Abstract
Circadian rhythms are near 24-hour patterns of physiology and behavior that are present independent of external cues including hormones, body temperature, mood, and sleep propensity. The term “circadian misalignment” describes a variety of circumstances, such as inappropriately timed sleep and wake, misalignment of sleep/wake with feeding rhythms, or misaligned central and peripheral rhythms. The predominance of early research focused on misalignment of sleep to the biological night. However, discovery of clock genes and the presence of peripheral circadian oscillators have expanded the definitions of misalignment. Experimental studies conducted in animal models and humans have provided evidence of potential mechanisms that link misalignment to negative outcomes. These include dysregulation of feeding behaviors, changes in appetite stimulating hormones, glucose metabolism and mood. This review has two foci: 1. To describe how circadian misalignment has been defined and evaluated in laboratory and field experiments, 2. To describe evidence linking different types of circadian misalignment to increased risk for physical (cardiovascular disease, diabetes, obesity, cancer) and psychiatric (depression, bipolar, schizophrenia, attention deficit) disorders. This review will describe the role of circadian misalignment as a risk factor for disease in the general population and in clinical populations, including circadian rhythm sleep disorders and psychiatric disorders.
1. Introduction
1.1. General
All organisms, ranging from single cell organisms, plants, sea slugs to humans demonstrate circadian rhythms or near 24 hour patterns which are present independent of environmental cues. Disruption of circadian rhythms, through extrinsic factors such as shift work and intrinsic factors such as circadian disorders has been associated with both physical and psychiatric consequences (Bass, 2012; Lam & Levitan, 2000). The goals of this review are to describe how circadian misalignment has been operationalized and then describe the role of circadian rhythm misalignment in the development of chronic illnesses including cardiovascular disease, diabetes obesity and cancer as well as psychiatric disorders such as mood disorders. This review has 4 main sections. In the first part of the review, we will describe the circadian system and the ways that circadian misalignment has been defined. Next, we will discuss how circadian misalignment has been studied in the laboratory. The third section of the article will review the field studies of populations at risk for circadian misalignment and related physical and psychiatric consequences. These populations include: transient misalignment associated with daylight saving time, chronotype and social jet lag. The final section will focus on circadian misalignment in clinical populations: circadian rhythm sleep disorders, night eating syndrome and psychiatric disorders.
1.2. Search Strategy
In order to prepare this article, the authors searched PubMed and selected articles to provide a broad overview of the field of circadian misalignment, focusing primarily on cardiovascular disease, diabetes, obesity, cancer, depression and bipolar disorder. Our search included the following terms: “circadian rhythm”, “circadian misalignment”, “chronotype”, “social jet lag”, “shift-work”, “jet lag”, “delayed sleep phase”, “advance sleep phase”, “irregular sleep”, “non-24 hour”, “free running”, “phase angle”, “night eating syndrome”, and “seasonal affective disorder”. This article is not a systematic review encompassing all published articles of circadian misalignment, physical and mental health. Although our review includes several important animal studies, this review mostly focuses on human studies.
1.3. Circadian Rhythms
In order to understand the consequences of misalignment, it is first necessary to have a basic understanding of circadian rhythms. The term circadian is derived from Latin to mean “about a day” and refers to the roughly 24 hour rhythm generated by the suprachiasmatic nucleus (SCN), located in the anterior hypothalamus (Schibler & Sassone-Corsi, 2002). Numerous physiological and behavioral processes demonstrate circadian rhythms, including body temperature, hormones such as cortisol and melatonin, as well as behavioral factors such as cognition and mood. The sleep-wake rhythm is one of the most important and observable circadian rhythms. The circadian clock is not exactly 24 hours, and will “free run” when away from external cues (Czeisler et al., 1999). Therefore, these external zeitgeibers or “time givers” such as light, endogenous melatonin and to a lesser extent physical and social activity are important factors for synchronizing the circadian rhythm to the 24-hour day (Baehr et al., 2003; Barger, Wright, Hughes, & Czeisler, 2004; Goel, 2005). The timing of these zeitgeibers is highly important, in that they will have a different effect on the circadian rhythm depending on the time of exposure (Khalsa, Jewett, Cajochen, & Czeisler, 2003; Kripke, Elliott, Youngstedt, & Rex, 2007; Lewy, 2007). For example, morning light will advance (move earlier) whereas evening light will delay (move later) the circadian rhythm (Duffy & Wright, 2005; Strogatz, 1990). Endogenous melatonin, released by the pineal gland under conditions of darkness, suppresses the signal of the SCN (Moore, 1996). Exogenous melatonin also shifts circadian phase, based on time of administration, but in the opposite direction to light exposure (Burgess, Revell, Molina, & Eastman, 2010; Lewy, Ahmed, Jackson, & Sack, 1992).
Circadian rhythms are coordinated by the SCN or “master clock”, however the molecular mechanism of the clock is present in every cell of the body. Circadian or clock genes (in humans- e.g. CLOCK, CRY, PER, BMAL) comprise an autoregulatory transcriptional-translational feedback loop which demonstrates a cycle every 24 hours (Hardin, Hall, & Rosbash, 1990; Loros & Dunlap, 1991). In addition to individual cells, rhythms are also generated among organs including the heart, stomach, liver and pancreas (Dibner, Schibler, & Albrecht, 2010). Circadian patterns are also present among physiological systems, including the cardiovascular and renal systems (Dibner et al., 2010; Gachon, Nagoshi, Brown, Ripperger, & Schibler, 2004). These peripheral rhythms have a unique phase relationship with the master clock which is coordinated through neuronal pathways, neuropeptides and hormones. Several excellent reviews have been published on this topic (Bass, 2012; Brown & Azzi, 2013; Zelinski, Deibel, & McDonald, 2014).
1.4. Circadian Misalignment
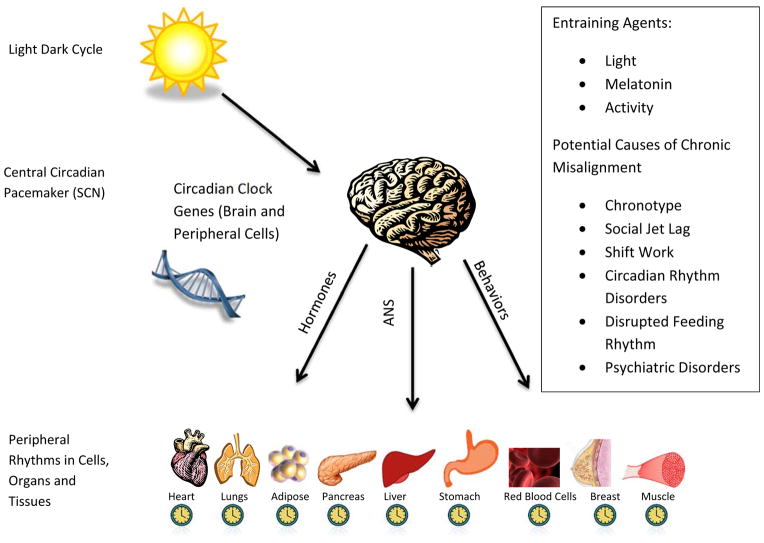
The term circadian misalignment can describe a variety of circumstances both in the laboratory and natural environment. By the Oxford Dictionary, misalignment refers to “the incorrect arrangement or position of something in relation to something else”. One of the most common types of misalignment studied is misalignment of the sleep-wake cycle in relation to the biological night. Other types of misalignment include misalignment of feeding rhythms to the sleep-wake or light-dark cycle, or internal misalignment of central and peripheral rhythms. Figure 1. presents a schematic depiction of the organization of the central and peripheral circadian rhythms. This diagram includes zeitbeibers (light, melatonin and activity) as well as the potential causes of chronic circadian misalignment. The timing of melatonin onset under dim light conditions (dim light melatonin onset or DLMO) and the core body temperature minimum are often used as markers of circadian timing (Benloucif et al., 2008). The timing of these circadian markers in relation to sleep-wake behaviors is commonly referred to as phase angle, and has also been used as a measure of circadian alignment (Figure 2). For example, the duration between circadian markers (DLMO or core body temperature minimum) and sleep timing (onset, midpoint or wake time) has been evaluated. Individuals with evening chronotype (preference for later timing of sleep and activity) have been shown in some studies to have a shorter phase angle between circadian markers and sleep, indicating that they sleep and wake earlier in their circadian phase (Duffy, Rimmer, & Czeisler, 2001; Mongrain, Carrier, & Dumont, 2006). One of the most significant and immediate consequences of misalignment of the sleep-wake cycle to the biological night is sleep disturbance and/or daytime sleepiness. Insomnia, difficulty waking in the morning and sleepiness caused by circadian misalignment are typical symptoms that lead patients to seek care in sleep clinics for circadian disorders. However, the physiological and psychological consequences are much broader than sleep-wake disturbance. These include changes in feeding patterns, metabolic function and risk for mood disorders. Other types of disruption include internal misalignment of rhythms, such as timing of central vs. peripheral clocks. For example, research in animal models has demonstrated that altering availability of food timing shifts peripheral (e.g. hepatic) but not central rhythms (Stokkan, Yamazaki, Tei, Sakaki, & Menaker, 2001).
Figure 1. Representation of Central and Peripheral Circadian Rhythms.
This figure depicts the relationship between central and peripheral circadian rhythms. Circadian genes are present in every cell in the body. The central circadian rhythm is generated by the suprachiasmatic nucleus. The light/dark cycle is one of the main entraining agents for the central rhythm. Peripheral rhythms are present in cells, organs and organ systems. The coordination between the central and peripheral rhythms is not fully understood but involves hormonal, neurologic and behavioral pathways. Misalignment can occur when the central rhythm is misaligned to the light/dark cycle or when central and peripheral rhythms are misaligned.
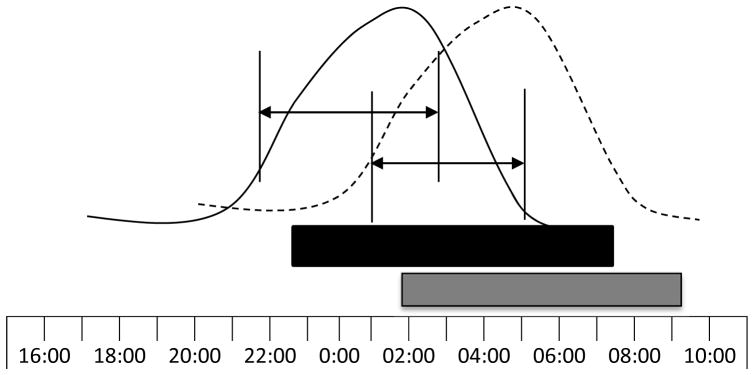
Figure 2. Circadian Phase and Phase Angle between Melatonin and Sleep.
The curved lines depict examples of the nocturnal melatonin rhythm in individuals with normal phase (solid line) and phase delay (dotted line). The bars below indicate the sleep periods in normal phase (black bar) and phase delay (gray bar). Phase angles between dim light melatonin onset (DLMO) and midpoint of sleep are depicted with lines and arrows. Compared with the normal alignment, the phase delayed example demonstrates a later DLMO and a shorter duration or phase angle between DLMO with sleep onset and midpoint of the sleep period.
2. Mechanisms
2.2 Laboratory Methods
In order to more precisely study the mechanisms linking circadian misalignment to physiological and psychiatric conditions, researchers have developed protocols to isolate the circadian rhythm from the sleep-wake cycle. Multiple studies have used temporary phase shifts to study the effect of misalignment and demonstrate changes in leptin rhythm and glucose response to meals (Hampton et al., 1996; Schoeller, Cella, Sinha, & Caro, 1997) as well as increased depressed mood (Surridge-David, MacLean, Coulter, & Knowles, 1987). However, these approaches are limited due to the associated sleep loss. One of the most commonly reported techniques is forced desynchrony (Czeisler et al., 1986; Dijk, Duffy, & Czeisler, 1992; Kleitman & Kleitman, 1953). In this protocol, individuals are scheduled for a “day” that is shorter or longer than 24 hours (typically 20 or 28 hours), and then sleep and meal timing is shifted either earlier or later around the clock. The circadian timing system cannot adjust to this “day” length and thus free runs at its own endogenous period, which is slightly longer than 24 hours in humans. This technique allows researchers to estimate sleep, mood, and performance at various degrees of circadian misalignment. These studies have also demonstrated that circadian misalignment causes disruptions in appetite regulating hormones, glucose metabolism and mood.
2.3 Laboratory Studies with Misalignment of the Sleep-Wake Rhythm
In a forced desynchrony protocol to simulate the experience of living on mars, individuals were assigned to a 24 hour day or a 24.6 hour day for 25 days. Interestingly, some individuals were capable of entraining their circadian rhythms and others were not (Gronfier, Wright, Kronauer, & Czeisler, 2007; Wright, Hughes, Kronauer, Dijk, & Czeisler, 2001). Results indicated that those who were non-entrained had poorer sleep quality, shorter sleep duration, and had lower leptin during wakefulness. Results from a later study also demonstrated decreased leptin and higher glucose response to meals despite higher insulin production associated with this misalignment (Scheer, Hilton, Mantzoros, & Shea, 2009). Most recently, a study compared 3 days of phase advance to three days of phase delay (21 hour day versus a 27 hour day), with a 4 week wash out period in between the two conditions (Gonnissen, Hursel, Rutters, Martens, & Westerterp-Plantenga, 2012). Results demonstrated that in the phase delay condition, total sleep time was related to levels of insulin and insulin resistance. In a later analysis, it was determined that specifically, decreased REM in the second half of the night was predictive of changes in cortisol, insulin and insulin resistance (Gonnissen et al., 2013). Since REM is modulated by the circadian clock, this suggests a role of circadian misalignment in the metabolic changes reported in this study.
The circadian rhythm of mood has been demonstrated in multiple studies (Wirz-Justice, 2008). These daily variations in mood have been studied in forced desynchrony and have been demonstrated to have a significant circadian pattern as well as an interaction with prior wakefulness, in that the trough of mood was lowest around the time of the core body temperature minimum and also deteriorated over the wake period (Boivin et al., 1997). This relationship has been demonstrated among healthy controls as well as individuals with seasonal affective disorder (Koorengevel, Beersma, den Boer, & van den Hoofdakker, 2003).
2.5. Laboratory Studies of Misalignment of Feeding Rhythms
Multiple experiments in animal models have demonstrated the effects of mistimed feeding on sleep, rhythms and cardiometabolic disease risk (Arble, Bass, Laposky, Vitaterna, & Turek, 2009; Fonken et al., 2010). Kohsaha and colleagues (2007) demonstrated that high fat feeding disrupts the circadian timing of mice, giving them a longer circadian period (greater than 24 hours) and also increased fragmentation of their sleep and feeding rhythms. Another study highlighted the possible deleterious effects of eating during the sleep period. Mice fed during the light period (biological night) consumed the same number of calories but gained twice as much weight as mice fed during the dark period (Arble et al., 2009). Further studies have demonstrated that light and feeding period also play a role. Fonken and colleagues (2010) demonstrated weight gain in mice kept in constant bright light compared with those kept in constant dim light and standard 12 hour light dark cycle. However, the effects of bright light were ameliorated when food was restricted only to the dark phase (the typical feeding time). Another study demonstrated that feeding mice high fat diets only during the dark phase prevented the development of metabolic syndrome (Hatori et al., 2012). At this point, only one study in humans has evaluated the metabolic effects of meal timing in an experimental design (Jakubowicz, Barnea, Wainstein, & Froy, 2013). In this study, women with polysyctic ovarian syndrome were assigned to two isocaloric weight loss diets for 12 weeks. Both diets were approximately 1400 kcal per day. One diet contained a large breakfast (700 kcal) moderate lunch (500 kcal) and small dinner (200 kcal). The other contained a small breakfast (200 kcal), moderate lunch (500 kcal) and large dinner (700 kcal). Results indicated that participants in the high calorie breakfast group demonstrated greater insulin sensitivity following the intervention. This study demonstrates that changing the timing of caloric intake has the potential to influence metabolic processes in humans. However, this study did not evaluate circadian alignment.
In summary, the effects of misalignment of sleep-wake patterns as well as misalignment of feeding with the light/dark cycles have been tested in experimental protocols in human and animal models. Results suggest potential mechanisms that may link misalignment to poor health are broad and include changes in mood, behavior, appetite regulating hormones and glucose metabolism.
3. Circadian Misalignment in Naturalistic Settings
Circadian misalignment occurs in many different “real world” settings. Some types of misalignment are transient, such as daylight saving time or time zone travel. Other types of misalignment are chronic, such as individuals’ preference for sleep-wake timing (i.e. chronotype) the change in schedule between weekend and weekdays (social jet lag) or circadian rhythm sleep disorders. We will first discuss misalignment that affects the population as a whole, including daylight saving time, chronotype and social jet lag. The final section of the paper will discuss clinical populations, including circadian rhythm sleep disorders, night eating syndrome and psychiatric disorders. Although shift work and jet lag do not always lead to clinical disorders, they will be discussed in the clinical disorders section.
3.1 Circadian Misalignment in Daylight Saving Time
Daylight saving time is a naturalistic experiment that allows researchers to observe a transient period of circadian misalignment as well as changes in sleep duration at the population level (Kantermann, Juda, Merrow, & Roenneberg, 2007). Multiple studies have demonstrated a small-to-modest increase in myocardial infarction risk after the one hour phase advance in the spring, with the greatest increase in risk during the first day or two after the shift. (Janszky et al., 2012; Janszky & Ljung, 2008). This relationship has also been documented in stroke (Foerch, Korf, Steinmetz, & Sitzer, 2008). Fewer studies have focused on the psychiatric effects of daylight saving time. There is a well-established link between spring DST with sleep loss and daytime sleepiness (Harrison, 2013; Lahti, Leppamaki, Lonnqvist, & Partonen, 2008). However, existing studies have found no relationship between psychiatric disturbance and DST. Two studies found no association between daylight saving time transition and incidence of manic episodes (Lahti, Haukka, Lonnqvist, & Partonen, 2008) or in psychiatric service utilization, including outpatient visits, inpatient admissions, or suicide attempts (Shapiro, Blake, Fossey, & Adams, 1990).
3.2. Chronotype and Social Jet Lag
Chronotype, or preference for timing of sleep and wake behaviors, is theorized to contribute to circadian misalignment in the population. Large epidemiologic surveys demonstrate that chronotype is a characteristic with a normal bell-shaped distribution that varies by age and gender, in that the young and old demonstrate earlier chronotype and adolescents and young adults demonstrate a later chronotype (Roenneberg et al., 2007). Additionally, women report slightly earlier chronotype than men across the lifespan. Chronotype can be evaluated as a continuous variable with morningness and eveningness being two sides of the same dimension. Someone can report “greater eveningness” or “less morningness” depending on the perspective of the researcher and the particular questionnaire. Additionally, individuals may be grouped at cut points into “morning types”, “neither morning nor evening” and “evening types”. Being either a late or early chronotype may cause misalignment because the individual’s preferences for sleep and activity are at odds with the typical workweek or social schedule. Chronotype is typically measured by questionnaires including the Morning Eveningness Questionnaire (Horne & Ostberg, 1976), the Munich Chronotype Questionnaire (Zavada, Gordijn, Beersma, Daan, & Roenneberg, 2005) or the Composite Scale of Morningness (Smith, Reilly, & Midkiff, 1989). The ease of self-report questionnaires compared to the measurement of objective circadian markers may explain why there are so many articles published about chronotype. However, there is evidence that chronotype is associated with timing of melatonin and core body temperature (Baehr, Revelle, & Eastman, 2000; Folkard, Monk, & Lobban, 1979; Waterhouse et al., 2001). There is some evidence of misalignment associated with chronotype, in that that those with an evening type preference (i.e. prefer to go to bed late and wake up late), sleep earlier in their biological rhythm, wake closer to core body temperature minimum (Duffy et al., 2001; Kerkhof & Lancel, 1991). Mongrain and colleagues (2006) also reported compared with morning types, evening types demonstrated a phase delay as well as a shorter phase angle between DLMO and sleep among some but not all evening types. Being an evening chronotype may predispose individuals to shorter sleep duration during workdays, due to the need to conform to typical work hours (Roenneberg et al., 2007). However, evening chronotypes also report longer sleep on free days, in order to compensate for shorter weekday sleep duration. The majority of studies of chronotype account for sleep duration in statistical models. However, few studies of chronotype and health measure circadian markers or phase angles,.
There are only a handful of studies evaluating chronotype and risk for cardiovascular disease and diabetes. This is possibly because circadian timing and alignment has more recently become of interest to researchers studying physical health. One of the earliest studies demonstrated in a cross sectional study that evening types were 2.5 times more likely to report their general health was poor or fair compared with morning types (Paine, Gander, & Travier, 2006). The study by Paine and colleagues did not control for sleep duration, however the majority of later studies include a measure of self-reported sleep duration in statistical models. In one of the only large epidemiologic studies of chronotype and objectively measured health outcomes, Merikanto and colleagues (2013) demonstrated in a cross-sectional study a 2.5 fold increase in type II diabetes prevalence among evening types and a 1.3 fold increase in hypertension prevalence. In another investigation, they reported greater risk for asthma among evening types (Merikanto et al., 2014). Reutrakul and colleagues (2013) demonstrated that later chronotype was associated with poorer glycemic control in a sample of patients with type II diabetes, independent of sleep duration and quality. Longitudinal research is needed to determine if chronotype is related to increased incidence of cardiovascular disease and diabetes over time.
Several studies have demonstrated greater risk for obesity in evening chronotypes (Baron, Reid, Kern, & Zee, 2011; Lucassen et al., 2013). Evening chronotype was associated with greater body fat among a sample of participants with bipolar disorder (Soreca, Fagiolini, Frank, Goodpaster, & Kupfer, 2009). One study reported higher BMI associated with later DLMO and shorter phase angle between DLMO and sleep among depressed peri or postmenopausal women (Meliska et al., 2011). This is the only study that draws a direct link between misalignment and increased risk of obesity among evening chronotypes.
Lifestyle factors are also suggested to play a role in this increased risk of obesity among evening chronotypes. Monk and colleagues (2004) reported morning types exhibit more lifestyle regularity compared with evening types. Multiple studies have reported larger dinner, poorer diet quality, and more calories consumed in the evening (Fleig & Randler, 2009; Sato-Mito, Shibata, Sasaki, & Sato, 2011). Greater eveningness was associated with higher scores on a binge eating disorder questionnaire (Harb et al., 2012). Evening chronotype has also been associated with smoking, alcohol use and lower physical activity (Kabrita, Hajjar-Muca, & Duffy, 2014; Urban, Magyarodi, & Rigo, 2011).
To date, there is only one study published evaluating chronotype and misalignment and cancer. In a recent study, breast cancer survivors had a shorter disease free interval in metastatic breast cancer if bedtimes were “misaligned”(Hahm et al., 2014) as well as in individuals with later chronotype. Both chronotype and misalignment were independent predictors of disease free interval. This study defined misalignment as a self-reported bedtime earlier or later than the preferred bedtime. Further research is needed to determine the role of chronotype in cancer incidence, disease progression and treatment outcomes.
3.3. Social Jet Lag
“Social jet lag” is another proposed cause of circadian misalignment in the general population (Wittmann, Dinich, Merrow, & Roenneberg, 2006). It is defined as the shift in schedule between workdays and free days, in that most individuals use an alarm clock to rise earlier on workdays/school days and sleep later on the weekends or days off from work. In a sense, social jetlag is a measure of misalignment between the individual’s schedule for work and their internal schedule. Epidemiologic research has demonstrated that most individuals have shifts in their sleep between workdays and free days (Roenneberg et al., 2007; Roenneberg, Wirz-Justice, & Merrow, 2003). Evening chronotypes are prone to larger social jet lag due to the need to conform to a conventional work schedule. In an analysis of a large database of 65,000 participants, social jetlag was associated with higher BMI above and beyond sleep duration and chronotype only for overweight individuals (Roenneberg, Allebrandt, Merrow, & Vetter, 2012). There was no relationship among normal weight individuals. Social jet lag has also been associated with smoking, alcohol consumption and caffeine consumption (Wittmann, Paulus, & Roenneberg, 2010).
3.4. Chronotype, Social Jet Lag and Psychiatric Disorders
There is a large literature demonstrating a greater risk of depression and higher depressive symptoms among evening chronotypes. Multiple studies in high school, college and medical school students as well as the general adult population demonstrate a positive association between evening chronotype and depressive symptoms (Chelminski, Ferraro, Petros, & Plaud, 1999; Hirata et al., 2007; Kim et al., 2010; Kitamura et al., 2010; Merikanto, Lahti, Kronholm, et al., 2013). Individuals diagnosed with major depressive disorder report greater eveningness than healthy controls (Drennan, Klauber, Kripke, & Goyette, 1991) and later chronotype is an indicator of greater depression severity among individuals diagnosed with major depressive disorder (Gaspar-Barba et al., 2009). One study demonstrated that social jet lag is associated with depressive symptoms above and beyond chronotype and sleep duration, suggesting that the chronic misalignment from weekdays to weekends is an additional risk factor (Levandovski et al., 2011).
Chronotype has also been linked to greater risk for other psychiatric disorders or greater severity of psychiatric symptoms including bipolar disorder and attention deficit hyperactivity disorder (ADHD). Multiple studies demonstrate greater evening type preference among individuals with bipolar disorder (both I and II) compared with healthy controls or individuals with schizophrenia or schizoaffective disorders. (Ahn et al., 2008; Chung et al., 2012; Giglio et al., 2010; Mansour et al., 2005; Wood et al., 2009). There is one study demonstrating that sleep timing and daytime sleepiness were significant predictors of symptom severity among individuals with ADHD, above and beyond sleep duration (Gamble, May, Besing, Tankersly, & Fargason, 2013). A limitation of this study is that it did not have a measure of preferred sleep timing or circadian markers, and therefore it is unknown the degree to which misalignment was associated with symptoms.
In summary, having an evening chronotype is associated with misalignment of the preferred sleep timing to external demands. Some studies have linked evening chronotype to misalignment of the sleep-wake period relative to circadian markers but few studies of diseae risk measure objective circadian markers. Chronotype has been linked to greater psychiatric symptoms (particularly depression) and poorer health behaviors in many studies. More recent studies are beginning to reveal the relationship between chronotype and risk for cardiovascular disease and diabetes. At this time, mechanisms by which chronotype affects health outcomes are poorly understood. Misalignment is hypothesized to play a role, but few studies include subjective or objective measures of circadian misalignment. Studies have demonstrated that clock genes, most notably PER3 polymorphisms, may contribute to the determination of chronotype through affecting sleep-wake regulation and intrinsic circadian period (Dijk & Archer, 2010). There has also been a relationship reported between some of the clock genes and psychiatric disorders (Bersani, Iannitelli, Pacitti, & Bersani, 2012; Etain, Milhiet, Bellivier, & Leboyer, 2011; Mansour et al., 2009; McCarthy & Welsh, 2012; Takahashi, Hong, Ko, & McDearmon, 2008). For example, alteration in circadian clock proteins in the circadian pacemaker is associated with mania-like behavior in mice (Roybal et al., 2007). In addition, in a few human genetic studies, there is evidence for weak associations between circadian gene polymorphisms and mood disorders (Takahashi et al., 2008). For example, a polymorphism in NPAS2 has been associated with seasonal affective disorder (Johansson et al., 2003), and PER3, BMAL1, CLOCK have been associated with bipolar disorder (Benedetti et al., 2008; Benedetti et al., 2003; Desan et al., 2000; Nievergelt et al., 2006). However, other circadian clock genes such as CLOCK and PER2 have shown no link with affective disorders or CRY1 with bipolar disorders (Bailer et al., 2005; Desan et al., 2000; Nievergelt et al., 2005). Examination of molecular changes in evening-types with and without psychiatric diagnosis may be extremely useful in elucidating the role of the circadian clock in mental health. These data suggest a shared genetic vulnerability for evening chronotypes to experience circadian phase delay, chronic misalignment and increased risk of psychiatric and medical disorders.
4. Misalignment in Clinical Populations
4.1 Circadian Rhythm Sleep Disorders (CRSDs)
Circadian rhythm disturbances are categorized by the International Classification of Sleep Disorders Second Edition (International Classification of Sleep Disorders, Second Edition: Diagnostic and Coding Manual, 2005) into extrinsic and intrinsic disorders. Extrinsic CRSDs include disorders with the internal rhythm is disrupted due to changes in the external environment, such as shift work and jet lag. Intrinsic CRSDs are defined as disruption in sleep and/or daytime function due to alteration in the internal circadian timing system, and include delayed sleep phase disorder, advanced sleep phase disorder, non-24 hour sleep-wake disorder, and irregular circadian rhythm sleep disorder. The following section will describe associations between circadian rhythm sleep disorders with physical and psychiatric disorders.
4.1.1 Shift work and Shift work Sleep Disorder
Shift work has become more common in the last century with at least 20% of the work force employed in a position requiring a shift work schedule. Although most shift workers experience circadian disruption and sleep curtailment, not all shift workers have the circadian rhythm sleep disorder shift work sleep disorder (SWD). SWD is characterized by complaints of insomnia and excessive sleepiness that are temporarily associated with a work schedule that occurs during the usual sleep period. Shift work is typically defined as work outside the hours of about 7am – 6pm, or in the case of the CRSD shift work sleep disorder, a work schedule that overlaps with the timing of the usual primary sleep period (.i.e. at least 50% of the work period is between 10pm –6am). Both night work and early morning start times (before 6am) are associated with the most sleep curtailment, typically anywhere between 1–4 hours less sleep per day compared to non-shift workers (Akerstedt, 1995; Knauth et al., 1980). It is estimated that, 10% of night and rotating shift workers and that 1% of the population meet criteria for SWD (Drake, Roehrs, Richardson, Walsh, & Roth, 2004). Those with SWD have shorter sleep duration, worse sleep quality and performance on memory tasks, greater prevalence of gastric ulcers and depressive symptoms than shift workers without SWD (Drake et al., 2004; Gumenyuk, Howard, Roth, Korzyukov, & Drake, 2014; Gumenyuk et al., 2010).
Much of the research on shift work and health has focused on the effects of shift work itself, rather than SWD. There is evidence to suggest that shift work is associated with a greater incidence of cardiovascular disease and risk factors, diabetes, obesity, cancer, triglycerides (Esquirol et al., 2011) and poor reproductive health (Labyak, Lava, Turek, & Zee, 2002; Nurminen, 1998). The cause of these poor health outcomes in shift workers is thought to be due to a complex combination of circadian misalignment, chronic sleep loss, increased light exposure at night, altered feeding patterns, restricted access to healthy foods, increases in smoking and other poor health behaviors.
The role of shift work as a risk factor for cardiovascular disease is controversial (Esquirol et al., 2011), some studies suggest an association but others do not. There is a similar case with diabetes risk. Further studies are required to definitively determine the role of shift work in these disorders. Although, the overall the consensus is that there is likely to be a negative impact of continuous circadian misalignment and sleep loss on health.
Shift work has been identified as a risk factor for cancer, particularly among women, although there is a clear need for further research using objective prospective exposure measures (Ijaz et al., 2013). Several studies, including the Nurses’ Health Study, report increased risk of cancer among shift workers including breast cancer (Schernhammer et al., 2001) and colorectal cancer (Schernhammer et al., 2003). The mechanism proposed in these studies is increased exposure to light at night resulting reduced levels of endogenous melatonin. More recently, a study from Canada with a diverse population of shift workers supports the increased risk of breast cancer in shift workers (Grundy et al., 2013). The data in this area is sufficient enough such that in 2007 the International Agency for Research on Cancer (the cancer arm of the World Health Organization) listed shift work as a possible carcinogen.
In addition to the impact on physical health outcomes, there is also evidence that shift work affects emotional well-being, such as increased divorce rates, social isolation (Barnes, 2010; Drake et al., 2004) and poor mood (Perry-Jenkins, Goldberg, Pierce, & Sayer, 2007).
4.1.2. Jet Lag and Jet Lag Disorder
Jet lag results from a misalignment between the internal circadian clock and the external environment as a result of rapid travel across multiple time zones. Similar to shift work, not all travelers will experience jet lag or meet the criteria for the CRSD, jet lag disorder. Symptoms of this transitory circadian rhythm sleep disorder range from difficulty sleeping, excessive daytime sleepiness, general malaise, impaired daytime function, and gastrointestinal upset (Boulos et al., 1995). Specific ICSD criteria include; a complaint of insomnia or excessive daytime sleepiness associated with jet travel across at least two time zones and associated impairment in daytime function, general malaise, or somatic symptoms such as gastrointestinal disturbance occurring within 1–2 days after travel (International Classification of Sleep Disorders, Second Edition: Diagnostic and Coding Manual, 2005). The ICSD criteria do not require a minimum number of jet lag occasions or a specific duration of symptoms, rather individuals who suffer from jet lag disorder will seek medical advice for the condition, if it is problematic. This is likely to be the case for individual who travel across multiple time zones on a consistent basis, although no prevalence data for jet lag disorder are available. The degree of sleep disruption and sleepiness associated with travel is variable, depending for example on the number of time zones crossed, direction of travel (east or west) and age. The functional impairment associated with jet lag disorder results from a combination of circadian misalignment and the associated sleep loss (for a review, see Reid, 2011). Currently, there are no published studies of the long term impact of frequent or chronic jet lag for cardiovascular disease, diabetes, obesity or mental health disorders.
4.1.3. Delayed sleep phase disorder (DSPD)
DSPD is an intrinsic CRSD characterized by sleep onset insomnia and/or morning sleepiness due to a 3–6 hour delay in the sleep-wake cycle. Individuals with DSPD typically report an evening chronotype, but the main distinction is that DSPD is characterized by sleep-wake dysfunction and significant distress as a result of these consequences (ICSD-2). It is estimated to be one of the most prevalent circadian rhythm disorders. Prevalence of DSPD has been reported between 0.2% and 10% depending on the population surveyed and criteria used for diagnosis (Regestein & Monk, 1995; Schrader, Bovim, & Sand, 1993; Weitzman et al., 1981). Misalignment between the biological timing and sleep-wake timing has been proposed as one of the mechanisms that causes the sleep-wake dysfunction in DSPD. Many studies have documented that sleep-wake timing and circadian markers are delayed in this population relative to healthy controls (Chang, Reid, Gourineni, & Zee, 2009; Ozaki, Uchiyama, Shirakawa, & Okawa, 1996; Shibui, Uchiyama, & Okawa, 1999; Uchiyama, Okawa, Shibui, Kim, et al., 2000). Some but not all studies have documented misalignment between core body temperature minimum and sleep compared with healthy controls (Uchiyama, Okawa, Shibui, Kim, et al., 2000; Uchiyama, Okawa, Shibui, Liu, et al., 2000). However, Chang and colleagues from our laboratory (2009) did not observe a difference in DLMO or core body temperature minimum and sleep timing among DSPD compared to healthy controls. Therefore, the extent to which misalignment is present and explains the health risks in DSPD is not completely understood at this time.
There have been only a few investigations of the effects of having DSPD on health, and majority has focused on obesity and health behaviors. There are no studies of cardiovascular disease diabetesor cancer. There is one case control study that reported higher BMI among DSPD versus control participants (33 vs. 30 kg/m2; Kripke et al., 2008). This study also reported the DSPD participants had greater medication use, particularly antacids and hypnotics. Several studies have observed poorer health behaviors among individuals with DSPD compared to controls. Among Norwegian high school students, a probable diagnosis of DSPD (difficulty falling asleep before 2 am) was associated with smoking and alcohol use (Saxvig, Pallesen, Wilhelmsen-Langeland, Molde, & Bjorvatn, 2012). Another study conducted in Australia used more stringent criteria to evaluate DSPD (objective rest/activity pattern monitoring via wrist actigraphy and clinical interview) and found that half of the sample of high school students met one of the criteria for DSPD but only 1.1% met the full ICSD-2 criteria (Lovato, Gradisar, Short, Dohnt, & Micic, 2013). In this study, there was higher caffeine and alcohol use and less sports participation among adolescents who met criteria for DSPD.
Multiple studies demonstrate higher depressive symptoms and greater prevalence of depressive disorders among individuals with DSPD. Even among those who meet criteria for DSPD, there is a relationship between later chronotype and depression scores (Abe et al., 2011). Among those diagnosed with DSPD, 64% had clinically significant depressive symptoms (Abe et al., 2011). Seasonal affective disorder is also reported to be higher among individuals with DSPD compared to controls (Lee, Rex, Nievergelt, Kelsoe, & Kripke, 2011). A study from our group compared prevalence of DSM-IV axis I disorders among individuals with evening chronotype and delayed sleep phase disorder (Reid et al., 2012). Results demonstrated no difference in rates of depressive disorders, either current or lifetime between evening chronotype and DSPD, The diagnosis of DSPD did not increase risk for depression above and beyond evening chronotype.
DSPD is also reported to be more prevalent among individuals with bipolar disorder, compared to unipolar depression and healthy controls (Robillard et al., 2013). There is one study that evaluated the sleep and circadian parameters of individuals with ADHD comorbid with DSPD. Compared with controls, individuals with DSPD demonstrated a more variable bedtime, shorter sleep duration on week days and a longer duration between dim light melatonin onset and sleep onset than control participants, suggesting they tended to sleep later in their circadian phase (Bijlenga et al., 2013).
4.1.4. Advanced Sleep Phase Disorder
There is much less known about the physical and emotional consequences of Advanced Sleep Phase Disorder (ASPD). This is likely due in part to lower prevalence of ASPD. It is estimated that the prevalence of ASPD is 1% of the general population (Ando, Kripke, & Ancoli-Israel, 2002). However, this number is likely to be an underestimation because individuals with ASPD may adapt their work and social schedules to accommodate early wake times. ASPD is an intrinsic CRSD where the individual’s sleep-wake timing is significantly earlier than desired (ICSD-2). Individuals often experience sleepiness in the early evening as well as early morning awakenings. Individuals with ASPD have demonstrated earlier timing of biological markers including melatonin (Reid et al., 2001). There are no published studies comparing the physical or psychiatric health of individuals with ASPD to healthy controls or other sleep disorders, therefore it is unknown whether ASPD predisposes individuals to health risk beyond that of sleep loss alone.
4.1.5. Non 24 hour sleep-wake disorder (N24HSWD)
N24HSWD is an intrinsic CRSD in which the individual’s sleep-wake cycle progressively delays each day, rather than entraining to a stable time (International Classification of Sleep Disorders, Second Edition: Diagnostic and Coding Manual, 2005). This leads to periods of insomnia and daytime sleepiness depending on where the individual is in her sleep-wake pattern as well as fragmented sleep if the individual is attempting to sleep when night-time is misaligned with internal timing. In the case of N24SWD, the individual is in a constant and changing degree of misalignment. N24HSWD is more commonly seen among blind people, and is the most common among totally blind, due to the role of the visual system in circadian phase entrainment (Lockley et al., 1997). However, it has been reported among sighted individuals as well (Hayakawa et al., 2005). Although N24HSWD is clearly disruptive to an individual’s social, emotional and occupational functioning, there are few reports of how this disorder affects risk for physical or psychiatric disease. Lockley and colleagues (2008) published a study of 52 blind participants with free-running type and demonstrated no differences overall in mood, but non-entrained individuals rated their mood as more worse when their melatonin peak occurred during their waking hours. Data from a large case series of sighted N24HSWD from Japan (57 participants) reports that psychiatric disorders preceded the onset of N24HSWD among 28% of the sample and 34% of the sample developed major depressive disorder after the onset of N24HSWD (Hayakawa et al., 2005).
4.1.6. Irregular sleep wake type
Irregular sleep wake type is an intrinsic CRSD in which the individual does not demonstrate a clear sleep-wake rhythm (ICSD-2) and has 3 or more sleep periods per 24 hour day. Individuals with this disorder report insomnia, daytime sleepiness and fragmented sleep. Much of the literature about irregular sleep-wake type focuses on sleep in the elderly. This sleep disorder is more common among institutionalized elderly and those with dementia (Hoogendijk et al., 1996; Witting, Kwa, Eikelenboom, Mirmiran, & Swaab, 1990). Decreased circadian rhythmicity is associated with incidence of dementia and mild cognitive impairment (Tranah et al., 2010). Circadian rhythmicity has been demonstrated to be strongly associated with all-cause mortality (Gehrman et al., 2004; Paudel et al., 2010; Tranah et al., 2011) and mortality from cardiovascular disease (Paudel et al., 2011).
4.2. Meal Timing and Pattern
One of the largest literatures on misalignment of eating in humans comes from night eating syndrome (NES), a disorder characterized by evening hyperphagia and nocturnal awakenings in which individuals wake and are unable to return to sleep without eating (Stunkard, Grace, & Wolff, 1955). Individuals with night eating syndrome demonstrate greater calorie intake at night, as well as delayed sleep patterns and delayed melatonin onset, leptin and insulin rhythms (Goel et al., 2009; O’Reardon et al., 2004). The prevalence of NES is higher among individuals who are overweight or obese (Calugi 2009;), bariatric surgery candidates (Adami, Meneghelli, & Scopinaro, 1999; Allison et al., 2008; Rand, Macgregor, & Stunkard, 1997) and individuals with psychiatric disorders (Lundgren et al., 2006). NES is associated with weight gain over time (Marshall, Allison, O’Reardon, Birketvedt, & Stunkard, 2004) and depression, particularly depressed mood in the evening (Birketvedt et al., 1999; Lundgren, Allison, O’Reardon, & Stunkard, 2008). Several, but not all studies have reported poorer weight loss outcomes in individuals with NES participating in behavioral weight loss programs (Gluck, Geliebter, & Satov, 2001; Stunkard et al., 1955). However one recent study reported similar weight loss outcomes in individuals with NES compared to control participants without NES (Dalle Grave, Calugi, Ruocco, & Marchesini, 2011).
There are several studies linking the timing of eating to weight regulation among participants without NES. Our group reported in a sample of healthy, non-depressed adults a correlation between calories consumed after 8:00 pm and BMI, which was independent of sleep timing and duration (Baron et al., 2011). Lucassen and colleagues (2013) reported in a sample of overweight individuals with short sleep duration, later chronotypes ate later and had higher BMIs. Two recent studies have highlighted the role of meal timing in weight loss. Garaulet and colleagues (2013) reported greater weight loss in participants enrolled in a weight loss program who ate their main meal before 3:00 pm. This study was conducted in Spain, where the main meal is lunch. Another study from this group reported greater weight loss outcomes associated with a more robust amplitude of their circadian rhythms and less fragmentation among obese women undergoing a weight loss program (Bandin, Martinez-Nicolas, Ordovas, Madrid, & Garaulet, 2013). These studies suggest that timing of eating is a potentially and understudied factor in weight regulation and weight loss outcomes.
4.3. Circadian Misalignment in Psychiatric Disorders
4.3.1. Depression and Seasonal Affective Disorder (SAD)
There is a long history of the investigation of circadian rhythms and misalignment in depression (Borbely, 1982; Germain & Kupfer, 2008). Early research in major depression noted the predominance of early morning awakenings and shortened REM latency. Based on these observations, researchers coined the “phase advance hypothesis” which posited that the biological rhythm was advanced in respect to the sleep-wake pattern (Kripke, Risch, & Janowsky, 1983; Wehr, Wirz-Justice, Goodwin, Duncan, & Gillin, 1979). Furthermore, changes in the light-dark schedule were thought to be linked to seasonal affective disorder going back to the earliest investigations into this disorder (Rosenthal et al., 1984). However, later work in this area demonstrated either no phase difference or a phase delay compared to non-depressed individuals (von Zerssen et al., 1985). This observation of phase differences, rather than an advance or delay, lead to the “phase shift hypothesis” (Lam & Levitan, 2000; Lewy et al., 2009; Lewy, Lefler, Emens, & Bauer, 2006).
Recent literature on depression and circadian rhythms has focused attention on the alignment of sleep to circadian rhythms, rather than a phase advance or delay. Two small studies have evaluated phase angle in major depressive disorder. Emens and colleagues (2009) demonstrated that a shorter phase angle between DLMO and sleep was correlated with higher depressive symptoms among participants with major depressive disorder. In another study, Hasler and colleagues (2010) did not find a relationship between DLMO and sleep with depressive symptoms. However, there were higher depressive symptoms in those with a shorter phase angle between midpoint of the sleep period and core body temperature minimum. Meliska and colleagues (2011) demonstrated a positive correlation between symptoms of atypical depression (appetite, hypersomnia) and later DLMO timing but no association between atypical depression symptoms and phase angle between melatonin and sleep timing. These studies suggest that rather than a phase advance or delay, it is possible that alignment of sleep to circadian markers may be an important marker of depression severity.
4.3.2. Bipolar Disorder
Disruption of sleep and activity are part of the key features that define bipolar disorder (Cassidy, Murry, Forest, & Carroll, 1998; Wehr, Sack, & Rosenthal, 1987). Multiple studies using wrist actigraphy have demonstrated that individuals with bipolar disorder have less stable circadian sleep-wake patterns, lower amplitude, lower daytime activity and more fragmented nocturnal sleep compared with those with unipolar depression or healthy controls, even inter-episode (Jones, Hare, & Evershed, 2005; Rock, Goodwin, Harmer, & Wulff, 2014; Salvatore et al., 2008). There is only one small study evaluating phase angle of circadian markers and sleep among individuals with bipolar disorder (Robillard et al., 2013). In this study, individuals with bipolar disorder on average had later DLMO than individuals with unipolar major depressive disorder. The results regarding phase angle were complicated. There were no overall differences in phase angle between the unipolar and bipolar groups. However, 10 participants (5 uniloplar/5 bipolar) demonstrated abnormally long phase angle, with DLMO occurring after their habitual sleep onset time or after the end of saliva sampling in this protocol (>2 hours after habitual sleep onset time). Results of these studies suggest that rhythms are important in bipolar disorder but the role of misalignment needs to be evaluated in further studies.
4.3.3. Other Psychiatric Disorders
Sleep problems and circadian disruption are present in many other psychiatric disorders beyond mood disorders. Significant circadian disruption has been reported among individuals with schizophrenia (Monti et al., 2013). Circadian rhythms of individuals with schizophrenia have been reported to be phase advanced (Rao et al., 1994), delayed or free running (Wulff, Dijk, Middleton, Foster, & Joyce, 2012) and misaligned (Afonso, Brissos, Figueira, & Paiva, 2011; Bromundt et al., 2011). Bromundt and colleagues (2011) reported bedtimes before DLMO were common, particularly among individuals with low amplitude of activity during the day. A delay of melatonin timing and activity rhythms have been reported among children and adolesents with ADHD (van der Heijden, Smits, & Gunning, 2005; Van Veen, Kooij, Boonstra, Gordijn, & Van Someren, 2010). In addition, differences in the timing of melatonin offset relative to wake time have been reported in ADHD compared to healthy controls (Novakova et al., 2011).
5. Conclusion
The timing and alignment of circadian rhythms are integral to the health and wellbeing of all organisms, including humans. Misalignment of circadian rhythms can occur when the individual’s sleep-wake cycle is inappropriately timed relative to the biological night, when eating is misaligned with other biological rhythms or there can even be misalignment between the central (SCN) and peripheral rhythms (e.g. organ or system). The consequences of circadian misalignment include changes in dietary behavior, appetite regulation, glucose regulation and mood. The experimental literature suggests that misalignment has profound effects on processes that affect risk for cardiovascular disease, diabetes, obesity and psychiatric conditions. However, much is still not understood about how misalignment contributes to disease risk. Furthermore, the etiology of how individuals become misaligned is not well understood. For example, in DSPD, misalignment has been demonstrated between sleep timing and circadian markers even when participants are sleeping at their preferred sleep-wake schedule, which suggests it is not only due to external factors such as work and social schedules. Treatments to shift and align circadian phase, such as bright light and melatonin have shown promise in the treatment of some conditions, such as depression and seasonal affective disorder (Boyce & Hopwood, 2013; Lewy et al., 2009; Pail et al., 2011). The application of aligning circadian phase to other conditions such as cardiovascular disease, obesity and diabetes has not been well studied. Experimental studies in animal models suggest that altering meal timing may be a promising intervention for weight regulation but this potential therapy is beginning to be explored. Further research is needed to understand the mechanisms that contribute to the development of circadian misalignment, the links between misalignment to disease development and finally, the role of improving circadian alignment in the management of chronic illness.
Acknowledgments
We are grateful to Phyllis Zee for comments on previous drafts of this manuscript. This project was supported by NIH Grant 1K23HL109110.
Footnotes
Declaration of Interest
Dr. Reid reports a grant from Philips that is urelated to the work presented in this review. Dr. Baron has no conflicts of interest to report. The authors alone are responsible for the content and writing of the paper.
References
- Abe T, Inoue Y, Komada Y, Nakamura M, Asaoka S, Kanno M, Takahashi K. Relation between morningness-eveningness score and depressive symptoms among patients with delayed sleep phase syndrome. Sleep Medicine. 2011;12(7):680–684. doi: 10.1016/j.sleep.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Adami GF, Meneghelli A, Scopinaro N. Night eating and binge eating disorder in obese patients. International Journal of Eating Disorders. 1999;25(3):335–338. doi: 10.1002/(sici)1098-108x(199904)25:3<335::aid-eat12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Afonso P, Brissos S, Figueira ML, Paiva T. Schizophrenia patients with predominantly positive symptoms have more disturbed sleep-wake cycles measured by actigraphy. Psychiatry Research. 2011;189(1):62–66. doi: 10.1016/j.psychres.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Ahn YM, Chang J, Joo YH, Kim SC, Lee KY, Kim YS. Chronotype distribution in bipolar I disorder and schizophrenia in a Korean sample. Bipolar Disorders. 2008;10(2):271–275. doi: 10.1111/j.1399-5618.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Akerstedt T. Work hours, sleepiness and the underlying mechanisms. Journal of Sleep Research. 1995;4(S2):15–22. doi: 10.1111/j.1365-2869.1995.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Allison KC, Engel SG, Crosby RD, de Zwaan M, O’Reardon JP, Wonderlich SA, Stunkard AJ. Evaluation of diagnostic criteria for night eating syndrome using item response theory analysis. Eating Behaviors. 2008;9(4):398–407. doi: 10.1016/j.eatbeh.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Kripke DF, Ancoli-Israel S. Delayed and advanced sleep phase symptoms. Israel Journal of Psychiatry and Related Sciences. 2002;39(1):11–18. [PubMed] [Google Scholar]
- Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17(11):2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr EK, Eastman CI, Revelle W, Olson SH, Wolfe LF, Zee PC. Circadian phase-shifting effects of nocturnal exercise in older compared with young adults. American Journal Physiology- Regulatory and Integrative Comparative Physiology. 2003;284(6):R1542–1550. doi: 10.1152/ajpregu.00761.2002. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. Journal of Sleep Research. 2000;9(2):117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Bailer U, Wiesegger G, Leisch F, Fuchs K, Leitner I, Letmaier M, Aschauer HN. No association of clock gene T3111C polymorphism and affective disorders. European Neuropsychopharmacology. 2005;15(1):51–55. doi: 10.1016/j.euroneuro.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Bandin C, Martinez-Nicolas A, Ordovas JM, Madrid JA, Garaulet M. Circadian rhythmicity as a predictor of weight-loss effectiveness. International Journal of Obesity. 2013 doi: 10.1038/ijo.2013.211. [DOI] [PubMed] [Google Scholar]
- Barger LK, Wright KP, Jr, Hughes RJ, Czeisler CA. Daily exercise facilitates phase delays of circadian melatonin rhythm in very dim light. American Journal of Physiology- Regulatory and Integrative Comparative Physiology. 2004;286(6):R1077–1084. doi: 10.1152/ajpregu.00397.2003. [DOI] [PubMed] [Google Scholar]
- Barnes M. Making time use explicit in an investigation of social exclusion in the UK. Swindon: 2010. RES-061-23-0122. [Google Scholar]
- Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19(7):1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- Bass J. Circadian topology of metabolism. Nature. 2012;491(7424):348–356. doi: 10.1038/nature11704. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Colombo C, Pirovano A, Marino E, Smeraldi E. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neuroscience Letters. 2008;445(2):184–187. doi: 10.1016/j.neulet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, Smeraldi E. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2003;123B(1):23–26. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Revell VL. Measuring melatonin in humans. Journal of Clinical Sleep Medicine. 2008;4(1):66–69. [PMC free article] [PubMed] [Google Scholar]
- Bersani FS, Iannitelli A, Pacitti F, Bersani G. Sleep and biorythm disturbances in schizophrenia, mood and anxiety disorders: a review. Rivista di Psichiatria. 2012;47(5):365–375. doi: 10.1708/1175.13027. [DOI] [PubMed] [Google Scholar]
- Bijlenga D, Van Someren EJ, Gruber R, Bron TI, Kruithof IF, Spanbroek EC, Kooij JJ. Body temperature, activity and melatonin profiles in adults with attention-deficit/hyperactivity disorder and delayed sleep: a case-control study. Journal of Sleep Research. 2013;22(6):607–616. doi: 10.1111/jsr.12075. [DOI] [PubMed] [Google Scholar]
- Birketvedt GS, Florholmen J, Sundsfjord J, Osterud B, Dinges D, Bilker W, Stunkard A. Behavioral and neuroendocrine characteristics of the night-eating syndrome. Journal of the American Medical Association. 1999;282(7):657–663. doi: 10.1001/jama.282.7.657. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, Waterhouse JM. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Archives of General Psychiatry. 1997;54(2):145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Boyce P, Hopwood M. Manipulating melatonin in managing mood. Acta Psychiatrica Scandinavica. Supplementum. 2013;(444):16–23. doi: 10.1111/acps.12175. [DOI] [PubMed]
- Bromundt V, Koster M, Georgiev-Kill A, Opwis K, Wirz-Justice A, Stoppe G, Cajochen C. Sleep-wake cycles and cognitive functioning in schizophrenia. British Journal of Psychiatry. 2011;198(4):269–276. doi: 10.1192/bjp.bp.110.078022. [DOI] [PubMed] [Google Scholar]
- Brown SA, Azzi A. Peripheral circadian oscillators in mammals. Handbook of Experimental Pharmacology. 2013;(217):45–66. doi: 10.1007/978-3-642-25950-0_3. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. Journal of Clinical Endocrinology and Metabolism. 2010;95(7):3325–3331. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy F, Murry E, Forest K, Carroll BJ. Signs and symptoms of mania in pure and mixed episodes. Journal of Affective Disorders. 1998;50(2–3):187–201. doi: 10.1016/s0165-0327(98)00016-0. [DOI] [PubMed] [Google Scholar]
- Chang AM, Reid KJ, Gourineni R, Zee PC. Sleep timing and circadian phase in delayed sleep phase syndrome. Journal of Biological Rhythms. 2009;24(4):313–321. doi: 10.1177/0748730409339611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelminski I, Ferraro FR, Petros TV, Plaud JJ. An analysis of the “eveningness-morningness” dimension in “depressive” college students. Journal of Affective Disorders. 1999;52(1–3):19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Chung JK, Lee KY, Kim SH, Kim EJ, Jeong SH, Jung HY, Joo EJ. Circadian Rhythm Characteristics in Mood Disorders: Comparison among Bipolar I Disorder, Bipolar II Disorder and Recurrent Major Depressive Disorder. Clinical Psychopharmacology Neuroscience. 2012;10(2):110–116. doi: 10.9758/cpn.2012.10.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sanchez R, Rios CD, Kronauer RE. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233(4764):667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Dalle Grave R, Calugi S, Ruocco A, Marchesini G. Night eating syndrome and weight loss outcome in obese patients. International Journal of Eating Disorders. 2011;44(2):150–156. doi: 10.1002/eat.20786. [DOI] [PubMed] [Google Scholar]
- Desan PH, Oren DA, Malison R, Price LH, Rosenbaum J, Smoller J, Gelernter J. Genetic polymorphism at the CLOCK gene locus and major depression. American Journal of Medical Genetics. 2000;96(3):418–421. doi: 10.1002/1096-8628(20000612)96:3<418::aid-ajmg34>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annual Review of Physiology. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Medicine Reviews. 2010;14(3):151–160. doi: 10.1016/j.smrv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. Journal of Sleep Research. 1992;1(2):112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27(8):1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- Drennan MD, Klauber MR, Kripke DF, Goyette LM. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. Journal of Affective Disorders. 1991;23(2):93–98. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behavioral Neuroscience. 2001;115(4):895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Wright KP., Jr Entrainment of the human circadian system by light. Journal of Biological Rhythms. 2005;20(4):326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Research. 2009;168(3):259–261. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Esquirol Y, Perret B, Ruidavets JB, Marquie JC, Dienne E, Niezborala M, Ferrieres J. Shift work and cardiovascular risk factors: new knowledge from the past decade. Archives Cardiovascual Diseases. 2011;104(12):636–668. doi: 10.1016/j.acvd.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Etain B, Milhiet V, Bellivier F, Leboyer M. Genetics of circadian rhythms and mood spectrum disorders. European Neuropsychopharmacology. 2011;21(Suppl 4):S676–682. doi: 10.1016/j.euroneuro.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Fleig D, Randler C. Association between chronotype and diet in adolescents based on food logs. Eating Behaviors. 2009;10(2):115–118. doi: 10.1016/j.eatbeh.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Foerch C, Korf HW, Steinmetz H, Sitzer M. Abrupt shift of the pattern of diurnal variation in stroke onset with daylight saving time transitions. Circulation. 2008;118(3):284–290. doi: 10.1161/CIRCULATIONAHA.108.771246. [DOI] [PubMed] [Google Scholar]
- Folkard S, Monk TH, Lobban MC. Towards a predictive test of adjustment to shift work. Ergonomics. 1979;22(1):79–91. doi: 10.1080/00140137908924591. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: from gene expression to physiology. Chromosoma. 2004;113(3):103–112. doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- Gamble KL, May RS, Besing RC, Tankersly AP, Fargason RE. Delayed sleep timing and symptoms in adults with attention-deficit/hyperactivity disorder: a controlled actigraphy study. Chronobiology International. 2013;30(4):598–606. doi: 10.3109/07420528.2012.754454. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. International Journal of Obesity. 2013;37(4):604–611. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar-Barba E, Calati R, Cruz-Fuentes CS, Ontiveros-Uribe MP, Natale V, De Ronchi D, Serretti A. Depressive symptomatology is influenced by chronotypes. Journal of Affective Disorders. 2009;119(1–3):100–106. doi: 10.1016/j.jad.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Gehrman P, Marler M, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. The timing of activity rhythms in patients with dementia is related to survival. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2004;59(10):1050–1055. doi: 10.1093/gerona/59.10.m1050. [DOI] [PubMed] [Google Scholar]
- Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Human Psychopharmacology. 2008;23(7):571–585. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio LM, Magalhaes PV, Andersen ML, Walz JC, Jakobson L, Kapczinski F. Circadian preference in bipolar disorder. Sleep and Breathing. 2010;14(2):153–155. doi: 10.1007/s11325-009-0301-3. [DOI] [PubMed] [Google Scholar]
- Gluck ME, Geliebter A, Satov T. Night eating syndrome is associated with depression, low self-esteem, reduced daytime hunger, and less weight loss in obese outpatients. Obesity Research. 2001;9(4):264–267. doi: 10.1038/oby.2001.31. [DOI] [PubMed] [Google Scholar]
- Goel N. Late-night presentation of an auditory stimulus phase delays human circadian rhythms. American Journal of Physiology- Regulatory and Integrative Comparative Physiology. 2005;289(1):R209–216. doi: 10.1152/ajpregu.00754.2004. [DOI] [PubMed] [Google Scholar]
- Goel N, Stunkard AJ, Rogers NL, Van Dongen HP, Allison KC, O’Reardon JP, Dinges DF. Circadian rhythm profiles in women with night eating syndrome. Journal of Biological Rhythms. 2009;24(1):85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. British Journal of Nutrition. 2012:1–9. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- Gonnissen HK, Mazuy C, Rutters F, Martens EA, Adam TC, Westerterp-Plantenga MS. Sleep architecture when sleeping at an unusual circadian time and associations with insulin sensitivity. PLoS ONE. 2013;8(8):e72877. doi: 10.1371/journal.pone.0072877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C, Wright KP, Jr, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(21):9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy A, Richardson H, Burstyn I, Lohrisch C, SenGupta SK, Lai AS, Aronson KJ. Increased risk of breast cancer associated with long-term shift work in Canada. Occupational and Environmental Medicine. 2013;70(12):831–838. doi: 10.1136/oemed-2013-101482. [DOI] [PubMed] [Google Scholar]
- Gumenyuk V, Howard R, Roth T, Korzyukov O, Drake CL. Sleep loss, circadian mismatch, and abnormalities in reorienting of attention in night workers with shift work disorder. Sleep. 2014;37(3):545–556. doi: 10.5665/sleep.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumenyuk V, Roth T, Korzyukov O, Jefferson C, Kick A, Spear L, Drake CL. Shift work sleep disorder is associated with an attenuated brain response of sensory memory and an increased brain response to novelty: an ERP study. Sleep. 2010;33(5):703–713. doi: 10.1093/sleep/33.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm BJ, Jo B, Dhabhar FS, Palesh O, Aldridge-Gerry A, Bajestan SN, Zeitzer JM. Bedtime misalignment and progression of breast cancer. Chronobiology International. 2014;31(2):214–221. doi: 10.3109/07420528.2013.842575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton SM, Morgan LM, Lawrence N, Anastasiadou T, Norris F, Deacon S, Arendt J. Postprandial hormone and metabolic responses in simulated shift work. Journal of Endocrinology. 1996;151(2):259–267. doi: 10.1677/joe.0.1510259. [DOI] [PubMed] [Google Scholar]
- Handbook of sleep medicine. Thorax. 2001;56(1):86. doi: 10.1136/thorax.56.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb A, Levandovski R, Oliveira C, Caumo W, Allison KC, Stunkard A, Hidalgo MP. Night eating patterns and chronotypes: a correlation with binge eating behaviors. Psychiatry Research. 2012;200(2–3):489–493. doi: 10.1016/j.psychres.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Hardin PE, Hall JC, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990;343(6258):536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- Harrison Y. The impact of daylight saving time on sleep and related behaviours. Sleep Med Rev. 2013;17(4):285–292. doi: 10.1016/j.smrv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Buysse DJ, Kupfer DJ, Germain A. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: further evidence for circadian misalignment in non-seasonal depression. Psychiatry Research. 2010;178(1):205–207. doi: 10.1016/j.psychres.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabolism. 2012;15(6):848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Uchiyama M, Kamei Y, Shibui K, Tagaya H, Asada T, Takahashi K. Clinical analyses of sighted patients with non-24-hour sleep-wake syndrome: a study of 57 consecutively diagnosed cases. Sleep. 2005;28(8):945–952. doi: 10.1093/sleep/28.8.945. [DOI] [PubMed] [Google Scholar]
- Hirata FC, Lima MC, de Bruin VM, Nobrega PR, Wenceslau GP, de Bruin PF. Depression in medical school: the influence of morningness-eveningness. Chronobiology International. 2007;24(5):939–946. doi: 10.1080/07420520701657730. [DOI] [PubMed] [Google Scholar]
- Hoogendijk WJ, van Someren EJ, Mirmiran M, Hofman MA, Lucassen PJ, Zhou JN, Swaab DF. Circadian rhythm-related behavioral disturbances and structural hypothalamic changes in Alzheimer’s disease. International Psychogeriatrics. 1996;8(Suppl 3):245–252. doi: 10.1017/s1041610297003426. discussion 269–272. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4(2):97–110. [PubMed] [Google Scholar]
- Ijaz S, Verbeek J, Seidler A, Lindbohm ML, Ojajarvi A, Orsini N, Neuvonen K. Night-shift work and breast cancer--a systematic review and meta-analysis. Scandinavian Journal of Work, Environment and Health. 2013;39(5):431–447. doi: 10.5271/sjweh.3371. [DOI] [PubMed] [Google Scholar]
- International Classification of Sleep Disorders, Second Edition: Diagnostic and Coding Manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- Jakubowicz D, Barnea M, Wainstein J, Froy O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity. 2013;21(12):2504–2512. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- Janszky I, Ahnve S, Ljung R, Mukamal KJ, Gautam S, Wallentin L, Stenestrand U. Daylight saving time shifts and incidence of acute myocardial infarction--Swedish Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA) Sleep Medicine. 2012;13(3):237–242. doi: 10.1016/j.sleep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Janszky I, Ljung R. Shifts to and from daylight saving time and incidence of myocardial infarction. New England Journal of Medicine. 2008;359(18):1966–1968. doi: 10.1056/NEJMc0807104. [DOI] [PubMed] [Google Scholar]
- Johansson C, Willeit M, Smedh C, Ekholm J, Paunio T, Kieseppa T, Partonen T. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28(4):734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disorders. 2005;7(2):176–186. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Kabrita CS, Hajjar-Muca TA, Duffy JF. Predictors of poor sleep quality among Lebanese university students: association between evening typology, lifestyle behaviors, and sleep habits. Nature and Science of Sleep. 2014;6:11–18. doi: 10.2147/NSS.S55538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantermann T, Juda M, Merrow M, Roenneberg T. The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Current Biology. 2007;17(22):1996–2000. doi: 10.1016/j.cub.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA, Lancel M. EEG slow wave activity, REM sleep, and rectal temperature during night and day sleep in morning-type and evening-type subjects. Psychophysiology. 1991;28(6):678–688. doi: 10.1111/j.1469-8986.1991.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. Joural of Physiology. 2003;549(Pt 3):945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee YJ, Kim H, Cho IH, Lee JY, Cho SJ. Age as a moderator of the association between depressive symptoms and morningness-eveningness. Journal of Psychosomatic Research. 2010;68(2):159–164. doi: 10.1016/j.jpsychores.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Hida A, Watanabe M, Enomoto M, Aritake-Okada S, Moriguchi Y, Mishima K. Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiology International. 2010;27(9–10):1797–1812. doi: 10.3109/07420528.2010.516705. [DOI] [PubMed] [Google Scholar]
- Kleitman N, Kleitman E. Effect of non-twenty-four-hour routines of living on oral temperature and heart rate. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1953;6(5):283–291. doi: 10.1152/jappl.1953.6.5.283. [DOI] [PubMed] [Google Scholar]
- Knauth P, Landau K, Droge C, Schwitteck M, Widynski M, Rutenfranz J. Duration of sleep depending on the type of shift work. International Archives of Occupational and Environmental Health. 1980;46(2):167–177. doi: 10.1007/BF00378195. [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metabolism. 2007;6(5):414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Koorengevel KM, Beersma DG, den Boer JA, van den Hoofdakker RH. Mood regulation in seasonal affective disorder patients and healthy controls studied in forced desynchrony. Psychiatry Research. 2003;117(1):57–74. doi: 10.1016/s0165-1781(02)00305-0. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Elliott JA, Youngstedt SD, Rex KM. Circadian phase response curves to light in older and young women and men. Journal of Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Rex KM, Ancoli-Israel S, Nievergelt CM, Klimecki W, Kelsoe JR. Delayed sleep phase cases and controls. Journal of Circadian Rhythms. 2008;6:6. doi: 10.1186/1740-3391-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Risch SC, Janowsky D. Bright white light alleviates depression. Psychiatry Research. 1983;10(2):105–112. doi: 10.1016/0165-1781(83)90109-9. [DOI] [PubMed] [Google Scholar]
- Labyak S, Lava S, Turek F, Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health Care for Women International. 2002;23(6–7):703–714. doi: 10.1080/07399330290107449. [DOI] [PubMed] [Google Scholar]
- Lahti TA, Haukka J, Lonnqvist J, Partonen T. Daylight saving time transitions and hospital treatments due to accidents or manic episodes. BMC Public Health. 2008;8:74. doi: 10.1186/1471-2458-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti TA, Leppamaki S, Lonnqvist J, Partonen T. Transitions into and out of daylight saving time compromise sleep and the rest-activity cycles. BMC Physiology. 2008;8:3. doi: 10.1186/1472-6793-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RW, Levitan RD. Pathophysiology of seasonal affective disorder: a review. Journal of Psychiatry and Neuroscience. 2000;25(5):469–480. [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Rex KM, Nievergelt CM, Kelsoe JR, Kripke DF. Delayed sleep phase syndrome is related to seasonal affective disorder. Journal of Affective Disorders. 2011;133(3):573–579. doi: 10.1016/j.jad.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, Allebrandt KV. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiology International. 2011;28(9):771–778. doi: 10.3109/07420528.2011.602445. [DOI] [PubMed] [Google Scholar]
- Lewy AJ. Melatonin and human chronobiology. Cold Spring Harbor Symposia on Quantitative Biology. 2007;72:623–636. doi: 10.1101/sqb.2007.72.055. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiology International. 1992;9(5):380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Emens JS, Songer JB, Sims N, Laurie AL, Fiala SC, Buti AL. Winter Depression: Integrating mood, circadian rhythms, and the sleep/wake and light/dark cycles into a bio-psycho-social-environmental model. Sleep Medicine Clinics. 2009;4(2):285–299. doi: 10.1016/j.jsmc.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(19):7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Dijk DJ, Kosti O, Skene DJ, Arendt J. Alertness, mood and performance rhythm disturbances associated with circadian sleep disorders in the blind. Journal of Sleep Research. 2008;17(2):207–216. doi: 10.1111/j.1365-2869.2008.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. Journal of Clinical Endocrinology and Metabolism. 1997;82(11):3763–3770. doi: 10.1210/jcem.82.11.4355. [DOI] [PubMed] [Google Scholar]
- Loros JJ, Dunlap JC. Neurospora crassa clock-controlled genes are regulated at the level of transcription. Molecular and Cellular Biology. 1991;11(1):558–563. doi: 10.1128/mcb.11.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovato N, Gradisar M, Short M, Dohnt H, Micic G. Delayed sleep phase disorder in an Australian school-based sample of adolescents. Journal of Clinical Sleep Medicine. 2013;9(9):939–944. doi: 10.5664/jcsm.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen EA, Zhao X, Rother KI, Mattingly MS, Courville AB, de Jonge L, Cizza G. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PLoS ONE. 2013;8(3):e56519. doi: 10.1371/journal.pone.0056519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren JD, Allison KC, Crow S, O’Reardon JP, Berg KC, Galbraith J, Stunkard AJ. Prevalence of the night eating syndrome in a psychiatric population. American Journal of Psychiatry. 2006;163(1):156–158. doi: 10.1176/appi.ajp.163.1.156. [DOI] [PubMed] [Google Scholar]
- Lundgren JD, Allison KC, O’Reardon JP, Stunkard AJ. A descriptive study of non-obese persons with night eating syndrome and a weight-matched comparison group. Eating Behaviors. 2008;9(3):343–351. doi: 10.1016/j.eatbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour HA, Talkowski ME, Wood J, Chowdari KV, McClain L, Prasad K, Nimgaonkar VL. Association study of 21 circadian genes with bipolar I disorder, schizoaffective disorder, and schizophrenia. Bipolar Disorders. 2009;11(7):701–710. doi: 10.1111/j.1399-5618.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour HA, Wood J, Chowdari KV, Dayal M, Thase ME, Kupfer DJ, Nimgaonkar VL. Circadian phase variation in bipolar I disorder. Chronobiology International. 2005;22(3):571–584. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- Marshall HM, Allison KC, O’Reardon JP, Birketvedt G, Stunkard AJ. Night eating syndrome among nonobese persons. International Journal of Eating Disorders. 2004;35(2):217–222. doi: 10.1002/eat.10241. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. Journal of Biological Rhythms. 2012;27(5):339–352. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Martinez LF, Lopez AM, Sorenson DL, Nowakowski S, Parry BL. Relationship of morningness-eveningness questionnaire score to melatonin and sleep timing, body mass index and atypical depressive symptoms in peri- and post-menopausal women. Psychiatry Research. 2011;188(1):88–95. doi: 10.1016/j.psychres.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikanto I, Englund A, Kronholm E, Laatikainen T, Peltonen M, Vartiainen E, Partonen T. Evening chronotypes have the increased odds for bronchial asthma and nocturnal asthma. Chronobiology International. 2014;31(1):95–101. doi: 10.3109/07420528.2013.826672. [DOI] [PubMed] [Google Scholar]
- Merikanto I, Lahti T, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Partonen T. Evening types are prone to depression. Chronobiology International. 2013;30(5):719–725. doi: 10.3109/07420528.2013.784770. [DOI] [PubMed] [Google Scholar]
- Merikanto I, Lahti T, Puolijoki H, Vanhala M, Peltonen M, Laatikainen T, Partonen T. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiology International. 2013;30(4):470–477. doi: 10.3109/07420528.2012.741171. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. Journal of Sleep Research. 2006;15(2):162–166. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Potts JM, DeGrazia JM, Kupfer DJ. Morningness-eveningness and lifestyle regularity. Chronobiology International. 2004;21(3):435–443. doi: 10.1081/cbi-120038614. [DOI] [PubMed] [Google Scholar]
- Monti JM, BaHammam AS, Pandi-Perumal SR, Bromundt V, Spence DW, Cardinali DP, Brown GM. Sleep and circadian rhythm dysregulation in schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;43:209–216. doi: 10.1016/j.pnpbp.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Moore RY. Entrainment pathways and the functional organization of the circadian system. Progress in Brain Research. 1996;111:103–119. doi: 10.1016/s0079-6123(08)60403-3. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, Kelsoe JR. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2006;141B(3):234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM, Kripke DF, Remick RA, Sadovnick AD, McElroy SL, Keck PE, Jr, Kelsoe JR. Examination of the clock gene Cryptochrome 1 in bipolar disorder: mutational analysis and absence of evidence for linkage or association. Psychiatric Genetics. 2005;15(1):45–52. doi: 10.1097/00041444-200503000-00008. [DOI] [PubMed] [Google Scholar]
- Novakova M, Paclt I, Ptacek R, Kuzelova H, Hajek I, Sumova A. Salivary melatonin rhythm as a marker of the circadian system in healthy children and those with attention-deficit/hyperactivity disorder. Chronobiology International. 2011;28(7):630–637. doi: 10.3109/07420528.2011.596983. [DOI] [PubMed] [Google Scholar]
- Nurminen T. Shift work and reproductive health. Scandinavian Journal of Work, Environment and Health. 1998;24(Suppl 3):28–34. [PubMed] [Google Scholar]
- O’Reardon JP, Ringel BL, Dinges DF, Allison KC, Rogers NL, Martino NS, Stunkard AJ. Circadian eating and sleeping patterns in the night eating syndrome. Obesity Research. 2004;12(11):1789–1796. doi: 10.1038/oby.2004.222. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Uchiyama M, Shirakawa S, Okawa M. Prolonged interval from body temperature nadir to sleep offset in patients with delayed sleep phase syndrome. Sleep. 1996;19(1):36–40. [PubMed] [Google Scholar]
- Pail G, Huf W, Pjrek E, Winkler D, Willeit M, Praschak-Rieder N, Kasper S. Bright-light therapy in the treatment of mood disorders. Neuropsychobiology. 2011;64(3):152–162. doi: 10.1159/000328950. [DOI] [PubMed] [Google Scholar]
- Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30–49 years) Journal of Biological Rhythms. 2006;21(1):68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
- Paudel ML, Taylor BC, Ancoli-Israel S, Blackwell T, Stone KL, Tranah G, Ensrud KE. Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiology International. 2010;27(2):363–377. doi: 10.3109/07420520903419157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel ML, Taylor BC, Ancoli-Israel S, Stone KL, Tranah G, Redline S, Ensrud KE. Rest/activity rhythms and cardiovascular disease in older men. Chronobiology International. 2011;28(3):258–266. doi: 10.3109/07420528.2011.553016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry-Jenkins M, Goldberg AE, Pierce CP, Sayer AG. Shift Work, Role Overload, and the Transition to Parenthood. J Marriage Fam. 2007;69(1):123–138. doi: 10.1111/j.1741-3737.2006.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand CS, Macgregor AM, Stunkard AJ. The night eating syndrome in the general population and among postoperative obesity surgery patients. International Journal of Eating Disorders. 1997;22(1):65–69. doi: 10.1002/(sici)1098-108x(199707)22:1<65::aid-eat8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Rao ML, Gross G, Strebel B, Halaris A, Huber G, Braunig P, Marler M. Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biological Psychiatry. 1994;35(3):151–163. doi: 10.1016/0006-3223(94)91147-9. [DOI] [PubMed] [Google Scholar]
- Regestein QR, Monk TH. Delayed sleep phase syndrome: a review of its clinical aspects. American Journal of Psychiatry. 1995;152(4):602–608. doi: 10.1176/ajp.152.4.602. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Chang AM, Dubocovich ML, Turek FW, Takahashi JS, Zee PC. Familial advanced sleep phase syndrome. Archives of Neurology. 2001;58(7):1089–1094. doi: 10.1001/archneur.58.7.1089. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Jaksa AA, Eisengart JB, Baron KG, Lu B, Kane P, Zee PC. Systematic evaluation of Axis-I DSM diagnoses in delayed sleep phase disorder and evening-type circadian preference. Sleep Medicine. 2012;13(9):1171–1177. doi: 10.1016/j.sleep.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL, Van Cauter E. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36(9):2523–2529. doi: 10.2337/dc12-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard R, Naismith SL, Rogers NL, Ip TK, Hermens DF, Scott EM, Hickie IB. Delayed sleep phase in young people with unipolar or bipolar affective disorders. Journal of Affective Disorders. 2013;145(2):260–263. doi: 10.1016/j.jad.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Rock P, Goodwin G, Harmer C, Wulff K. Daily rest-activity patterns in the bipolar phenotype: A controlled actigraphy study. Chronobiology International. 2014;31(2):290–296. doi: 10.3109/07420528.2013.843542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Current Biology. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. Epidemiology of the human circadian clock. Sleep Medicine Reviews. 2007;11(6):429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. Journal of Biological Rhythms. 2003;18(1):80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Wehr TA. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Archives of General Psychiatry. 1984;41(1):72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, McClung CA. Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(15):6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore P, Ghidini S, Zita G, De Panfilis C, Lambertino S, Maggini C, Baldessarini RJ. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disorders. 2008;10(2):256–265. doi: 10.1111/j.1399-5618.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- Sato-Mito N, Shibata S, Sasaki S, Sato K. Dietary intake is associated with human chronotype as assessed by both morningness-eveningness score and preferred midpoint of sleep in young Japanese women. International Journal of Food Sciences and Nutrition. 2011;62(5):525–532. doi: 10.3109/09637486.2011.560563. [DOI] [PubMed] [Google Scholar]
- Saxvig IW, Pallesen S, Wilhelmsen-Langeland A, Molde H, Bjorvatn B. Prevalence and correlates of delayed sleep phase in high school students. Sleep Medicine. 2012;13(2):193–199. doi: 10.1016/j.sleep.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. Journal of the National Cancer Institute. 2001;93(20):1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses’ health study. Journal of the National Cancer Institute. 2003;95(11):825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111(7):919–922. doi: 10.1016/s0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. Journal of Clinical Investigation. 1997;100(7):1882–1887. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader H, Bovim G, Sand T. The prevalence of delayed and advanced sleep phase syndromes. Journal of Sleep Research. 1993;2(1):51–55. doi: 10.1111/j.1365-2869.1993.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Shapiro CM, Blake F, Fossey E, Adams B. Daylight saving time in psychiatric illness. Journal of Affective Disorders. 1990;19(3):177–181. doi: 10.1016/0165-0327(90)90089-q. [DOI] [PubMed] [Google Scholar]
- Shibui K, Uchiyama M, Okawa M. Melatonin rhythms in delayed sleep phase syndrome. Journal of Biological Rhythms. 1999;14(1):72–76. doi: 10.1177/074873049901400110. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989;74(5):728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Soreca I, Fagiolini A, Frank E, Goodpaster BH, Kupfer DJ. Chronotype and body composition in bipolar disorder. Chronobiology International. 2009;26(4):780–788. doi: 10.1080/07420520902929060. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291(5503):490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Strogatz SH. Interpreting the human phase response curve to multiple bright-light exposures. Journal of Biological Rhythms. 1990;5(2):169–174. doi: 10.1177/074873049000500208. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome; a pattern of food intake among certain obese patients. American Journal of Medicine. 1955;19(1):78–86. doi: 10.1016/0002-9343(55)90276-x. [DOI] [PubMed] [Google Scholar]
- Surridge-David M, MacLean A, Coulter ME, Knowles JB. Mood change following an acute delay of sleep. Psychiatry Research. 1987;22(2):149–158. doi: 10.1016/0165-1781(87)90102-8. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature Reviews Genetics. 2008;9(10):764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranah GJ, Blackwell T, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Stone KL. Circadian activity rhythms and mortality: the study of osteoporotic fractures. Journal of the American Geriatrics Society. 2010;58(2):282–291. doi: 10.1111/j.1532-5415.2009.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, Yaffe K. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Annals of Neurology. 2011;70(5):722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama M, Okawa M, Shibui K, Kim K, Tagaya H, Kudo Y, Takahashi K. Altered phase relation between sleep timing and core body temperature rhythm in delayed sleep phase syndrome and non-24-hour sleep-wake syndrome in humans. Neuroscience Letters. 2000;294(2):101–104. doi: 10.1016/s0304-3940(00)01551-2. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Okawa M, Shibui K, Liu X, Hayakawa T, Kamei Y, Takahashi K. Poor compensatory function for sleep loss as a pathogenic factor in patients with delayed sleep phase syndrome. Sleep. 2000;23(4):553–558. [PubMed] [Google Scholar]
- Urban R, Magyarodi T, Rigo A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiology International. 2011;28(3):238–247. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden KB, Smits MG, Gunning WB. Sleep-related disorders in ADHD: a review. Clinical Pediatrics. 2005;44(3):201–210. doi: 10.1177/000992280504400303. [DOI] [PubMed] [Google Scholar]
- Van Veen MM, Kooij JJ, Boonstra AM, Gordijn MC, Van Someren EJ. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biological Psychiatry. 2010;67(11):1091–1096. doi: 10.1016/j.biopsych.2009.12.032. [DOI] [PubMed] [Google Scholar]
- von Zerssen D, Dirlich G, Doerr P, Emrich HM, Lund R, Ploog D. Are biological rhythms disturbed in depression? Acta Psychiatrica Belgica. 1985;85(5):624–635. [PubMed] [Google Scholar]
- Waterhouse J, Folkard S, Van Dongen H, Minors D, Owens D, Kerkhof G, Tucker P. Temperature profiles, and the effect of sleep on them, in relation to morningness-eveningness in healthy female subjects. Chronobiology International. 2001;18(2):227–247. doi: 10.1081/cbi-100103188. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Sack DA, Rosenthal NE. Sleep reduction as a final common pathway in the genesis of mania. American Journal of Psychiatry. 1987;144(2):201–204. doi: 10.1176/ajp.144.2.201. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Wirz-Justice A, Goodwin FK, Duncan W, Gillin JC. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206(4419):710–713. doi: 10.1126/science.227056. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Czeisler CA, Coleman RM, Spielman AJ, Zimmerman JC, Dement W, Pollak CP. Delayed sleep phase syndrome. A chronobiological disorder with sleep-onset insomnia. Archives of General Psychiatry. 1981;38(7):737–746. doi: 10.1001/archpsyc.1981.01780320017001. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A. Diurnal variation of depressive symptoms. Dialogues Clin Neurosci. 2008;10(3):337–343. doi: 10.31887/DCNS.2008.10.3/awjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biological Psychiatry. 1990;27(6):563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiology International. 2006;23(1–2):497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Paulus M, Roenneberg T. Decreased psychological well-being in late ‘chronotypes’ is mediated by smoking and alcohol consumption. Substance Use and Misuse. 2010;45(1–2):15–30. doi: 10.3109/10826080903498952. [DOI] [PubMed] [Google Scholar]
- Wood J, Birmaher B, Axelson D, Ehmann M, Kalas C, Monk K, Nimgainkar VL. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Research. 2009;166(2–3):201–209. doi: 10.1016/j.psychres.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(24):14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM. Sleep and circadian rhythm disruption in schizophrenia. British Journal of Psychiatry. 2012;200(4):308–316. doi: 10.1192/bjp.bp.111.096321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavada A, Gordijn MC, Beersma DG, Daan S, Roenneberg T. Comparison of the Munich Chronotype Questionnaire with the Horne-Ostberg’s Morningness-Eveningness Score. Chronobiology International. 2005;22(2):267–278. doi: 10.1081/cbi-200053536. [DOI] [PubMed] [Google Scholar]
- Zelinski EL, Deibel SH, McDonald RJ. The trouble with circadian clock dysfunction: Multiple deleterious effects on the brain and body. Neuroscience and Biobehavioral Reviews. 2014;40C:80–101. doi: 10.1016/j.neubiorev.2014.01.007. [DOI] [PubMed] [Google Scholar]