Abstract
RNA interference/silencing mechanisms triggered by double-stranded RNA (dsRNA) have been described in many eukaryotes, including fungi. These mechanisms have in common small RNA molecules (siRNAs or microRNAs) originating from dsRNAs that, together with the effector protein Argonaute, mediate silencing. The genome of the fungal pathogen Candida albicans harbours a well-conserved Argonaute and a non-canonical Dicer, essential members of silencing pathways. Prototypical siRNAs are detected as members of the C. albicans transcriptome, which is potential evidence of RNA interference/silencing pathways in this organism. Surprisingly, expression of a dsRNA a hairpin ADE2 dsRNA molecule to interfere with the endogenous ADE2 mRNA did not result in down-regulation of the message or produce adenine auxotrophic strains. Cell free assays showed that the hairpin dsRNA was a substrate for the putative C. albicans Dicer, discounting the possibility that the nature of the dsRNA trigger affects silencing functionality. Our results suggested that unknown cellular events govern the functionality of siRNAs originating from transgenes in RNA interference/silencing pathways in C. albicans.
Keywords: Candida albicans, dsRNA, RNAi, RNase assay, Argonaute, Dicer
Introduction
RNA silencing pathways are conserved across many species. Two essential components of these pathways, Argonaute and Dicer, participate in cellular processes such as transposon silencing, viral defence, DNA elimination, heterochromatin formation, protection of the genome from repetitive elements and posttranscriptional gene silencing and regulation (Cerutti and Casas-Mollano, 2006; Drinnenberg et al., 2009; Malone and Hannon, 2009). The prototypical RNA silencing pathway, RNA interference (RNAi), is triggered by double-stranded RNA (dsRNA) that can arise endogenously by transcription of repetitive DNA, for example, or be experimentally introduced into the cell. The trigger dsRNA is cleaved by Dicer, a specialized endonuclease, into small interfering RNAs (siRNAs), which are subsequently loaded onto Argonaute and act as guides for silencing cognate mRNA molecules. Another silencing pathway commonly found in metazoans utilizes small non-coding microRNAs that are made as short stem-loop dsRNA precursors, preprocessed by a nuclear RNase III complex (Drosha-DGCR8), before final processing into microRNAs in the cytoplasm by Dicer (Rana, 2007).
RNA silencing pathways have been described in all four eukaryotic kingdoms, and many reports support an ancestral role of RNA silencing pathways in protection of the genome (Almeida and Allshire, 2005). In fungi, the RNA silencing machinery has undergone diversification and, in some cases loss, during evolution (Nakayashiki et al., 2006). RNA silencing pathways have been well described in the model fungi Neurospora crassa (Catalanotto et al., 2004; Cecere and Cogoni, 2009; Lee et al., 2009; Pickford et al., 2002) and Schizosaccharomyces pombe (Ekwall, 2004; Martienssen et al., 2005; Raponi and Arndt, 2003; Volpe et al., 2002, 2003; White and Allshire, 2008) but are notably absent in Saccharomyces cerevisiae (Drinnenberg et al., 2009). The genome of the fungal pathogen, Candida albicans, harbours a typical Argonaute homologue (orf19.2903; www.candidagenome.org), and a non-canonical Dicer (orf19.3796; Drinnenberg et al., 2009) lacking both a helicase and a PAZ domain. Recently, siRNAs were found as part of the transcriptome of C. albicans suggesting that RNAi pathways may exist in this organism. The authors also demonstrated Dicer activity in C. albicans extracts, but RNAi activity within cells was not addressed (Drinnenberg et al., 2009). RNAi in C. albicans cells has not been reported to date. Because important cellular processes are carried out by RNA silencing pathways and RNAi has been used as a tool to downregulate gene expression, we investigated the possibility of RNAi within C. albicans cells.
Materials and methods
Strains and growth conditions
The wild-type strain, SC5314 (Gillum et al., 1984) and the Ura-derivative, CAI4 (hisG::ura3::hisG/hisG::ura3::hisG; Fonzi and Irwin, 1993) were routinely maintained on yeast peptone dextrose medium (1% yeast extract, 2% peptone, 2% glucose; YPD). When necessary, uridine was added to a final concentration of 80 μg/ml to support the growth of CAI4.
Construction of the dsRNA ADE2-yEGFP3-ADE2 expressing plasmid
Generation of pEAGA was performed in several steps essentially as outlined in Liu et al. (2002). The oligonucleotides used in amplifications are listed in Table 1. Nucleotides +1 to +414 of the ADE2 gene were amplified by PCR utilizing an in-house recombinant Taq polymerase expressed in E. coli (Rustad et al., 2002) with pCRW3 (Srikantha et al., 1996) as source of ADE2 sequences, and primers ADEFP and ADERG to add a PstI restriction site at the 5′ end and a portion of yEGFP3 to the 3′ end of the amplicon (fragment A). Fragment A was used as template with primers ADEFH and ADERVH in a PCR to incorporate HindIII sites at each end and an EcoRV site at the 3′ end (fragment B). One hundred base pairs (+1 to +100) of yEGFP3 were amplified using primers ADEaG and GFPRH with pENO1GFP3 (Staab et al., 2003) as source of yEGFP3 sequences to add a HindIII restriction site at the 3′ of the amplicon (fragment C). Fragments A and C were used in a patch-PCR with primers ADEFP and GFPRH to generate a 530 bp amplicon (fragment D). Fragment D was digested with PstI and HindIII and cloned into pENO1GFP3 after removing the yEGFP3 open reading frame following digestion with PstI and HindIII. Fragment B digested with HindIII was subsequently cloned at the HindIII site of the fragment D-containing plasmid, pENOGA. The relative orientation of the ADE2 sequences in pENOGA clones was assessed by digesting with EcoRV and PstI. Those recombinant plasmids (pEAGA) in which the ADE2 sequences were in opposite orientation were kept for subsequent studies. The final ADE2-yEGFP3-ADE2 construct had the constitutive enolase promoter driving expression of a hairpin dsRNA molecule and an URA3 gene for positive selection in the Ura− strain CAI4 (Fonzi and Irwin, 1993).
Table 1.
Oligonucleotides used in this study
| Primer | Sequence | Description |
|---|---|---|
| ADEFP | 5′-AACTGCAGATGGATAGCAAAACTGTTGG-3′ | dsRNA construct. ADE2 nt 1–20; PstI site (underlined) |
| ADERG | 5′-GAATAATTCTTCACCTTTAGACATCCCCAATGTGTAACAAGTCATCG-3′ | dsRNA construct. yEGFP3 nt 24–1 antisense (underlined); ADE2 nt 414–392 antisense |
| ADEFH | 5′-CCCAAGCTTATGGATAGCAAAACTGTTGG-3′ | dsRNA construct. ADE2 nt 1–20; HindIII site (underlined) |
| ADERVH |
|
dsRNA construct. ADE2 nt 414–392 antisense; HindIII site (underlined) and EcoRV site (double underlined) |
| ADEaG | 5′-CGATGACTTGTTACACATTGGGGATGTCTAAAGGTGAAGAATTATTC-3′ | dsRNA construct. ADE2 nt 392–414 sense (bold), yEGFP3 nt 1–20 |
| GFPRH | 5′-CCCAAGCTTCACCTTCACCGGAGACAGAAAATTTG-3′ | dsRNA construct. yEGFP3 nt 100–73 antisense; HindIII site (underlined) |
| ADE2-35 | 5′-GGAGGTGGCCAATTAGGTCGTATG-3′ | RT-PCR. ADE2 nt 35–58 sense |
| ACT237 | 5′-GAGAGTTGCTCCAGAAGAACATCC-3′ | RT-PCR. ACT1 nt 943–966 sense |
| ACT994 | 5′-CCAAGATAGAACCACCAATCCAGAC-3′ | RT-PCR. ACT1 nt 1700–1676 antisense |
| T7-A131 | 5′-CCAAGCTTCTAATACGACTCACTATAGGGTCTAGACGATCACGTTGATGGATCG-3′ | ADE2 sense oligo nt 131–155 with T7 promoter (bold) and HindIII site (underlined) |
| A414 | 5′-CCCCAATGTGTAACAAGTCATCGAC-3′ | ADE2 antisense oligo nt 414–389 |
| A131 | 5″-TCTAGACGATCACGTTGATGGATCG-3′ | ADE2 sense oligo nt 131–155 |
| T7-141 | 5′-CCAAGCTTCTAATACGACTCACTATAGGGCCCCAATGTGTAACAAGTCATCGAC-3′ | ADE2 antisense oligo nt 414–389 with T7 promoter (bold) and HindIII site (underlined) |
C. albicans transformation
Linearized pEAGA was used to transform CAI4 spheroplasts as previously (Staab et al., 2003). Ura+ transformants were selected on yeast nitrogen base [0.17% yeast nitrogen base without amino acids (Sigma), 0.5% ammonium sulfate, 50 mM glucose; YNB] agar plates without uridine. Stable transformants were verified for integration of the pEAGA at the ENO1 locus by Southern blotting (Staab et al., 2003).
Northern blot analysis
ADE2 message levels in EAGA3 (pEAGA CAI4 transformant) were analysed by northern blotting. Total RNA (RNeasy RNA isolation Kit, Qiagen) was isolated from cultures grown in liquid YNB to mid-log phase at 30 °C. Ten micrograms of RNA from wild-type (SC5314), parent (CAI4) and EAGA3 were separated in standard formaldehyde gels and transferred to nitrocellulose (Brown et al., 2004). The immobilized RNAs were probed with a 32P-labelled (Promega, Prime-a-Gene kit) PCR amplicon comprising bp +1 to +414 of ADE2 (fragment B, see above). The hybridized membrane was washed three times in 0.2× SSC, 0.1% SDS at room temperature, and exposed to X-ray film at −80 °C for 4 days.
For detection of CaAGO expression, 4 μg of RNA from SC5314 grown in YNB was separated in formaldehyde gels and immobilized onto nitrocellulose as described above. The membrane was first probed with a 32P-labelled (Promega, Prime-a-Gene kit) PCR (Platinum Taq, Invitrogen) product (bp +1 to +1427 relative to the ATG of orf19.2903) generated with oligonucleotides SI 5′-ATGAGTGATTTGGTTAAATTTTCAACACCAACC-3′ and B7, 5′-GGTTTCCCCATTGGTTTAGTGAG-3′ bp with SC5314 genomic DNA as template. The hybridized membrane was washed and exposed to X-ray film as above for 4 days at −80 °C. The membrane was stripped of the CaAGO probe (Sambrook et al., 1989), and hybridized with a PCR product generated from the ACT1 locus (GenBank AACQ01000009, region 219902..220984) using the same oligonucleotides as for RT-PCR of the actin message (see below; for ACT237 and ACT994, see Table 1). The membrane was washed, and exposed to X-ray film at −80 °C for 2 days.
Detection of ADE2 hairpin and CaAGO expression by reverse transcriptase-PCR (RT-PCR)
Expression of the ADE2 dsRNA hairpin molecule in strain EAGA3 was detected by a reverse transcriptase reaction followed by PCR (RT-PCR) using a single oligonucleotide predicted to anneal twice within the construct, and generate an amplicon. Total RNA was prepared (RNeasy RNA isolation kit, Qiagen) from CAI4 (parental strain) and EAGA3 (pEAGA CAI4 transformant) grown in YPD at 30 °C to mid-log phase. Approximately 600 ng of DNAse I (Promega)-treated RNA was used in RT reactions with 500 ng oligo (dT)12 – 18, 10 nmol each dNTP, 50 mM Tris-HCl pH 8.3, 75 mM KCl, 3 mM MgCl2, 10 mM DTT, and 200 U of Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (Invitrogen). PCR utilizing a recombinant Taq polymerase (Rustad et al., 2002) was performed with one tenth of the RT reaction and the single oligonucleotide ADE2-35 (Table 1), a sense primer of ADE2 nt +35 to +58. Control PCRs were performed with oligonucleotides to actin, ACT237 and ACT994 (Table 1).
CaAGO expression was detected in RNA isolated from SC5314 grown in YNB to mid-log phase by first performing an RT reaction with 500 ng of DNase I-treated total RNA, followed by PCR (Platinum Taq polymerase, Invitrogen) with one-tenth of the RT reaction and oligonucleotides UF1, 5′-CCCTACCACGTCTCTGGC-3′ and B8, 5′-CGCTGGGGTTGGATTCAAATTG-3′ comprising bp −37 to +741 relative to the putative ATG in orf19.2903. Positive control reactions amplified a region of the actin message with oligonucleotides ACT237 and ACT994. The products of the RT-PCRs were subsequently separated in agarose gels and stained with ethidium bromide as per standard methods (Sambrook et al., 1989).
Cell-free RNase assays
The generation of siRNAs from radiolabelled dsRNA was performed essentially as described previously (Drinnenberg et al., 2009) except for the use of both a hairpin and a blunt-ended dsRNA substrates. SC5315 extracts from cells grown in YPD to OD600 1.4 at 30 °C served as a source of Dicer activity. The hairpin substrate was produced by T7 transcription of the internal XbaI fragment of the ADE2-yEGFP3-ADE2 construct (Fig. 1A) cloned at the XbaI site of pBluescript SK- (Stratagene/Agilent Technologies) and digested with NotI. NotI digests the plasmid once immediately downstream of the insert. The blunt-ended substrate (same ADE2 sequences found from the XbaI site to nt 414) was the result of transcription from two amplicons bearing T7 promoter sequences at either end (Lanar and Kain, 1994). Plasmid pENOGA (see above) was used as template for two separate PCRs (High Fidelity Platinum Taq, Invitrogen) utilizing oligonucleotides T7-131 and A414, and T7-414 and A131 (Table 1). Radiolabelled transcripts were generated from the linearized plasmid and the T7 promoter-ADE2 amplicons with use of a MAXIscript Kit (Ambion/Applied Biosystems) and a molar ratio of UTP to (α32P)UTP of 32 : 1. The labelled substrates were gel-purified from a 6% acrylamide, 7 M urea gel (Invitrogen). The 20 μl reactions [5 μg cell extract, 40K cpm dsRNA substrate in reaction buffer (Drinnenberg et al., 2009) with 80U of Protector RNase inhibitor (Roche Applied Science)] were incubated at 30 °C for 2 h, followed by quenching with 50 mM sodium acetate, 10 mM EDTA, pH 5.5 and extraction with equal volume of acidic phenol–chloroform/isoamyl alcohol (25 : 24:1). One set of reactions was incubated at 4 °C to determine the requirement for ATP hydrolysis (Ye and Liu, 2008). The products of the RNase reactions were resolved by electrophoresis in 15% acrylamide, 7 M urea gels (Invitrogen). The size of the products was determined by comparing their migration to RNA standards (Decade Markers, Ambion/Applied Biosystems).
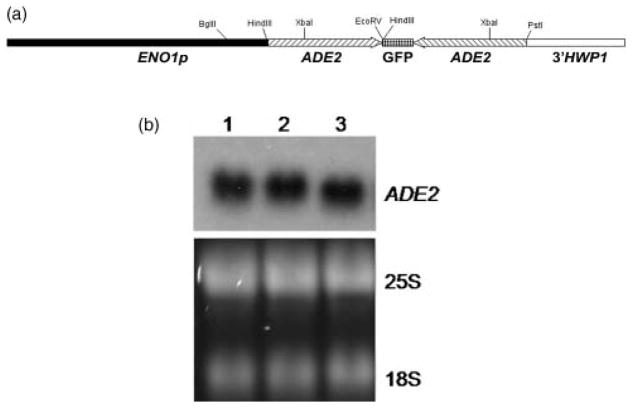
Figure 1.
Plasmid construct for expression of hairpin ADE2 dsRNA within cells to interfere with the ADE2 message. (a) Schematic of the ADE2 hairpin construct. ADE2 sequences from nt +1 to +414 were cloned in opposite orientation flanking 100 bp of yEGFP3, downstream of the enolase promoter (ENO1p) and with the 3′ untranslated region of HWP1 (3′ HWP1) in pENO1GFP3. Arrows indicate 5′ to 3′ of ADE2. Relevant restriction endonuclease sites are shown above the construct. (b) Endogenous ADE2 mRNA levels are not affected in a strain bearing the dsRNA plasmid construct. Top panel: northern blot analysis of the wild-type strain SC5314, lane 1; parental strain (CAI4), lane 2; and EAGA (CAI4 transformed with pEAGA), lane 3; probed for ADE2 sequences with a 32P-labelled PCR amplicon. Bottom panel: equal loading of total RNA was assessed by ethidium bromide staining of rRNA bands separated in standard formaldehyde gels used in northern blot analysis
Results and discussion
To test the possibility of RNAi in C. albicans, a plasmid was generated that expresses a dsRNA hairpin molecule that interferes with the ADE2 message as previously described (Liu et al., 2002; Table 1). ADE2 was chosen as a target because disruption of this gene is non-lethal, and mutant colonies are readily distinguished on agar plates by their red colour (Poulter and Rikkerink, 1983). The plasmid bearing the ADE2 hairpin construct (pEAGA; Fig. 1A) was integrated at the ENO1 locus in CAI4 (ura3 Δ::λimm434/ura3Δ::λmm434; Fonzi and Irwin, 1993; Staab et al., 2003) by electroporation (Reuss et al., 2004). Verified transformants [via Southern blot analysis (Sambrook et al., 1989), data not shown] were analysed for adenine auxotrophy. We tested multiple transformants for growth in the absence of adenine on YNB minimal medium and determined that the colonies grew normally and did not exhibit the typical reddish colour of adenine auxotrophs (data not shown). Furthermore, the growth rate of these transformants on media lacking adenine did not differ from the parental strain or the Ura+ wild-type strain, SC5314 (data not shown). Adenine auxotrophy is not a phenotype of ADE2/ade2 strains but slowed growth has been reported for single ADE2 deletions propagated in the absence of added adenine (Donovan et al., 2001), suggesting that ADE2 message abundance must fall at or below the levels conferred by a heterozygous null genotype to observe any phenotype. Northern blot analysis showed that ADE2 message levels were not affected by expression of the ADE2 hairpin construct (Fig. 1B), consistent with the lack of an ADE2-related phenotype. We were confident that the ADE2 probe detected the native message because we were unable to detect the ADE2-yEGFP3-ADE2 hairpin (approximately 1.0 kb in length) using conventional formaldehyde gels and a probe consisting of yEGFP3 sequences that separate the inverted ADE2 repeats (data not shown), probably due to the extensive secondary structure of the predicted transcript. In addition, the migration of the single hybridized band at approximately 2.2 kb correlated well with the calculated size of the ADE2 open reading frame (1707 bp; www.candidagenome.org, orf19.5906). Taken together, the results were consistent with the lack of RNAi in the cells.
Other laboratories have attempted RNAi in C. albicans by expressing 1.0 kb of inverted repeats of TUP1 or EFG1 driven by the MAL2 promoter (unpublished results; personal communication, Richard Bennett, Brown University). Bidirectional transcription of the same TUP1 and EFG1 1.0 kb fragments from opposing MAL2 promoters also failed to produce transformants with defects associated with decreased TUP1 or EFG1 message. In addition, expressing a short transcript consisting of inverted repeats of 33 bp of TUP1 separated by a short sequence of thymidines did not yield transformants having altered filamentation patterns that would suggest a decrease in message abundance. Bennett’s negative results are congruent with ours, and suggest a general inability to induce RNAi by cellular expression of dsRNA in C. albicans. Others have achieved RNAi of the ADE2 gene in Cryptococcus neoformans (Liu et al., 2002) and in Histoplasma capsulatum (Rappleye et al., 2004) via the expression of long hairpin dsRNAs. Thus, in other fungi, the highly conserved ADE2 is amenable to depletion through an RNAi mechanism, implying that the ADE2 target gene is unlikely to be the culprit for RNAi failure. In summary, both published and published results support our findings of a lack of RNAi functionality in C. albicans triggered by exogenously introduced dsRNA.
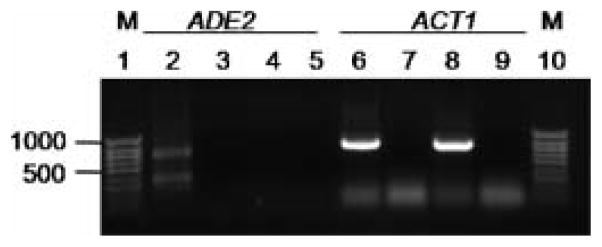
Failure of dsRNA expression in the cells may explain the negative RNAi results. To investigate this possibility, we performed reverse transcription of total RNA prepared from EAGE3 (CAI4 transformed with pEAGA) followed by PCR (RT-PCR) utilizing a single oligonucleotide hybridizing within the ADE2 gene fragments. A single oligonucleotide (ADE2-35, Table 1) was expected to hybridize twice, once within each inverted ADE2 repeat, and generate an amplicon if the hairpin construct was transcribed. The RT-PCR confirmed hairpin expression (Fig. 2), as an amplicon of the predicted size together with a faster migrating DNA molecule (presumably a hairpin of one strand of DNA, the result of the secondary structure of the inverted repeats) was detected only in the EAGA RNA sample (Fig. 2, lane 2). Thus the lack of RNAi did not correlate with the failure to express the hairpin dsRNA. Based on our findings, we concluded that the absence of RNAi was probably related to the inability of the hairpin dsRNA to induce silencing of the cognate ADE2 RNA in C. albicans.
Figure 2.
Detection of ADE2 dsRNA by RT-PCR. Total RNA from EAGA (lanes 2, 3, 6 and 7) and the parental strain, CAI4 (lanes 4, 5, 8 and 9), was reverse-transcribed and used in PCRs with a single oligonucleotide of ADE2 sequences (ADE2-35; lanes 2, 3, 4, and 5). A PCR amplicon was detected only in reactions with RT templates from EAGA in addition to a faster migrating band probably due to secondary structure (hairpin) of the inverted repeat DNA product (lane 2). Controls: no RT reactions to exclude DNA contamination (lanes 3, 5, 7 and 9), and the amplification of an actin amplicon (lanes 6, 7, 8 and 9) that served as positive control for the RT-PCR. M, DNA size markers (HyperLadder IV, Bioline). The migration of the 1000 and 500 bp bands are indicated on the left
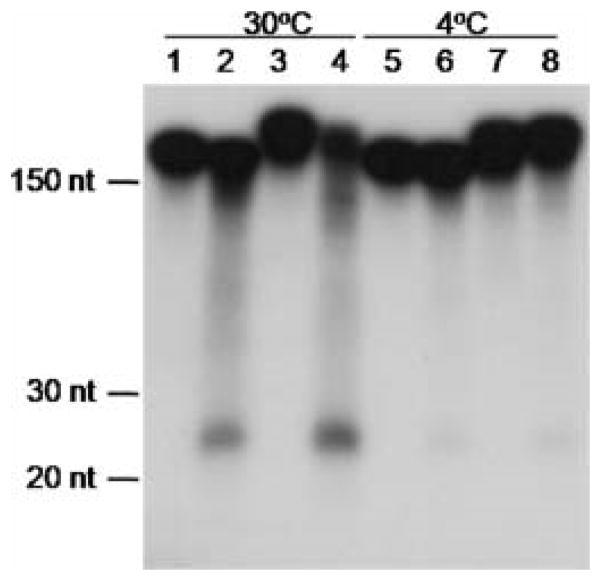
Our results were puzzling in light of the recent report of evidence of RNAi products (siRNA) in C. albicans and other yeasts (Drinnenberg et al., 2009). Reconstitution of transgene RNAi in Saccharomyces cerevisiae is possible by introducing Saccharomyces castellii AGO1 (Argonaute) and DCR1 (Dicer) into the model yeast’s genome (Drinnenberg et al., 2009). Analysis of the small-RNA libraries from S. castellii and C. albicans reveal that 68% of the sequence reads from C. albicans map to rRNA loci and ~6% of siRNAs map to retroelements, while in S. castellii, 41% map to retrotransposons (Ty elements) and repetitive elements, consistent with the predicted function of transposon control in the latter yeast (Drinnenberg et al., 2009). Although the abundance of rRNA siRNAs in C. albicans is not understood in terms of function, it may suggest that the RNA silencing pathway in C. albicans is not orthologous to that in S. castellii. Perhaps the nature of the dsRNA impacts subsequent RNAi functionality in C. albicans; the substrate used for RNase assays was a blunt-end dsRNA and not a hairpin molecule. To determine if C. albicans Dicer utilizes hairpin dsRNA as a substrate, we performed dsRNase assays with wild-type SC5314 (Drinnenberg et al., 2009). Uniformly radiolabelled hairpin and blunt-ended dsRNAs were tested as substrates for the generation of siRNAs (Fig. 3). SC5314 extracts (source of Dicer activity) accumulated siRNAs of approximately 23 nt in length when presented with both hairpin and blunt dsRNAs (Fig. 3), and this reaction was ATP-dependent (Fig. 3), as has been shown for other Dicers (Colmenares et al., 2007; Ye and Liu, 2008). The processing of the substrate dsRNAs into approximately 23 nt length fragments was specific to Dicer activity as the assays were performed in the presence of an RNase inhibitor to prevent RNase A, RNase B and RNase T2 activities (Roche Applied Science). These results showed that C. albicans is capable of utilizing hairpin dsRNA as substrate to generate putative siRNAs, and recapitulated Drinnenberg and colleagues’ results utilizing a blunt-ended dsRNA substrate (Drinnenberg et al., 2009). Together, the data suggest that the presence of Argonaute and Dicer in C. albicans is insufficient to perform RNAi trigged by exogenously introduced dsRNA. Although we can conclude from our data that RNAi activity is lacking in C. albicans, the possibility exists that a form of RNAi is viable in this organism based on the isolation of small non-coding siRNAs typical of Argonaute-associated guide RNAs (Drinnenberg et al., 2009). Perhaps C. albicans RNAi pathways are specific to the rDNA locus and transgene dsRNAs cannot serve as substrates. Further studies are needed to elucidate the significance of predominantly rDNA siRNAs in C. albicans.
Figure 3.
Cell extract processing of blunt and hairpin dsRNA templates. A hairpin (ADE2-GFP-ADE2) or blunt (ADE2 sequences only) radiolabelled substrate was incubated with SC5314 cell extracts, and the product resolved in a 15% acrylamide, 7 M urea gel. Lanes 1, 2, 5 and 6 were incubated with the blunt dsRNA; lanes 3, 4, 7 and 8 were incubated with the hairpin dsRNA. Odd-numbered lanes lacked cell extract. The RNase reaction is dependent upon ATP hydrolysis (4 °C reactions). The migration of RNA size markers is indicated on the left (Decade Markers, Ambion/Applied Biosystems)
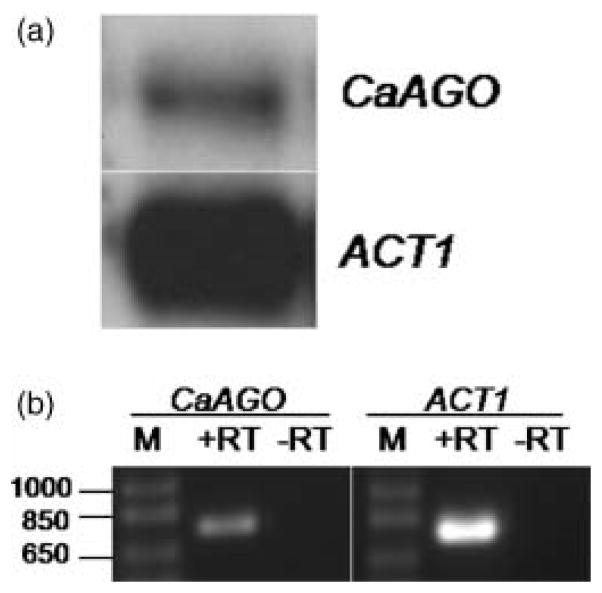
Although RNase assays demonstrated the production of siRNAs from hairpin dsRNA, other factors must influence the functionality of these molecules to induce RNAi within the cells. The C. albicans Argonaute (CaAGO) is unlikely to be a pseudogene because transcription was detected by northern blotting and RT-PCR (Fig. 4). Because the biological functions of Argonaute and Dicer are unknown in C. albicans, the lack of endogenous RNAi triggered by the exogenous introduction and expression of dsRNA remains unclear. The genome of C. albicans is missing an RNA-dependent RNA polymerase (RdRP) homologue (Nakayashiki et al., 2006), an important component of silencing pathways in nematodes, plants and fungi. An identifiable homologue of an RdRP is also missing from the S. castellii genome and this organism is still capable of performing RNAi of transgenes, thus it appears that Argonaute and Dicer are sufficient for RNAi activity in S. castellii but not for C. albicans. This together with our negative data may imply that RNA silencing in C. albicans has devolved to an atypical, species-specific pathway that is no longer triggered by exogenously introduced dsRNA.
Figure 4.
Expression analysis of C. albicans Argonaute (CaAGO, orf19.2903). (a) Total RNA from SC5314 was probed with radiolabelled CaAGO and ACT1 sequences by northern blotting. (b) Detection of CaAGO mRNA by RT-PCR. Amplification of a region of ACT1 served as positive control for the RT reactions. Reactions lacking RT (–RT) controlled for DNA contamination of the samples. M, DNA size markers (1 kb Plus DNA Ladder, Invitrogen). The identity of the size markers in base pairs are indicated on the left
Acknowledgments
We thank Richard Bennett for making available unpublished data, and Judy Berman, Brian Wong and Paula Sundstrom for helpful discussions. This work was supported by grant R21DE015749 awarded from the National Institute of Dental and Craniofacial Research (NIH) to J.F.S.
References
- Almeida R, Allshire RC. RNA silencing and genome regulation. Trends Cell Biol. 2005;15:251–258. doi: 10.1016/j.tcb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Brown T, Mackey K, Du T. Current Protocols in Molecular Biology. John Wiley & Sons; New York: 2004. Preparation and Analysis of RNA; pp. 4.9.1–4.9.19. [DOI] [PubMed] [Google Scholar]
- Catalanotto C, Pallotta M, ReFalo P, et al. Redundancy of the two dicer genes in transgene-induced posttranscriptional gene silencing in Neurospora crassa. Mol Cell Biol. 2004;24:2536–2345. doi: 10.1128/MCB.24.6.2536-2545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecere G, Cogoni C. Quelling targets the rDNA locus and functions in rDNA copy number control. BMC Microbiol. 2009;9:44. doi: 10.1186/1471-2180-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti H, Casas-Mollano JA. On the origin and functions of RNA-mediated silencing: from protists to man. Curr Genet. 2006;50:81–99. doi: 10.1007/s00294-006-0078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenares SU, Buker SM, Buhler M, Dlakic M, Moazed D. Coupling of double-stranded RNA synthesis and siRNA generation in fission yeast RNAi. Mol Cell. 2007;27:449–461. doi: 10.1016/j.molcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Donovan M, Schumuke JJ, Fonzi WA, et al. Virulence of a phosphoribosylaminoimidazole carboxylase-deficient Candida albicans strain in an immunosuppressed murine model of systemic candidiasis. Infect Immun. 2001;69:2542–2548. doi: 10.1128/IAI.69.4.2542-2548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinnenberg IA, Weinberg DE, Xie KT, et al. RNAi in budding yeast. Science. 2009;326:544–550. doi: 10.1126/science.1176945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall K. The roles of histone modifications and small RNA in centromere function. Chromosome Res. 2004;12:535–542. doi: 10.1023/B:CHRO.0000036584.40567.e5. [DOI] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Lanar DE, Kain KC. Expression-PCR (E-PCR): overview and applications. PCR Meth Appl. 1994;4:S92–6. doi: 10.1101/gr.4.2.s92. [DOI] [PubMed] [Google Scholar]
- Lee HC, Chang SS, Choudhary S, et al. qiRNA is a new type of small interfering RNA induced by DNA damage. Nature. 2009;459:274–277. doi: 10.1038/nature08041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Cottrell TR, Pierini LM, Goldman WE, Doering TL. RNA interference in the pathogenic fungus Cryptococcus neoformans. Genetics. 2002;160:463–470. doi: 10.1093/genetics/160.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone CD, Hannon GJ. Small RNAs as guardians of the genome. Cell. 2009;136:656–668. doi: 10.1016/j.cell.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martienssen RA, Zaratiegui M, Goto DB. RNA interference and heterochromatin in the fission yeast Schizosaccharomyces pombe. Trends Genet. 2005;21:450–456. doi: 10.1016/j.tig.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Nakayashiki H, Kadotani N, Mayama S. Evolution and diversification of RNA silencing proteins in fungi. J Mol Evol. 2006;63:127–135. doi: 10.1007/s00239-005-0257-2. [DOI] [PubMed] [Google Scholar]
- Pickford AS, Catalanotto C, Cogoni C, Macino G. Quelling in Neurospora crassa. Adv Genet. 2002;46:277–303. doi: 10.1016/s0065-2660(02)46010-5. [DOI] [PubMed] [Google Scholar]
- Poulter RT, Rikkerink EH. Genetic analysis of red, adenine-requiring mutants of Candida albicans. J Bacteriol. 1983;156:1066–1077. doi: 10.1128/jb.156.3.1066-1077.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 2007;8:23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- Raponi M, Arndt GM. Double-stranded RNA-mediated gene silencing in fission yeast. Nucleic Acids Res. 2003;31:4481–4489. doi: 10.1093/nar/gkg484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye CA, Engle JT, Goldman WE. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol Microbiol. 2004;53:153–165. doi: 10.1111/j.1365-2958.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- Reuss O, Vik A, Kolter R, Morschhauser J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene. 2004;341:119–127. doi: 10.1016/j.gene.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Rustad TR, Stevens DA, Pfaller MA, White TC. Homozygosity at the Candida albicans MTL locus associated with azole resistance. Microbiology. 2002;148:1061–1072. doi: 10.1099/00221287-148-4-1061. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Srikantha T, Klapach A, Lorenz WW, et al. The sea pansy Renilla reniformis luciferase serves as a sensitive bioluminescent reporter for differential gene expression in Candida albicans. J Bacteriol. 1996;178:121–129. doi: 10.1128/jb.178.1.121-129.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab JF, Bahn YS, Sundstrom P. Integrative, multifunctional plasmids for hypha-specific or constitutive expression of green fluorescent protein in Candida albicans. Microbiology. 2003;149:2977–2986. doi: 10.1099/mic.0.26445-0. [DOI] [PubMed] [Google Scholar]
- Volpe TA, Kidner C, Hall IM, Teng G, Grewal SI, Martienssen RA. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- Volpe T, Schramke V, Hamilton GL, et al. RNA interference is required for normal centromere function in fission yeast. Chromosome Res. 2003;11:137–146. doi: 10.1023/a:1022815931524. [DOI] [PubMed] [Google Scholar]
- White SA, Allshire RC. RNAi-mediated chromatin silencing in fission yeast. Curr Top Microbiol Immunol. 2008;320:157–183. doi: 10.1007/978-3-540-75157-1_8. [DOI] [PubMed] [Google Scholar]
- Ye X, Liu Q. Expression, purification, and analysis of recombinant Drosophila Dicer-1 and Dicer-2 enzymes. Methods Mol Biol. 2008;442:11–27. doi: 10.1007/978-1-59745-191-8_2. [DOI] [PMC free article] [PubMed] [Google Scholar]