Abstract
Background
Trichomonas vaginalis is the most prevalent nonviral sexually transmitted infection in the United States, affecting 3.1% of women of reproductive age. Infection is associated with HIV acquisition and pelvic inflammatory disease. In the United States, Centers for Disease Control and Prevention guidelines recommend testing all women with vaginal discharge for T. vaginalis, but except for HIV-infected women, there are no national guidelines for screening asymptomatic persons. The objective of this analysis is to assess testing and screening practices for T. vaginalis among symptomatic and asymptomatic women in the sexually transmitted disease (STD) clinic setting.
Methods
We analyzed data on demographics, clinical presentation, and laboratory testing for all women visiting a clinician in 2010 to 2011 at any of 15 STD clinics participating in the STD Surveillance Network. Prevalence of laboratory-confirmed T. vaginalis infection was calculated among symptomatic women tested and among asymptomatic women screened.
Results
A total of 59,176 women visited STD clinicians: 39,979 were considered symptomatic and 19,197 were considered asymptomatic for T. vaginalis infection, whereas 211 were HIV-infected. Diagnostic practices varied by jurisdiction: 4.0% to 96.1% of women were tested or screened for T. vaginalis using any laboratory test. Among 17,952 symptomatic women tested, prevalence was 26.2%. Among 3909 asymptomatic women screened, prevalence was 6.5%. Among 92 HIV-infected women tested/screened, prevalence was 29.3%.
Conclusions
Trichomoniasis is common among STD clinic patients. In this analysis, most STD clinics tested symptomatic women seeking care, in accordance with national guidelines. All HIV-infected women should be screened annually. Additional evidence and national guidance are needed regarding potential benefits of T. vaginalis screening in other asymptomatic women.
Trichomonas vaginalis is the most prevalent curable sexually transmitted infection in the world.1 In the United States, according to the 2000 to 2004 National Health and Nutrition Examination Survey, an estimated 3.1% of women of reproductive age are infected with T. vaginalis, making this parasitic infection more prevalent than either chlamydia or gonorrhea.2,3 A disproportionately high burden of disease has been observed among older women and non-Hispanic black women.2,4 Infection with T. vaginalis has been associated with serious adverse outcomes such as pelvic inflammatory disease5,6 and 1.5 to 2.7 times greater risks of HIV acquisition and transmission.7–10
An estimated 70% to 85% of infected women are asymptomatic.2,11 Symptoms of trichomoniasis range from mild to severe inflammation and genital pruritis and may also include cervicovaginal and/or urethral discharge. A single oral dose of a nitroimidazole antimicrobial (ie, 2 g of metronidazole or tinidazole) is generally sufficient treatment to cure infection.12 Although in vitro antimicrobial resistance has been documented in 4% of vaginal culture specimens collected from sexually transmitted disease (STD) clinics, in vivo antimicrobial resistance has remained infrequent to date.13,14
The Centers for Disease Control and Prevention (CDC) STD Treatment Guidelines recommend that all women seeking care for symptoms of vaginal discharge should be tested for T. vaginalis infection.12 As for screening asymptomatic persons, however, these guidelines recommend routine annual screening only among HIV-infected women and currently do not include specific recommendations for T. vaginalis screening among the general population or among other high-risk groups.12 Although approximately 71% of male sexual partners of women with trichomoniasis are also infected with T. vaginalis, clinical testing for this parasite is not commonly conducted for male patients.15
Traditional diagnostic testing is by wet mount microscopy, in which a specimen of genital (eg, cervicovaginal) fluid is collected by a clinician during a pelvic examination and interpreted at the point of care; the test is inexpensive but operator dependent, and sensitivity is generally poor at 51% to 65%.16,17 Additional testing options include culture, which does not provide immediate results and is less commonly performed; recently developed rapid antigen tests, which may be performed at the point of care; and nucleic acid amplification tests (NAATs), the first of which was cleared for use in symptomatic or asymptomatic women by the US Food and Drug Administration in 2011, with high sensitivity (95%–100%) and specificity (95%–100%) for detecting T. vaginalis in urine specimens, endocervical swabs, or vaginal swabs.16,18,19
Little is known about current diagnostic testing and screening practices for this very common STD among persons presumably at high risk for infection, such as patients presenting at STD clinics.20 The objective of this analysis was to assess T. vaginalis prevalence among symptomatic and asymptomatic women tested or screened at a diverse group of STD clinics in the United States.
MATERIALS AND METHODS
Demographic, clinical, and laboratory data were collected from all patients visiting geographically diverse, publicly funded, urban STD clinics participating in the STD Surveillance Network (SSuN). Established by the CDC in 2005, SSuN is a sentinel surveillance system comprising collaborating state and local health departments that follow similar protocols for collecting and analyzing enhanced STD surveillance data. The purpose of SSuN is to improve the capacity of national, state, and local STD programs to detect, monitor, and respond rapidly to trends in STDs.21 SSuN data collection is a public health surveillance activity. Analysis of de-identified SSuN data does not constitute research involving human subjects, so institutional review board review was not required.
We analyzed data on demographics, clinical presentation including signs and symptoms of trichomoniasis, and laboratory testing for T. vaginalis among all women visiting a clinician at each of 15 participating STD clinics from January 1, 2010, through December 31, 2011.
These 15 STD clinics were located in 6 states: Alabama (1 clinic), California (1 clinic), Colorado (1 clinic), New York (9 clinics), Pennsylvania (2 clinics), and Washington (1 clinic). Jurisdictions were included if at least 1 STD clinic site routinely collected clinical data on signs and symptoms of trichomoniasis as well as laboratory test results for T. vaginalis. Women were included if they had at least 1 clinician visit because T. vaginalis testing could be performed only by clinicians at these clinics; women were excluded if their only visit types were express or rapid visits in which T. vaginalis testing could not have been performed.
Patient characteristics (eg, demographics) were collected during any one of multiple visits and summed across all visits. Age was defined as age at the latest clinician visit. HIV infection was determined by at least 1 positive laboratory test result for HIV and/or self-report of HIV infection at any visit during the analytic period. A nonspecific symptom of trichomoniasis was a subjective report of vaginal discharge, odor, or itching; a nonspecific sign of trichomoniasis was an objective finding of vaginal discharge observed during physical examination by a clinician. For this analysis, women were classified as “symptomatic” if they had any relevant subjective symptom and/or objective sign, and as “asymptomatic” if no relevant symptom or sign was reported. Among women receiving a diagnostic test for T. vaginalis, symptomatic women were considered to have received testing, whereas asymptomatic women were considered to have received screening. Women were considered to have T. vaginalis infection only if they had a positive result on any type of laboratory test (ie, wet mount, culture, NAAT, or any other type of test) for T. vaginalis infection at a participating STD clinic within the analytic time frame; clinical diagnoses made by the clinician were not considered. Jurisdictions also provided local information regarding their current clinic policies and procedures regarding testing and screening for T. vaginalis.
Descriptive statistics were calculated using SAS (version 9.2, SAS Institute, Cary, NC). Testing and screening practices for T. vaginalis were assessed by jurisdiction of the participating STD clinics. Prevalence of laboratory-confirmed infection detected was calculated both among symptomatic women tested and among asymptomatic women screened for T. vaginalis infection. In addition, testing and screening practices were assessed for the subgroup of women who were known to be HIV-infected.
RESULTS
A total of 233,101 people, including 90,432 women, visited 1 of the 15 participating STD clinics at least once in 2010 to 2011; excluding express visits and other nonclinician visit types, 59,176 women made at least 1 visit to an STD clinician. Demographic characteristics of these women visiting STD clinicians are summarized both overall and by jurisdiction in Table 1. The largest proportion of women was in the 20- to 29-year age group both overall (52.2%) and also within each jurisdiction (range, 48.0%–54.3%). As for race/ethnicity, overall the largest proportion of women identified as non-Hispanic black (55.6%), but this proportion varied between jurisdictions (range, 21.0%–85.9%).
TABLE 1.
Demographic Characteristics of Women Visiting an STD Clinician—6 States (15 STD Clinics), 2010 to 2011
| Characteristic | Alabama (1 Clinic), n (%) | California (1 Clinic), n (%) | Colorado (1 Clinic), n (%) | New York (9 Clinics), n (%) | Pennsylvania (2 Clinics), n (%) | Washington (1 Clinic), n (%) | Overall (15 Clinics), N (%) |
|---|---|---|---|---|---|---|---|
| Women with ≥1 visit to an STD clinician | 7253 (100) | 3762 (100) | 4266 (100) | 34,553 (100) | 6041 (100) | 3301 (100) | 59,176 (100) |
| Age at latest visit, years | |||||||
| ≤19 | 962 (13.3) | 244 (6.5) | 654 (15.3) | 6362 (18.4) | 1034 (17.1) | 237 (7.2) | 9493 (16.0) |
| 20–29 | 3618 (49.9) | 2041 (54.3) | 2219 (52.0) | 18,464 (53.4) | 2899 (48.0) | 1668 (50.5) | 30,909 (52.2) |
| 30–39 | 1599 (22.1) | 934 (24.8) | 884 (20.7) | 5813 (16.8) | 970 (16.1) | 741 (22.4) | 10,941 (18.5) |
| 40–49 | 749 (10.3) | 370 (9.8) | 373 (8.7) | 2733 (7.9) | 769 (12.7) | 426 (12.9) | 5420 (9.2) |
| 50+ | 325 (4.5) | 173 (4.6) | 136 (3.2) | 1181 (3.4) | 369 (6.1) | 229 (6.9) | 2413 (4.1) |
| Race/Ethnicity | |||||||
| Black, non-Hispanic | 6228 (85.9) | 791 (21.0) | 963 (22.6) | 19,211 (55.6) | 4888 (80.9) | 836 (25.3) | 32,917 (55.6) |
| Hispanic | 121 (1.7) | 618 (16.4) | 1671 (39.2) | 9577 (27.7) | 240 (4.0) | 227 (6.9) | 12,454 (21.0) |
| White, non-Hispanic | 872 (12.0) | 1567 (41.7) | 1273 (29.8) | 3092 (8.9) | 321 (5.3) | 1598 (48.4) | 8723 (14.7) |
| Other* | 32 (0.4) | 786 (20.9) | 359 (8.4) | 2673 (7.7) | 592 (9.8) | 640 (19.4) | 5082 (8.6) |
Other race/ethnicity includes American Indian and Alaska Native, Asian, Multi-race, Pacific Islander and Hawaiian, and other/unknown.
Among the 59,176 women visiting clinicians at STD clinics, 39,979 had signs or symptoms that could be consistent with trichomoniasis (67.6%; range, 38.4%–87.3% between jurisdictions), and the other 19,197 women did not have signs or symptoms of trichomoniasis (32.4%; range, 12.7%–61.6% between jurisdictions). A total of 211 women were known to be HIV-infected. Testing and screening practices among symptomatic, asymptomatic, and HIV-infected women are described in Table 2.
TABLE 2.
Testing and Screening Practices for T. vaginalis (TV) Among Symptomatic, Asymptomatic, and HIV-Infected Women Visiting an STD Clinician—6 States (15 STD Clinics), 2010 to 2011
| Characteristic | Alabama (1 Clinic), n (%) | California (1 Clinic), n (%) | Colorado (1 Clinic), n (%) | New York (9 Clinics), n (%) | Pennsylvania (2 Clinics), n (%) | Washington (1 Clinic), n (%) | Overall (15 Clinics), N (%) |
|---|---|---|---|---|---|---|---|
| Women with ≥1 visit to an STD clinician | 7253 (100) | 3762 (100) | 4266 (100) | 34,553 (100) | 6041 (100) | 3301 (100) | 59,176 (100) |
| Symptomatic | 5889 (81.2) | 1444 (38.4) | 3726 (87.3) | 22,444 (65.0) | 4791 (79.3) | 1685 (51.0) | 39,979 (67.6) |
| Asymptomatic | 1364 (18.8) | 2318 (61.6) | 540 (12.7) | 12,109 (35.0) | 1250 (20.7) | 1616 (49.0) | 19,197 (32.4) |
| Symptomatic women | 5889 (100) | 1444 (100) | 3726 (100) | 22,444 (100) | 4791 (100) | 1685 (100) | 39,979 (100) |
| Tested for TV | 5814 (98.7) | 1384 (95.8) | 3672 (98.6) | 1202 (5.4) | 4279 (89.3) | 1601 (95.0) | 17,952 (44.9) |
| Positive for TV | 2915 (50.1) | 126 (9.1) | 342 (9.3) | 103 (8.6) | 1079 (25.2) | 135 (8.4) | 4700 (26.2) |
| Asymptomatic women | 1364 (100) | 2318 (100) | 540 (100) | 12,109 (100) | 1250 (100) | 1616 (100) | 19,197 (100) |
| Screened for TV | 1156 (84.8) | 803 (34.6) | 113 (20.9) | 191 (1.6) | 607 (48.6) | 1039 (64.3) | 3909 (20.4) |
| Positive for TV | 173 (15.0) | 13 (1.6) | 2 (1.8) | 13 (6.8) | 30 (4.9) | 23 (2.2) | 254 (6.5) |
| HIV-infected women | 45 (100) | 20 (100) | 5 (100) | 109 (100) | 23 (100) | 9 (100) | 211 (100) |
| Tested or screened | 44 (97.8) | 12 (60.0) | 5 (100) | 6 (5.5) | 17 (73.9) | 8 (88.9) | 92 (43.6) |
| Positive for TV | 20 (45.5) | 1 (8.3) | 1 (20.0) | 0 (0) | 3 (17.6) | 2 (25.0) | 27 (29.3) |
| TV test method/s | 6970 (100) | 2187 (100) | 3785 (100) | 1393 (100) | 4886 (100) | 2640 (100) | 21,861 (100) |
| Wet mount only | 6962 (99.9) | 2069 (94.6) | 3263 (86.2) | 1393 (100) | 4681 (95.8) | 2541 (96.3) | 20,909 (95.6) |
| Culture and wet mount | 6 (<0.1) | 0 (0) | 520 (13.7) | 0 (0) | 184 (3.8) | 98 (3.7) | 808 (3.7) |
| Culture only | 2 (<0.1) | 0 (0) | 2 (<0.1) | 0 (0) | 21 (0.4) | 1 (<0.1) | 26 (0.1) |
| NAAT only | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other method | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unknown method | 0 (0) | 118 (5.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 118 (0.5) |
Among the 39,979 symptomatic women, 17,952 (44.9% overall; range, 5.3%–98.7%) were tested for T. vaginalis infection; however, at least 89.3% of symptomatic women were tested in all jurisdictions except one (New York), where only 5.3% of symptomatic women were tested. Of symptomatic women tested for T. vaginalis, 4700 (26.2% overall; range, 8.4%–50.1%) were found to be infected.
Among the 19,197 women without signs or symptoms, 3909 (20.4% overall; range, 1.6%–84.8%) were screened for T. vaginalis infection. After excluding the New York jurisdiction, where 1.6% of asymptomatic women were screened, screening practices still varied considerably by jurisdiction (52.5% overall; range, 20.9%–84.8%). Of asymptomatic women who were screened, 254 (6.5% overall; range, 1.6%–15.0%) were found to be infected with T. vaginalis.
Our population included 211 women known to be HIV-infected. Of these, only 92 (43.6% overall; range, 5.5%–100%) were tested or screened for T. vaginalis infection at the participating STD clinics; these practices varied considerably by jurisdiction, with only 1 clinic reporting that 100% of HIV-infected women were screened at least once during the 2-year period. Among these 211 HIV-infected women, 121 (57.3%) had symptoms or signs consistent with trichomoniasis, whereas 90 (42.7%) were asymptomatic. Testing and screening rates were low in both subgroups: among the 121 symptomatic HIV-infected women, 56 (46.3%) were tested, and among the 90 asymptomatic HIV-infected women, 36 (40.0%) were screened. T. vaginalis infection was confirmed in 27 HIV-infected women, corresponding to 29.3% of those who were tested or screened.
There were 4954 women with at least 1 positive laboratory test result for T. vaginalis during this period. Wet mount was the laboratory method used almost exclusively at all sites; culture was used rarely, and no clinic reported using rapid antigen testing or NAAT testing for T. vaginalis during this period.
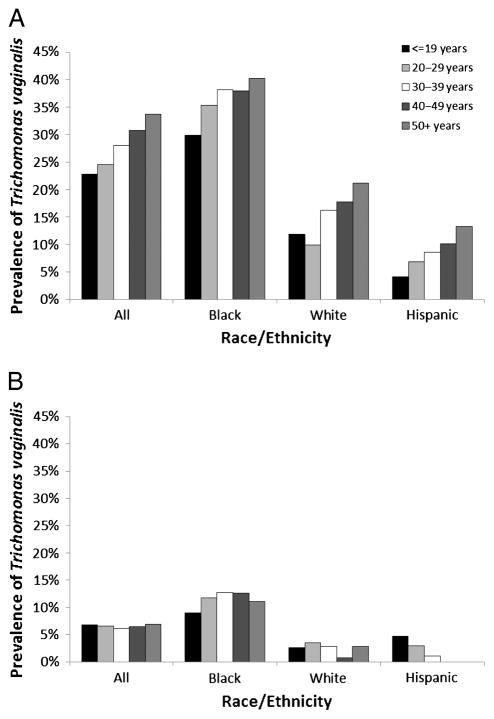
The prevalence of infection among women tested or screened for T. vaginalis in the STD clinic setting is summarized by age and race/ethnicity in Figure 1. Among symptomatic women who were tested, the prevalence of laboratory-confirmed trichomoniasis increased in a stepwise fashion with increasing age, from 22.8% in women 19 years or younger, up to 33.7% in women 50 years or older. Prevalence was higher among non-Hispanic black women tested, ranging from 29.9% in black women 19 years or younger up to 40.3% in black women 50 years or older. Among asymptomatic women screened, the prevalence of T. vaginalis infection was 6.5% overall, without much variation by age. Prevalence was higher among non-Hispanic black women screened than among non-Hispanic white or Hispanic women screened. These observed disparities are consistent with previously published population-based reports on the epidemiology of T. vaginalis infections.2
Figure 1.
Prevalence of infection among women tested or screened for T. vaginalis in the STD clinic setting, by age group and race/ethnicity among symptomatic women tested (A) and among asymptomatic women screened (B)—6 states (15 STD clinics), 2010 to 2011.
Clinics reported several notable differences in their policies and procedures with respect to testing and screening for T. vaginalis. At STD clinics in some states, clinicians both collect and interpret wet mounts, whereas in others, microscopy is performed only by laboratory personnel. At the clinics in Alabama and Pennsylvania, screening is routinely performed for all women receiving a pelvic examination regardless of symptoms, a practice that exceeds CDC recommendations. (In 2012, NAAT testing for T. vaginalis was implemented at the STD clinic in Alabama.) At the clinics in Colorado, California, and Washington, wet mount testing is generally performed only for symptomatic women who receive a pelvic examination, consistent with CDC recommendations. However, at the New York clinics, owing to financial restrictions, the diagnosis of trichomoniasis is made on clinical grounds,* and wet mount is performed only on women for whom a clinical diagnosis is in doubt or in the event of treatment failure.
DISCUSSION
In this analysis of 59,176 women visiting STD clinicians at 15 STD clinics, clinics in most jurisdictions tested symptomatic women for trichomoniasis, in accordance with national guidelines, yet screening practices varied widely. Consistent with CDC recommendations, at least 89% of symptomatic women were tested for T. vaginalis in all jurisdictions—except the largest one, New York, where budget limitations have nearly eliminated wet mount testing from STD clinics. Among symptomatic women visiting an STD clinician and receiving a laboratory test for T. vaginalis, the prevalence of infection was high at 26.2%. Even among asymptomatic women screened, prevalence of T. vaginalis was 6.5%, more than double the 3.1% prevalence estimated for US women overall. In Alabama, where nearly universal testing was conducted, detected prevalence was particularly high, for reasons that are only partly understood. Differences between jurisdictions in demographic characteristics including race may play an important role. In addition, given the low sensitivity of wet mount testing used in all jurisdictions, some additional cases may have been missed. These results are concerning in light of previously published smaller studies that have reported that T. vaginalis prevalence may be 13% to 15% among women at STD clinics.22,23
Screening practices for T. vaginalis among asymptomatic women varied widely between jurisdictions. Differences in local testing policies may have contributed to vastly different testing rates: for example, some clinics had instituted T. vaginalis screening for all women receiving a pelvic examination. We observed that STD clinics performing more screening tests detected a higher prevalence of T. vaginalis infections (an unusual pattern because increasing screening often leads to a reduction in observed disease prevalence rates, even as the number of detected cases increases). This lack of consistent practices might be addressed by implementing national screening guidelines for T. vaginalis among asymptomatic women. Routine screening regardless of symptomatic status may be worthwhile in populations with a high prevalence of infection and where treatment can prevent adverse outcomes.
Given the national recommendation that all HIV-infected women should be screened for T. vaginalis infection upon entry to care and at least annually thereafter, regardless of symptoms,12 the finding that only 43.6% of HIV-infected women were tested or screened over a 2-year period, after visiting STD clinicians in an STD clinic setting, revealed a probable room for improvement in most jurisdictions. Clinicians should assess and document whether HIV-infected women have been screened for trichomoniasis within the past year and should offer this recommended service if needed.
This analysis is subject to several important limitations. First, patients at these 15 STD clinics may not be representative of all US STD clinics or other broader segments of the population. Although most patients were seen in a jurisdiction where diagnostic testing for T. vaginalis was rarely performed (New York), positivity rates among women who were tested and screened in this jurisdiction were comparable with other sites. Second, any trichomoniasis testing or screening that these patients may have received in other practice settings beyond the STD clinic, such as primary care clinics, could not be assessed. Third, cultures for T. vaginalis performed at some sites during 2010 were for a research project and did not reflect usual clinic practice, although the small number of culture tests performed had little impact on the results of this analysis. In addition, adequacy of treatment provided could not be assessed for any patients or their sexual partners; because treatment data are not reported in SSuN, patients at some sites may have received appropriate empiric treatment based on clinical presentation alone, whether or not a positive laboratory test result for T. vaginalis was documented.
Historically, public health resources for STDs have focused on nationally notifiable diseases, including chlamydia, gonorrhea, syphilis, and HIV infection.3,24 Despite the high prevalence of infection, observed health disparities, and communicability of infection, trichomoniasis is not currently a reportable disease in any state, nor is it a nationally notifiable condition, largely because of a lack of consistent evidence for severity of disease, associated costs, preventability of adverse outcomes, or perceived public interest.25 Prevalence estimates commonly used to derive cost-effectiveness thresholds for other STDs are as low as a population prevalence of 3% for chlamydia26 or 0.1% for HIV,27 above which screening asymptomatic persons has been recommended to minimize sequelae. Further research is needed to clarify whether T. vaginalis infection causes sequelae even if asymptomatic, and to determine the cost-effectiveness and affordability of expanded screening. Additional evidence would be helpful to clarify when T. vaginalis screening should be recommended for asymptomatic individuals at high risk, such as STD clinic patients.
Where microscopy is not available or desirable, providers may want to assess the net benefits of introducing highly sensitive NAATs or other same-day tests as options for expediting T. vaginalis screening, either alone or as part of an algorithm when wet mount testing is negative.17 However, the feasibility of expanding use of these technologies under funding constraints is unclear. Nationally, a key criterion for recommending screening is the ability to intervene successfully to reduce poor health outcomes. Nitroimidazole antimicrobials are a simple, effective, inexpensive, and widely available treatment for T. vaginalis infection, yet they are the only drug class available, and the potential development of antimicrobial-resistant parasites is an ominous prospect.12,28 Also, several studies have found that treating T. vaginalis infection reduces genital shedding of HIV RNA,29–31 yet it has not been demonstrated that the successful treatment of trichomoniasis reduces the risk of HIV transmission. Furthermore, health consequences of asymptomatic T. vaginalis infections remain poorly defined. Although trichomoniasis has been identified as an independent risk factor for pelvic inflammatory disease and HIV infection, women visiting clinicians in the STD clinic setting may have additional risk factors for these serious adverse events.5,6 Future research may clarify the role T. vaginalis infection plays in the ongoing HIV epidemic, especially among racial/ethnic minority populations in the United States. Answers to such questions might provide evidence to support revisions of national screening recommendations.
Acknowledgments
The authors thank Darlene Davis, Robert Kirkcaldy, Ellen Klinger, Lauri Markowitz, Tremeka Sanders, Mark Stenger, Elizabeth Torrone, and Kimberly A. Workowski.
Source of funding: The Centers for Disease Control and Prevention provided funding support for this work. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Laboratory tests for trichomoniasis were reported from all 9 New York clinics in both 2010 and 2011; 1 of these clinics reported that additional wet mount testing was conducted for a special project during the first 3 quarters of 2010 and did not reflect usual clinic practice.
Conflicts of interest: Jane Schwebke has received research support from Embil Pharmaceuticals and Gen-Probe. For the remaining authors, no conflicts of interest were declared.
References
- 1.World Health Organization. Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: Methods and results used by WHO to generate 2005 estimates. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 2.Sutton M, Sternberg M, Koumans EH, et al. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis. 2007;45:1319–1326. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2010. Atlanta: U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 4.Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the APTIMA Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601–2608. doi: 10.1128/JCM.00748-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherpes TL, Wiesenfeld HC, Melan MA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006;33:747–752. doi: 10.1097/01.olq.0000218869.52753.c7. [DOI] [PubMed] [Google Scholar]
- 6.Moodley P, Wilkinson D, Connolly C, et al. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis. 2002;34:519–522. doi: 10.1086/338399. [DOI] [PubMed] [Google Scholar]
- 7.McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 8.Laga M, Alary M, Nzila N, et al. Condom promotion, sexually transmitted diseases treatment, and declining incidence of HIV-1 infection in female Zairian sex workers. Lancet. 1994;344:246–248. doi: 10.1016/s0140-6736(94)93005-8. [DOI] [PubMed] [Google Scholar]
- 9.Van Der Pol B, Kwok C, Pierre-Louis B, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197:548–554. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1–serodiscordant couples. J Infect Dis. 2012;205:358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterman TA, Tian LH, Metcalf CA, et al. High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: A case for rescreening. Ann Intern Med. 2006;145:564–572. doi: 10.7326/0003-4819-145-8-200610170-00005. [DOI] [PubMed] [Google Scholar]
- 12.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR. 2010;59:1–110. [PubMed] [Google Scholar]
- 13.Kirkcaldy RD, Augostini P, Asbel LE, et al. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD Surveillance Network, 2009–2010. Emerg Infect Dis. 2012;18:939–943. doi: 10.3201/eid1806.111590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosserman EA, Helms DJ, Mosure DJ, et al. Utility of antimicrobial susceptibility testing in Trichomonas vaginalis–infected women with clinical treatment failure. Sex Transm Dis. 2011;38:983–987. doi: 10.1097/OLQ.0b013e318224db39. [DOI] [PubMed] [Google Scholar]
- 15.Seña AC, Miller WC, Hobbs MM, et al. Trichomonas vaginalis infection in male sexual partners: Implications for diagnosis, treatment, and prevention. Clin Infect Dis. 2007;44:13–22. doi: 10.1086/511144. [DOI] [PubMed] [Google Scholar]
- 16.Huppert JS, Mortensen JE, Reed JL, et al. Rapid antigen testing compares favorably with transcription-mediated amplification assay for the detection of Trichomonas vaginalis in young women. Clin Infect Dis. 2007;45:194–198. doi: 10.1086/518851. [DOI] [PubMed] [Google Scholar]
- 17.Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol. 2009;200:188.e1–188.e7. doi: 10.1016/j.ajog.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Andrea SB, Chapin KC. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification assay and BD Affirm VPIII for detection of T. vaginalis in symptomatic women: Performance parameters and epidemiological implications. J Clin Microbiol. 2011;49:866–869. doi: 10.1128/JCM.02367-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective US clinical trial. J Clin Microbiol. 2011;49:4106–4111. doi: 10.1128/JCM.01291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachmann LH, Hobbs MM, Seña AC, et al. Trichomonas vaginalis genital infections: Progress and challenges. Clin Infect Dis. 2011;53(suppl 3):S160–S172. doi: 10.1093/cid/cir705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rietmeijer CA, Donnelly J, Bernstein KT, et al. Here comes the SSuN: Early experiences with the STD Surveillance Network. Public Health Rep. 2009;124:72–77. doi: 10.1177/00333549091240S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helms DJ, Mosure DJ, Metcalf CA, et al. Risk factors for prevalent and incident Trichomonas vaginalis among women attending three sexually transmitted disease clinics. Sex Transm Dis. 2008;35:484–488. doi: 10.1097/OLQ.0b013e3181644b9c. [DOI] [PubMed] [Google Scholar]
- 23.Johnson HL, Ghanem KG, Zenilman JM, et al. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner city public sexually transmitted diseases clinics. Sex Transm Dis. 2011;38:167–171. doi: 10.1097/OLQ.0b013e3181f2e85f. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. HIV surveillance—United States, 1981–2008. MMWR. 2011;60:689–693. [PubMed] [Google Scholar]
- 25.Hoots BE, Peterman TA, Torrone EA, et al. A trich-y question: Should Trichomonas vaginalis infection be reportable? Sex Transm Dis. 2013;40:113–116. doi: 10.1097/OLQ.0b013e31827c08c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honey E, Augood C, Templeton A, et al. Cost effectiveness of screening for Chlamydia trachomatis: A review of published studies. Sex Transm Infect. 2002;78:406–412. doi: 10.1136/sti.78.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR. 2006;55:1–17. [PubMed] [Google Scholar]
- 28.Forna F, Gülmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev. 2003:CD000218. doi: 10.1002/14651858.CD000218. [DOI] [PubMed] [Google Scholar]
- 29.Wang CC, McClelland RS, Reilly M, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis. 2001;183:1017–1022. doi: 10.1086/319287. [DOI] [PubMed] [Google Scholar]
- 30.Kissinger P, Amedee A, Clark RA, et al. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis. 2009;36:11–16. doi: 10.1097/OLQ.0b013e318186decf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson BL, Firnhaber C, Liu T, et al. Effect of trichomoniasis therapy on genital HIV viral burden among African women. Sex Transm Dis. 2012;39:638–642. doi: 10.1097/OLQ.0b013e31825725ad. [DOI] [PMC free article] [PubMed] [Google Scholar]