with different blood glucose levels by either method. However, the H-G groups showed a shorter survival trend with the increase of follow-up time.
Table 5.
Mean and Median CRC Survival and blood glucose levels.
| Groups | Mean* | Median | ||||||
|---|---|---|---|---|---|---|---|---|
| 95% Confidence Interval | 95% Confidence Interval | |||||||
| Estimate | Std. error | Lower bound | Upper bound | Estimate | Std. error | Lower bound | Upper bound | |
| DM | 37.28 | 2.40 | 32.57 | 41.98 | 33.00 | 2.72 | 27.67 | 38.33 |
| IGT | 33.28 | 4.25 | 24.93 | 41.62 | 26.00 | 3.53 | 19.07 | 32.93 |
| IFG | 38.68 | 3.08 | 32.62 | 44.73 | 36.00 | 3.16 | 29.80 | 42.19 |
| N-G | 36.89 | 1.32 | 34.29 | 39.49 | 31.00 | 1.95 | 27.18 | 34.82 |
| Overall | 36.96 | 1.06 | 34.88 | 39.05 | 32.00 | 1.46 | 29.13 | 34.87 |
Estimation is limited to the largest survival time if it is censored.
DM – diabetes mellitus; IGT – impaired glucose tolerance; IFG – impaired fasting glucose; H-G – high glucose groups including DM, IGT and IFG; N-G – normal blood glucose group.
We also analyzed patient survival using several other statistical methods. No significant differences in overall survival between the 4 glucose groups were found by log-rank (Mantel-Cox) with χ2=0.757, DF=1, p=0.384; Breslow (generalized Wilcoxon), with χ2=0.115, DF=1, p=0.734; or Tarone-Ware analysis, with χ2=0.062, DF=1, p=0.804.
Discussion
Studies showed that T2DM increase the risk of colorectal cancer [3–5]. Larsson [17] conducted a meta-analysis of 15 related studies (6 case-control and 9 cohort) that included 2 593 935 patients. Their analysis showed that diabetes increased CRC risk by 30% (RR=1.30, 95%CI=1.20–l.40) compared with non-diabetes patients. Another study found that serum glucose level correlates with colorectal cancer incidence in woman after menopause [18]. In our study, we investigated the glucose levels of 391 CRC patients, including patients with DM and impaired glucose regulation: IFG and IGT, 2 pre-diabetes stages. Our study showed that 13.41% of patients had co-existing DM and CRC, 4.32% of patients had both IGT and CRC, and 9.32% of patients had both IFG and CRC. Thus, a total of about 27.05% of CRC patients had above-normal glucose. Therefore, it seems that high glucose level plays a role in the development of CRC. However, the mechanism of how high glucose level contributes to CRC development is currently unclear.
Our result showed that, compared to patients with normal glucose level, high glucose and diabetes groups had larger tumor diameter, lower differentiation (thus higher malignancy), higher percentage of ulcerative tumor, and more advanced TNM stages. The 2 pre-diabetes stages (IGT and IFG) had less effect on tumor malignancy compared to DM patients (Table 3). Although no statistical difference was found between the 4 patient groups with different glucose levels in survival curve analysis, patients in the H-G group exhibited a shorter survival trend at later follow-up time. Our data suggest that glucose level affects the local malignancy of CRC and might affect patient overall survival if given longer follow-up time.
Research shows that antidiabetic medication affects the risk of CRC in patients with diabetes mellitus [19,20]. Singh conducted a systematic evaluation of 15 studies including 13 871 patients with diabetes mellitus and assessed their risk for CRC. Their meta-analysis concluded that a protective effect of metformin use and CRC risk, although no significant association was observed with insulin or sulfonylurea use [19]. However, a harmful effect of insulin therapy and CRC risk among T2DM patients has also been reported [20]. We analyzed the DM patients enrolled in our study and their diabetes-controlling treatment. Our result showed that DM patients who received INS and hypoglycemic drug treatment had significantly larger tumor diameter and more advanced TNM stage compared to patients with normal glucose. However, no significant difference was found in clinicopathological features and overall survival between patients with INS and hypoglycemic drug treatment, patients with oral hypoglycemic only, and patients with DM but received no diabetes controlling treatment. DM patients, regardless of whether they received INS treatment or not, had more severe tumor malignancy compared to patients with normal glucose. Our results indicate that diabetes-controlling treatment in our patient set had no significant effect on CRC development. However, our DM patient cohort was small and a larger scale study is needed to confirm the observations.
Conclusions
We observed a positive association between T2DM and the local malignancy of CRC. Glucose level is a significant risk factor correlates with the development of CRC. Early diagnosis and personalized treatment is critical for colorectal cancer patients with high blood glucose and diabetes.
Footnotes
Source of support: This work was funded by Public Welfare Technical Applied Research Project of Zhejiang Province (2013C37031), Public Welfare Technical Applied Research Major Project of Huzhou City (2013GZ14), and Public Welfare Technical Applied Research Project of Huzhou City (2013GY19)
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.International diabetes federation. IDF Diabetes Atlas. 4th ed. Brussels, Belgium: International Diabetes Federation; 2009. http://www.idf.org. [Google Scholar]
- 2.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 3.Mills KT, Bellows CF, Hoffman AE, et al. Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Dis Colon Rectum. 2013;56:1304–19. doi: 10.1097/DCR.0b013e3182a479f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giouleme O, Diamantidis MD, Katsaros MG. Is diabetes a causal agent for colorectal cancer? Pathophysiological and molecular mechanisms. World J Gastroenterol. 2011;17:444–48. doi: 10.3748/wjg.v17.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berster JM, Goke B. Type 2 diabetes mellitus as risk factor for colorectal cancer. Arch Physiol Biochem. 2008;114:84–98. doi: 10.1080/13813450802008455. [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Yu S. Diabetes mellitus is an independent risk factor for colorectal cancer. Dig Dis Sci. 2012;57:1586–97. doi: 10.1007/s10620-012-2059-x. [DOI] [PubMed] [Google Scholar]
- 7.Chen KH, Shao YY, Lin ZZ, et al. Type 2 diabetes mellitus is associated with increased mortality in chinese patients receiving curative surgery for colon cancer. Oncologist. 2014;19:951–58. doi: 10.1634/theoncologist.2013-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan Y, Huang XD, Zhang H, et al. Association between serum HbA1c levels and adenomatous polyps in patients with the type 2 diabetes mellitus. Minerva Endocrinol. 2015;40(3):163–67. [PubMed] [Google Scholar]
- 9.Shin HY, Jung KJ, Linton JA, Jee SH. Association between fasting serum glucose levels and incidence of colorectal cancer in Korean men: the Korean Cancer Prevention Study-II. Metabolism. 2014;63:1250–56. doi: 10.1016/j.metabol.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Jullumstro E, Kollind M, Lydersen S, Edna TH. Diabetes mellitus and outcomes of colorectal cancer. Acta Oncol. 2009;48:361–67. doi: 10.1080/02841860802637765. [DOI] [PubMed] [Google Scholar]
- 11.Kramer HU, Muller H, Stegmaier C, et al. Type 2 diabetes mellitus and gender-specific risk for colorectal neoplasia. Eur J Epidemiol. 2012;27:341–47. doi: 10.1007/s10654-012-9686-6. [DOI] [PubMed] [Google Scholar]
- 12.La Vecchia C, Negri E, Decarli A, Franceschi S. Diabetes mellitus and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 1997;6:1007–10. [PubMed] [Google Scholar]
- 13.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–50. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Flood A, Strayer L, Schairer C, Schatzkin A. Diabetes and risk of incident colorectal cancer in a prospective cohort of women. Cancer Causes Control. 2010;21:1277–84. doi: 10.1007/s10552-010-9555-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang ZJ, Zheng ZJ, Kan H, et al. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:2323–28. doi: 10.2337/dc11-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–74. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 17.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 18.Kabat GC, Kim MY, Strickler HD, et al. A longitudinal study of serum insulin and glucose levels in relation to colorectal cancer risk among postmenopausal women. Br J Cancer. 2012;106:227–32. doi: 10.1038/bjc.2011.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S, Singh H, Singh PP, et al. Antidiabetic medications and the risk of colorectal cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2258–68. doi: 10.1158/1055-9965.EPI-13-0429. [DOI] [PubMed] [Google Scholar]
- 20.Yin S, Bai H, Jing D. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients: a systemic review and meta-analysis. Diagn Pathol. 2014;9:91. doi: 10.1186/1746-1596-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]