Abstract
Equatorial populations of marine species are predicted to be most impacted by global warming because they could be adapted to a narrow range of temperatures in their local environment. We investigated the thermal range at which aerobic metabolic performance is optimum in equatorial populations of coral reef fish in northern Papua New Guinea. Four species of damsel fishes and two species of cardinal fishes were held for 14d at 29, 31, 33, and 34°C, which incorporated their existing thermal range (29–31°C) as well as projected increases in ocean surface temperatures of up to 3°C by the end of this century. Resting and maximum oxygen consumption rates were measured for each species at each temperature and used to calculate the thermal reaction norm of aerobic scope. Our results indicate that one of the six species, Chromisatripectoralis, is already living above its thermal optimum of 29°C. The other five species appeared to be living close to their thermal optima (approximately 31°C). Aerobic scope was significantly reduced in all species, and approached zero for two species at 3°C above current-day temperatures. One species was unable to survive even short-term exposure to 34°C. Our results indicate that low-latitude reef fish populations are living close to their thermal optima and may be more sensitive to ocean warming than higher-latitude populations. Even relatively small temperature increases (2–3°C) could result in population declines and potentially redistribution of equatorial species to higher latitudes if adaptation cannot keep pace.
Keywords: global warming, oxygen consumption, latitudinal gradient, Pomacentridae, Apagonidae, coral reef fish
Introduction
Species living at extreme latitudes (equator and poles) may be particularly sensitive to global warming because they evolved in a relatively stable thermal environment and are expected to exhibit narrow thermal tolerance ranges (Tewksbury et al., 2008). The thermal range for optimum aerobic performance (thermal optima; Topt) in such species may only span a few degrees, whereas temperate species need to perform over a much wider range of temperatures (Deutsch et al., 2008, Somero, 2010, Nguyen et al., 2011, Sunday et al., 2011). Further, theory suggests that populations living at thermally stable latitudes (e.g. near the equator or poles) have less capacity for thermal acclimatization (i.e. physiological, anatomical, or biochemical modifications that occur within a lifetime to cope with natural changes in temperature) than populations living in more thermally variable, high-latitude locations (Pörtner, 2002, Stillman, 2003, Somero, 2010, Neuheimer et al., 2011, Nguyen et al., 2011). This latitudinal pattern is predicted because the physiological costs to acclimatize to a wide temperature range may outweigh any potential benefits if those temperatures are rarely experienced (Angilletta, 2009). Moreover, there may be no selection pressure to tolerate temperatures outside of what the populations are regularly experiencing. Equatorial populations of marine species are predicted to be especially sensitive to rising temperature because they experience an even narrower temperature range than equatorial populations ofter restrial species (due to the large heat storage capacity of water). Additionally, their geographical ranges more closely conform to organismal thermal tolerance limits than the geographical ranges of terrestrial species, especially near equatorial boundaries (Sunday et al., 2011, Sunday et al., 2012). Consequently the 2–3°C warming of tropical oceans projected to occur by the end of this century due to climate change (IPCC, 2007, Poloczanska et al., 2007, Ganachaud et al., 2011) could have serious impacts on equatorial populations of many marine organisms.
Coral reef ecosystems are already facing a biodiversity crisis that is exacerbated by global climate change (Hughes et al., 2003, Bellwood et al., 2004). For coral reef fishes, rising ocean temperatures may not be immediately lethal (Mora & Ospína, 2001), but could have significant impacts on individual performance that will ultimately affect population demography and biogeographic distributions (Munday et al., 2008b, Nilsson et al., 2009). For example, an increase in average temperature of 2–3°C significantly compromises growth and reproduction of some species (Munday et al., 2008a, Donelson et al., 2010, Pankhurst & Munday, 2011, Rushworth et al., 2011, Zarco-Perelló et al., 2012). The mechanistic explanation for the effects on individual performance may be related to how temperature influences O2 uptake, transport, and delivery, also known as oxygen- and capacity-limited thermal tolerance (OCLTT) (Pörtner, 2001, Pörtner & Knust, 2007, Pörtner and Farrell, 2008, Eliason et al., 2011). The physiological scope for aerobic performance (aerobic scope), calculated as the difference in O2 consumption between resting and maximal performance (Fry & Hart, 1948, Priede, 1977), represents the oxygen available – in excess of that required for basic maintenance of the organism – for activities essential to support biological fitness (e.g. activity, feeding and reproduction). In principle, a species may perform optimally at a particular temperature (Topt); above or below Topt, the aerobic scope narrows and therefore performance falls (Pörtner & Farrell, 2008). The temperature range of optimal performance spans Topt and is delimited by upper and lower pejus temperatures (Frederich & Pörtner, 2000). The pattern has been demonstrated for several temperate species, such as Atlantic cod, Sockeye salmon, pink salmon, and banded morwong (Sylvestre et al., 2007, Farrell et al., 2008, Clark et al., 2011, Eliason et al., 2011, Neuheimer et al., 2011) and for an increasing number of tropical species. For example, aerobic scope may be reduced by as much as 65% in tropical reef fishes acclimated to temperatures only 2°C above their Topt (Nilsson et al., 2009, Gardiner et al., 2010, Johansen & Jones, 2011). Temperature-induced reductions in aerobic scope have ecological consequences because less energy is available for vital life history processes that shape population dynamics and community structure (Pörtner & Peck, 2010).
While experimental studies show that reef fish populations can be sensitive to small increases in water temperature (Nilsson et al., 2009, Johansen & Jones, 2011), many species also have large geographical ranges that span temperature ranges that are greater than the projected increase in ocean temperature due to global warming (Munday et al., 2008b). This suggests that populations are acclimatized or adapted to their local thermal environment along a latitudinal gradient. Adaptation to the very narrow equatorial temperature range may cause equatorial populations of reef fishes to be far more sensitive to global warming than populations of the same species from higher latitudes. The aim of this study was two-fold: 1) to determine if the temperature increases projected for the end of the century due to global warming have significant effects on the aerobic performance of equatorial reef fish populations, and 2) to determine whether their optimal temperature range for performance differs from that of higher latitude populations, suggesting local adaptation to higher average and maximum summer temperatures. Many reef fish species are numerically abundant across wide latitudes (Randall et al., 1997), and so we chose populations that may be experiencing the highest and narrowest range of environmental temperatures when compared to higher latitude counterparts. We also chose species for which data were already available from higher latitude populations (Nilsson et al., 2009, Gardiner et al., 2010, Johansen & Jones, 2011). Specifically, we selected four widely distributed species of damselfishes (family Pomacentridae), an abundant and diverse guild of coral reef fishes (Allen, 1991), and two species of cardinal fishes (family Apogonidae) that have been found to exhibit marked temperature sensitivity in previous studies (Gardiner et al., 2010). Species were also chosen based on their similar habitat preferences, all being strongly coral associated. The damselfishes are all planktivorous, whereas the cardinal fishes are nocturnal predators, feeding on small invertebrates. Despite dietary differences, we expected all species to exhibit similar metabolic requirements, given that they all utilize the median paired fin locomotory mode – predominantly using their pectoral fins for swimming and manoeuvringon the reef (Fulton, 2007). We predicted that: (1) the thermal range where aerobic scope is optimum would be closely associated with the temperature range equatorial populations presently experience (28–31°C), (2) aerobic scope would decline sharply at elevated temperatures consistent with global warming scenarios (2–3°C above present day) and (3) the optimal thermal range of each species would be narrower for equatorial populations compared to populations of the same species, or sister species, from higher latitudes.
Materials and Methods
Study site and species
This study was conducted at Nago Island, New Ireland Province, Papua New Guinea (2° 35.765' S; 150° 46.193' E; PNG). Nago Island is the closest near-equatorial location that is both readily accessible and at approximately the same longitude to previous studies on the thermal sensitivity of reef fishes conducted on the Great Barrier Reef (GBR) (Nilsson et al., 2009, Gardiner et al., 2010), thereby enabling comparisons with studies conducted at higher latitudes. Four damselfish species (mean mass ± SD; mean standard length ± SD), Dascyllusmelanurus (3.02±0.87g; 39.5±3.8mm), Chromisatripectoralis (2.62 ±0.65g; 43.7 ±5.1mm), Pomacentrus moluccensis (2.94 ±0.97g; 40.4 ±3.8mm), and Acanthochromis polyacanthus (4.43 ±1.31g; 47.8 ±5.5mm) were selected to compare to previously investigated species (Nilsson et al., 2009, Gardiner et al., 2010). The exception was D. melanurus, which has a distribution centred at the equator and might be expected to have higher thermal optima than its congener from higher latitudes (D. aruanus) investigated in previous studies. Two cardinal fish species (mean mass ± SD; mean standard length ± SD), Zoramialeptacantha (1.02 ±0.16g; standard lengths unavailable) and Cheilodipterus quinquelineatus (3.31 ±0.83g; 54.4±5.1mm) were also selected, the former specifically for its equatorial distribution and the latter as a more broadly distributed species (Randall, 2005).
Fish were collected from shallow reefs near Nago Island using a barrier net or hand nets and clove oil anaesthetic (Munday & Wilson, 1997). Fish were maintained at the National Fisheries Authority’s Nago Island Mariculture and Research Facility inaquaria supplied with flow-through seawater at ambient summer temperatures (30°C) for 3–4d. When normal feeding behaviour had resumed, fish were separated into four temperature treatment groups. Fish were fed to satiation twice daily with aquaculture pellets (damselfishes)(NRD pellets, INVE Aquaculture, Salt Lake City, USA) or hatched Artemia spp. (cardinal fishes). Food was withheld 24h prior to experimentation to ensure a post-absorptive state (Niimi & Beamish, 1974), a time we determined sufficient for damselfish and cardinal fish of this size (Rummer, unpublished data). Furthermore, holding tanks were maintained free from algae to ensure feeding only occurred during prescribed times (e.g. grazing on tank algae could not substantially contribute to metabolic demand). All animal care and experimental protocols complied with James Cook University ethics regulations (permit: A1722).
In August of 2011, a submersible temperature logger (Odyssey™ Dataflow Systems PTY Limited, Christchurch, New Zealand) was deployed (2° 39.904' S; 150° 44.006' E) at a depth of 1m near the areas where fish were collected for this study and set to record water temperatures every 30min until the logger was retrieved in March 2012. This timeframe spans the coolest and warmest seasonal temperatures in the region. Data from that temperature logger were used to estimate the maximum, minimum, and average temperatures these particular populations experience in their natural habitat.
Temperature treatments
Four temperature treatments were selected (29, 31, 33 and 34°C) that represented the range of summer ocean temperatures experienced at the study location (approximately 29–31°C) and a possible 2–3°C increase in temperature due to global change (33 and 34°C). Six aquaria were assigned to each temperature treatment and 10–12 fish per species were distributed evenly among aquaria. Aquaria were supplied with a constant flow of seawater, aerated by means of an electric air pump, and heated using 300-watt submersible heaters (EHEIM GmbH& Co. KG, Deizisau, Germany). Because ambient outside air temperature was on average 30°C, additional aquaria were set up in an air-conditioned room (adjusting lights for a 12:12 photoperiod) to maintain water temperatures at the lowest temperature treatment (29°C). All other aquaria were maintained under shelter outside the laboratory. Temperature in treatment aquaria was increased or decreased at a rate of 0.5°C per day until the desired temperature was reached. Fish were then maintained at treatment temperatures for 12–14d prior to experimentation, which is thought to be sufficient time for metabolic acclimation to warmer temperatures (Barrioneuvo and Fernandes, 1998; Nilsson et al., 2010). Temperatures in each aquarium were measured four times daily and maintained within ±0.2°C of the desired temperature. All fish survived and continued feeding at all temperatures investigated (29, 31, 33, and 34°C) with the exception of A. polyacanthus. After 2d at 34°C, A. polyacanthus individuals stopped eating, and we recorded 100% mortality within 7d. All other species continued eating throughout the 34°C exposure period, although we observed feeding to noticeably decrease at 34°C when compared to the lower holding temperatures.
Resting and maximum oxygen consumption
The use of resting respirometry chambers has been found to provide a reliable estimate of standard or resting metabolic rates (Clark et al., 2013; Roche et al., 2013). Therefore, intermittent-flow respirometry was used to determine resting O2 consumption rates (ṀO2Rest) for 8 individuals of each species at their respective temperature treatments. Respirometry was only performed during daylight hours (on average 3 trials per day), and species and temperature treatments were randomized. Fish were placed individually into 285-ml darkened respirometry chambers submerged in a temperature-controlled aquarium at the same temperature as their experimental treatments and allowed 90min to habituate to the chamber. In preliminary experiments, we could see (but the fish could not see us or their neighbors) that these small, warm water fishes calmed inside the covered resting chambers very quickly, and that 90min was ample time to ensure O2 consumption rates had reached the lowest possible values, beyond which O2 consumption rates did not significantly vary. After 90min., the O2 consumption rates no longer deviated or continued to decrease for at least 8 hours (Supp. Info., Fig. S1.). Submersible pumps supplied a constant water flow (150 l h−1) from the aquaria into and through the chambers while a peristaltic pump (Gilson, Inc. MINIPULS® 3) maintained flow within each chamber (maximum, 5340 ml h−1). Then, water flow into each chamber was stopped for 15min every 30min over a period of at least 90min. The time the water flow was interrupted was short enough to ensure O2 did not fall below 80% saturation (see best practices in Clark et al., 2013). Temperature-compensated O2 concentration (mg l−1) vs. time (s) of the water within each chamber was continuously recorded (1 s−1) using oxygen-sensitive REDFLASH dye on contactless spots (2mm) adhered to the inside of each chamber and linked to a Firesting Optical Oxygen Meter (Pyro Science e. K., Aachen, Germany) via fibre-optic cables. Data were analyzed using LabChart version 6.1.3, (AD Instruments, Colorado Springs, CO, USA). The first and last minute of each slope were excluded from analysis to maintain consistency. Then, ṀO2Rest (mg kg−1 h−1) was calculated from the average of the last three slopes of O2 consumed per second (R2 ≥ 0.90), minus the background O2 consumption. Background O2 consumption was determined for each chamber at the start of each trial prior to introducing a fish into a chamber, and again at the end of each trial after removing the fish. Initial background O2 consumption as well as a proportion of the additional background O2 consumption that had (assumed linearly) accumulated during the trial was subtracted from each slope. Whenever background O2 consumption rates exceeded 5% of the ṀO2Rest of the fish, we rinsed the chambers with a 10% bleach solution and then soaked them in clean water. Temperature quotients (Q10) were calculated using mean ṀO2Rest values (R1 and R2) for each species at low (T1 = 29°C) and high (T2 = 34°C) temperature using the following equation: Q10 = (R2/R1)[10/(T2−T1)].
Following ṀO2Rest fish were held in individual mesh baskets for up to 1h prior to determining maximum O2 consumption rates (ṀO2Max). Fish were then placed individually into a 770-ml sealed cylinder submerged in a temperature-controlled aquarium at the same temperature as their experimental treatment. A water current within the cylinder was created using a magnetic stirring bar and plate (below the cylinder), and the water speed was increased over a period of ~1 min. to the maximum speed at which the fish could sustain a steady position (defined as maximum sustained swimming speed; see (Nilsson et al., 2007) for further details). If the fish could no longer hold position in the chamber, the speed was decreased slightly until steady swimming resumed. The decrease in O2 concentration in the cylinder was monitored with an oxygen probe (WTW OXI 340i, Germany) while the fish was swimming for a period of 6 min., which was enough time to obtain a maximum rate of O2 consumption but short enough such that the water O2 in the chamber did not fall below 80% saturation. The ṀO2Max was calculated as the decrease in oxygen concentration over that period of time when the fish was swimming maximally, but otherwise as described above for ṀO2Rest. Absolute (ṀO2Max − ṀO2Rest) aerobic scope was calculated for each fish.
Latitudinal temperature variations and aerobic scope comparisons
Historical temperature data were obtained from the Integrated Global Ocean Services System (IGOSS) satellite sea surface temperatures taken in 1° latitude/longitude grids near Kavieng (2.5° S; 150.5° E) monthly from November 1982 until April 2012. Temperature data were also compiled from near two other research stations within approximately the same longitude for a total latitudinal range of approximately 2,321 km (courtesy of J. Lough, Australian Institute of Marine Science). Specifically, Lizard Island (14.5° S; 145.5° E) and Heron Island (23.5° S; 152° E) GBR, Australia, were chosen because data exist for aerobic scope Topt for several of the same fish species examined in the current study (Nilsson et al., 2009, Gardiner et al., 2010). The summer maximum, average, and minimum as well as the annual average and winter minimum sea surface temperatures were plotted for each location for comparison.
Local temperatures and the thermal range where aerobic scope was greatest were compiled for 5 species of damselfishes represented by 3 populations. In addition to the present results from PNG, data for C. atripectoralis, P. moluccensis, and A. polyacanthus were also investigated at Lizard Island (experimental temperatures ranging 29–33°C) and Heron Island (27–33°C) during two previous studies (Nilsson et al., 2009, Gardiner et al., 2010). However, D. melanurus was only investigated at the equatorial site (PNG). The sister species, D. aruanus, was investigated at Lizard (29–33°C) and Heron (27–32°C) Islands and was therefore used for those locations. Data from the two previous studies (Nilsson et al., 2009, Gardiner et al., 2010) were collected in an almost identical manner as in this study with the exception of respirometry chamber size. Raw data for ṀO2Rest and ṀO2Max from both of these previous studies in addition to further data collected to increase sample sizes and add temperature groups (unpublished data, N. Gardiner) were used to compare to data collected in the current study (Fig. S2). To do this, all aerobic scope values were recalculated and ranked from highest to lowest within each species from each location. In addition to the 32 measurements for each species (24 for A. polyacanthas) we derived from the present study, we used 40, 34, 34, and 16 measurements from Heron Island data and 38, 33, 31, and 32 measurements from Lizard Island data for P. moluccensis, A. polyacanthus, C. atripectoralis, and D. aruanus respectively. The top 25% of those values were selected and the corresponding acclimation temperatures at which those fish performed were reported as mean ± SD. This represented the temperature range for optimal aerobic performance for each species at each location. Optimal performance range data were superimposed onto the historical temperature data for each location. The data from the past experiments (Nilsson et al., 2009, Gardiner et al., 2010) are directly comparable to the findings of the present study as nearly the same experimental protocol and comparable equipment were used to assess the different fish populations representing different latitudes and climatologies.
Statistical analyses
Data are presented as mean ± SEM or SD as specified. One-way ANOVAs and Holm-Sidak post-hoc tests (as necessary) were used to compare aerobic scope between treatment temperatures for each species post-exercise. For the latitudinal comparison between species and locations, a one-way ANOVA was used to compare the mean of the optimal performance temperature range and Brown-Forsythe and Bartlett's tests used to compare SD. Statistical analyses were conducted using SigmaPlot for Windows 11.0.0.77 (Systat Software, Inc., Chicago, IL, USA) and Prism 6 (GraphPad Software, Inc. La Jolla, CA, USA).
Results
Resting and maximum oxygen consumption
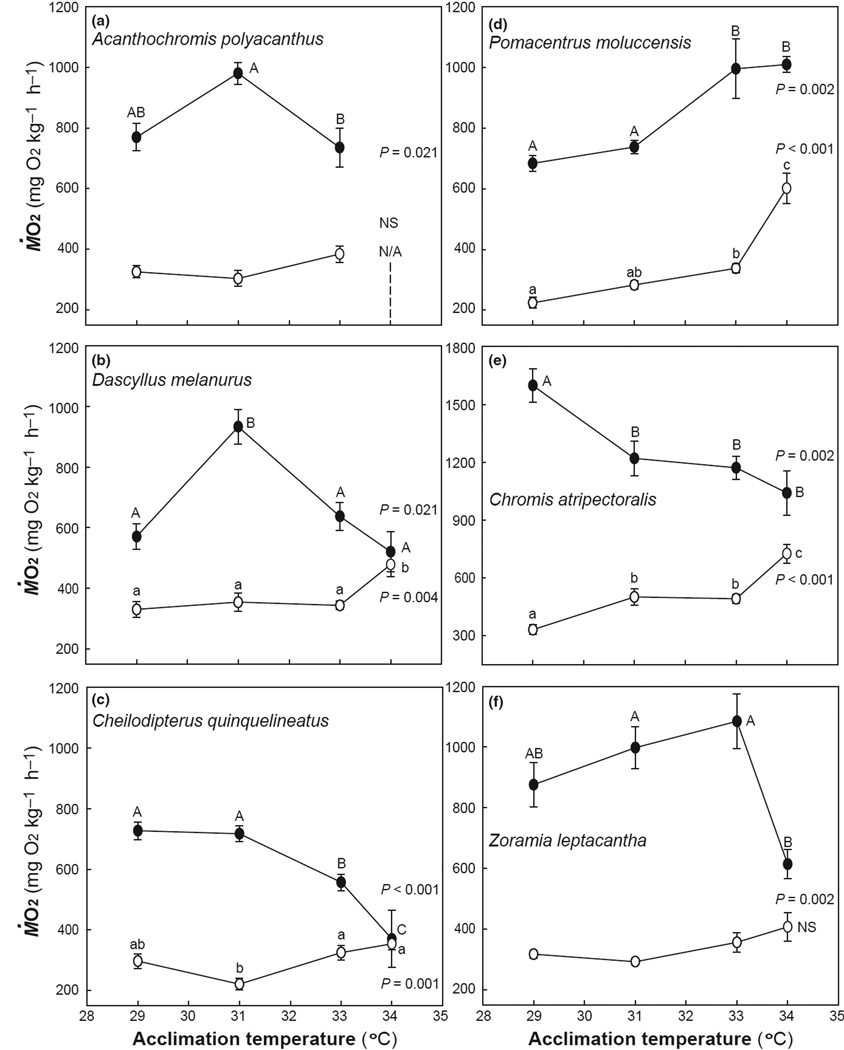
Of the six coral reef fish species investigated, three exhibited a significant increase in ṀO2Rest between 33 and 34°C (Fig. 1). In one species, C. atripectoralis, ṀO2Rest was significantly elevated between 29 and 31°C and then further elevated between 33 and 34°C (Fig. 1e). Two species exhibited no significant change in ṀO2Rest with elevated temperatures (Figs. 1a, 1f). Temperature quotients (Q10) were calculated to characterize the influence of temperature on metabolic rate, which typically doubles or triples (i.e., Q10 = 2–3) with each 10°C increase in ambient temperature. For D. melanurus, the Q10 calculated for ṀO2Rest between 29 and 34°C was 2.1, whereas the response to temperature was greater in P. moluccensis and C. atripectoralis (Q10 = 4.8 and 7.2, respectively). All remaining calculated Q10 values were <2.0 (Table 1).
Fig. 1.
Resting (open circles; ṀO2Rest) and maximum (filled circles; ṀO2Max) O2 consumption rates for six coral reef fishes investigated upon acclimation to 29, 31, 33, and 34°C. Measurements were not possible for A. polyacanthus at 34°C (indicated by N/A). All graphs are scaled identically with the exception of C. atripectoralis. All data are means ± S.E.M., n=8 for each species and each temperature. Within a species, significant differences between resting values are demarcated by lower-case letters; whereas, significant differences between maximum values are demarcated by upper-case letters. Statistical significance (one-way ANOVA) is indicated by the P-value. NS indicates a non-significant relationship.
Table 1.
Temperature quotients (Q10) calculated for the changes in ṀO2Rest between acclimation temperatures.
| Species | Q10 values | ||
|---|---|---|---|
| 29–31°C | 29–33°C | 29–34°C | |
| A. polyacanthus | 0.7 | 1.5 | n/a |
| D. melanurus | 1.4 | 1.1 | 2.1 |
| C. quinquelineatus | 0.2 | 1.3 | 1.4 |
| P. moluccensis | 3.2 | 2.8 | 7.2 |
| C. atripectoralis | 7.8 | 2.7 | 4.8 |
| Z. leptacantha | 0.7 | 1.3 | 1.7 |
Note: A. polyacanthus did not survive when held at 34°C. Therefore, no ṀO2Rest values are available for calculating the 29–34°C Q10 for that species.
For four of the six species ṀO2Max was highest at 29 or 31°C, and above 31°C ṀO2Max was reduced significantly (Fig. 1). The exceptions were Z. leptacantha and P. moluccensis. The ṀO2Max of Z. leptacantha remained unchanged between 29 and 33°C, but fell at 34°C; whereas, the ṀO2Max of P. moluccensis increased between 31 and 33°C and remained elevated at 34°C (Figs. 1b and f). ṀO2Max ranged from approximately 700 to 1,100 mg O2 kg−1 h−1 for all species except for C. atripectoralis, which had a ṀO2Max ranging from approximately 1,000 to 1,600 mg O2 kg−1 h−1 (Fig. 1e).
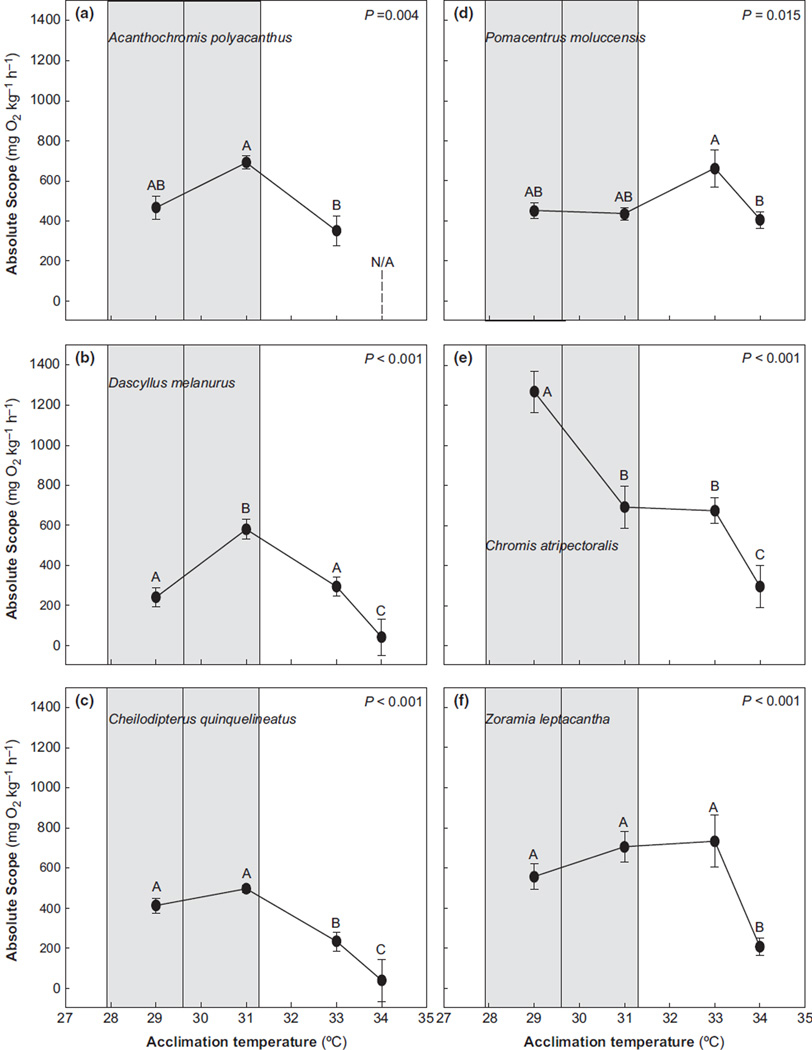
Collectively, the changes in ṀO2Rest and ṀO2Max resulted in significant peaks (Topt) or plateaus in absolute aerobic scope at a maximum temperature of 31°C for A. polyacanthus, D. melanurus, and C. quinquelineatus and 33°C for Z. leptacantha. The highest aerobic scope value for C. atripectoralis was calculated at 29°C, but because we did not test fish at lower temperatures, we do not know if this is a peak or plateau (Fig. 2e). The other five species, on average, increased aerobic scope from 29°C by at least 20%, and by as much as 140%, at their optimal temperatures for aerobic scope. Beyond 31°C, scope decreased significantly by 49% in A. polyacanthus and D. melanurus (Fig. 2a,b), and by 53% in C. quinquelineatus (Fig. 2c), whereas a significant reduction in scope did not occur until 33–34°C in P. moluccensis (39%; Fig. 2d) and Z. leptacantha (72%; Fig. 2f). In contrast, aerobic scope significantly declined by 45% between 29 and 31°C in C. atripectoralis (Fig. 2e).
Fig. 2.
Absolute aerobic scope (ṀO2Max - ṀO2Rest) for six coral reef fishes investigated upon acclimation to 29, 31, 33, and 34°C and the range of temperatures recorded for the site near where fish were collected. Measurements were not possible for A. polyacanthus at 34°C (indicated by N/A). All data are means ± S.E.M., n=8 for each species and each temperature. Within a species, significant differences between values are demarcated by lower-case letters; whereas, significant differences between maximum values are demarcated by upper-case letters. Statistical significance is indicated by the P-value. The shaded gray area demarcates the minimum, average, and maximum temperatures recorded between August 2011 and March 2012 by data loggers deployed at a depth of 1m near where fish were collected.
Absolute aerobic scope was plotted for each of the six species in conjunction with the minimum (27.9°C), average (29.6°C), and maximum (31.3°C) temperatures logged between August 2011 and March 2012 near where fish were collected (Fig. 2). For A. polyacanthus, D. melanurus, C. quinquelineatus and C. atripectoralis, aerobic scope was found to benumerically highest (Topt) at temperatures immediately within the 3.4°C range recorded during the 8 month temperature logging period. By comparison, the Topt for aerobic scope of P. moluccensis and Z. leptacantha fell above the maximum temperature. The numerically highest aerobic scope for P. moluccensis and Z. leptacantha was 33°C, but Z. leptacantha also maintained an elevated aerobic scope (no significant differences from 33°C) at 29 and 31°C (Fig. 2f).
Latitudinal temperature variations and aerobic scope
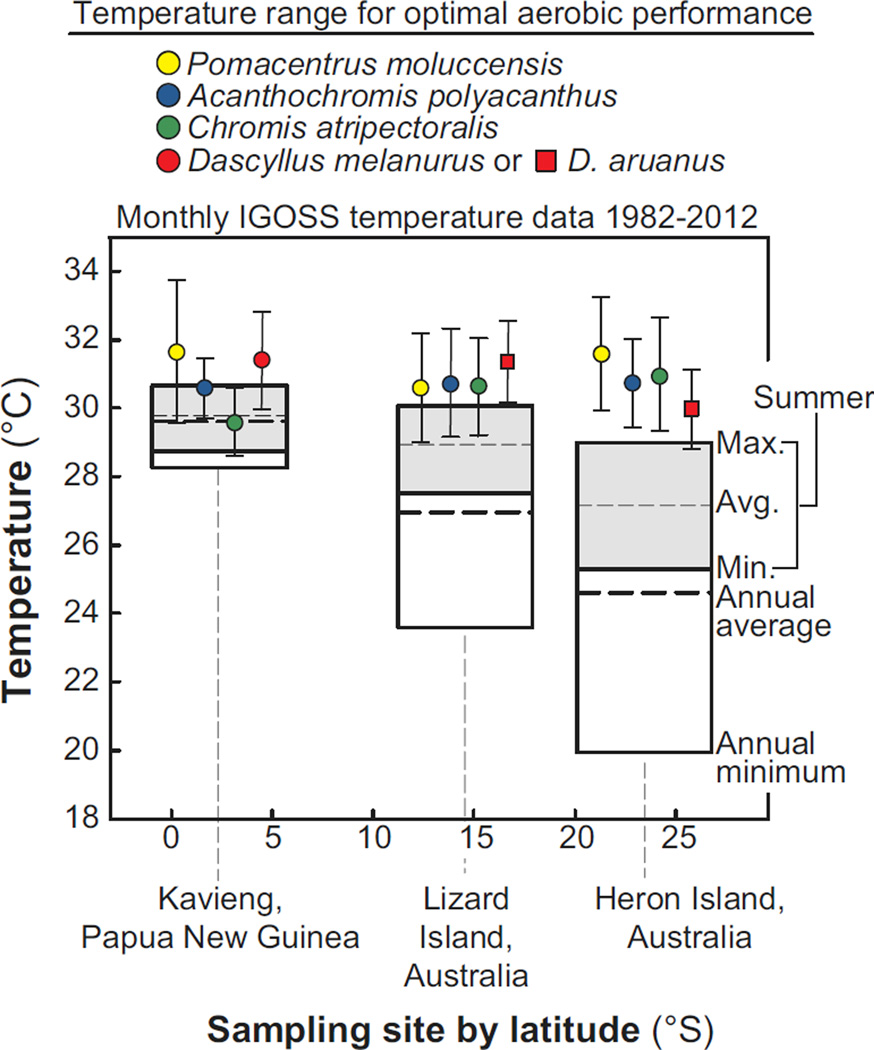
The optimal performance range for the four damselfish species was plotted in conjunction with data compiled from previous studies for the same species investigated at sites near Lizard Island (experimental temperatures ranging 29–33°C) and Heron Island (27–33°C), GBR, Australia (Nilsson et al., 2009, Gardiner et al., 2010) (Fig. S2) and the IGOSS satellite sea surface temperatures taken near all three locations between November 1982 and April 2012 (Fig. 3). The only exception was that D. melanurus was investigated in the current study, whereas the congener, D. aruanus, was studied at both Lizard and Heron Islands. There were no statistical differences in the mean of the optimal performance range (one-way ANOVA) or SD (Brown-Forsythe test and Bartlett's test) among populations in any of the species investigated. Consequently, when optimal performance range (i.e. mean ± SD) was plotted in conjunction with the historical temperature data, distinct differences were apparent between populations. For the equatorial populations, the optimal performance range mean was below the average summer temperature for only one of the species examined (C. atripectoralis), and the range of optimal performance overlapped with the span of the minimum and maximum summer temperatures for all species examined. By contrast, at the highest latitude, Heron Island, not only the mean, but the optimal performance range exceeded the maximum summer temperature in all but one species (D. aruanus). The pattern at Lizard Island was intermediate to PNG and Heron Island. None of the means of the optimal performance range were below the average or maximum summer temperatures, but the optimal performance range for three of the four species overlapped the maximum summer temperatures.
Fig. 3.
Local temperatures and the temperature range for optimal aerobic performance for 5 species of damselfishes represented by 3 populations spanning 2,321 km from the southern Great Barrier Reef (GBR) to Papua New Guinea. Sampling sites are represented by their latitudinal position (°S). Each species is represented by a unique colour. Note: D. melanurus was only investigated at the equatorial site; whereas, D. aruanus was investigated only at Lizard and Heron Islands. All individuals tested within each species and population were ranked by maximal aerobic scope. The top performers (the top 25% of aerobic scope values) within each species and population were selected, and the corresponding temperatures were plotted on this graph as mean ± SD to represent the range of optimal performance. Gray rectangles represent the summer maximum, average, and minimum sea surface temperatures for each site. A thin, hashed line and a thick, hashed line represent each site’s annual summer average and overall average, respectively. The temperature blocks extend to include each location’s annual minimum sea surface temperatures as well. Sea surface temperatures are from monthly IGOSS data collections between 1982 and 2012 (see Materials and Methods for further details).
Discussion
Equatorial populations of four of the six species investigated in this study appear to be living at or above their optimal temperatures for aerobic performance. One of these species (C. atripectoralis) exhibited a decrease in aerobic scope by 45% between 29 and 31°C, temperatures that encompass the current-day annual range for the study site. Interestingly, the other two species (P. moluccensis and Z. leptacantha) maintained aerobic scope when held at temperatures approximately 2°C above summer maxima. However, for all species held at 3°C above current-day temperatures, aerobic scope declined and even approached zero in two species, D. melanurus and C. quinquelineatus. One species, A. polyacanthus, was unable to survive short-term exposure to 34°C. This species is a direct developer, which is not the norm among reef fishes, and is known to exhibit strong genetic structure among populations (Bay et al., 2008). Consequently, A. polyacanthus populations may exhibit stronger local adaptation of their critical thermal limits than other species, leading to a very steep decline in performance at the higher temperatures. Aerobicscope has been classically referred to as a proxy for individual performance (Fry, 1971); although, potential limitations with this idea have been outlined in a recent review (Clark et al. 2013). Still, the significant temperature-induced reductions in aerobic scope observed here in all species at 3°C above current-day temperatures may be expected to have ecological consequences because less energy may be available for vital life history processes such as growth, reproduction, and predator-prey interactions (Pörtner & Peck, 2010). Our results are consistent with the hypothesis that equatorial populations of marine organisms that experience a narrow range of seasonal temperatures may be especially vulnerable to elevated temperatures due to global warming.
Resting and maximum oxygen consumption
A decreased capacity to perform aerobically (reduced aerobic scope) is predicted by some to be key in determining the response of marine fishes to increased ocean temperature (Pörtner, 2001, Pörtner & Knust, 2007). Our results suggest that equatorial populations may have optimized the rate functions that sustain metabolism to within or below the narrow temperature range they experience annually (27.9–31.3°C). Within this range, resting O2 consumption rates (a proxy for basal metabolic needs) remain low, while maximum O2 consumption rates remain high. Beyond this temperature range, however, an increase in ṀO2Rest,a decrease in ṀO2Max, or both contribute to decreasing aerobic scope by 39–72% for four of the species we investigated. Our results highlight the need to understand how temperature influences ṀO2Rest and ṀO2Max individually, as well as combined (ṀO2Max–ṀO2Rest) as aerobic scope.
Resting metabolic rate, like many biological rate functions, exhibits a predictable response to temperature (an approximate doubling or tripling for every 10°C increase, Q10≈2–3) (Schmidt-Nielsen, 1990, Clarke & Johnston, 1999). When ṀO2Rest increases with temperature, the minimum energy required for basal maintenance of the organism is increased. In two of the damselfish species investigated here, the increase in ṀO2Rest between 29 and 34°C was dramatic (Q10 = 7.2, 4.8, Table 1). In other words, at 34°C, over twice as much energy is required for these species to maintain routine metabolic processes than at 29 or 31°C. Interestingly, this occurred in these two species with little change in ṀO2Max. We hypothesize that the high Q10 values could be a product of the stable, narrow thermal range experienced by these small tropical fishes. If tropical fishes are locally adapted to their thermal environment, do not regularly experience temperature fluctuations, and do not move far from these microhabitats, there would be no drive to possess such metabolic compensation. Therefore, the surprise might actually be the species exhibiting a Q10 of ~2. For example, D. melanurus held at 34°C exhibited a moderate 45% increase in resting metabolic rates (Q10 = 2.1, Table 1); however, ṀO2Max decreased such that no differences could be detected between ṀO2Rest and ṀO2Max and aerobic scope neared zero (Fig. 1b, 2b). Thus, in D. melanurus, the sharp decrease in ṀO2Max contributed more to the diminished aerobic scope at 34°C than the increase in ṀO2Rest (Supp. Info.). Along with D. melanurus, three other reef fishes we investigated exhibited Q10 values less than 2 for ṀO2Rest (Table 1) and thus, in theory, the capacity to largely offset the temperature-induced increase in resting metabolic rates within the temperature range tested. The cost for these species, however, may be at the level of maximal performance, as ṀO2Max was largely temperature-dependent. The behaviour and functional role of each species on the reef will dictate at which level saving energy will be most important. For example, the trade-off for some species may a loss of maximum aerobic performance in order to save energy at rest, whereas others may survive increased costs at rest as long as they can maintain maximum aerobic performance. It is therefore important to investigate both the trends in resting and maximum metabolic rates and each species’ behaviour and functional role within its ecosystem when interpreting what is driving declines in their aerobic scope.
Thermal specialization in equatorial species/populations
We observed variations in thermal sensitivity and potentially metabolic efficiency among closely related species. Our findings reflect a mix of equatorial specialists and geographically widespread species (thermal generalists) at our study site in northern PNG. Near the equator, D. aruanus is largely replaced with its congener D. melanurus (Allen, 1991). With a high Q10 and the most bell-shaped aerobic scope thermal reaction norm (Fig. 2b), D. melanurus may be an equatorial specialist, predominating more thermally stable, low latitude habitats, without the extensive latitudinal range of congener, D. aruanus. The geographically widespread C. atripectoralis, a species that occurs all the way to the most southerly coral reefs in Australia, exhibited a decrease in aerobic scope across all temperatures, the lowest Topt and optimal performance range of the six species investigated and a high Q10, suggesting some level of temperature sensitivity in equatorial populations of this species. In contrast, we observed that the notably thermally-tolerant sister species damselfish, C. viridis (Nilsson et al., 2009), was largely absent from our study site. The lemon damsel, P. moluccensis, is also geographically widespread and exhibited an optimal performance range ranging from just under 30 to almost 34°C, which extends beyond current day maximum temperatures at all latitudes (Nilsson et al., 2009, Gardiner et al., 2010). Compared to damselfishes, cardinal fishes were expected to exhibit marked temperature sensitivity (Gardiner et al., 2010). However, both species investigated exhibited relatively wide thermal optima, centered around 31°C for the widely distributed C. quinquelineatus but ranging 29–33°C for the tropical specialist, Z. leptacantha. Whole organism performance clearly declined, if not diminished at 34°C in at least five of the species we examined; notably, we were not able to hold A. polyacanthus at 34°C, even for a short period of time. There have been other instances in the literature where a species’ aerobic scope optimal temperature is very close to lethal temperatures (Clark et al., 2013), and this warrants further investigation. It may be that A. polyacanthus, especially equatorial populations, will be especially vulnerable to ocean temperatures projected to occur by the end of this century. Our result show that the Topt for aerobic performance may closely resemble local temperature ranges for equatorial populations, but may also indicate that the area is not comprised strictly of equatorial specialists.
Latitudinal temperature variations and aerobic scope
We compared the thermal range for which the aerobic scope was the greatest between the three latitudinally distinct populations of damselfishes to determine whether each population’s temperature range for optimal performance matched its local thermal environment. Two important patterns emerged from this comparison. First, all three populations seem to have approximately the same mean range of optimal performance despite 21° of latitudinal separation (Fig. 3). Second, the optimal performance range only closely matched the range of summer temperatures experienced for the equatorial populations; whereas, the optimal performance range extended beyond local temperature maxima for higher latitude populations. At Heron Island, the site at the highest latitude, optimal performance range exceeded the maximum summer temperature experienced in all but one species. Similar patterns have been described for terrestrial ectotherms, and suggest that, indeed, low latitude species are already living close to their thermal optima and will be most vulnerable to a changing climate (Deutsch et al., 2008, Tewksbury et al., 2008, Nguyen et al., 2011) if adaptation cannot keep pace with increasing temperatures. In contrast, higher latitude species are living in climates currently cooler than their aerobic scope thermal optima and may therefore possess a wider thermal safety margin; climate change increases in sea surface temperatures may even enhance fitness in high latitude populations.
The similar optimal performance range among populations may suggest a lack of local adaptation in aerobic scope; however, our latitudinal comparisons did come with several assumptions, and there are alternative conclusions (Supp. Info.). For example, because aerobic scope declined at temperatures lower than the highest experimental temperature used in the current study (34°C), we assume we captured the upper limits to aerobic performance for the species we investigated. For some of the Lizard and Heron Island populations, however, the variation around the mean of the optimal performance range extended to the maximum experimental temperature (33°C), suggesting that the upper thermal limit for aerobic performance could be even higher and therefore the optimal performance range broader in some of the high latitude populations. Furthermore, high latitude populations might be adapted to a greater range of seasonal temperatures that they would normally experience compared with low latitude populations, leading to improved capacity to perform at a range of temperatures, including higher temperatures outside the normal range experienced. This could produce an apparent mismatch between the average temperatures experienced in high latitude populations and the temperatures where aerobic scope is greatest. Consequently, our results could indicate that reef fish populations are more closely adapted to seasonal temperature variations than they are to the average temperatures they experience.
Due to less thermal variability and thus, narrower thermal safety margins, the impact of global warming on coral reef fishes could be the greatest at low latitudes. If adaptation cannot keep pace with climate change, even relatively minor ocean warming (2–3°C) could result in population declines and potentially the redistribution of equatorial populations to higher latitudes (Nilsson et al., 2009, Nguyen et al., 2011, Sunday et al., 2012). Thermal acclimation (plasticity) may assist some species and populations in coping with future temperature increases; however, thermal performance ranges of stenothermal (e.g. tropical, low latitude, polar) species are expected to beless plastic than eurythermal (often temperate) species (Nguyen et al., 2011). Previous data exist to support minimal short-term capacity for acclimation in adults of two of the species we investigated, A. polyacanthus and P. moluccensis (Nilsson et al., 2010, Donelson et al., 2011). However, there could be an ontogenetic or trans-generational component, which may be more relevant to keeping pace with the rate of change occurring due to climate change. Indeed, new research has demonstrated developmental acclimation of resting O2 consumption rates and other life history metrics in A. polyacanthus reared for their entire life cycle at current and 3°C above current temperatures (Donelson et al., 2011). Exposure to elevated temperatures during the early stages of development may condition enzymatic processes to operate more efficiently later as adults (Nilsson et al., 2010), but there may be a cost at the level of diminished growth rates (Munday et al., 2008a, Donelson et al., 2011). A. polyacanthus has also been found to exhibit pronounced trans-generational thermal acclimation (Donelson et al., 2012), suggesting that the parental environment plays a role in the offspring’s response. Whether equatorial populations of reef fish, including A. polyacanthus, have the capacity for developmental and/or trans generational acclimation, however, is unknown and will be a topic of future studies.
Implications and conclusions
In an era of rapid climate change, understanding the link between organisms and environment will be increasingly important in developing management strategies for the conservation of marine biodiversity and the sustainable use of marine fisheries, particularly in the context of food security for dependent human communities. We have demonstrated here that equatorial populations of coral reef species are already living at temperatures close to their thermal optimum for aerobic scope and that even a small amount of ocean warming could cause a significant decline in aerobic scope in these populations. This is a significant advancement to our understanding of global change ecology for marine species. If aerobic scope is closely linked to individual performance (Pörtner and Farrell 2008; reviewed in Clark et al., 2013), then declines in this trait could have implications for long-term sustainability of equatorial fish populations and could drive a range shift of species away from their equatorial boundary (Munday et al., 2008b, Nguyen et al., 2011, Sunday et al., 2012). In addition to the implications for marine diversity, population declines and range shifts would also have significant consequences for human societies in the equatorial zone. There is a concentration of developing countries in the equatorial zone where fish are crucial to the livelihoods and survival of millions of people (FAO, 2012). Climate change may lead to redistribution of global catch potential, with the most significant declines likely to occur near the equator (Cheung et al., 2010). Thus, understanding the effect of global warming on reef fishes across their geographical ranges is critical for predicting likely changes in fisheries productivity. This will also be important indetermining the most ecologically and economically susceptible geographic locations and countries, and ultimately, tailoring mitigation, management and conservation strategies to effectively conserve the biodiversity of tropical marine environments and the ecosystems services they provide.
Supplementary Material
Acknowledgments
Funding for this study was from the Australian Research Council (J.L.R., P.L.M.), James Cook University (N.M.G.), the University of Oslo (G.E.N., J.A.W.S., C.S.C.) and the U.S. National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103395 (J.A.W.S). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Sylvia Kinch and the technical staff at the National Fisheries Authority’s Nago Island Mariculture and Research Facility for logistical support. Gratitude is also due to R. Neumann for technical/field assistance, I. McLeod for deploying and retrieving temperature loggers on site, and Dr. Sjannie Lefevre for editorial assistance. We also greatly appreciate the advice and access to temperature data from Prof. Janice Lough (Australian Institute of Marine Science).
References
- Allen GR. Damselfishes of the World. Melle, Germany: Mergus Publishers; 1991. [Google Scholar]
- Angilletta MJ. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford University Press; 2009. [Google Scholar]
- Barrionuevo WR, Fernandes MN. Time-course of respiratory metabolic adjustments of a South American fish, Prochilodus scrofa, exposed to low and high temperatures. Journal of Applied Ichthyology. 1998;14:37–41. [Google Scholar]
- Bay LK, Caley MJ, Crozier RH. Meta-population structure in a coral reef fish demonstrated by genetic data on patterns of migration, extinction and re-colonisation. BMC Evolutionary Biology. 2008;8:248. doi: 10.1186/1471-2148-8-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellwood DR, Hughes TP, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- Cheung WWL, Lam VWY, Sarmiento JL, Kearney K, Watson REG, Zeller D, Pauly D. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Global Change Biology. 2010;16:24–35. [Google Scholar]
- Clark TD, Sandblom E, Jutfelt F. Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. The Journal of Experimental Biology. 2013;216:2771–2782. doi: 10.1242/jeb.084251. [DOI] [PubMed] [Google Scholar]
- Clark TD, Jeffries KM, Hinch SG, Farrell AP. Exceptional aerobic scope and cardiovascular performance of pink salmon (Oncorhynchus gorbuscha) may underlie resilience in a warming climate. The Journal of Experimental Biology. 2011;214:3074–3081. doi: 10.1242/jeb.060517. [DOI] [PubMed] [Google Scholar]
- Clarke A, Johnston NM. Scaling of metabolic rate with body mass and temperature in teleost fish. Journal of Animal Ecology. 1999;68:893–905. [Google Scholar]
- Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelson JM, Munday PL, Mccormick MI, Nilsson GE. Acclimation to predicted ocean warming through developmental plasticity in a tropical reef fish. Global Change Biology. 2011;17:1712–1719. [Google Scholar]
- Donelson JM, Munday PL, Mccormick MI, Pankhurst NW, Pankhurst PM. Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Marine Ecology Progress Series. 2010;401:233–243. [Google Scholar]
- Donelson JM, Munday PL, Mccormick MI, Pitcher CR. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nature Clim. Change. 2012;2:30–32. [Google Scholar]
- Eliason EJ, Clark TD, Hague MJ, et al. Differences in thermal tolerance among sockeye salmon populations. Science. 2011;332:109–112. doi: 10.1126/science.1199158. [DOI] [PubMed] [Google Scholar]
- Eme J, Bennett WA. Critical thermal tolerance polygons of tropical marine fishes from Sulawesi, Indonesia. Journal of Thermal Biology. 2009b;34:220–225. [Google Scholar]
- FAO. The state of world fisheries and aquaculture (SOFIA) In: Food and Agriculture Organization of the United Nations, editor. FAO, Rome; 2012. [Google Scholar]
- Farrell AP, Hinch SG, Cooke SJ, Patterson DA, Crossin GT, Lapointe M, Mathes MT. Pacific salmon in hot water: Applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Physiological and Biochemical Zoology. 2008;81:697–709. doi: 10.1086/592057. [DOI] [PubMed] [Google Scholar]
- Frederich M, Pörtner HO. Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;279:R1531–R1538. doi: 10.1152/ajpregu.2000.279.5.R1531. [DOI] [PubMed] [Google Scholar]
- Fry FEJ. The effect of environment factors on the physiology of fish. In: Hoar WS, Randall DJ, editors. Fish physiology: Environmental relations and behavior. New York: Academic Press; 1971. [Google Scholar]
- Fry FEJ, Hart JS. The relation of temperature to oxygen consumption in the goldfish. The Biological Bulletin. 1948;94:66–77. [PubMed] [Google Scholar]
- Fulton CJ. Swimming speed performance in coral reef fishes: field validations reveal distinct functional groups. Coral Reefs. 2007;26:217–228. [Google Scholar]
- Ganachaud AS, Gupta AS, Orr JC, et al. Observed and expected changes to the tropical Pacific Ocean. In: Bell JD, Johnson JE, Hobday AJ, editors. Vulnerability of Tropical Pacific Fisheries and Aquaculture to Climate Change. Noumea, New Caledonia: Secretariat of the Pacific Community; 2011. [Google Scholar]
- Gardiner NM, Munday PL, Nilsson GRE. Counter-gradient variation in respiratory performance of coral reef fishes at elevated temperatures. PLoS ONE. 2010;5:e13299. doi: 10.1371/journal.pone.0013299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TP, Baird AH, Bellwood DR, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- IPCC. Climate change 2007: the physical science basis. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, 2007. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Johansen JL, Jones GP. Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Global Change Biology. 2011;17:2971–2979. [Google Scholar]
- Mora CM, Ospína AO. Tolerance to high temperatures and potential impact of sea warming on reef fishes of Gorgona Island (tropical eastern Pacific) Marine Biology. 2001;139:765–769. [Google Scholar]
- Munday P, Kingsford M, O’callaghan M, Donelson J. Elevated temperature restricts growth potential of the coral reef fish Acanthochromis polyacanthus. Coral Reefs. 2008a;27:927–931. [Google Scholar]
- Munday PL, Jones GP, Pratchett MS, Williams AJ. Climate change and the future for coral reef fishes. Fish and Fisheries. 2008b;9:261–285. [Google Scholar]
- Munday PL, Wilson SK. Comparative efficacy of clove oil and other chemicals in anaesthetization of Pomacentrus amboinensis a coral reef fish. Journal of Fish Biology. 1997;51:931–938. [Google Scholar]
- Neuheimer AB, Thresher RE, Lyle JM, Semmens JM. Tolerance limit for fish growth exceeded by warming waters. Nature Climate Change. 2011;1:110–113. [Google Scholar]
- Nguyen KDT, Morley SA, Lai C-H, Clark MS, Tan KS, Bates AE, Peck LS. Upper temperature limits of tropical marine ectotherms: Global warming implications. PLoS ONE. 2011;6:e29340. doi: 10.1371/journal.pone.0029340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi AJ, Beamish FWH. Bioenergetics and growth of largemouth bass (Micropterus salmoides) in relation to body weight and temperature. Canadian Journal of Zoology. 1974;52:447–456. doi: 10.1139/z74-056. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Crawley N, Lunde IG, Munday PL. Elevated temperature reduces the respiratory scope of coral reef fishes. Global Change Biology. 2009;15:1405–1412. [Google Scholar]
- Nilsson GE, Östlund-Nilsson S, Munday PL. Effects of elevated temperature on coral reef fishes: Loss of hypoxia tolerance and inability to acclimate. Comparative Biochemistry and Physiology - Part A. Molecular and Integrative Physiology. 2010;156:389–393. doi: 10.1016/j.cbpa.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Östlund-Nilsson S, Penfold R, Grutter AS. From record performance to hypoxia tolerance: respiratory transition in damselfish larvae settling on a coral reef. Proceedings of the Royal Society B. Biological Sciences. 2007;274:79–85. doi: 10.1098/rspb.2006.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst NW, Munday PL. Effects of climate change on fish reproduction and early life history stages. Marine and Freshwater Research. 2011;62:1015–1026. [Google Scholar]
- Poloczanska ES, Babcock RC, Butler A, et al. Climate change and Australian marine life. Oceanography and Marine Biology. 2007;45:407–478. [Google Scholar]
- Pörtner H-O. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88:137–146. doi: 10.1007/s001140100216. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comparative Biochemistry and Physiology - Part A. Molecular and Integrative Physiology. 2002;132:739–761. doi: 10.1016/s1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O, Farrell AP. ECOLOGY: Physiology and climate change. Science. 2008;322:690–692. doi: 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O, Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science. 2007;315:95–97. doi: 10.1126/science.1135471. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O, Peck MA. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. Journal of Fish Biology. 2010;77:1745–1779. doi: 10.1111/j.1095-8649.2010.02783.x. [DOI] [PubMed] [Google Scholar]
- Priede IG. Natural selection for energetic efficiency and the relationship between activity level and mortality. Nature. 1977;267:610–611. doi: 10.1038/267610a0. [DOI] [PubMed] [Google Scholar]
- Randall JE. Reef and shore fishes of the South Pacific: New Caledonia to Tahiti and the Pitcairn Islands. Honolulu: University of Hawai'i Press; 2005. [Google Scholar]
- Randall JE, Allen GR, Steene RC. Fishes of the Great Barrier Reef and Coral Sea. Honolulu: University of Hawai'i Press; 1997. [Google Scholar]
- Roche DG, Binning SA, Bosiger Y, Johansen JL, Rummer JL. Finding the best estimates of metabolic rates in a coral reef fish. The Journal of Experimental Biology. 2013;216:2103–2110. doi: 10.1242/jeb.082925. [DOI] [PubMed] [Google Scholar]
- Rushworth KJW, Smith SDA, Cowden KL, Purcell SW. Optimal temperature for growth and condition of an endemic subtropical anemonefish. Aquaculture. 2011;318:479–482. [Google Scholar]
- Schmidt-Nielsen K. Animal Physiology: Adaptation and Environment. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- Somero GN. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. The Journal of Experimental Biology. 2010;213:912–920. doi: 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- Stillman JH. Acclimation capacity underlies susceptibility to climate change. Science. 2003;301:65. doi: 10.1126/science.1083073. [DOI] [PubMed] [Google Scholar]
- Sunday JM, Bates AE, Dulvy NK. Global analysis of thermal tolerance and latitude in ectotherms. Proceedings of the Royal Society B. Biological Sciences. 2011;278:1823–1830. doi: 10.1098/rspb.2010.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday JM, Bates AE, Dulvy NK. Thermal tolerance and the global redistribution of animals. Nature Climate Change. 2012;2:686–690. [Google Scholar]
- Sylvestre E-L, Lapointe D, Dutil J-D, Guderley H. Thermal sensitivity of metabolic rates and swimming performance in two latitudinally separated populations of cod, Gadus morhua L. Journal of Comparative Physiology B. Biochemical, Systemic, and Environmental Physiology. 2007;177:447–460. doi: 10.1007/s00360-007-0143-x. [DOI] [PubMed] [Google Scholar]
- Tewksbury JJ, Huey RB, Deutsch CA. Putting the heat on tropical animals. Science. 2008;320:1296–1297. doi: 10.1126/science.1159328. [DOI] [PubMed] [Google Scholar]
- Zarco-Perelló S, Pratchett M, Liao V. Temperature-growth performance curves for a coral reef fish, Acanthochromis polyacanthus. Galaxea, Journal of Coral Reef Studies. 2012;14:1–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.