Abstract
Using the Chinese cabbage (Brassica campestris) cultivar ‘Chun-goang’ as a host and turnip mosaic virus (TuMV) as a pathogen, we studied the effects of ambient temperature (13°C, 18°C, 23°C, 28°C and 33°C) on disease intensity and the speed of systemic infection. The optimal temperature for symptom expression of TuMV was 18–28°C. However, symptoms of viral infection were initiated at 23–28°C and 6 days post infection (dpi). Plants maintained at 33°C were systemically infected as early as 6 dpi and remained symptomless until 12 or 22 dpi, depending on growth stage at the time of inoculation. It took 45 days for infection of plants grown at 13°C. Quantitative real-time polymerase chain reaction (q-PCR) results showed that the accumulation of virus coat protein was greater in plants grown at 23–28°C. The speed of systemic infection increased linearly with rising ambient temperature, up to 23°C. The zero-infection temperature was 10.1°C. To study the effects of abruptly elevated temperatures on systemic infection, plants inoculated with TuMV were maintained at 10°C for 20 d; transferred to a growth chamber at temperatures of 13°C, 18°C, 23°C, 28°C, or 33°C for 1, 2, or 3 d; and then moved back to 10°C. The numbers of plants infected increased as duration of exposure to higher temperatures and dpi increased.
Keywords: chinese cabbage, disease symptoms, temperature, TuMV
Chinese cabbage is a widely cultivated crop in Korea, where it occupies approximately 35,513 ha annually (Food, Agriculture, Forestry and Fisheries Statistical Yearbook, 2012). The cropping system is divided into three types: spring cabbage (planted in April, harvested in June), autumn cabbage (planted in September, harvested in November), and summer cabbage (planted in May, harvested in September). Summer cabbage is cultivated in the alpine mountainous area of Gangwon Province, above 400 m altitude.
Viral diseases that affect Chinese cabbage in Korea include cucumber mosaic virus (CMV), turnip mosaic virus (TuMV), and ribgrass mosaic virus (RMV; Cho et al., 2003; Choi, 1995; Yoon et al., 1995). TuMV is the most prevalent virus, followed by CMV (Choi, 1995). However, double infections are common; for example, one study (Yoon et al., 1995) found that infection with TuMV was 13.9% and infection with RMV was 4.5%, but double infection with both viruses was 31.9%.
TuMV infects most cruciferous plants, but it is particularly damaging to Chinese cabbage, turnip, mustard, and radish (Chivasa et al., 2001; Nguyen et al., 2013). It also attacks beets, spinach, and tobacco (Provvidenti, 1981; Walsh and Jenner, 2002), with a host range of at least 318 species representing 156 genera and 43 families. TuMV occurs in many parts of the world, including temperate, subtropical, and tropical regions of Africa, Asia, Europe, Oceania, and North and South America (Ha et al., 2008; Ohshima et al., 2002; Spence, 1999). TuMV causes stunted, coarsely mottled, and distorted symptoms in Chinese cabbage (Cho et al., 2003). Black spots develop on leaves, which subsequently drop prematurely. There is also yellowing along the leaf veins, leading to early senescence (Tomlinson, 1970).
In recent years, as summers have tended to begin earlier, viral damage has increased in summer cabbage cultivated in highlands, reducing the production of marketable cabbage in Korea. Plant viruses and their vectors are strongly influenced by weather and climate. Climate changes are expected to affect the establishment, spread, and reproduction potential of viruses (Kido et al., 2008; Tamada and Harrison, 1981). In the present study, we investigated the effects of temperature on systemic infection and symptom severity by varying the temperature at the time of infection of TuMV in Chinese cabbage.
Material and Methods
Virus inoculation and temperature treatment
Seeds of the Chinese cabbage cultivar ‘Chun-goang’, which is generally known as a susceptible cultivar to TuMV, were sowed in a commercial soil ‘Barok’ and were grown in the insect proof glasshouse until using. At the two- (14-day-old) and four- (35-day-old) true-leaf stages, seedlings were mechanically inoculated with TuMV Virus inoculum was obtained from infected Chinese cabbage plants and prepared by pulverizing infected leaves with a mortar and pestle in 0.1 M PBS buffer (pH 7.2) at an approximate dilution of 1:5 (w/v). A constant volume of crude sap of the inoculum was rubbed on each test plant after dusting the plant with carborundum. TuMV-inoculated plants were immediately transferred to a growth chamber, set at 16-h day length and constant temperature treatments of 13°C, 18°C, 23°C, 28°C and 33°C. Fifteen plants were included in each temperature treatment. Inoculated plants were monitored regularly, over a period of 30 d, for symptom development. To study the effects of an abrupt increase in temperature on systemic infection by TuMV, inoculated plants were initially maintained at 10°C. After 20 d, they were transferred to a growth chamber at a temperature of 13°C, 18°C, 23°C, 28°C or 33°C for 1, 2, or 3 d, and then they were moved back to 10°C.
Detection of virus infection
To study systemic infection, upper leaves of TuMV-inoculated plants were collected at intervals of 2 d over a 30-d period, starting at 6 days post infection (dpi). Systemic infection was determined based on ELISA results. The ELISA procedure was performed according to the manufacturer’s instructions (Agdia, USA).
Assessment of disease severity
The severity of viral symptoms of infected plants was scored as the symptoms started appearing at 6 dpi. The symptom severity score was rated on a 4.0-point scale: 0 = symptomless, 0.5 = slight leaf stunt, 1.0 = slight leaf stunt with mottle, 1.5 = clear leaf stunt with leaf deformation, 2 = leaf stunt with some chlorosis or yellow spots, 2.5 = leaf chlorosis half of the leaflets, 3 = leaf stunt and severe chlorosis over all of the leaflets, 3.5 = severe necrosis, 4 = withering. The SAS 4.2 statistical package (SAS Inc., Cary, NC, USA) was used for data analysis.
Speed of systemic infection
The mean systemic infection rate was expressed as the reciprocal of the systemic infection time (days) as suggested by Wagner et al. (Wagner et al., 1984). A linear equation fitted to the systemic infection rate showed a linear relationship in the range of 13–23°C. The best-fit linear lines were obtained using the TableCurve 2D program (Jandel Scientific, 1996). The infection zero temperature was calculated by solving the –intercept/slope of the fitted equation.
Quantitative Real-Time PCR
Accumulation of virus coat protein (CP) was measured by real-time qPCR analysis at 10, 17, and 22 dpi under the five temperature treatments, following inoculation with TuMV at the two-true-leaf stage. RNA was extracted with an RNeasy mini kit (Qiagen, German, Hilden) and 200 ng RNA was used for cDNA synthesis. A total of 1 μl cDNA was used for qPCR analysis. Reactions were performed in a C1000 Touch thermal cycler (Bio-Rad, USA) using the SYBR-Green method (Universal SYBR-Green Supermix, Bio-Rad) according to the following protocol: 1 cycle at 95°C for 30 s, 39 cycles at 95°C for 10 s, 60°C for 30 s, and 65–95°C in increments of 0.5°C at intervals of 5 s. The q-PCR primers (Supplementary Table S2) were designed using Integrated DNA technology (www.idtdna.com). The gene fragments amplified were TuMV-122 (accession no. NC_002509, nucleotides 177 to 299) and ACT1 (accession no. FJ969844, nucleotides 170 to 279). TuMV-RNA (target) was normalized to ACT1 expression (internal reference) to calculate the normalized target gene expression using CFX Manager v3.0 (Bio-rad, USA).
Results
Time required for systemic infection
A higher proportion of plants inoculated at the four-true-leaf stage were infected compared to those inoculated at the two-true-leaf stage (Table 1). The time until systemic infection did not vary at temperatures from 23°C to 33°C or from 8 to 10 dpi (Table 1). At 13°C, plants were not infected systemically until 45 dpi.
Table 1.
The time taken for systemic infection as affected by the ambient temperature maintained after inoculation of TuMV in Chinese cabbage
| Growth stage of plants | Temperature (°C) | No. of plants inoculated | Days for systemic infection | ||
|---|---|---|---|---|---|
|
| |||||
| Mean±SE | Median | Rate (%) | |||
| 2 true leaf | 13 | 15 | 44.3±0.38 a* | 45 | 20.0 |
| 18 | 15 | 15.7±0.80 b | 15 | 40.0 | |
| 23 | 15 | 8.9±0.21 c | 8 | 73.3 | |
| 28 | 15 | 8.2±0.20 c | 8 | 66.6 | |
| 33 | 15 | 8.1±0.11 c | 8 | 100.0 | |
|
| |||||
| 4 true leaf | 13 | 15 | 44.0±0.58 a | 45 | 20.0 |
| 18 | 15 | 13.7±0.51 b | 14 | 100.0 | |
| 23 | 15 | 9.8±0.19 cd | 10 | 93.3 | |
| 28 | 15 | 8.1±0.13 cde | 8 | 93.3 | |
| 33 | 15 | 7.4±0.08 de | 8 | 100.0 | |
Within a column of each growth stage, means with same letters are not significantly different (Tukey’s studentized range test at P > 0.05).
Systemic infection speed
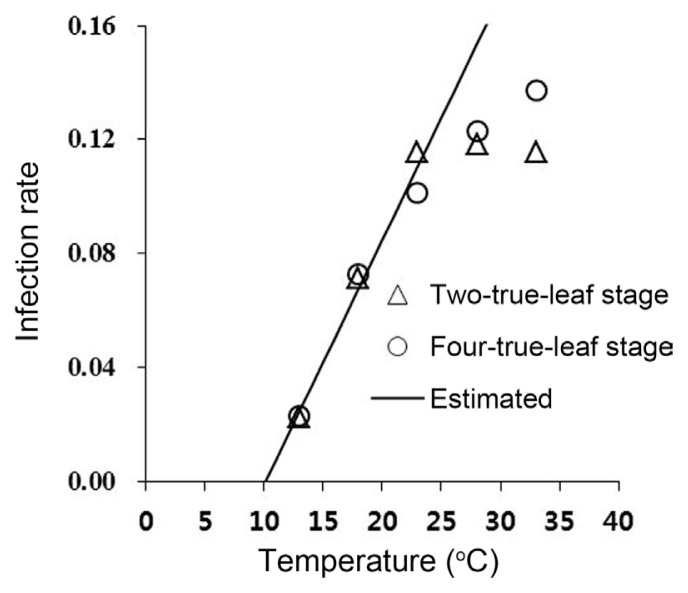
The speed of systemic infection increased linearly as the temperature increased to 23°C (Fig. 1). A linear model (Y = aX + b) was fitted to the infection rate curve and the estimated parameters a and b were 0.0858 and −0.0866, respectively. The linear model described the systemic infection rate well in the range 13–23°C (γ2 = 0.97; d.f. = 1, P = 0.00016; Fig. 1). The zero-infection temperature was calculated to be 10.1°C.
Fig. 1.
Effects of ambient temperature maintained after inoculation of TuMV on systemic infection rate in Chinese cabbage. A linear model (Y = a × X + b) was fitted to the infection rate curve and estimated parameters a and b were 0.0858 and −0.0866, respectively (γ2 = 0.97; d.f. = 1, P = 0.00016). The linear model fit is denoted by the solid line. Arrows indicate the zero-infection temperature (10.1°C). The systemic infection rate was calculated as the inverse of the number of days required for systemic infection.
Symptom severity
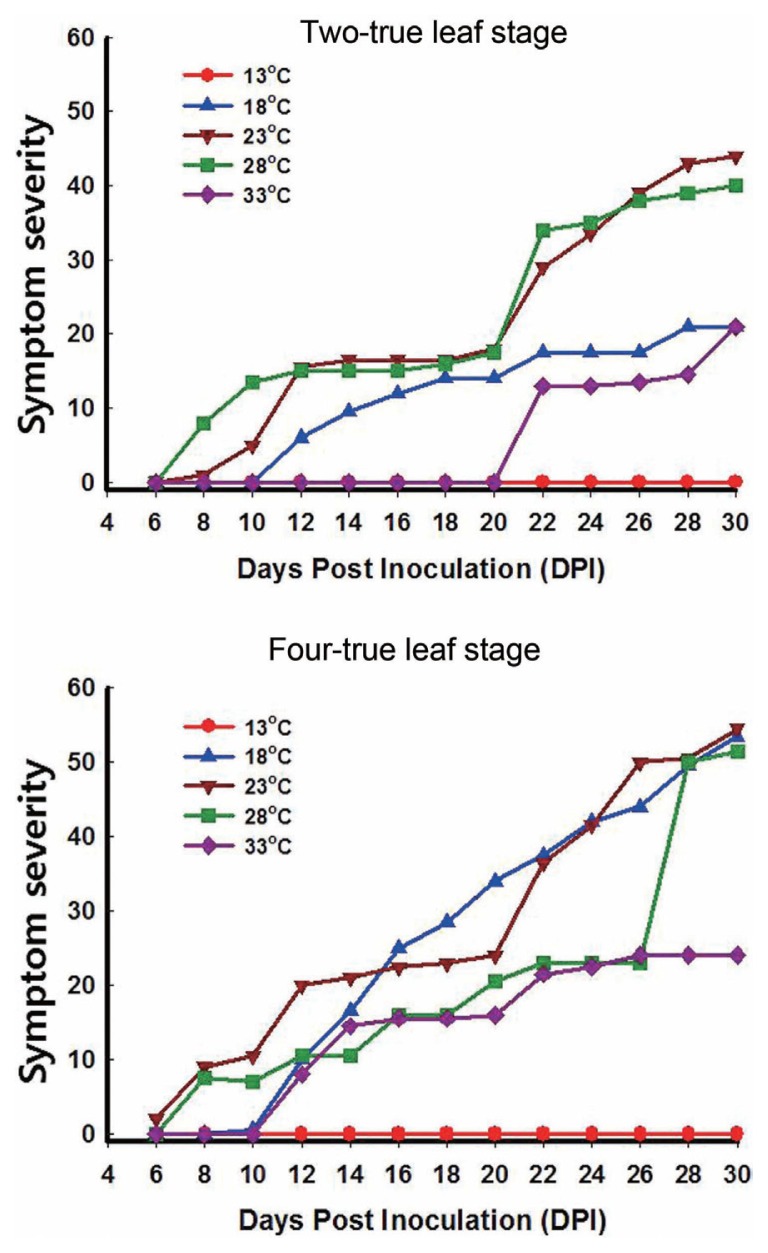
The optimal temperature for symptom expression of TuMV was within the 18–28°C range. Symptom severity was much higher in plants inoculated at the four-true-leaf stage than in those inoculated at the two-true-leaf stage. The low proportion of plants infected at the two-true-leaf stage was attributed to the difference in symptom severity between the two-true-leaf and four-true-leaf stages. This was particularly the case for plants maintained at 18°C (Fig. 2). At 33°C, plants inoculated at the two-true-leaf stage did not show symptoms until 22 dpi, whereas those inoculated at the four-true-leaf stage started to show symptoms at 12 dpi. For plants inoculated at the two- or four-true-leaf stages and maintained at 23–28°C, symptoms started to appear within 6–8 dpi, reaching a maximum severity (i.e., severe necrosis, withering) at 26–30 dpi (Supplementary Table S1). At 18°C, symptom appearance was delayed by about 4 d compared to the 23–28°C range, regardless of plant growth stage at the time of inoculation. Symptom expression increased quickly thereafter, and plants inoculated at the four-true-leaf stage achieved maximum symptom expression at 28 dpi (Supplementary Table S1).
Fig. 2.
The symptom severity of Chinese cabbage maintained under five temperature regimes after inoculation with TuMV at the two- or four-true-leaf stages. The symptom severity is given as the sum of the scores of 15 plants representing a given treatment.
TuMV caused slight leaf stunting, with mottling, at an early stage of infection, and leaf chlorosis or yellow spots appeared at 12 dpi (Fig. 3). Necrosis was visible at 24–26 dpi and withering was observed at 18–28°C (Fig. 3). Yellows spots were observed only at 18°C. At 33°C, the symptoms were no longer typical for TuMV and the appearance of symptoms was delayed. Mottling was observed at 22 dpi and persisted until 30 dpi (Fig. 3).
Fig. 3.
Symptom expression of Chinese cabbage under five different temperature regimes (13°C, 18°C, 23°C, 28°C or 33°C) after inoculation with TuMV at the two- (A) or four- (B) true-leaf stages.
Accumulation of CP
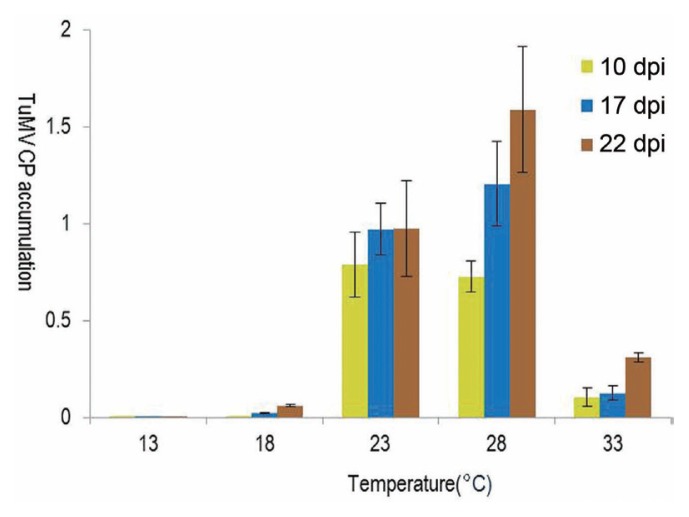
Real-time qPCR results showed that plants maintained at 23–28°C had higher levels of CP than those maintained at 13–18°C or 33°C, starting at 10 dpi (Fig. 4). At 13°C, virus accumulation was not detected until 22 dpi.
Fig. 4.
Accumulation of TuMV CP as determined by real-time qPCR analysis with the passage of time as influenced by the temperature maintained after inoculation with TuMV at the two-true-leaf stage in Chinese cabbage. Bars indicate SE values of five plants.
Abrupt temperature increase
To study the effects of abrupt temperature increases on systemic infection of TuMV, two- and four-true-leaf-stage plants inoculated with TuMV were maintained at 10°C for 20 d. After 20 d, the plants were transferred to a growth chamber with different temperature settings (13°C, 18°C, 23°C, 28°C and 33°C) for 1, 2, or 3 d, and then they were moved back to 10°C. Virus infection was determined at 6, 10, and 28 dpi after completing the treatments (Table 2). More plants inoculated at the two-true-leaf stage were infected compared with those inoculated at the four-true-leaf stage. However, plants maintained at 10°C were not infected. The virus symptoms were detected at temperatures of 18°C or higher in plants inoculated at the two-true-leaf stage and exposed to higher temperatures (Table 2). The number of plants infected increased with increased exposure to each temperature regime and with increases in temperature. This phenomenon was even more pronounced in plants inoculated at the two-true-leaf stage (Table 2).
Table 2.
The number of plants that developed systemic infection with TuMV as affected by temperature, and the hours of exposure to each temperature, after being maintained at 10ºC for 20 d after virus inoculation
| Growth stage of plantsa | Temperature elevated (°C) | No. of plants tested per each hours | No. of plants developed systemic infection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 24 hb | 48 h | 72 h | Total | |||||||||
|
|
|
|
||||||||||
| 6c | 10 | 28 | 6 | 10 | 28 | 6 | 10 | 28 | ||||
| Two-true leaf | 10 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 18 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| 23 | 5 | 0 | 0 | 0 | 1 | 3 | 3 | 2 | 2 | 5 | 8 | |
| 28 | 5 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 2 | 4 | 6 | |
| 33 | 5 | 1 | 2 | 4 | 1 | 2 | 4 | 1 | 3 | 4 | 12 | |
|
| ||||||||||||
| Four-true leaf | 10 | 5 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 13 | 5 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| 18 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 23 | 5 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 3 | |
| 28 | 5 | 0 | 0 | 1 | 0 | 0 | 4 | 0 | 1 | 3 | 9 | |
| 33 | 5 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 3 | |
Plant growth stage at the time of inoculation of TuMV
Duration of exposure of plants to a temperature elevated
Days until ELISA test after moving back to 10°C chamber
Discussion
Using the Chinese cabbage cultivar Chun-goang as a host and TuMV as a pathogen, we studied the effects of temperature (13°C, 18°C, 23°C, 28°C and 33°C) on the intensity of disease symptoms and speed of systemic infection, which increased linearly with temperature up to 23°C. A linear model was fitted to the infection rate curve to elucidate the timing of the onset of systemic infection; the zero-infection temperature was calculated to be 10.1°C. Plants maintained at 10°C after inoculation were not infected systemically. This result supports the utility of the infection rate model. Variation in the time until systemic infection was statistically non-significant from 23°C to 33°C, regardless of plant age at the time of inoculation. However, at 33°C, a complete loss of typical TuMV symptoms was observed and the appearance of symptoms was delayed. The first visual symptoms were observed at 22 dpi. Symptoms included mottling and persisted until 30 dpi. This was probably attributed to the defense responses of the host plants to TuMV being much stronger at 33°C than below 28°C. Viral replication may have been negatively affected by the higher temperature. Inhibition of symptoms at 33°C was associated with low accumulation of CP. It was assumed that extremely high temperatures prohibit virus replication and movement. Similar results have been reported for potato leaf roll virus at elevated temperatures (Tamada and Harrison, 1981). It has been demonstrated that the temperature at which virus transmission occurs affects the efficiency of pathogen multiplication (Feil and Purcell, 2001) and the establishment of infection in the host (Chu and volety, 1997). In protoplasts of Nicotiana benthamiana inoculated with Tomato bushy stunt virus (TBSV) genomic RNA and one of its defective interfering (DI) RNAs, replication of both genomic RNAs and DI RNAs were attenuated at elevated temperatures (32°C), and they concluded that symptom atteunation of TBSV at elevated temperatures are primarily the result of reduced viral replication (Jones et al., 1990). N. benthamiana plants inoculated with Cymbidium ring spot virus (CymRSV) and grown at different temperatures died within 2 weeks at 15, 21 and 24°C, however at 27°C CymRSV symptoms were ‘heat masked’, and revealed that the attenuated symptoms were associated with reduced virus level (Szittya et al., 2003). RNA silencing plays a natural role in plant defense against molecular parasites, including viruses (reviewed in Voinnet, 2001). Szittya et al. (2003) showed that RNA silencing is a temperature dependent as it is activated and the amount of siRNAs gradually increased with rising temperature from 21 to 27°C.
Plants grown at 18°C showed symptoms later, but over time the symptoms became severe, with a maximum severity of necrosis at 28 dpi. Virus replication or movement was slow at 18°C during the early stages of infection, thus more time was needed to establish a systemic infection. One to three days of short exposure of Chinese cabbage (inoculated with TuMV and maintained at 10°C) to the higher temperatures would be sufficient to show symptoms. Plants exposed to 33°C for 24 h showed symptoms 28 d after being moved back to the 10°C chamber. We also found that a greater number of plants were infected when inoculated at the two-true leaf stage than when inoculated at the four-true-leaf stage.
This results indicate that young plants are more susceptible to virus infection than old plants with short time exposure to high temperature. This was probably because the defense responses of host plants to virus infection were much stronger in older plants, which explains the apparent need for more exposure time to establish infection. An example of this age effect is seen when okra plants are infected with virus at growth stages earlier than four weeks has more severe effect on the physiological performance of okra plant (Fajinmi and Fajinmi, 2010).
Plant age affected the time of symptom appearance of Chinese cabbage with TuMV infection. The time needed to show systemic infection was similar in plants inoculated at the two- and four-true-leaf stages, but symptoms appeared earlier in plants inoculated at the four-true-leaf stage. It is presumed that, although the same amount of virus was inoculated for each plant, symptom development might be faster in older plants growing quickly, with concurrent proliferation of the virus throughout the plants. Direct evidence in support of this hypothesis is lacking, but higher infection rate of four-true-leaf stage plants in Table 1 shows consistency. During host–virus interactions, virus establishment in the host is affected by several factors, including growth stage, temperature, virus replication, virus movement, and RNA silencing (Chellappan et al., 2005; Soler et al., 1998; Szittya et al., 2003; Zhang et al., 2012; Zitter and Murphy, 2009). The involment of the developmental stage of the host plant in systemic infection has been mainly examined with cauliflower mosaic virus (CaMV) in turnip (Leisner et al., 1992). It was demonstrated that CaMV moved passively through the plant within the phloem, following the flow of photoassimilates from source to sink leavesm, and the flow fluctuates depending on the growth and developmental conditions of the plant. The developmental stage of the host plant can have a strong impact on vascular movement of certain viruses. Thus, further studies are required to clarify the effects of developmental stage of Chinese cabbage on systemic infection of TuMV..
According to a previous report, early exposure of cabbage to TuMV results in plants being 50% lighter than plants that have not been infected, but later infection is less damaging (Spence et al., 2007). In the present study, temperature at the time of TuMV infection had a strong effect on the productivity of Chinese cabbage. Average temperatures of 20–24°C at the time of planting summer Chinese cabbage in alpine areas of Korea are suitable for TuMV symptom expression. Though virus infection of plants are affected by several other factors including virus strains and plant cultivars besides temperature, as the spring temperatures rise, if aphids emerge earlier, viral damage is expected to increase. If growers cannot control aphids, they must destroy plants infected with TuMV. These plants may include weed host plants growing around fields. Another option is to move to higher altitudes, where aphids are not present.
Supplementary Material
Acknowledgments
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ010246)” Rural Development Administration, Republic of Korea.
References
- Chellappan P, Vanitharani R, Ogbe F, Fauquet CM. Effect of temperature on geminivirus-induced RNA Silencing in Plants. Plant Physiol. 2005;138:1828–1841. doi: 10.1104/pp.105.066563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivasa S, Ekpo EJA, Hicks RGT. New hosts of Turnip mosaic virus in Zimbabwe. New Dis Rep. 2001;4:5. [Google Scholar]
- Cho JD, Choi HS, Kim JS, Kim KH, Kim KS. Symptom variances in mixed infections of six turnip mosaic virus and one ribgrass mosaic virus isolates in crucifers. Plant Pathol J. 2003;19:111–116. doi: 10.5423/PPJ.2003.19.2.111. [DOI] [Google Scholar]
- Choi JK. Annual report. Ministry of Agriculture, Forestry and Fisheries; 1995. [Google Scholar]
- Chu F-LE, Volety AK. Disease processes of the parasite Perkinsus marinus in eastern oyster Crassostrea virginica: minimum dose for infection initiation, and interaction of temperature, salinity and infective cell dose. Dis Aquat Organ. 1997;28:61–68. doi: 10.3354/dao028061. [DOI] [Google Scholar]
- Fajinmi AA, Fajinmi OB. Incidence of okra mosaic virus at different growth stages of okra plants (Abelmoschus esculentus (L.) Moench) under tropical condition. J Gen Mol Virol. 2010;2:28–3. [Google Scholar]
- Feil H, Purcell AH. Temperature-dependent growth and survival of Xylella fastidiosa in vitro and in potted grapevines. Plant Dis. 2001;85:1230–1234. doi: 10.1094/PDIS.2001.85.12.1230. [DOI] [PubMed] [Google Scholar]
- Ha C, Revill P, Harding RM, Vu M, Dale JL. Identification and sequence analysis of potyviruses infecting crops in Vietnam. Arch Virol. 2008;153:45–60. doi: 10.1007/s00705-007-1067-1. [DOI] [PubMed] [Google Scholar]
- Jandel Scientific. Automated curve fitting and equation discovery: Version 4.0. Jandel Scientific; Sam Rafael, CA, USA: 1996. TableCurve 2D. [Google Scholar]
- Jones RW, Jackson AO, Morris TJ. Defective-interfering RNAs and elevated temperatures inhibit replication of tomato bushy stunt virus in inoculated protoplasts. Virology. 1990;176:539–545. doi: 10.1016/0042-6822(90)90024-L. [DOI] [PubMed] [Google Scholar]
- Kido K, Tanaka C, Mochizuki T, Kubota K, Ohki T, Ohnishi J, Knight LM, Tsuda S. High temperatures activate local viral multiplication and cell-to-cell movement of Melon necrotic spot virus but restrict expression of systemic symptoms. Phytopathology. 2008;98:181–186. doi: 10.1094/PHYTO-98-2-0181. [DOI] [PubMed] [Google Scholar]
- Leisner SM, Turgeon R, Howell SH. Long distance movement of califlower mosaic virus infected turnip plant. Mol Plant-Microbe Interact. 1992;5:41–47. doi: 10.1094/MPMI-5-041. [DOI] [Google Scholar]
- Nguyen HD, Tomitaka Y, Ho SYM, Duchéne S, Vetten HJ, Lesemann D, Walsh JA, Gibbs AJ, Ohshima K. Turnip mosaic potyvirus probably first spread to eurasian brassica crops from wild orchids about 1000 years Ago. PLoS One. 2013;8:e55336. doi: 10.1371/journal.pone.0055336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima K, Yamaguchi Y, Hirota R, Hamamoto T, Tomimura K, Tan Z, Sano T, Azuhata F, Walsh JA, Fletcher J, Chen J, Gera A, Gibbs AJ. Molecular evolution of Turnip mosaic virus: evidence of host adaptation, genetic recombination and geographical spread. J Gen Virol. 2002;83:1511–1521. doi: 10.1099/0022-1317-83-6-1511. [DOI] [PubMed] [Google Scholar]
- Provvidenti R. Turnip mosaic virus. Plant Viruses Online. Descriptions and Lists from the VIDE Database 1981 [Google Scholar]
- Soler S, Diez MJ, Nuez F. Effect of temperature regime and growth stage interaction on pattern of virus presence in TSWV-resistant accessions of Capsicum chinense. Plant Dis. 1998;82:1199–1204. doi: 10.1094/PDIS.1998.82.11.1199. [DOI] [PubMed] [Google Scholar]
- Spence NJ. Final Technical Report for DFID CPP Project ZA0272. 1999. Survey of viruses of vegetable crops in the peri-urban production system of Kenya. [Google Scholar]
- Spence NJ, Phiri NA, Hughes SL, Mwaniki A, Simons S, Oduor G, Chacha D, Kuria A, Ndirangu S, Kibata GN, Marris GC. Economic impact of Turnip mosaic virus, Cauliflower mosaic virus and Beet mosaic virus in three Kenyan vegetables. Plant Physiol. 2007;56:317–323. [Google Scholar]
- Szittya G, Silhavy D, Molnár A, Havelda Z, Lovas A, Lakatos L, Bánfalvi Z, Burgyán J. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 2003;22:633–640. doi: 10.1093/emboj/cdg74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada T, Harrison BD. Quantitative studies on the uptake and retention of potato leafroll virus by aphids in laboratory and field conditions. Ann Appl Biol. 1981;98:261–276. doi: 10.1111/j.1744-7348.1981.tb00759.x. [DOI] [Google Scholar]
- Tomlinson JA. Description of plant viruses. Association of Applied Biologists; 1970. Turnip mosaic virus. [Google Scholar]
- Voinnet O. RNA silencing as a plant immune system against viruses. Trends Genet. 2001;17:449–459. doi: 10.1016/S0168-9525(01)02367-8. [DOI] [PubMed] [Google Scholar]
- Wagner TL, Wu H, Sharpe PJH, Schoolfield RM, Coulson R. Modeling insect development rates: a literature review and application of a biophysical model. Ann Entomol Soc Am. 77(1984):208–225. [Google Scholar]
- Walsh JA, Jenner CE. Turnip mosaic virus and the quest for durable resistance. Mol Plant Pathol. 2002;3:289–300. doi: 10.1046/j.1364-3703.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- Yoon MK, Kim JY, Choi GS, Kim JS. Soil transtnission, screening of resistant variety and incidence of Ribgrass mosaic virus occurring in Chinese cabbage in autumn growing season. Kor J Plant Pathol. 1995;11:191. (Abst.) [Google Scholar]
- Zhang X, Zhang X, Singh J, Li D, Qua F. Temperature-dependent survival of Turnip crinkle virus-infected Arabidopsis plants relies on an RNA silencing-based defense that requires DCL2, AGO2, and HEN1. J Virol. 2012;12:6847–6854. doi: 10.1128/JVI.00497-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitter TA, Murphy JF. Cucumber mosaic. The Plant Health Instructor. 2009 doi: 10.1094/PHI-I-2009-0518-01. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.