Abstract
Objectives
Hypertension (HTN) is a common cause of left ventricular hypertrophy (LVH). Sustained pressure overload induces a permanent myocardial switch from fatty-acid to glucose metabolism. In this study we tested the hypothesis that metabolic remodeling, characterized by increased myocardial glucose uptake precedes structural and functional remodeling in HTN induced LVH.
Methods
We recruited 31 patients: 11 with HTN only, 9 with HTN and LVH, and 11 normotensive controls without LVH. Transthoracic echocardiography was performed to assess left ventricular (LV) function, LV mass, LV wall thickness, and LV diastolic function. PET imaging was performed and the rate of myocardial 2-deoxy-2-[18F]fluoro-D-glucose uptake, Ki, was determined using a 3-compartment kinetic model.
Results
The mean Ki values were significantly higher in HTN patients compared to HTN with LVH (p<0.001) and control (p=0.003) patients. The unexpected decrease in Ki with LVH may be secondary to a decreased Ki with diastolic dysfunction (DD), 0.039±0.032 versus 0.072±0.013, p=0.004. Also, there was a significant stepwise decrease in Ki with increasing DD grade(p=0.04).
Conclusion
Glucose metabolic remodeling is detectable in hypertensive patients before the development of LVH. Furthermore, lower glucose uptake rates are observed in patients with DD. The mechanism for this last finding requires further investigation.
Keywords: Metabolic imaging, Positron emission tomography, Left ventricular hypertrophy, Echocardiogram, 18F-FDG
INTRODUCTION
Acute pressure overload prompts the heart muscle to switch from fatty acids to glucose as its main source for oxidative energy production[1]. This adaptive response is beneficial for cardiac function in the short-term. However, sustained pressure overload reactivates the fetal gene program [2] increases intracellular glucose 6-phosphate levels (G6P), activates the mechanistic Target of Rapamycin and makes the metabolic “remodeling” from fatty-acid to glucose metabolism permanent. Understanding the progression of metabolic and structural changes in hypertension (HTN)-induced left ventricular hypertrophy (LVH), as well as defining precise thresholds when metabolic changes become maladaptive can help define a window for aggressive therapy to prevent progression to LVH, left ventricular dysfunction and failure.
Positron emission tomography (PET) imaging identifies metabolic substrate utilization non-invasively[3]. However, myocardial glucose uptake rate estimates may be inaccurate due to partial volume (PV) effects and spill-over (SP) of radioactivity from the myocardium into the LV blood pool and vice versa. We developed a novel quantitative method, wherein the blood input function with SP and PV coefficients and the metabolic rate constants in a compartment model are simultaneously estimated from attenuation-corrected, dynamic, gated 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) PET images of mouse heart in vivo[4]. Using this refined method in humans, we tested the hypothesis that metabolic remodeling precedes structural changes in hypertension-induced LVH.
MATERIALS AND METHODS
In this prospective study, the electronic medical records of the University of Virginia (UVA) cardiology clinics between July 2012 and 2013 were reviewed. A cohort of hypertensive patients was recruited, and subjects were classified according to the presence of LVH on transthoracic echocardiography (TTE). Volunteer patients from the same clinic without hypertension or LVH on TTE were included as controls.
Patients with the following characteristics were excluded from the study: history of congenital heart disease or other structural heart disease; prior myocardial infarction; known or suspected infiltrative heart disease; evidence of or symptoms concerning for myocardial ischemia; severe valvular disease on echocardiogram; history of chemotherapy administration in cardiotoxic doses; current corticosteroid therapy; atrial fibrillation or other significant arrhythmia; hypersensitivity to 18F-FDG; pregnancy or current breastfeeding.
Systemic hypertension was defined as a blood pressure ≥140/90mmHg on two or more separate occasions or anti-hypertensive medications use for the purposes of blood pressure control[5].
The study was approved by the Institutional Review Board of the University of Virginia. Informed consent was obtained from all individual participants included in the study.
Demographic data
Patients characteristics were collected through chart review, and included: comorbidities; duration, treatment, and control of HTN; current cardiac medications; and fasting blood glucose levels.
Transthoracic echocardiography (TTE)
Transthoracic echocardiograms were performed to determine left ventricular (LV) mass, wall thickness, left ventricular ejection fraction (LVEF), and diastolic function parameters within 6 months of study enrollment. LVH was considered present in the setting of an end-diastolic LV interventricular septum or posterior wall dimension ≥1.2cm measured after closure of the mitral valve. Heart weight (HW) was calculated using the Devereaux formula[6] and was divided by the body weight (BW) to compute HW/BW ratios. Diastolic dysfunction (DD) was identified and graded according to the latest American Society of Echocardiography guidelines [7]. The mitral E/A ratio, annular and lateral e’, deceleration time (DT) and left atrial (LA) size were calculated to assess diastolic function. Diastolic dysfunction diagnostic criteria included left atrial enlargement (>34 mL/m2) and septal e’ velocities <8 cm/s. Mild diastolic dysfunction was defined as an E/A ratio <0.8 and DT >200ms. Moderate diastolic dysfunction was classified as an E/A ratio of 0.8–1.5 and DT 160–200ms. Severe diastolic dysfunction was defined as an E/A ratio ≥2 and DT <160ms.
2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography
PET imaging was performed after an overnight fast. We aimed to determine glucose uptake under normal, steady-state conditions rather than facilitating glucose uptake with a hyperinsulinemic euglycemic clamp. All patients received a computed tomographic (CT) scan for attenuation correction followed by a 30 minute dynamic PET scan with cardiac gating on a Siemens Biograph mCT PET-CT scanner. Imaging was initiated a few seconds before the slow administration of 10–15 mCi intravenous 18F-FDG over 30 seconds. Images were reconstructed using an iterative reconstruction algorithm with the following dynamic frames: 12 10-second frames; 8 30-second frames; 8 60-second frames; 2 180-second frames and 2 300-second frames. Regions of interest (ROIs) were drawn in the areas corresponding to the LV blood pool and the myocardium (one region corresponding to the left anterior descending artery (LAD), left circumflex artery (LCX) and right coronary artery (RCA)), and average time activity curves were generated. A novel model [4] was used to determine the global rate of myocardial FDG uptake: Ki (ml/min/g).
Statistical Analyses
Continuous variables are given as means ± standard deviation and were compared using ANOVA with Tukey’s studentized range testing. Categorical variables are given as percentages and were compared using Chi-square or Fisher’s exact testing when appropriate. SigmaStat 3.0 software was used for statistical analysis. A P value of <0.05 was considered statistically significant.
RESULTS
The study population included 31 patients: 11 with HTN without LVH (HTN no LVH), 9 with HTN and LVH (HTN+LVH), and 11 controls with neither LVH nor HTN (controls). The general characteristics of the patient population are presented in Table 1. The mean age was 53±9 years, with women comprising 61% of the patient population. The mean age was lower in the control group compared with the hypertensive groups. Gender distribution was similar in controls and HTN no LVH groups (54% each), but the percentage of females was greater in the HTN+LVH group (78%, p<0.001). Heart rate was significantly lower in controls patients. No significant differences in comorbid conditions (hyperlipidemia, smoking, stroke), fasting hyperglycemia, BMI or BSA were found between the three groups. Between the HTN and HTN+LVH groups there was no significant difference in the type of antihypertensive used.
Table 1.
Comparison of patients’ baseline characteristics between the three groups; HTN+LVH, HTN no LVH, and controls.
| HTN and LVH (N=9) |
HTN no LVH (N=11) |
Controls (N=11) |
P value | |
|---|---|---|---|---|
| Age (years) | 59 ± 13 | 57 ± 12 | 43 ± 13 | 0.017 |
| Female-No.(%) | 7 (78%) | 6 (54%) | 6 (54%) | <0.001 |
| Height (inches) | 64.7 ± 4.5 | 66.9 ± 4.4 | 66.8 ± 5 | NS (0.56) |
| Weight (lbs) | 223 ± 58 | 219 ± 47 | 188 ± 40 | NS (0.21) |
| BMI (kg/m2) | 35.5 ± 6.5 | 34.4 ± 7.3 | 29.3 ± 5.9 | NS (0.09) |
| BSA | 2.1 ± 0.3 | 2.1 ± 0.2 | 1.9 ± 0.3 | NS (0.18) |
| Duration of HTN | ≥7 years | ≥2 years | None | |
| Pulse (beats/min) | 79.3±17.9 | 72.5 ± 15.9 | 55.7 ± 7.4 | 0.002 |
| BP at time of enrollment (mm Hg): | ||||
| Systolic | 146±18 | 150±23 | 115±12 | <0.001 |
| Diastolic | 78±14 | 81±10 | 76±7 | NS (0.54) |
| Fasting glucose (mg/dl) | 114 ± 27 | 105 ± 25 | 91 ± 8 | NS (0.07) |
| Diabetes Mellitus-No.(%) | 3 (33%) | None | None | NS |
| Hyperlipidemia | 2 (22%) | 6 (54%) | 1 (9%) | NS |
| Smoking | NS | |||
| Current | 2 (22%) | 2 (18%) | 0 | |
| Past | 2 (22%) | 2 (18%) | 2 (18%) | |
| HTN meds: | ||||
| HCTZ | Yes (4) | Yes (2) | No | NS |
| ACE inhibitors/ARB | Yes (8) | Yes (5) | No | NS |
| Beta blockers | Yes (2) | Yes (5) | No | NS |
| ASA | Yes (2) | Yes (5) | No | NS |
| LVEF | 60–65% | 60–65% | 60–65% | NS |
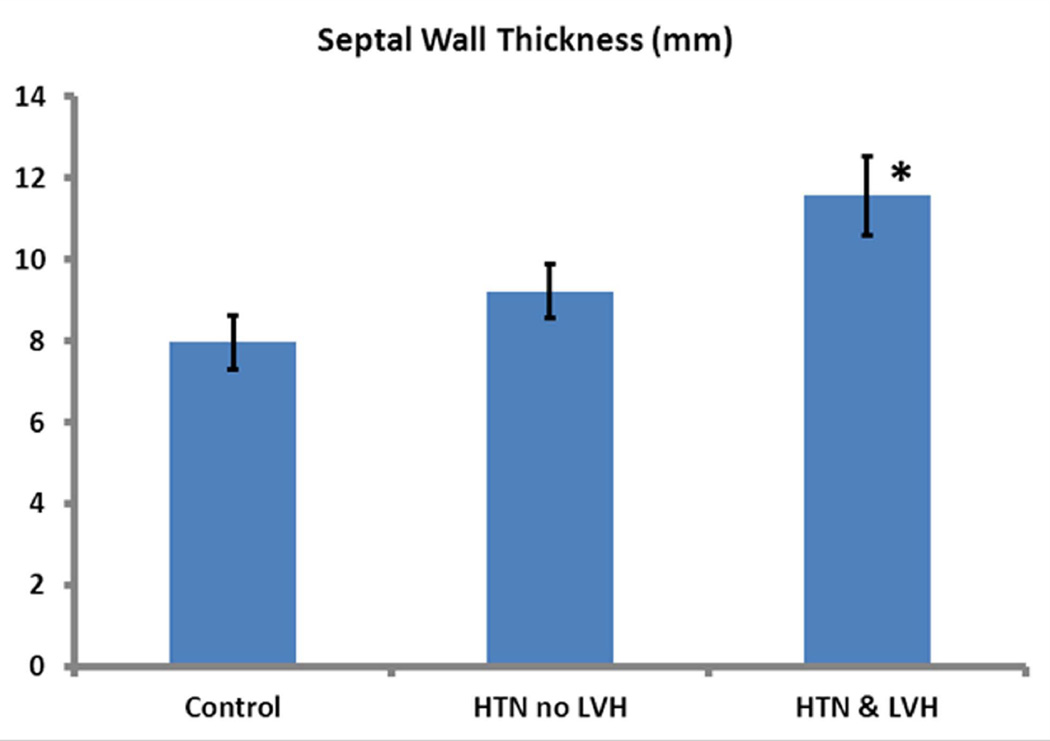
| LV septal wall thickness (mm) | 11.6 ± 2.9 | 9.2 ± 1.8 | 7.9 ± 2.3 | 0.009 |
Transthoracic echocardiographic findings
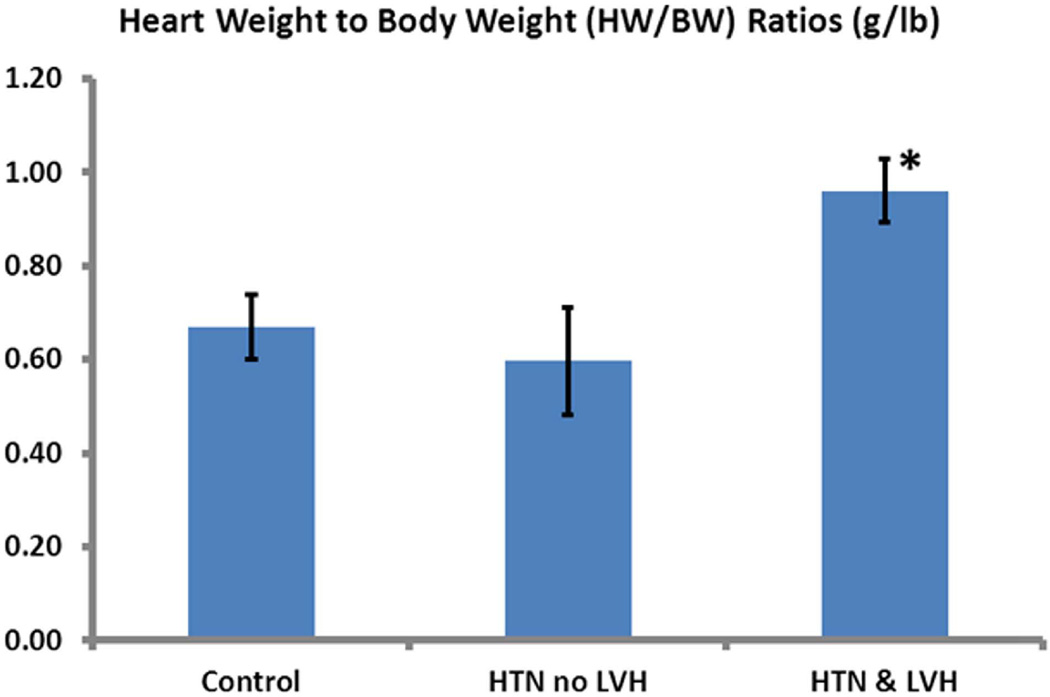
All patients had normal baseline LV systolic function with an ejection fraction of 60-65% on transthoracic echocardiography. The mean septal wall thickness differed significantly between the groups (p=0.009) (Table 1); it was higher in HTN+LVH patients than in the control group (11.5±2.9 mm vs 7.9±2.3 mm, p=0.006). The mean wall thickness for the HTN patients did not differ significantly from the control patients (p=0.523), but was significantly lower than in the HTN+LVH group (p<0.05) (Figure 1). The mean HW/BW was also greater in the HTN+LVH (0.96±0.2 g/lb) than in the HTN (0.6±0.3 g/lb, p=0.021) and control (0.67±0.3 g/lb, p=0.034) patient populations. There was no significant difference in HW/BW ratios between the HTN and control (p=0.809) patients (Figure 2).
Figure 1. Comparison of septal wall thickness (mm) measured by transthoracic echocardiogram between the study groups.
The HTN+LVH population exhibited significantly greater septal wall thickness compared to the control and HTN groups (P<0.05). Data are shown as Mean ± SE.
Figure 2. Comparison of heart weight to body weight ratio (HW/BW) in g/lbs between the study groups.
The HTN+LVH group exhibited significantly greater HW/BW ratios compared to the control and HTN groups (P<0.05). Data are shown as Mean ± SE.
PET Metabolic imaging
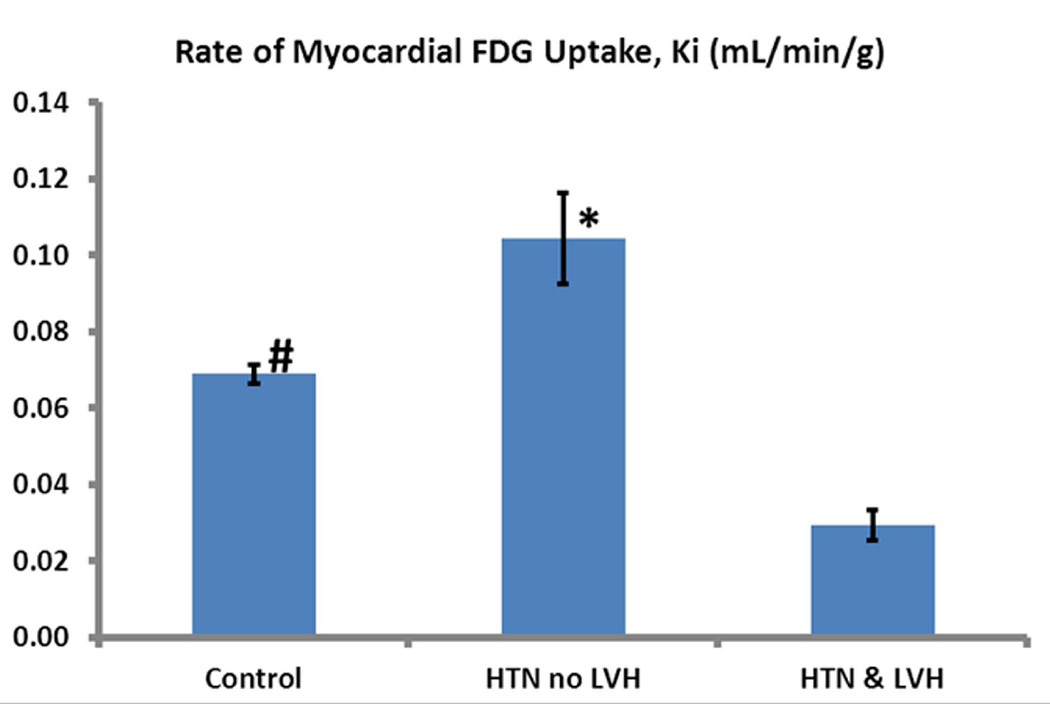
The mean Ki value (ml/min/g) for glucose uptake in the 3 groups differed significantly (p<0.001). It was higher in the HTN cohort (0.10±0.032) compared to the HTN+LVH (0.024±0.011) (p<0.001) and control (0.067±0.013) (p=0.003) cohorts. The mean Ki was significantly lower in the HTN+LVH group compared with the control group (p<0.001) (Figures 3A–B).
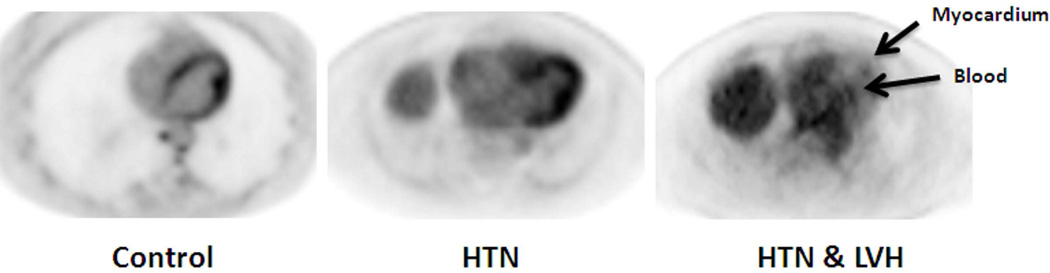
Figure 3. Measured rates of myocardial FDG uptake, Ki.
(A) Representative FDG PET axial slices of the heart in the 3 patient populations at the last time frame. The limited contrast between the left ventricular cavity and myocardium in the HTN+LVH image is due to the minimal FDG uptake in the myocardium, leading to limited differentiation from background blood pool levels. This finding is not due to poor image quality. Arrows indicate the myocardium and the blood pool in the HTN+LVH image. (B) The rate of FDG uptake in the HTN group is significantly higher than the control and the HTN+LVH group (P<0.05). We also observe significantly greater rate of FDG uptake in the control group compared to the HTN+LVH group (P<0.05). Data are shown as Mean ± SE.
Diastolic function and Ki
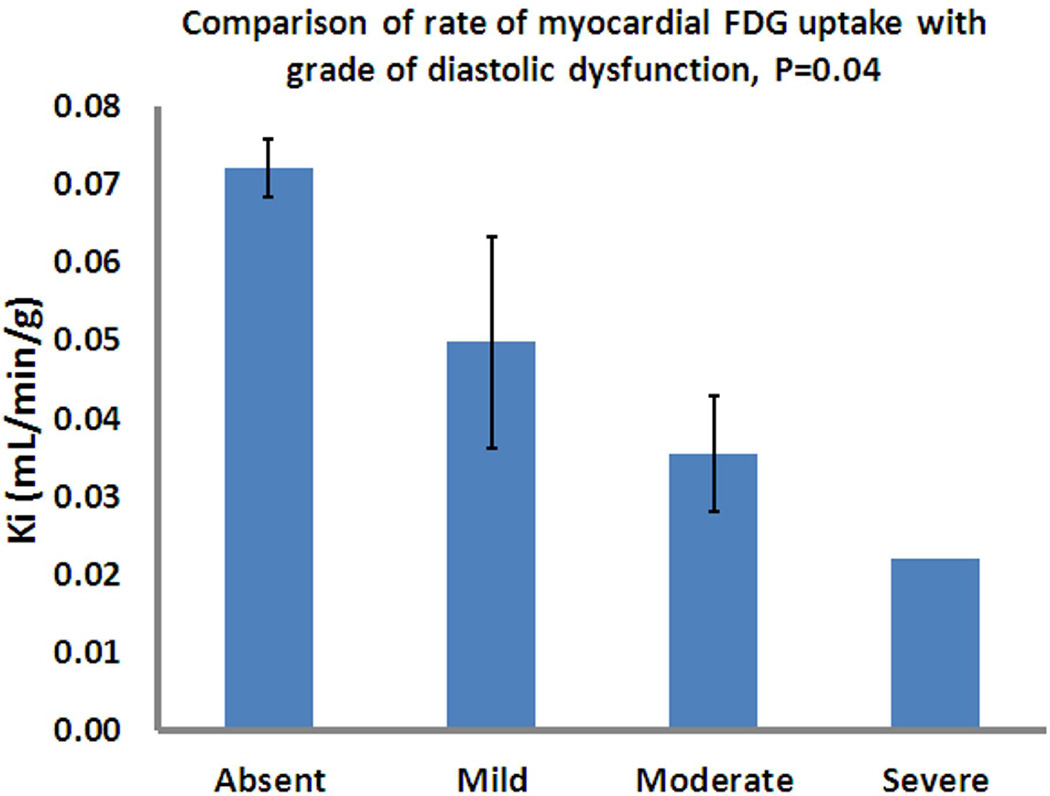
Diastolic function was assessed by echocardiography in 28 of 31 subjects (90.3%); diastolic function data was unavailable in 1 control subject, 2 with HTN no LVH, and 1 subject with HTN + LVH. Overall, the mean Ki was significantly lower in those patients with DD (0.039±0.032 versus 0.072±0.013, p=0.004). There was a significant stepwise decrease in Ki (p=0.04) as the grade of DD increased (see Figure 4). Diastolic function was normal in all control subjects. DD was present in 5/9 (55.6%) of those with HTN and no LVH. One of these subjects had moderate DD, the rest were mild. However, there was no significant difference in Ki in the HTN group between patients with and without DD (0.072±0.032 versus 0.096±0.039, p=0.304). DD was present in all patients with HTN and LVH. One of the subjects had severe DD, 2 had moderate DD, and the rest were mild.
Figure 4. Rate of myocardial FDG uptake (Ki) according to grade of diastolic dysfunction (DD).
There was a significant stepwise decrease in Ki as the grade of DD increased (p=0.04). Diastolic function was normal in all control subjects. DD was present in 5/9 (55.6%) of those with HTN and no LVH. One of these subjects had moderate DD, the remainder were mild. DD was present in all patients with HTN and LVH. One of these subjects had severe DD, 2 moderate DD, and the 5 were mild. Data are shown as Mean ± SE.
DISCUSSION
The findings in this study indicate that glucose uptake increases in patients with HTN but no LVH compared to control subjects. These results are consistent with previous findings in animal models of LVH [4,8,9] and suggest that metabolic changes in the human heart precede the development of structural remodeling in the form of LVH. Although derangements in fatty acid metabolism in hypertensive heart disease have been previously studied [10], this is the first study that addresses myocardial glucose uptake using 18F-FDG PET in human subjects with HTN prior to the development of LVH. The increase in the rate of myocardial FDG uptake, Ki, in early hypertension could be an adaptive response to maintain function with no change in LV structure. It is consistent with our prior finding that glucose metabolic remodeling occurs in the heart of hypertensive rabbits[11]. Subsequent studies with serial measurements in the same patient population is the next step to take in order to further understand the metabolic remodeling in human heart.
An interesting and unexpected finding of our study was the decreased 18F-FDG uptake rate in patients with HTN+LVH compared to HTN patients without LVH. The presence and severity of DD correlates and may explain, at least partially this finding. Those with DD in the HTN no LVH group had a lower Ki, however did not reach statistical significance. Furthermore, there was a stepwise decrease in Ki with increasing DD grade in all patients with HTN. The pathophysiology for this finding is unclear and will require further studies. A possible explanation could be increased levels of myocardial fibrosis in the HTN patients with LVH due to structural remodeling (see Figures 1–2) resulting in severe DD and lower Ki. Other potential explanations for this finding are gender differences in metabolic and structural remodeling induced by hypertension [12] and the presence of diabetic patients in the HTN+LVH group. Diabetes may affect 18F-FDG uptake through downregulation of the transcription factor myocyte enhancer factor 2c and its controlled genes, including the glucose transporter 4[13]. There is a growing body of literature, including published work from one of us and others, showing that insulin resistance protects the heart from fuel overload [14,15][16]. In addition to diabetes, the duration of HTN may play a role in the reduced myocardial glucose uptake in the LVH population. Also, based on previous findings from the coinvestigators’ lab [17], in human heart there is a negative correlation between plasma free fatty acids and diastolic function.
Beta blockers can attenuate myocardial FDG uptake, as also observed in our recent animal studies[4]. However, in the present study, it is unlikely that differences in treatment with beta blockers explain these findings, as there was no significant difference in the percentage of patients treated with these drugs in the HTN and HTN+LVH groups (Table 1). Furthermore, in patients with idiopathic dilated cardiomyopathy the heart changes from predominantly fat metabolism to glucose metabolism in response to beta blocker treatment[18,19]. These reports make the issue of substrate preference in human heart muscle even more complex.
CONCLUSIONS
Metabolic remodeling of the left ventricle, as indicated by an increased rate of 18F-FDG uptake, in hypertensive patients precedes the development of structural remodeling as assessed by LVH. This finding may encourage protocols for non-invasive detection of early metabolic changes to indicate early aggressive therapy to prevent disease progression. Hypertensive subjects with LVH have an unexpected decreased rate of 18F-FDG uptake, and this finding appears to correlate with decreased 18F-FDG uptake in subjects with DD. Further research is warranted to identify the underlying mechanisms of this finding.
ACKNOWLEDGEMENTS
This work was supported by the University of Virginia Thelma R. Swortzel Award (to BKK and JMB) and in part by NHLBI (to BKK). Work in Dr. Taegtmeyer’s lab is supported in part by the NHLBI.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Reference List
- 1.Bishop SP, Altschuld RA. Increased glycolytic metabolism in cardiac hypertrophy and congestive failure. Am J Physiol. 1970;218:153–159. doi: 10.1152/ajplegacy.1970.218.1.153. [DOI] [PubMed] [Google Scholar]
- 2.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail. Rev. 2007;12:331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 3.Gropler RJ. Recent advances in metabolic imaging. J Nucl. Cardiol. 2013;20:1147–1172. doi: 10.1007/s12350-013-9786-z. [DOI] [PubMed] [Google Scholar]
- 4.Zhong M, Alonso CE, Taegtmeyer H, Kundu BK. Quantitative PET Imaging Detects Early Metabolic Remodeling in a Mouse Model of Pressure-Overload Left Ventricular Hypertrophy In Vivo. J Nucl. Med. 2013;54:609–615. doi: 10.2967/jnumed.112.108092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James PA, Oparil S, Carter BL, Cushman WC, nison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 6.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 7.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am. Soc. Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Sen S, Kundu BK, Wu HC, Hashmi SS, Guthrie P, Locke LW, Roy RJ, Matherne GP, Berr SS, Terwelp M, Scott B, Carranza S, Frazier OH, Glover DK, Dillmann WH, Gambello MJ, Entman ML, Taegtmeyer H. Glucose Regulation of Load-Induced mTOR Signaling and ER Stress in Mammalian Heart. J Am. Heart Assoc. 2013;2:e004796. doi: 10.1161/JAHA.113.004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kundu BK, Zhong M, Sen S, Davogustto G, Keller SR, Taegtmeyer H. Remodeling of Glucose Metabolism Precedes Pressure Overload-Induced Left Ventricular Hypertrophy: Review of a Hypothesis. Cardiology. 2015;130:211–220. doi: 10.1159/000369782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de las FL, Soto PF, Cupps BP, Pasque MK, Herrero P, Gropler RJ, Waggoner AD, vila-Roman VG. Hypertensive left ventricular hypertrophy is associated with abnormal myocardial fatty acid metabolism and myocardial efficiency. J. Nucl. Cardiol. 2006;13:369–377. doi: 10.1016/j.nuclcard.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension. 1988;11:416–426. doi: 10.1161/01.hyp.11.5.416. [DOI] [PubMed] [Google Scholar]
- 12.Petrov G, Dworatzek E, Schulze TM, Dandel M, Kararigas G, Mahmoodzadeh S, Knosalla C, Hetzer R, Regitz-Zagrosek V. Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC. Cardiovasc. Imaging. 2014;7:1073–1080. doi: 10.1016/j.jcmg.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Razeghi P, Young ME, Cockrill TC, Frazier OH, Taegtmeyer H. Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation. 2002;106:407–411. doi: 10.1161/01.cir.0000026392.80723.dc. [DOI] [PubMed] [Google Scholar]
- 14.Harmancey R, Lam TN, Lubrano GM, Guthrie PH, Vela D, Taegtmeyer H. Insulin resistance improves metabolic and contractile efficiency in stressed rat heart. FASEB J. 2012;26:3118–3126. doi: 10.1096/fj.12-208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taegtmeyer H, Beauloye C, Harmancey R, Hue L. Insulin resistance protects the heart from fuel overload in dysregulated metabolic states. Am. J Physiol Heart Circ. Physiol. 2013;305:H1693–H1697. doi: 10.1152/ajpheart.00854.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolan CJ, Ruderman NB, Kahn SE, Pedersen O, Prentki M. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes. 2015;64:673–686. doi: 10.2337/db14-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leichman JG, Aguilar D, King TM, Vlada A, Reyes M, Taegtmeyer H. Association of plasma free fatty acids and left ventricular diastolic function in patients with clinically severe obesity. Am. J Clin. Nutr. 2006;84:336–341. doi: 10.1093/ajcn/84.1.336. [DOI] [PubMed] [Google Scholar]
- 18.Eichhorn EJ, Heesch CM, Barnett JH, Alvarez LG, Fass SM, Grayburn PA, Hatfield BA, Marcoux LG, Malloy CR. Effect of metoprolol on myocardial function and energetics in patients with nonischemic dilated cardiomyopathy: a randomized, double-blind, placebo-controlled study. J Am. Coll. Cardiol. 1994;24:1310–1320. doi: 10.1016/0735-1097(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 19.Wallhaus TR, Taylor M, Degrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2001;103:2441–2446. doi: 10.1161/01.cir.103.20.2441. [DOI] [PubMed] [Google Scholar]