Abstract
Purpose
To evaluate adverse drug reactions (ADRs) experienced by chronic myeloid leukemia (CML) patients during per oral tyrosine kinase inhibitor (TKI) treatment and correlation of ADR symptoms with medication adherence and perceived quality of life (QoL).
Patients and methods
Eighty-six adult, chronic-phase CML patients who had been on TKI treatment (79% on imatinib, 10.5% dasatinib, and 10.5% nilotinib) for at least 6 months participated in the study (mean age: 57.8 years, 52% males). The mean time from diagnosis was 5.1 years. All patients were interviewed, and patient-reported ADRs were obtained using a structured list. Adherence was assessed using Morisky’s 8-item Medication Adherence Scale (MMAS). The symptoms’ interference with patient’s daily QoL was measured by asking patients about the influence of symptom(s) on their mood, general condition, enjoyment of life, walking, relationships, and work.
Results
Ninety-seven percent of the patients were suffering from at least one ADR. The mean number of different symptoms was seven (range: 0–15, median 6). The most commonly perceived ADRs were muscle soreness or cramp (69/86, 80%); swelling of hands, legs, feet, or around the eyes (59/86, 69%); and fatigue (43/86, 50%). No correlation was found between adherence and ADRs, because symptoms were equally common in each MMAS adherence class. Half of the patients felt that the ADRs had a negative influence on their daily QoL. A quarter of the patients reported that ADRs affected either their mood, general condition, or enjoyment of life. The incidence of almost all ADRs was much higher among patients reporting negative influence of ADRs on their daily life compared to total study population (P=0.016).
Conclusion
TKI-related ADRs were common among CML patients irrespective of patient’s adherence level. Patients who reported that ADRs had a negative influence on their daily QoL perceived more ADRs than those who did not experience a negative influence.
Keywords: chronic myeloid leukemia, tyrosine kinase inhibitors, adverse drug reaction, adherence, quality of life, patient-reported outcomes
Introduction
Chronic myeloid leukemia (CML) is a clonal disease of hematopoietic stem cells and is characterized by the presence of the Philadelphia chromosome and its oncogenic product p210 (Bcr-Abl).1 The treatment of CML has dramatically changed over the last decade with the development of targeted therapy tyrosine kinase inhibitors (TKIs). CML patients treated with TKIs have good survival rates: patients treated with imatinib have been shown to have an estimated overall survival rate of 85% after 8-years’ follow-up.2 Patients treated with TKIs need to continue treatment on a daily basis for their entire life to control the disease.3 With current TKI therapies, the average rate of progression is approximately 1% per year.2,4–7 This means that the symptom burden associated with TKI therapy generally has a greater effect on the patients’ daily life than the symptom burden of this progressive disease.8 Several adverse drug reactions (ADRs) related to TKI therapy in CML are common to all TKIs, including myelosuppression, rash, nausea, diarrhea, fatigue, and musculoskeletal pain/arthralgia/myalgia, which occur at varying frequencies depending on the TKI in question.4–6
The need for continuous, potentially lifelong treatment with TKIs requires a high degree of patient perseverance for long-term disease control. The levels of adherence to TKI therapy among patients with CML have been found to be low.9–13 When asked about their poor adherence to imatinib therapy, CML patients report numerous reasons, both unintentional (forgetfulness, prescribing error, drug availability), and intentional (side effects, social events, travel, temporary illness, negative feelings, medication taste).14 Eliasson et al14 have also found that patients who reported intentional reasons for nonadherence had greater symptom severity than patients who reported unintentional reasons. Marin et al10 found significantly lower rates of adherence to imatinib among patients who reported ADRs.
A standardized collection of health-related quality of life (QoL) data and other patient-reported outcomes (PROs) has contributed to a better understanding of overall treatment effectiveness in patients with solid tumors,15,16 but such evidence is lacking in patients with leukemia.17,18 PROs are defined by the FDA as “a measurement of any aspect of a patient’s health status that comes directly from the patient” (ie, without the interpretation of the patient’s responses by a physician or anyone else).19 Documenting QoL and the adverse effects of CML treatments from the patients’ perspective is necessary to evaluate overall treatment effectiveness and the net clinical benefits of newer therapeutic strategies.20 A patient-centered approach to determining the symptoms most relevant to patients with CML is supported by recent findings showing that health-care providers tend to underestimate the intensity of symptoms felt by patients with advanced cancer.21 While the impact of TKIs from the patient’s perspective has been little investigated, PROs could be critical for making more informed treatment decisions, as all TKIs seem to provide similar excellent clinical outcomes.4,6 More attention has been placed on understanding the impact of symptom burden on patient QoL. At the moment, however, validated instruments to measure QoL in CML patients are not widely available or regularly used in clinical research or routine practice.
The aim of this study was to investigate patient-reported ADRs and their influence on adherence and QoL among CML patients on per oral TKI treatment.
Patients and Methods
Patient population
The study period was from June 2012 to September 2013. Eighty-six adult chronic-phase CML patients who had been on TKI treatment for at least 6 months were enrolled from eight secondary and tertiary care hospitals in Finland. All recruited patients gave their written informed consent before participating in the study. The study protocol was approved by HUS Ethics Committee of Medicine, and the ethics committees of the other concerned hospitals.
Patient interviews
All patients were interviewed in person by one of the researchers (MK), using a structured interview form. Each patient’s demographic data were collected during the interview. The interviews were digitally recorded, transcribed verbatim, and the results analyzed.
Adherence
Patient adherence was measured at the beginning of the interview using Morisky’s 8-item Medication Adherence Scale (MMAS)22 validated for Finnish speakers.12 MMAS is a structured questionnaire validated to estimate adherence to treatment and is widely used in chronic diseases.22 The 8-item scale consists of seven questions with “yes” or “no” alternatives and one item (the last one) with a 5-point Likert scale. MMAS evaluates items addressing the circumstances surrounding adherence behavior.22 Each item measures a specific medication-taking behavior and not a determinant of adherence behavior. MMAS scores can range from 0 to 8 and have been classified into three levels of adherence: high adherence (score 8), medium adherence (score 6–7.75), and low adherence (score <6).22 MMAS questions related to intentional adherence and symptoms were separately analyzed.
ADRs
Patient-reported ADRs were assessed during the interview using a structured questionnaire. The questionnaire consisted of a list of 19 CML and TKI treatment-specific symptoms, and six items assessing the symptoms’ interference with the patient’s daily life (QoL). Patients were asked about the ADRs they experienced at the time of the study using a list of symptoms collected from the most common ADRs caused by TKIs in Phase III studies. The interviewer followed the standardized symptom inventory questionnaire, and every symptom was investigated by asking, “After the start of the TKI treatment have you suffered or are you currently suffering from the (mentioned) symptom?” The following alternatives were given: 1) not applicable; 2) has suffered before, but not at the moment (these answers were not included in the analysis); or 3) yes, I am currently suffering from this symptom (included in the analysis). The number of patient-reported ADRs was considered as a “symptom score”, each reported symptom yielding one score (score range: 0–1).
QoL
The patient interview also included six structured questions assessing functional impairment associated with TKI treatment (QoL). QoL was assessed as a negative influence of patient-reported ADRs on six items: mood, general condition, enjoyment of life, walking, relationships, and work in general. Each item scored one point (ie, the patient answered “yes”), leading to a maximum score of 6. This was considered as a QoL measure. If the patient did not report any negative influence on his/her daily QoL, then the score was “0”. The correlation between “QoL score” and “symptom score” was measured.
Statistical analysis
Each patient’s MMAS score was compared with the QoL score and symptom score. The statistical analysis was performed using IBM SPSS Statistics for MAC version 21.0 (IBM Corporation, Armonk, NY, USA). Spearman’s rho test was used to evaluate the difference between adherence, QoL, and ADRs. For statistical analysis, an α-value of 0.05 was considered statistically significant. Descriptive statistics were calculated as frequencies, percentages, means, and medians.
Results
Patients
A total of 120 chronic-phase CML patients were contacted between June 2012 and September 2013 in eight secondary or tertiary care hospitals in Finland. Of these, 86 participated in the study. The mean age was 57.8 years and 52% were male. Of the patients, 79.1% were using imatinib, 10.5% dasatinib, and 10.5% nilotinib. Patient characteristics are shown in Table 1.
Table 1.
Characteristics of the CML patients on TKI medication involved in the study (n=86)
| Variables | n (%) |
|---|---|
| Sex, n (%) | |
| Male | 45 (52.3) |
| Female | 41 (47.7) |
| Agea (years) | |
| Mean (SD) | 57.8 (12.1) |
| Median | 59.0 |
| Range | 25.0–83.0 |
| Age at diagnosis (years) | |
| Mean (SD) | 52.7 (12.3) |
| Median | 52.0 |
| Range | 19.0–79.0 |
| Time from diagnosis (years) | |
| Mean (SD) | 5.1 (3.7) |
| Median | 4.0 |
| Range | 0.5–17.0 |
| TKI medication-related factors | |
| TKI medication, n (%) | |
| Imatinib | 68 (79.1) |
| Dasatinib | 9 (10.5) |
| Nilotinib | 9 (10.5) |
| Line, n (%) | |
| First | 47 (54.7) |
| Second | 25 (29.1) |
| Third | 13 (15.1) |
| Fourth | 1 (1.2) |
| Number of TKI doses per day, n (%) | |
| One | 72 (83.7) |
| Two | 14 (16.3) |
| Comorbidities (n) | |
| Mean | 1.6 |
| Median | 1 |
| Range | 0–8 |
| Other medications (n) | |
| Mean | 2.2 |
| Median | 2 |
| Range | 0–10 |
| Quality of Life Scoreb | |
| Mean | 1 |
| Median | 0 |
| Range | 0–5 |
| Patient-reported ADRs (n) | |
| Mean | 7 |
| Median | 6 |
| Range | 0–15 |
Notes:
At the time of adherence evaluation;
Range 0–6; score 0, best; score 6, worst.
Abbreviations: ADRs, adverse drug reactions; CML, chronic myeloid leukemia; SD, standard deviation; TKI, tyrosine kinase inhibitor.
Patient-reported ADRs and their influence on medication adherence
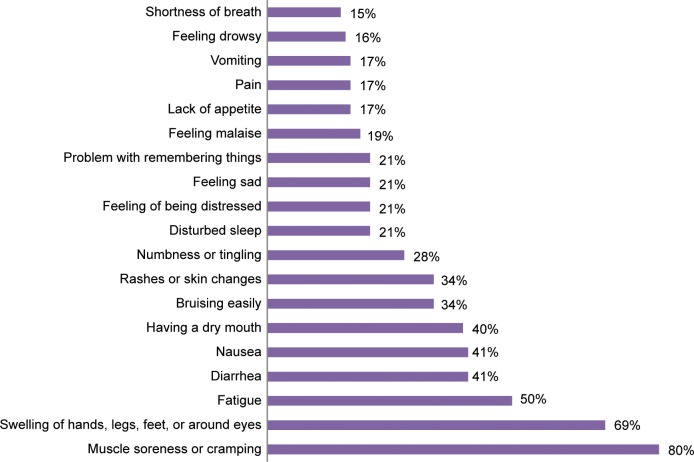
The incidence of patient-reported ADRs was high (Figure 1). At the time of the study, 97% of the patients reported suffering from at least one ADR which had started after the start of TKI treatment. The most commonly experienced ADRs were muscle soreness or cramp (69/86, 80%), swelling of hands, legs, feet, or around the eyes (59/86, 69%), and fatigue (43/86, 50%). There were also differences in ADR profiles between the three different therapies (Table 2). The incidence of different ADRs at different adherence levels is shown in Table 3. No correlation was found between adherence and patient-reported ADRs, because symptoms were equally common in each MMAS adherence class (high, medium, and low).
Figure 1.
Prevalence of each patient-reported ADR in the study population (% of the patients, n=86).
Abbreviation: ADR, adverse drug reaction.
Table 2.
CML patients’ self-reported ADRs with regard to the TKI in use
| ADRs | Total (n=86) n (%) |
Imatinib (n=68) n (%) |
Dasatinib (n=9) n (%) |
Nilotinib (n=9) n (%) |
|---|---|---|---|---|
| Muscle soreness or cramping | 69 (80) | 60 (88) | 3 (33) | 6 (67) |
| Swelling of hands, legs, feet, or around the eyes | 59 (69) | 51 (75) | 4 (44) | 4 (44) |
| Fatigue | 43 (50) | 34 (50) | 3 (33) | 6 (67) |
| Diarrhea | 35 (41) | 34 (50) | 1 (11) | 0 |
| Nausea | 35 (41) | 34 (50) | 1 (11) | 0 |
| Having a dry mouth | 34 (40) | 30 (44) | 3 (33) | 1 (11) |
| Bruising easily | 29 (34) | 24 (35) | 3 (33) | 2 (22) |
| Rashes or skin changes | 29 (34) | 22 (32) | 2 (22) | 5 (56) |
| Numbness or tingling | 24 (28) | 19 (28) | 3 (33) | 2 (22) |
| Disturbed sleep | 18 (21) | 16 (24) | 2 (22) | 0 |
| Feeling of being distressed | 18 (21) | 13 (19) | 3 (33) | 2 (22) |
| Feeling sad | 18 (21) | 14 (21) | 2 (22) | 2 (22) |
| Problem with remembering things | 18 (21) | 13 (19) | 1 (11) | 4 (44) |
| Feeling malaise | 16 (19) | 15 (22) | 0 | 1 (11) |
| Lack of appetite | 15 (17) | 12 (18) | 2 (22) | 1 (11) |
| Pain | 15 (17) | 13 (19) | 0 | 2 (22) |
| Vomiting | 15 (17) | 15 (22) | 0 | 0 |
| Feeling drowsy | 14 (16) | 12 (18) | 2 (22) | 0 |
| Shortness of breath | 13 (15) | 12 (18) | 1 (11) | 0 |
Abbreviations: CML, chronic myeloid leukemia; TKI, tyrosine kinase inhibitor; ADRs, adverse drug reactions.
Table 3.
CML patients’ self-reported ADRs compared to their adherence to TKIs measured by MMAS (n=86)
| ADRs | Total (n=86) n (%) |
MMAS score 8 (n=20) (high adherence) n (%) |
MMAS score 6–7.75 (n=48) (medium adherence) n (%) |
MMAS score <6 (n=18) (low adherence) n (%) |
|---|---|---|---|---|
| Muscle soreness or cramping | 69 (100) | 15 (22) | 39 (57) | 15 (22) |
| Swelling of hands, legs, feet, or around the eyes | 59 (100) | 14 (24) | 35 (59) | 10 (17) |
| Fatigue | 43 (100) | 11 (26) | 19 (44) | 13 (30) |
| Diarrhea | 35 (100) | 10 (29) | 21 (60) | 4 (11) |
| Nausea | 35 (100) | 7 (20) | 21 (60) | 7 (20) |
| Having a dry mouth | 34 (100) | 8 (24) | 20 (59) | 6 (18) |
| Bruising easily | 29 (100) | 9 (31) | 17 (59) | 3 (10) |
| Rashes or skin changes | 29 (100) | 6 (21) | 15 (52) | 8 (28) |
| Numbness or tingling | 24 (100) | 8 (33) | 12 (50) | 4 (17) |
| Disturbed sleep | 18 (100) | 5 (28) | 8 (44) | 5 (28) |
| Feeling of being distressed | 18 (100) | 2 (11) | 13 (72) | 3 (17) |
| Problem with remembering things | 18 (100) | 5 (28) | 11 (61) | 2 (11) |
| Feeling sad | 18 (100) | 3 (17) | 10 (56) | 5 (28) |
| Feeling malaise | 16 (100) | 5 (31) | 7 (44) | 4 (25) |
| Lack of appetite | 15 (100) | 4 (27) | 7 (47) | 4 (27) |
| Pain | 15 (100) | 3 (20) | 9 (60) | 3 (20) |
| Vomiting | 15 (100) | 2 (13) | 12 (80) | 1 (7) |
| Feeling drowsy | 14 (100) | 3 (21) | 7 (50) | 4 (29) |
| Shortness of breath | 13 (100) | 4 (31) | 6 (46) | 3 (23) |
Abbreviations: ADRs, adverse drug reactions; CML, chronic myeloid leukemia; TKI, tyrosine kinase inhibitor; MMAS, Morisky’s 8-item Medication Adherence Scale.
Influence of ADRs on patients’ QoL
More than half of the patients felt that the ADRs had a negative influence on their daily QoL (Table 4). A quarter of the patients reported that the symptoms had a negative influence either on their mood, general condition, or enjoyment of life. Patients who felt that their symptoms negatively affected their QoL suffered from an average of eight different symptoms (range: 3–15, median 8).
Table 4.
The negative influence of ADRs to patients’ quality of life in different adherence levels
| The perceived influence of ADR to QoL variables | MMAS 8 (n=20) % (n) |
MMAS 6–7.75 (n=48) % (n) |
MMAS <6 (n=18) % (n) |
MMAS total (n=86) % (n) |
|---|---|---|---|---|
| Has some influence (total) | 23 (10) | 55 (24) | 23 (10) | 100 (44) |
| Influence in mood | 17 (4) | 63 (15) | 21 (5) | 100 (24) |
| Influence in general condition | 30 (7) | 43 (10) | 23 (5) | 100 (22) |
| Influence in enjoying life | 23 (5) | 55 (12) | 23 (5) | 100 (22) |
| Influence in walking | 30 (3) | 40 (4) | 30 (3) | 100 (10) |
| Influence in relationships | 40 (2) | 40 (2) | 20 (1) | 100 (5) |
| Influence in working | 33 (1) | 33 (1) | 33 (1) | 100 (3) |
Abbreviations: ADR, adverse drug reaction; QoL, quality of life; MMAS, Morisky’s 8-item Medication Adherence Scale.
Compared with the total study population, the incidence of all symptoms other than nausea and vomiting was higher among patients who said that their symptoms negatively affected their daily life than among those who reported no such influence (Table 5). More men reported their symptoms to have a negative influence on their daily life than women (53% vs 44%). More than half of the imatinib users (54%), one-third of the dasatinib users (33%), and one-fifth of the nilotinib users (22%) experienced symptoms that had an unwanted influence on their daily life.
Table 5.
ADRs of the patients who reported that symptoms had negative influence to daily life (n=44) vs ADRs of all patients (n=86)
| ADRs | Negative influence (n=44) % (n) |
All (n=86) % (n) |
|---|---|---|
| Muscle soreness or cramping | 82 (36) | 80 (69) |
| Swelling of hands, legs, feet, or around the eyes | 77 (34) | 69 (59) |
| Fatigue | 59 (26) | 50 (43) |
| Diarrhea | 48 (21) | 41 (35) |
| Nausea | 39 (17) | 41 (35) |
| Having a dry mouth | 43 (19) | 40 (34) |
| Bruising easily | 36 (16) | 34 (29) |
| Rashes or skin changes | 43 (19) | 34 (29) |
| Numbness or tingling | 30 (13) | 28 (24) |
| Disturbed sleep | 30 (13) | 21 (18) |
| Feeling of being distressed | 30 (13) | 21 (18) |
| Feeling sad | 34 (15) | 21 (18) |
| Problem with remembering things | 32 (14) | 21 (18) |
| Feeling malaise | 30 (13) | 19 (16) |
| Lack of appetite | 23 (10) | 17 (15) |
| Pain | 27 (12) | 17 (15) |
| Vomiting | 11 (5) | 17 (15) |
| Feeling drowsy | 20 (9) | 16 (14) |
| Shortness of breath | 20 (9) | 15 (13) |
Abbreviation: ADRs, adverse drug reactions.
Ceased medication or reduced dose
Nine (10%) patients spontaneously reported that ADRs had influenced their medication taking, ie, they had stopped taking the medication or reduced the dose when feeling worse (MMAS Question no 3). These patients also sometimes unintentionally forgot to take their medication. All of them reported that they were suffering from disturbed sleep, 89% (n=8) reported swelling, and 89% (n=8) cramp. Five (56%) of them felt that the symptoms influenced their daily life. Four patients had low adherence and five had medium adherence according to MMAS. Eight of them were using imatinib and one, dasatinib. The knowledge of the disease and its treatment was poor in this patient group,12 except for one patient who scored 4 out of 5 points. Six of these patients scored 0 and two patients scored 1 in the knowledge test.
Intentional nonadherence and patient-reported symptoms
Twenty-six (30%) patients in the study reported intentional nonadherence (ie, not taking medication for some reason other than forgetting). On average, these patients suffered from six different symptoms (range: 0–11, median 7). Intentional nonadherence was more common among women than men (37% vs 24%), and among dasatinib and nilotinib users than imatinib users (44%, 44% vs 26%). Half of the patients taking the medication twice daily reported intentional nonadherence compared with 26% of patients taking the medication on a single daily dose.
Discussion
In this study, the prevalence of patient-reported ADRs during TKI treatment was high and much higher than in clinical trials, which in most cases study the efficacy and safety of treatments. Even though the ADRs did not influence adherence, they had a significant influence on patients’ QoL.
It has been reported in previous studies that symptom burden is cited as a primary reason for poor adherence to TKI therapy, and poor adherence has been linked to unsatisfactory treatment response and increased health-care resource utilization.9,10,14 Only 10% of the patients in the present study spontaneously reported that ADRs had influenced their medication taking, ie, they had stopped taking the medication or reduced the dose when feeling worse. Patients were willing to take the medication even though they were reporting ADRs. We were unable to find a clinical correlation between these symptoms and patient adherence, but there was a significant correlation between higher number of symptoms and a negative impact on the patient’s QoL.
Results from previous randomized controlled trials suggest that treatment decisions influenced by QoL considerations may be beneficial in some patients.23 It has been shown in a previous study that treatment with TKIs generally does not adversely affect – and may even improve – patient QoL.24 As stated by the FDA, some “treatment effects are known only to the patient”, and such information can be lost when the patient’s perspective “is filtered through a clinician’s evaluation of the patient’s response to clinical interview questions”.19 Thus, it is likely that robust QoL evidence in this area will help physicians to make more tailored treatment decisions. In some therapeutic areas, symptom-specific rating scales have been found to be valuable tools for assessing the effects of an intervention on treatment-related symptoms. In general, patients report symptoms earlier and more frequently than clinicians. From these studies, it appears that clinicians may be better at recognizing ADRs with potentially serious consequences, whereas patients may be better at assessing more subtle changes that affect their overall QoL.25
The emergence of treatment-related ADRs, although potentially detrimental to patient QoL, can be effectively managed in most cases because ADRs are mostly mild to moderate in severity and generally consistent (ie, predictable) over time and across lines of therapy. Furthermore, the number of TKIs currently approved increases the likelihood that patients found to be intolerant to one TKI can switch to another, better tolerated alternative.
As CML patients come to expect increasingly longer survival with TKI therapy, the importance of managing symptom burden related to the disease and its treatment will also increase. The complex interplay between symptom burden, adherence, response to TKI therapy, and health-care utilization highlights the need for regular symptom burden assessment in CML as a means to identify potential adherence problems before they affect the patients’ response to TKI treatment. Information on disease and treatment-related effects from the patient’s perspective crucially provides the additional knowledge needed for both patients and physicians to make informed treatment decisions. Many patients do not exhibit disease symptoms at diagnosis and therefore may be irritated by the ADRs caused by the treatment. The ADRs which had the most negative effect on patients’ QoL in the present study were swelling, rashes, disturbed sleep, feeling sad or depressed, a problem with remembering things, and a feeling of malaise. Identifying these symptoms could help in treatment follow-up designed to manage the ADRs and patients to be able to successfully continue their treatment. QoL is necessary to support the proper use of TKI therapy in CML.
Limitations
The present study has some limitations. The instruments used for assessing ADRs and QoL were not validated. At the time this study was started, no validated QoL assessment instruments specific to leukemia or CML were available. Recently, three leukemia- and CML-specific QoL instruments have been validated: the Functional Assessment of Cancer Therapy – Leukemia,15 the M.D. Anderson Symptom Inventory-CML,26,27 and the EORTC QLQ-CML24.28 Further development and validation of leukemia- or CML-specific QoL measurement tools could improve the overall management of CML.
The study was a cross-sectional study (only one interview done). The ADRs were only reported by the patient and not compared with the physician’s assessment. It would be interesting to evaluate the severity of the symptoms in future studies. It may also be argued whether ADRs are the reason for a deterioration in QoL. ADRs may affect QoL, but it may also be argued that persons reporting impaired QoL may be those experiencing ADRs more frequently than others.
Conclusion
TKI-related ADRs were common among CML patients irrespective of patients’ adherence level. Patients who reported that ADRs had a negative influence on their daily QoL perceived more ADRs than those who did not experience a negative influence.
Acknowledgments
We are grateful to all the patients who participated in this study. We thank Dr Kimmo Talvensaari, MD, PhD; Professor Anne Juppo; Perttu Koskenvesa, MD; and Professor Kimmo Porkka for their help in making the study design. We would like to thank Professor Kari Remes; Dr Marjut Kauppila, MD, PhD; Dr Venla Terävä, MD, PhD; Dr Hanna Ollikainen, MD, PhD; Dr Sakari Kakko, MD, PhD; Antti Koponen, MD; Dr Maija Mikkola, MD, PhD; Anu Kutila MD; and Kirsi Launonen, MD for their help in recruiting the patients for the study. Our warm thanks go to study nurses Minna Pajuportti, Raija Aaltonen, Marjut Yrjönen, Ulla Pietilä, Erja Silventoinen, Leena Lahdenmaa, and Riitta Saavalainen for their help with patient contacts.
Footnotes
Disclosure
Meri Kekäle has received a grant from University Pharmacy, Helsinki, for this research. The authors report no other conflicts of interest in this work.
References
- 1.Goldman J, Melo J. Chronic myeloid leukemia – advances in biology and new approaches to treatment. N Engl J Med. 2003;349(15):1451–1464. doi: 10.1056/NEJMra020777. [DOI] [PubMed] [Google Scholar]
- 2.Deininger MW, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow-up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [abstract 1126] Blood. 2009;114 [Google Scholar]
- 3.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the firstline treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 8.Cella D, Nowinski CJ, Frankfurt O. The impact of symptom burden on patient quality of life in chronic myeloid leukemia. Oncology. 2014;87:133–147. doi: 10.1159/000362816. [DOI] [PubMed] [Google Scholar]
- 9.Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113:5401–5411. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 10.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117:3733–3736. doi: 10.1182/blood-2010-10-309807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kekäle M, Talvensaari K, Koskenvesa P, Porkka K, Airaksinen M. Chronic myeloid leukemia patients’ adherence to per oral tyrosine kinase inhibitors compared with adherence as estimated by their physicians. Patient Prefer Adherence. 2014;8:1619–1627. doi: 10.2147/PPA.S70712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yood MU, Oliveria SA, Cziraky M, et al. Adherence to treatment with second-line therapies, dasatinib and nilotinib, in patients with chronic myeloid leukemia. Curr Med Res Opin. 2012;28(2):213–219. doi: 10.1185/03007995.2011.649849. [DOI] [PubMed] [Google Scholar]
- 14.Eliasson L, Clifford S, Barber N, Marin D. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res. 2011;35:626–630. doi: 10.1016/j.leukres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Trask PC, Cella D, Powell C, et al. Health related quality of life in chronic myeloid leukemia. Leuk Res. 2013;37:9–13. doi: 10.1016/j.leukres.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Phillips KM, Pinilla-Ibarz J, Sotomayor E, et al. Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a controlled comparison. Support Care Cancer. 2013;21(4):1097–1103. doi: 10.1007/s00520-012-1630-5. [DOI] [PubMed] [Google Scholar]
- 17.Efficace F, Breccia M, Saussele S, et al. Which health-related quality of life aspects are important to patients with chronic myeloid leukemia receiving targeted therapies and to health care professionals? GIMEMA and EORTC quality of life group. Ann Hematol. 2012;91:1371–1381. doi: 10.1007/s00277-012-1458-6. [DOI] [PubMed] [Google Scholar]
- 18.Aziz Z, Iqbal J, Aaqib M, et al. Assessment of quality of life with imatinib mesylate as first-line treatment in chronic phase-chronic myeloid leukemia. Leuk Lymphoma. 2011;52:1017–1023. doi: 10.3109/10428194.2011.560310. [DOI] [PubMed] [Google Scholar]
- 19.US Food Drug Administration: Guidance for Industry . Patient-reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Washington, DC: U.S. Department of Health and Human Services, Food and Drug Administration; 2009. [Google Scholar]
- 20.Efficace F, Cocks K, Breccia M, et al. Time for a new era in the evaluation of targeted therapies for patients with chronic myeloid leukemia: inclusion of quality of life and other patient-reported outcomes. Crit Rev Oncol Hematol. 2012;81:123–135. doi: 10.1016/j.critrevonc.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Laugsand EA, Sprangers MA, Bjordal K, et al. Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes. 2010;8:104. doi: 10.1186/1477-7525-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Guyatt GH, Ferrans CE, Halyard MY, et al. Exploration of the value of health-related quality-of-life information from clinical research and into clinical practice. Mayo Clin Proc. 2007;82:1229–1239. doi: 10.4065/82.10.1229. [DOI] [PubMed] [Google Scholar]
- 24.Hahn EA, Glendenning GA, Sorensen MV, et al. Quality of life in patients with newly diagnosed chronic phase chronic myeloid leukemia on imatinib versus interferon alfa plus low-dose cytarabine: results from the IRIS Study. J Clin Oncol. 2003;21:2138–2146. doi: 10.1200/JCO.2003.12.154. [DOI] [PubMed] [Google Scholar]
- 25.Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101:1624–1632. doi: 10.1093/jnci/djp386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.M.D. Anderson Cancer Center: The MD Anderson Symptom Inventory Chronic Myeloid Leukemia Module (MDASI-CML) [Accessed March 18, 2014]. Available from: http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/mdasi-cml.html.
- 27.Williams L, Garcia Gonzalez A, Ault P, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood. 2013;122(5):641–647. doi: 10.1182/blood-2013-01-477687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efficace F, Baccarani M, Breccia M, et al. International development of an EORTC questionnaire for assessing health-related quality of life in chronic myeloid leukemia patients: the EORTC QLQ-CML24. Qual Life Res. 2014;23(3):825–836. doi: 10.1007/s11136-013-0523-5. [DOI] [PubMed] [Google Scholar]