Abstract
The study was designed to explore the feasibility of increasing the delivery of gemcitabine-HCL (Gem), a poor membrane permeable and short half-life drug, through PEGylated thermosensitive liposomal nanoparticles (TSLnps) delivery system followed by mild hyperthermia (mTH) at 42°C. In vitro release pattern of Gem-TSLnps showed a significant Gem release (60%, p<0.01) at 42°C compared to that released at 37°C (29%). Cell viability and clonogenic assay demonstrated significant inhibition of MiaPaCa-2 cells growth by Gem-TSLnps + mHT compared to Gem alone. Further, IC50 value of Gem treated cells was (0.077μM) 1.2 fold higher compared to that treated with Gem-TSLnps + mHT (0.063 μM). mHT treated cells showed moderate inhibition of cell growth compared to controls. For cellular uptake studies, flow cytometric analysis and confocal imaging revealed higher uptake of Rho-TSLnps compared to Rho-PE or untreated cells. Tumor volume of mice treated with Gem alone was 1.8 fold higher compared to the group treated with Gem-TSLnps + mHT. Further, tumor regression of Gem-TSLnps + mHT treated group was significantly higher (p<0.01) compared to Gem-TSLnps or Gem. No significant elevated liver enzymes were observed when serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level of control group was compared to that of Gem or Gem-TSLnps+mHT treated groups. However, serum level of alkaline phosphatase (ALP) of Gem or Gem-TSLnps+ mHT treated group was significantly elevated (p<0.05) when compared to the control group. In conclusion, TSLnps increased the delivery of Gem to tumor cells and also enhanced significantly the antitumor activity of Gem when combined with heat.
Keywords: Pancreatic cancer, Gemcitabine-HCL, Liposomes, Thermosensitive, Anti-tumor, Liver enzymes
INTRODUCTION
Pancreatic cancer is the fourth-leading cause of cancer-related deaths in the United States after breast, colon and lung cancers. The most common type of cancer of the pancreas is adenocarcinoma a tumor that arises from the cells that line the duct of the pancreas. Only about 20 to 40% of patients with adenocarcinoma of the pancreas have a tumor that is confined to the pancreas at the time of diagnosis. This year 48,960 people are estimated to be diagnosed with pancreatic cancer of which 40,560 people are estimated to be killed1. The 5-year survival rate is 7.2%1. Although, Gem is used as the first-line drug for treatment of pancreatic cancer, therapeutic outcome is disappointing as a result of tumor resistance to drug delivery as demonstrated in clinical and preclinical trials which has recorded a median survival between 5.0-7.2 months post treatment2, 3.
Cardinal features of pancreatic tumors confer drug resistance include discontinuous, leaky and dysfunctional vasculature with high interstitial pressure impeding transport of chemotherapeutic agents to the extracellular compartment3. Pancreatic tumors are characteristically hypoxic and undergo a series of stromal reaction resulting in desmoplasia of the extracellular compartment. These processes have been recognized to augment the survival and antagonistically confer resistance to drug therapy2, 3. Gem, a pyrimidine nucleoside analog, is transported into the intracellular region by nucleoside transporter and catalytically converted to Gem- mono, di and triphosphate by deoxycytidine kinase which subsequently blocks ribonucleotide reductase and deoxyribonucleic acid (DNA) synthesis4, 5. Though it has been proven to be clinically effective in the management of pancreatic cancer, it has demonstrated a low metabolic stability with a short half-life of approximately 8-17 min5. Effective delivery of Gem will therefore require a delivery system which has the capacity to deliver discriminately a i) high payload of Gem to tumors, ii) protects Gem from enzymatic degradation with concomitant increase in residence time, and iii) minimizes unwanted systemic toxicity. A promising strategy that has gained tremendous attention is the entrapment of anticancer drugs in the core of liposomes, a non-toxic, biocompatible and biodegradable, and has been approved by Food and drug administration (FDA) as a carrier for doxorubicin hydrochloride6.
mHT involves the heating of tumors to temperatures of up to 42°C and is usually combined with chemotherapy or radiation to enhance the therapeutic outcome of the treatment. HT has a major impact on several tumor pathophysiological parameters such as perfusion and vascular permeability on liposome extravasation in tumor areas7. However, uptake of intravenously administrated liposomes by macrophages in reticuloendothelial system (RES) as a result of opsonization leads to decrease drug distribution and low therapeutic efficacy of anticancer drugs 8, 9.
Opsonization could be averted by the conjugation of polyethylene glycol (PEG) polymers to liposomal surface leading to reduce steric interaction of liposomes with plasma opsonin such as serum albumin. Conferring “stealth” properties to liposomal carrier increases its residence time in systemic circulation which may lead to increase concentration of entrapped drug in tumor environment8. Upon arrival in the tumor area, pegylated thermosensitive liposomes, which composed of temperature sensitive phospholipids, undergo a gel-to-liquid crystalline phase transition at temperatures around 42°C, a process which causes significant release of liposome-entrapped water-soluble drugs10. Report also indicates that mild HT may synergistically enhance the antitumor effect of anticancer agent11.
Other studies on delivery of Gem through thermosensitive liposomes have been published12-14, however the use of waterbath or light as a source of heat to disrupt the liposomes brings into question its translational potential. The reason is that neither waterbath (immersion of the flank-bearing tumor in water) nor light can provide sufficient heat for deep seated tumors. Our study seek to address this problem by employing special thermocouple heating device made by Omega Engineering which has the capacity to provide heat to deep seated orthotopic tumor model such as pancreatic cancer.
In this study, we report the feasibility of employing TSLnps as delivery system and mild HT to increase the delivery of Gem, which is poorly membrane permeable and improve its anti-cancer activity in pancreatic cancer tumor. The study was conducted in both in vitro and in vivo.
Materials and Methods
Dipalmitoylphosphatidilcholine (DPPC), 1-myristoyl-2-palmitoyl-sn-glycero-3-phosphocholine (MPPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol) 2000 (DSPE PEG2000), and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt) (Rho-PE) were purchased from Avanti Polar Lipids (Alabaster, AL). Gemcitabine hydrochloride (2′-Deoxy-2′,2′-difluorocytidine) and reagentsfor alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) determination were purchased from Sigma-Aldrich (St. Louis, MO). MiaPaCa-2 cell line was purchased from American Type Culture Collection (ATCC) (Manassas, VA). All solvents used were of analytical grade.
Preparation of Gem-TSLnps
A Gem-TSLnps was formulated as described in our previous publication 15. Briefly, Gem-TSLnps was prepared using DPPC:MPPC:DSPE-PEG2000 in a molar ratio of 90:10:4 by thin film hydration and extrusion method at 60 °C. A combination of 50 mg of phospholipids was dissolved in chloroform and mixed until a clear solution was observed. Chloroform was then removed by passing a thin stream of nitrogen gas through the solution under a fume hood. Lipid mixture was further dried under vacuum for 24 hr to remove residual chloroform. The thin film formed was subsequently hydrated with 10 mM of Gem-HCl dissolved in phosphate buffered saline (PBS, 1×) at pH 7.2. It was further subjected to intermittent vortexing for 15 min to form multilaminar vesicles (MLV). The lipid vesicle solution was extruded through 200 nm polycarbonate membranes for 15 times. Unencapsulated Gem was removed by dialysis against PBS for 24 hr16-18 (Spectra/Por® Dialysis Membrane MWCO 3500, Spectrum, Rancho Dominguez, CA). Similarly, Rho-PE-TSLnps was prepared with DPPC: MPPC: Rho-PE: DSPE-PEG2000 in a molar ratio of 90:10:0.5:3.5 by the method described above. The final products, Gem-TSLnps and Rho-TSLnps, were lyophilized using 5.0 % (w/v) mannitol as a cryoprotectant, kept in sealed cryovials and stored at 4°C in wrapped aluminium foil. All the formulations were filtered through 0.22 μmbefore use. Size was determined by dynamic light scattering using Particle Sizing systems, Santa Barbara, California.
Determination of Gem entrapment in TSLnps
Lyophilized Blank TSLnps and Gem-TSLnps were prepared as described above. Fifty mg of physical mixture made up of Gem, DPPC, MPPC and DSPE-PEG2000 was also prepared. Fourier Transform-infrared spectroscopy (FTIR) spectra of the formulations and physical mixture were determined (750-4000 cm−1) on the FTIR spectrophotometer (PerkinElmer Life and Analytical Sciences, Connecticut). The obtained spectra of pure Gem, blank TSLnps and Gem-TSLnps and that of physical mixture were compared to identify characteristic peak that might indicate the presence of Gem in TSLnps19.
Entrapment efficiency (EE)
The amount of Gem entrapped in TSLnps was determined by disrupting 1ml of Gem-TSLnps suspension with 100 μl of 30% triton X-100. The solution was increased to 3 ml with mobile phase (mobile phase 10 mM phosphate buffer, pH 3.0 containing 5% of acetonitrile). The solution was then vortexed for 1 min and centrifuged at 10,000 rpm (11,630 rcf) for 10 min. Internal standards and obtained supernatant solutions were analyzed using Water's high performance liquid chromatography (HPLC) with photodiode array (2998 UV/Vis) detector (column = C18, 4.6 × 250 mm, flow rate of 1.0 ml/min, injection volume 20μl)20. The EE (%) of Gem was calculated as follows:
| (1) |
In vitro release of Gem
Percent release of Gem from TSLnps was determined at different temperatures (26, 34, 37, 39, 42, 45, and 50 °C) as previously described15. Cumulative release of Gem at 37 °C was determined at different time points (5, 10, 15, 30, 60, 120, 240, 360, 720 and 1,440 min). Twenty milligram of lyophilized Gem-TSLnps was suspended in 1 ml of PBS and transferred into a washed dialysis bag (MWCO: 12, 000 daltons) that was subsequently placed in a glass bottle containing 5 ml of PBS and sealed tightly with a screw cap. The temperature of the system was maintained at 37 °C with continuous stirring (80 rpm). One milliliter of the receiver solution was removed at each time point and replaced with fresh PBS kept at 37 °C. Amount of Gem released into receiver chamber at each time point was determined by HPLC as described above. Gem in 20 mg of lyophilized Gem-TSLnps also was determined by disrupting TSLnps with 30% Triton X-100 and diluted to appropriate volume.
Cell viability assay
Cytotoxic effects of Gem and Gem-TSLnps were determined by trypan blue assay method. MiaPaCa-2 cells were initially grown in DMEM (Dulbecco's Modified Eagle's Medium) supplemented with25 mM HEPES buffer, 10% fetal bovine serum (FBS) and5ml of Penicillin-streptomycin (10000 UI Pen/10000μg Strep per 1ml). MiaPaCa-2 cells were cultured in 75 cm3 culture flask at optimum growth conditions (pCO2, 5%, humidity, 95%) and incubated at 37°C. Cells were seeded in 12-well plates in triplicates at a density of 2.0 × 104 cells per well. The groups were treated with different concentrations of either Gem or Gem-TSLnps and incubated for 24 hr. After 24 hr, Gem-TSLnps treated groups were exposed to heat at 42°C (± 0.02°C) for 10 min (after reaching thermal equilibrium at 42°C) and incubated further for 24 hr at 37°C. Cells were stained with 0.4% trypan blue and counted using Biorad automated cell counter. Percent live cells were then calculated as follows:
| (2) |
Clonogenic Assay
Survival of MiaPaCa-2 cells post treatment was determined by seeding cells at 1.5-2.0 × 105 in 25 cm3 culture flask and incubated at optimum growth conditions of (pCO2, 5%; humidity, 95%; 37°C) and allowed to grow to a confluence level of 70%. Cells were then treated with 0.001, 0.01, 0.1, 1.0, 10 and 100 μM of Gem or Gem-TSLnps. After 24 hr, cells treated with Gem-TSLnps were exposed to mild HT at 42°C for 10 min in an isotemp incubator and placed back into CO2 incubator at 37°C. Cell growth was terminated on the 7th day and re-plated in 6-well plates at 1,000 live cells per well and incubated for 10 days until cluster of colonies were formed. Colonies were washed 2 times with PBS, fixed using methanol and acetic acid in ratio of 7:1. The colonies were stained with 0.5% methylene blue (dissolved in 50% ethanol) for 5 min and thoroughly washed with running water and air dry. Colonies were later visualized and counted using stereomicroscope. Cluster of 50 or more cells were counted as one colony.
Confocal imaging and flow cytometric analysis
Confocal imaging and flow cytometric analysis were employed to determine the extent of cellular uptake of TSLnps by MiaPaCa-2 pancreatic cancer cell lines. MiaPaCa-2 cells were cultured on cover slides at a density of 1.5×105 at optimum growth conditions for 24 hr as reported previously 15. In confocal studies, MiaPaCa-2 cells were exposed to 1 ml of 0.5mol % Rho-TSLnps in growth medium and incubated for 4 hr at optimum growth conditions. Cells were adequately washed with PBS and incubated with Hoechst dye (5μg/ml) for 30 min. After 30 min, cell monolayers were washed 3 times with PBS, fixed with 4.0% paraformaldehyde solution overnight and later examined by a confocal laser microscope 21.
For flow cytometric analysis, MiaPaCa-2 cells were plated in duplicates in a 6-well plate as described 15. Cells were seeded at 2.0×105 cells per well and treated with 0.5 mol% Rho-PE and Rho-PE-TSLnps (0.5 mol% of Rho-PE) after the cell growth reached 75 % confluence. After 24 hr incubation growth medium was removed, cells washed twice with PBS and detached using 0.25 % trypsin-EDTA. The trypsin was then neutralized with growth medium and cell suspension centrifuged at 2,500 rpm for 3 min. Cell pellets were fixed with 4% paraformaldehyde and rewashed 3 times with PBS to remove any traces of paraformaldeyde. The fixed pellets were re-suspended in 500 μl of PBS and analyzed using fluorescence-activated cell sorting (FACS) caliber flow cytometer.
Animals and tumor cells
Female athymic nude (Nu/Nu) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) at 6 to 8 weeks of age. The animals were housed in a virus-free, indoor, light- and temperature controlled barrier environment and were provided ad libitum access to food and water. All procedures with animals were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Florida A&M University Animal Care and Use Committee. MiaPaCa-2 cells were cultured in DMEM medium supplemented with 10% FBS and 1% penstrep (100 U/mL penicillin and 100μg/mL streptomycin) and incubated in 5% CO2 and humidified environment of 95% air at 37 °C. Cells were (5.0 × 10−6 in 100μl PBS) injected subcutaneously in the lower flanks of nude mice, and tumors of 7 mm in diameter were used.
Antitumor activity study
Mice were injected intraperitoneally (i.p) with Gem (20.0 mg/kg), Gem-TSLnps and Gem-NTSLnps (equivalent dose of Gem, 10.0 mg/kg) and followed up with the same dose of the formulations every other day for 2 weeks. IP injection was preferred to intravenous (IV) injections because we wanted to prevent vascular damage due to the repeated injections (3 injections per week) and also to avoid severe stress to the animal, which needed to be restrained if not anesthetized during injection.
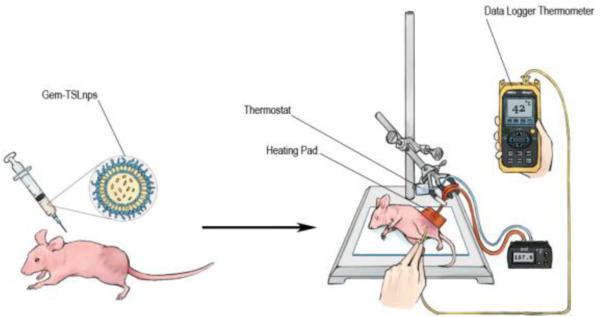
In localized hyperthermia, heat was exposed to tumor area of Gem-TSLnps treated mice where needle thermocouples (Omega Engineering) were positioned into the tumor core and at the tumor surface for 10 min once a week for 2 consecutive weeks (timing was started after thermal equilibration of tumor core has reached 42 ± 1.0 °C) Figure 1. Tumor measurements of tumors including untreated or control group were taken every other day for 24 days using a digital Vernier caliper (measuring both longer axis and shorter axis of tumor). Animal with tumor size equal to 2 cm was euthanized to prevent pain and suffering. Tumor volume was calculated based on the equation below.
| (3) |
Figure 1.
Schematic diagram showing the injection of Gem-TSLnps in tumor bearing mouse followed by tumor heating.
Where L is tumor length and W is tumor width.
Toxicological Analysis
Weight of mice: All mice in the treatment groups as well as the control group were weighed every other day until the end of the experiments to assess the effect of Gem or Gem-TSLnps on mouse body weight.
Measurement of liver function: Blood samples were collected post treatment from mouse through the tail in the control, Gem and Gem-TSLnps + mHT treated groups to determine the effect of treatment on liver. The blood samples were centrifuged to separate the serum from blood clot at 3,500 rpm for 5 min. Serum were serially diluted and tested for ALT, AST and ALP enzyme activity according to manufacturer's protocol to assess liver integrity. The ALT, AST and ALP enzyme activities were measured colorimetrically22.
The enzymes were calculated based on the equations below:
For ALT
| (4) |
Where;
B = Amount (nmole) of pyruvate generated between Tinitial and Tfinal
Tinitial=Time of first reading in minutes
Tfinal=Time of penultimate reading in minutes
V = Sample volume (ml) added to well.
For AST
| (5) |
Where,
B = Amount (nmole) of glutamate generated between Tinitial and Tfinal
Reaction Time = Tfinal – Tinitial (minutes)
V = Sample volume (ml) added to well.
For ALP
| (6) |
Where,
df = Dilution Factor
VF = Volume (in militers of assay)
18.5 = Millimolar extinction coefficient of p-Nitrophenyl Phosphate (pNPP) at 450 nm
VE= Volume (in milliliters) of enzyme used
Statistics
The difference between Gem and Gem-TSLnps treated groups were analyzed using Student's t-test (paired) and considered significant at p < 0.05. All experiments were performed at least in triplicate and analyzed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA)
Results
Characterization of TSLnps
Particle size and zeta potential
Gem entrapment efficient in both TSLnps and NTSLnps was found to be 41.10 ± 2.02 % and 7.73 ± 0.26 % respectively. While Gem-TSLnp hydrodynamic size was 216.10 ± 0.57 nm, Gem-NTSLnp size was 285.00 ± 0.442 nm slightly larger than that of Gem-TSLnps although NTSLnps entrapped lesser amount of Gem. For zeta potential values, Gem-TSLnps and Gem-NTSLnps were determined −0.047 ± 0.117 mV0.018 ± 0.678 mV respectively (Table 1).
Table 1.
| Formulation | Lipid composition | Molar Ratio | Mean Particle size (nm) | Zeta potential (mV) | Entrapment Efficiency (%) |
|---|---|---|---|---|---|
| GEM-TSLnps | DPPC: MPPC: DSPC-PEG2000 | 90:10:4 | 216.10 ± 0.565 | −0.047 ± 0.117 | 41.1 ± 2.022 |
| GEM-NTSLnps | HSPC: CHOL: DSPC-PEG2000 | 75:50:3 | 285.00 ± 0.442 | 0.018 ± 0.678 | 7.73 ± 0.258 |
Lipid composition, % EE and physicochemical characterization of Gem-TSLnps and Gem-NTSLnps. Data expressed as mean ± S.D, n = 3
FTIR analysis
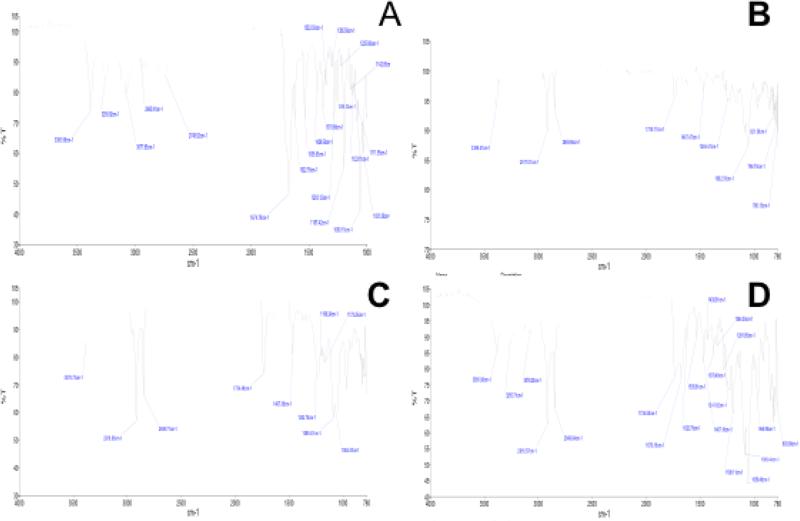
FTIR is not a confirmatory analysis to determine drug entrapment in a carrier or nanoparticle however; it could be used to assess the association or interaction of drug and lipid by way of bond formation between drug and the carrier. In this study, FTIR analysis was conducted to determine an interaction between the Gem and the liposomal carrier. Careful examinations of the absorption peaks of Gem spectrum (Fig. 2A) and that of Gem-TSLnps (Fig. 2B) were completely different. Further, blank TSLnps(Fig. 2C) absorption peaks were not similar to that of Gem-TSLnps absorption peaks. The unique features of Gem-TSLnps peaks clearly show a close association between Gem and Gem-TSLnps. FTIR spectra of pure Gem showed characteristic peaks at 3387.6, 1674.79, 1535.63, 1087.72, 1060.15, and 1197.42 cm−1, however, Gem-TSLnps demonstrated comparable prominent sharp peaks at 3389.41 and 1062.35 cm−1 respectively which showed an association of Gem andTSLnps.
Figure 2.
FTIR spectra for (A) pure Gem, (B) Gem-TSLnps, (C) blank TSLnps and (D) physical mixture (DPPC, MPPC, DSPE-PEG2000 and Gem).
In vitro release of Gem from TSLnps at different temperatures
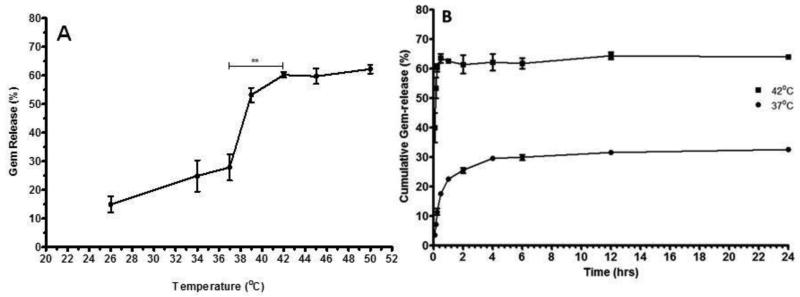
The release of Gem from Gem-TSLnps was determined at different set of temperatures as observed in Fig 3A. Gem release was up to 25 % with increasing temperature from 26°C to 37°C, however, sharp increase in Gem release (60 %) was observed between 38°C to 42°C after which Gem release remained fairly constant from 42°C to 50 °C. Overall, Gem released at 42°C (~ 60%) was significant compared to that at 37°C (**p<0.01) however, the fast release of Gem from 26°C to 37°C could be attributed to Gem that were adsorbed to the surface of the liposomal nanoparticles.
Figure 3.
Gem-release at varying at temperature and time: (A) TSLnps release behavior of Gem from TSLnps with increasing temperature, Gem-TSLnps was kept at each temperature for 10 min (**p < 0.01 comparison of Gem release at 37°C vrs 42°C). (B) in vitro cumulative release of Gem at 37°C and 42°Cover period of 24 hr. Data expressed as mean ± SEM, n = 3
Release of Gem from TSLnps at different time points
For optimal TSLnps-based drug delivery, it is imperative that the nanoparticles retain their content as they move through the circulatory system before reaching the heated tumor. In figure 3B, Gem release during the 24hr of incubation at 37°C and 42°C was presented. As previously observed with 25% Gem release at 37°C (Fig. 2), we observed a similar trend of Gem release of 28% at 37°C within the first 4 hr (Fig. 3) followed by insignificant Gem release for the next 20 hr. This trend of release could be largely attributed to the adsorbed Gem on TSLnps surface during the formulation process, despite rigorous effort to remove the unentrapped Gem. Another release study at 42°C led to a rapid burst of Gem release (60 %) from TSLnps within the first 10 min after which the release was maintained as the time increased for over period of 24 hr.
In vitro viability studies
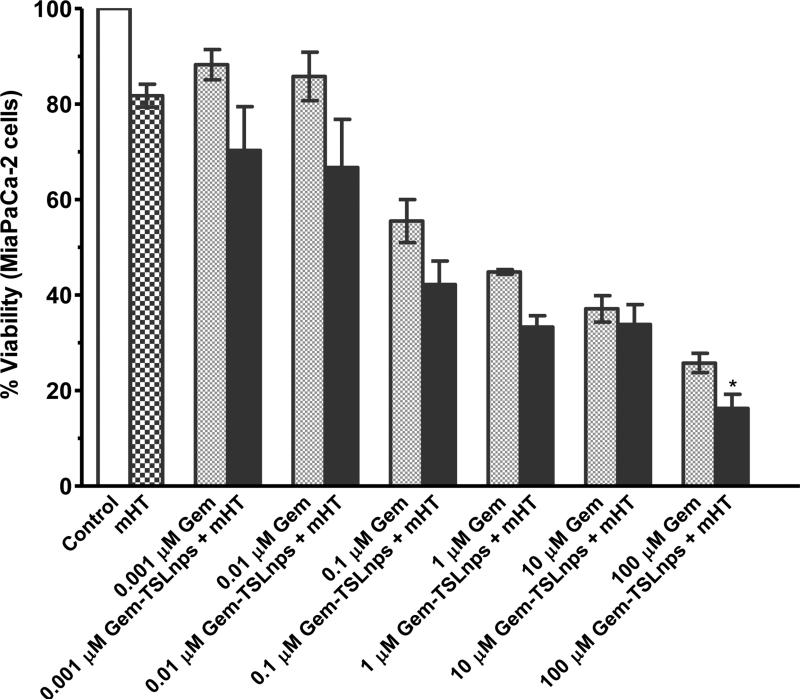
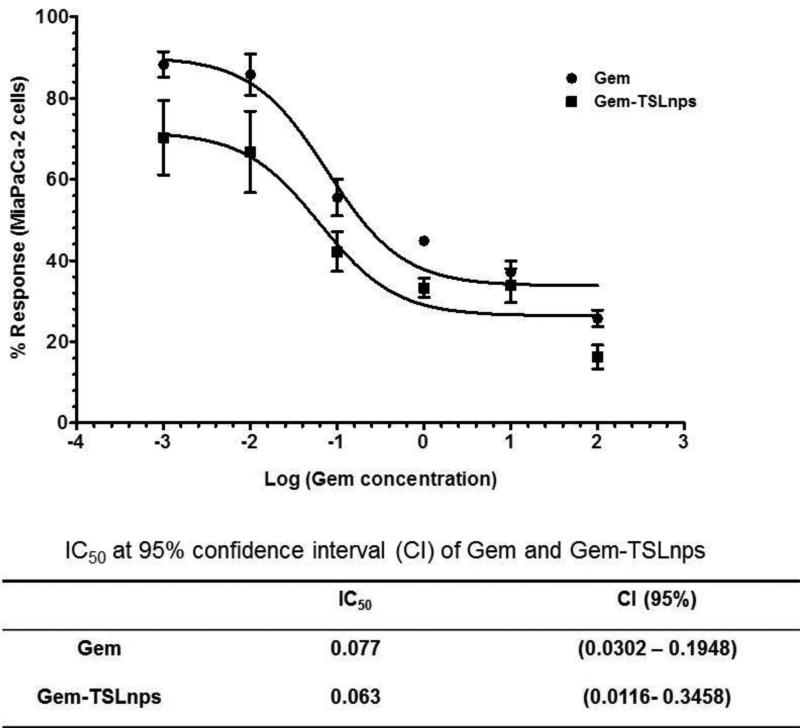
Cytotoxic effects of Gem-TSLnps and Gem were investigated on MiaPaCa-2 cells at varying concentrations: 0.001, 0.01, 0.1, 1.0, 10.0 and 100.0 μM (Fig.4). No significant difference was observed between cells exposed to mHT and the control (no treatment) groups. We observed decline in cell viability (%) of Gem-TSLnps + mHT treated group compared to Gem treated group but marked decline in cell viability was noticed at 100μM (*p < 0.05) where Gem-TSLnps + mHT was more effective against MiaPaCa-2 cells. As expected, estimates of IC50 showed a lower inhibitory concentration for Gem-TSLnps + mHT treated group compared to Gem treated group (0.063 vrs 0.077 μM) (Fig. 5).
Figure 4.
Percent cell viability of MiaPaCa-2 cells against varying concentration of Gem and Gem-TSLnps(*p < 0.05, 100 μM Gem vrs 100 μM Gem-TSLnps) p-value was calculated by Student's t-test. Data expressed as mean ± SEM, n = 3
Figure 5.
Non-linear dose response curve of percentage live MiaPaCa-2 cells against different concentrations of Gem (common logs). Data expressed as mean ± SEM, n = 3.
Clonogenic Survival Assay
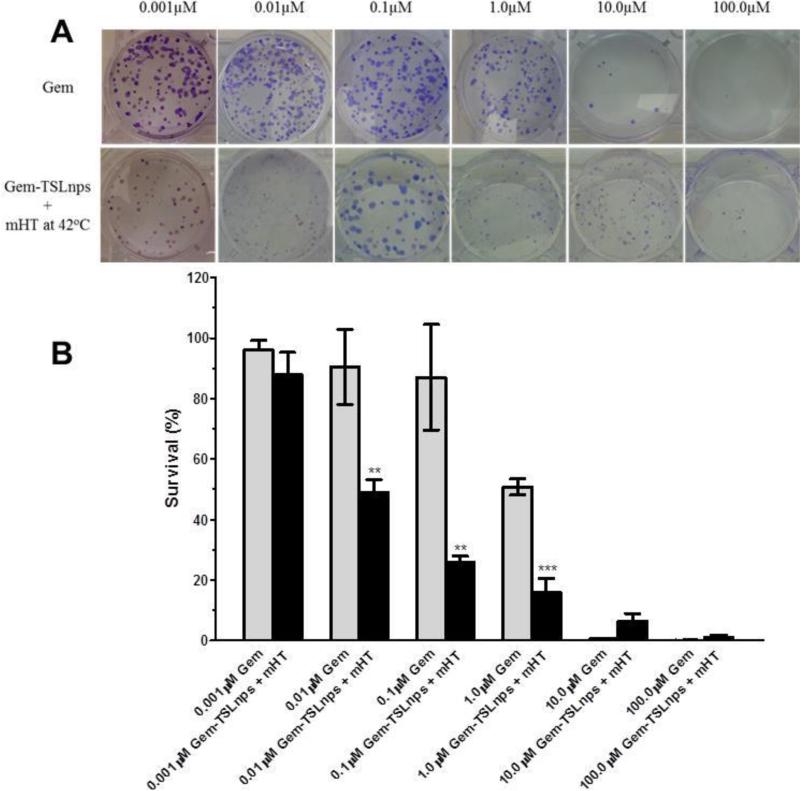
In vitro cell survival was determined via clonogenic assay. The primary aim was to determine the resilience and proliferative ability of MiaPaCa-2 after treatment with Gem or Gem-TSLnps + mHT. As shown in Fig 6B, there was a marked decrease in percentage survival of MiaPaCa-2 cells after treatment with Gem-TSLnps + mHT compared to that of Gem treated. This was observed as a reduction of number of colonies formed in a dose dependent manner of Gem or Gem-TSLnps treated MiaPaCa 2 cells (Fig. 6A). Percentage survival of MiaPaCa-2 cells declined remarkably in groups treated with Gem-TSLnps + mHT compared to Gem from0.01, through to1.0 μM (p** < 0.01; p*** < 0.001).
Figure 6.
Survival data of MiaPaCa-2 cells post treatment: (A) Plates showing colonies of MiaPaCa-2 cells after treatment with Gem or Gem-TSLnps + mHT, (B) % Survival of MiaPaCa-2 cells against different concentrations of Gem and Gem-TSLnps + mHT. Data expressed as mean ± S.D, n = 3, (*p<0.05, **p < 0.01, ***p < 0.01, Gem vs Gem-TSLnps treated MiaPaCa-2 cells). P-values were calculated by Student's t-test.
Confocal imaging
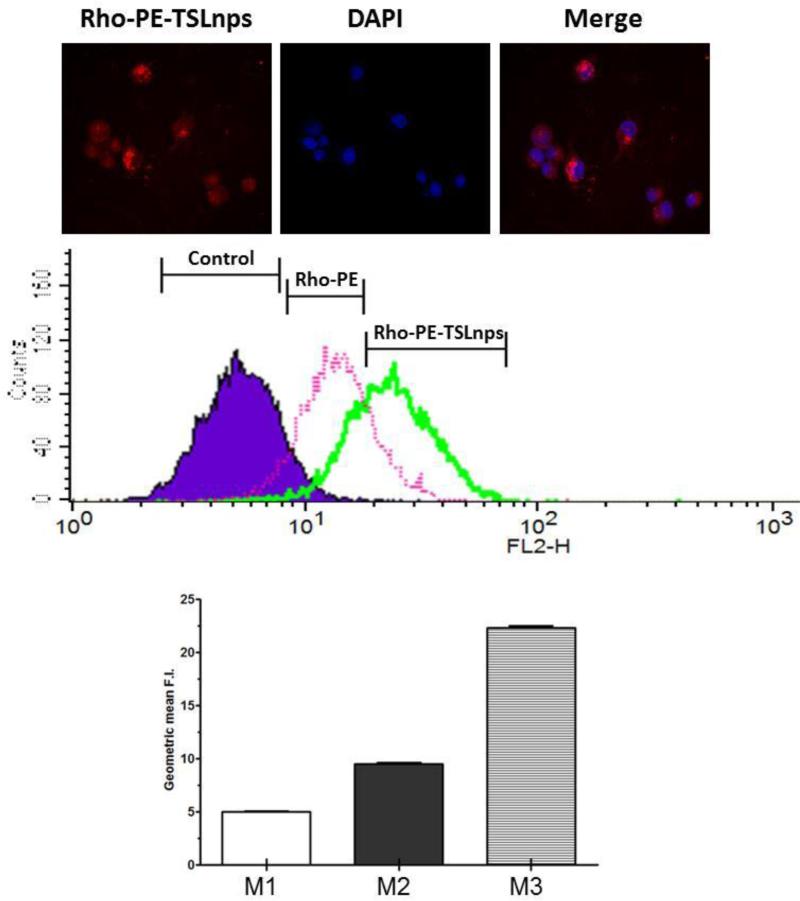
The intracellular uptake of Rho-TSLnps by MiaPaCa-2 monolayer cells was observed as bright red fluorescence while nuclei counterstained with DAPI/Hoechst dye appeared in blue fluorescence as shown in figure 7A. The merged image provided a co-localization of Rho-TSLnps and nuclei of the cells. This provides a clue of Rho-TSLnps’ location relative to the cells’ nuclei.
Figure 7.
Cellular uptake efficiency of TSLnps by MiaPaCa-2 cells assessed by confocal microscopy and flow cytometry: (A) Confocal image showing the accumulation of Rho-PE-TSLnps (red fluorescence) and cells nuclei counterstained with DAPI (blue florescence) in MiaPaca-2 cells. Merge image shows co-localization of Rho-PE-TSLnps and nucleus. (B) Data show histogram representative of cellular uptake of Rho-PE-TSLnps and Rho-PE in MiaPaCa-2 cells. (C) GMFI representing untreated cells (M1), Rho-PE (M2) and Rho-PE-TSLnps (M3). (p<0.01, M2 vrs M3) p-value was calculated by Student's t-test. Data expressed as mean + SEM.
Flow cytometric analysis
Cellular uptake of TSLnps was determined by flow cytometric analysis. MiaPaCa-2 monolayer cells were treated with either TSLnps tagged with Rho (Rho-TSLnps) or Rho alone as shown in Fig 7B. Using geometric mean fluorescence intensity (GMFI) value of untreated cells as the baseline, both Rho-PE and Rho-PE-TSLnps shown increased cellular uptake however, the uptake potentially of Rho-PE-TSLnps was 2.4-fold higher (**p <0.01) than that of Rho-PE as observed in GMFI values (Fig. 7C).
Toxicological Analysis
Mouse body weight
The toxicity occurring in the mice treated with Gem, Gem-TSLnps and Gem-NTSLnps formulations was monitored by measuring changes in body weight. None of the mice treated with Gem, Gem-TSLnps and Gem-NTSLnps or control group exhibited any body weight loss.
Measurement of liver function
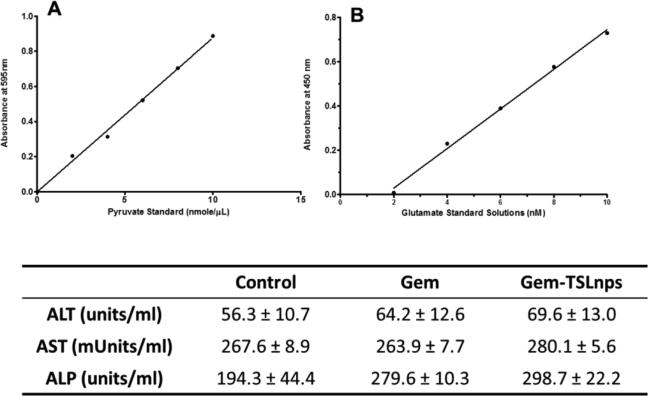
To evaluate the toxicity of our formulation TSLnps, serum levels of AST, ALT and ALP were determined to assess the effect that it may have on liver. As shown in figure 8, AST and ALT levels of Gem and Gem-TSLnps treated mice were comparable and also not statistically different from that of the control (untreated) group. However, ALP serum level of either Gem or Gem-TSLnps treated mice was significantly higher compared to ALP level of the control group (*p<0.05). Further, ALP level of Gem-TSLnps treated group was moderately higher than that of Gem treated group.
Figure 8.
Liver enzyme measurement: (A) glutamate standard curve for AST assay (y = 0.0894× −0.149, r2=0.996); (B) pyruvate standard curve for ALT assay (y = 0.0878× −0.0006, r2=0.995). The table shows the ALT, AST and ALP serum levels of control (untreated), Gem and Gem-TSLnps + mHT mice. Data expressed as mean ± S.D, n = 3.
Antitumor activity studies
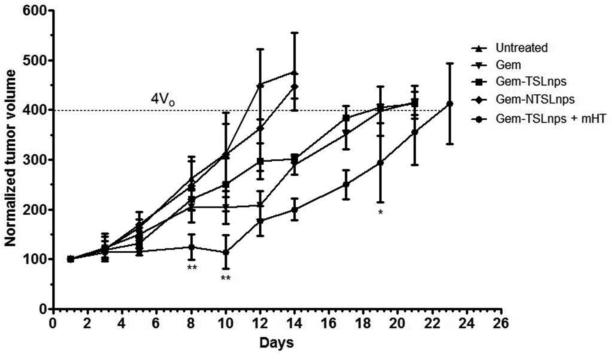
The antitumor efficacy of Gem, Gem-TSLnps and Gem-NTSLnps formulations were monitored after i.p. administration. The Gem-TSLnps treated group with heated tumor showed a lower tumor volume compared to Gem (Fig. 9), despite a relatively lower drug dose: Gem 20 mg/kg, GemTSLnps 10 mg/kg. Further, Gem-TSLnps + mHTmarkedly inhibited tumor growth 1.7 fold-lower (**p<0.01) and 1.8 fold-lower (**p<0.01) compared to Gem treated group on the 8th and 10th days respectively. Also on day 19th day, Gem-TSLnps + mHT significantly regressed tumor to 1.4 fold (*p<0.05) compared to that of Gem treated tumor. As expected, Gem-TSLnps and Gem-NTSLnps did not significantly tumor growth.
Figure 9.
In vivo antitumor activity of Gem, Gem-TSLnps + mHT, Gem-TSLnps and Gem-NTSLnps against MiaPaCa-2 tumors in nude mice. (*p< 0.05 and **p< 0.01; Gem vrs Gem-TSLnps + mHT on the 8th, 10th and 20th days on MiaPaCa-2 tumor growth curves) p-values were calculated by Student's t-test. Data reported as mean ± S.D., n = 5.
Discussion
Pancreatic cancer, a ductal adenocarcinoma continues to be a critical health problem due lack of early detection and its resistance to chemotherapy or radiation23. Gem is currently the standard drug of choice in the management of pancreatic cancer, however the therapeutic application of Gem is clinically presented with low metabolic stability and poor cell membrane permeability. TSLnps, a biocompatible drug delivery system that allows for protection and targeted delivery of both hydrophilic and hydrophobic drugs has currently evolved as a promising strategy in recent times 24.
Although studies on the delivery of Gem through thermosensitive liposomes have been published12-14, however the use of waterbath or light as a source of heat to disrupt liposomes in these published studies brings into question its translational potential. The reason is that neither waterbath (immersion of the flank-bearing tumor in water) nor light can provide sufficient heat to disrupt thermosensitive liposomes in deep seated tumors. Our study however, is unique in the sense that we employed special thermocouple heating device made by Omega Engineering which has the capacity to provide heat to deep seated orthotopic tumor model such as pancreatic cancer. In this current study, we investigated cytotoxicity effect of Gem-TSLnps + mHT on MiaPaCa-2 pancreatic cancer cells.
Lipid composition have been found to possibly influence liposome size and entrapment efficiency25. The particle size of our formulation and in increase in pore size of tumor vasculature due to localized heating tumor are more likely to influence the respond of tumor to nanoparticle based drug that relies on EPR for delivery26, 27. As shown in Table 1, both Gem-TSLnps and Gem-NTSLnps demonstrated low zeta potential possibly due to pegylation of the liposomes by DSPE-PEG20008. Nonetheless, pegylation of liposomes could confer “stealth” properties and rendered liposomes less likely to undergo opsonization and uptake by reticuloenothelial system (RES)8, 26.
Although FTIR studies do not confirmed entrapment of Gem in TSLnps, FTIR analysis have been conducted on drug interaction with delivery system to determine an association between drug-carrier complex 28, 29. Our FTIR obtained spectra revealed distinct and sharp peaks that were characteristics of blank or empty TSLnps, Gem and Gem-TSLnps. However, careful comparison of these absorption peaks clearly suggests the presence of Gem in TSLnps. The sharp prominent peaks were possibly due to free –OH (3389.41 cm−1) and –C-O (1062.35 cm−1) stretch in Gem entrapped in TSLnps (Gem-TSLnps)30.
Once Gem entrapped in TSLnps was confirmed by FTIR, we proceeded to study the release behavior of Gem from TSLnps at different temperatures and different time points. TSLnps displayed a maximum Gem release of 28% at 37 °C over period of 24 hr while 60% of Gem was released within 10 min by TSLnps after exposure to mHT (42 °C) over 24 hr. The data suggest that TSLnps was largely stable at 37 °C but highly unstable at 42 °C leading to rapid release of Gem. Also, the 28% Gem release at 37 °C was suspected to be those that were adsorbed on the surface of TSLnps during the formulation process, although significant effort was made to remove the free Gem.
It has been reported that release of drugs in thermosensitive liposomes depended largely on melting phase transition temperature (Tm) of lipid composition18. At Tm phase, a liquid-gel phase intermediate co-exists that allow the release of Gem from TSLnps. DPPC with Tm of 41.5°C constituted about 87% of lipid composition of our TSLnps formulation and previous studies suggest that Gem release is significantly influence by DPPC at 42.0 °C15, 18, 31.
To evaluate the efficacy of Gem-TSLnps, in vitro cytotoxicity of Gem and Gem-TSLnps + mHT against MiaPaCa-2 cells was compared. We observed that cytotoxic effect of Gem-TSLnps + mHT was stronger compared to Gem treated cells in dose dependent fashion. Further, IC50 of Gem-TSLnps + mHT was 1.17 fold high compared to that of Gem. It was worth noting that the initial purpose of mHT application was to disruptTSLnps and release Gem, we also noticed that the 10 min exposure of mHT to the cells slightly inhibited the cells’ growth when compared to control cells. This indicated a dual action of mHT by: i) disrupting TSLnps, and ii) sensitizing cells to Gem. We further conducted clonogenic cell survival assay to determine the effectiveness of Gem and Gem-TSLnps + mHT on the survival and proliferation ofMiaPaCa-2 cells. Our findings revealed a significant loss in the proliferative ability of surviving cells that were previously treated with Gem-TSLnps plus mHT compared to that of Gem treated cells. This is an indicative of a loss of long term survival of Gem-TSLnps + mHT treated MiaPaCa-2 cells which is imperative in therapeutic intervention32.
To assess the ability of TSLnps to deliver poor membrane permeable drugs such as Gem, intracellular uptake of Rho-PE and Rho-PE-TSLnps was evaluated using flow cytometric analysis. Although Rho usually has high cellular uptake, our data showed a higher uptake of Rho-PE-TSLnps compared to that of Rho-PE. The fact that Rho-PE uptake was greatly increased when entrapped in TSLnps suggests that TSLnps was a good delivery system. To confirm the flow cytometry data, confocal imaging was performed on previously Rho-PE-TSLnps treated cells. The data obtained was consistent with that of flow cytometry suggesting that TSLnps could deliver significant amount of Gem into tumor cells by overcoming the energetically unfavorable endocytosis process for the drug33.
In the MiaPaCa-2 tumor animal model, Gem delivery to heated tumor by the TSLnps was greatly enhanced as these tumors were significantly regressed compared to tumors treated with Gem or Gem-NTSLnps, suggesting that Gem released under hyperthermia could rapidly permeate into the tumor and efficiently be taken up by the tumor cells11, 12, 18. While it took 24 days for tumor volume of Gem-TSLnps treated mice to quadruple (4Vo), the Gem treated group took 20 days. This result suggests that TSLnps approach can be used for the successful delivery of Gem.
It should be emphasized here that Gem in TSLnps was half the dose of Gem used in Gem-treated group. We could not use equivalent amount of Gem in TSLnps because of injection volume (100 μL) restriction, each mouse was required to receive 100 μL of either free Gem or Gem-TSLnps irrespective of Gem concentration. In contrast, groups treated with Gem-TSLnps without mHT or Gem-NTSLnps demonstrated poor antitumor activity. It was therefore apparent that the application of mHT played a pertinent role in the therapeutic outcome of Gem-TSLnps treatment. Our findings were consistent with studies performed by others13. The toxicity of Gem-TSLnps was investigated by analyzing liver enzymes post treatment, but no significant elevated levels of AST, ALT or ALP were observed. The slightly increased levels of AST and ALP suggest moderate liver uptake of Gem-TSLnpsas reported by others34, 35.
This study was conducted to evaluate the feasibility of delivering Gem a short half-life and poor membrane permeable drug through TSLnp delivery system. In our future studies, we plan to use orthotopic tumor model with magnetic resonance guided focused ultrasound to assess fully the application of TSLnps as a Gem delivery system in the treatment of pancreatic cancer using mHT.
Conclusion
We have successfully formulated Gem loaded thermosensitive liposomes. These liposomes showed a temperature sensitive Gem release in vitro and showed significant tumor regression compared to free Gem in MiaPaCa-2 tumor model. Despite the relatively lower Gem dose in TSLnps (Gem-TSLnps 10 mg/kg) compared to free Gem (20mg/kg), tumor regression of Gem-TSLnps group was significantly high. The findings provide strong evidence in support of a plausible therapeutic application of thermosensitive liposomes as Gem delivery system for the effective treatment of pancreatic cancer.
Acknowledgement
Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities (NIMHD) of the National Institutes of Health (NIH) under award numbers G12MD007582-28 and U54MD008149.
References
- 1.American Cancer Society. 2015 [Google Scholar]
- 2.Zhao X, Li F, Li Y, Wang H, Ren H, Chen J, Nie G, Hao J. Biomaterials. 2015;46:13–25. doi: 10.1016/j.biomaterials.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Koay EJ, Truty MJ, Cristini V, Thomas RM, Chen R, Chatterjee D, Kang Y, Bhosale PR, Tamm EP, Crane CH, Javle M, Katz MH, Gottumukkala VN, Rozner MA, Shen H, Lee JE, Wang H, Chen Y, Plunkett W, Abbruzzese JL, Wolff RA, Varadhachary GR, Ferrari M, Fleming JB. The Journal of clinical investigation. 2014;124(4):1525–36. doi: 10.1172/JCI73455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon C, He C, Liu D, Lu K, Lin W. Journal of controlled release : official journal of the Controlled Release Society. 2015;201:90–9. doi: 10.1016/j.jconrel.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Immordino ML, Brusa P, Rocco F, Arpicco S, Ceruti M, Cattel L. Journal of controlled release : official journal of the Controlled Release Society. 2004;100(3):331–46. doi: 10.1016/j.jconrel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.2013 http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm370803.htm.
- 7.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Journal of controlled release : official journal of the Controlled Release Society. 2000;65(1-2):271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 8.Federico C, Morittu VM, Britti D, Trapasso E, Cosco D. International journal of nanomedicine. 2012;7:5423–36. doi: 10.2147/IJN.S34025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peer Dan, Karp Jeffrey M., Hong4 Seungpyo, C. O, Farokhzad, Margalit Rimona, Langer R. Nature Nanotechnology. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 10.Li L, ten Hagen TL, Schipper D, Wijnberg TM, van Rhoon GC, Eggermont AM, Lindner LH, Koning GA. Journal of controlled release : official journal of the Controlled Release Society. 2010;143(2):274–9. doi: 10.1016/j.jconrel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Kirui DK, Koay EJ, Guo X, Cristini V, Shen H, Ferrari M. Nanomedicine : nanotechnology, biology, and medicine. 2014;10(7):1487–96. doi: 10.1016/j.nano.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.May JP, Ernsting MJ, Undzys E, Li SD. Molecular pharmaceutics. 2013;10(12):4499–508. doi: 10.1021/mp400321e. [DOI] [PubMed] [Google Scholar]
- 13.Lim SK, Shin DH, Choi MH, Kim JS. Drug development and industrial pharmacy. 2014;40(4):470–6. doi: 10.3109/03639045.2013.768631. [DOI] [PubMed] [Google Scholar]
- 14.Limmer S, Hahn J, Schmidt R, Wachholz K, Zengerle A, Lechner K, Eibl H, Issels RD, Hossann M, Lindner LH. Pharmaceutical research. 2014;31(9):2276–86. doi: 10.1007/s11095-014-1322-6. [DOI] [PubMed] [Google Scholar]
- 15.Affram K, Udofot O, Agyare E. Integrative Cancer Science and Therapeutics. 2015;2(2):133–142. [PMC free article] [PubMed] [Google Scholar]
- 16.Achim M, Precup C, Gonganăuniţu D, Barbu-Tudoran L, Silvia Porfire Alina, Scurtu R, Ciuce C. Farmacia. 2009;57(6):703–10. [Google Scholar]
- 17.Li L, ten Hagen TL, Hossann M, Suss R, van Rhoon GC, Eggermont AM, Haemmerich D, Koning GA. Journal of controlled release : official journal of the Controlled Release Society. 2013;168(2):142–50. doi: 10.1016/j.jconrel.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. Cancer Research. 2000;(60):1197–1201. [PubMed] [Google Scholar]
- 19.Agyare EK, Jaruszewski KM, Curran GL, Rosenberg JT, Grant SC, Lowe VJ, Ramakrishnan S, Paravastu AK, Poduslo JF, Kandimalla KK. Journal of controlled release : official journal of the Controlled Release Society. 2014;185:121–9. doi: 10.1016/j.jconrel.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanz C, Fruh M, Thormann W, Cerny T, Lauterburg BH. Journal of separation science. 200730(12):1811–20. doi: 10.1002/jssc.200600534. [DOI] [PubMed] [Google Scholar]
- 21.Huth US, Schubert Rolf, Peschka-Süss R. Journal of Controlled Release. 2006;(110):490–504. doi: 10.1016/j.jconrel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Samy A, Elmowafy M, Arafa M, Kari O, Viitala T, Kassem A, Abu-Elyazid SK, Ibrahim H, Yliperttula M. Journal of Life Medicine. 2014;2(1):15–24. [Google Scholar]
- 23.Murata M, Narahara S, Kawano T, Hamano N, Piao JS, Kang JH, Ohuchida K, Murakami T, Hashizume M. Molecular pharmaceutics. 2015;12(5):1422–30. doi: 10.1021/mp5007129. [DOI] [PubMed] [Google Scholar]
- 24.Yang T, Cui FD, Choi MK, Cho JW, Chung SJ, Shim CK, Kim DD. International journal of Pharmaceutics. 2007;338(1-2):317–26. doi: 10.1016/j.ijpharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Kneidl B, Peller M, Winter G, Lindner LH, Hossann M. International journal of nanomedicine. 2014;9:4387–98. doi: 10.2147/IJN.S49297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ta T, Porter TM. Journal of Controlled Release. 2013;(169):112–125. doi: 10.1016/j.jconrel.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mody N, Tekade RK, Mehra NK, Chopdey P, Jain NK. AAPS PharmSciTech. 2014;15(2):388–99. doi: 10.1208/s12249-014-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chitkara D, Mittal A, Behrman SW, Kumar N, Mahato RI. Bioconjugate chemistry. 2013;24(7):1161–73. doi: 10.1021/bc400032x. [DOI] [PubMed] [Google Scholar]
- 29.Hosseinzadeh H, Atyabi F, Dinarvand R, Ostad SN. International journal of nanomedicine. 2012;7:1851–63. doi: 10.2147/IJN.S26365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yalcin S, Erkan M, Unsoy G, Parsian M, Kleeff J, Gunduz U. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2014;68(6):737–43. doi: 10.1016/j.biopha.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Grull H, Langereis S. Journal of controlled release : official journal of the Controlled Release Society. 2012;161(2):317–27. doi: 10.1016/j.jconrel.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 32.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Nature protocols. 2006;1(5):2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 33.Cai D, Gao W, He B, Dai W, Zhang H, Wang X, Wang J, Zhang X, Zhang Q. Biomaterials. 2014;35(7):2283–94. doi: 10.1016/j.biomaterials.2013.11.088. [DOI] [PubMed] [Google Scholar]
- 34.Paolino D, Cosco D, Celano M, Moretti S, Puxeddu E, Russo D, Fresta M. Nanomedicine (Lond) 2013;8(2):193–201. doi: 10.2217/nnm.12.101. [DOI] [PubMed] [Google Scholar]
- 35.Graeser R, Bornmann C, Esser N, Ziroli V, Jantscheff P, Unger C, Hopt UT, Schaechtele C, von Dobschuetz E, Massing U. Pancreas. 2009;38(3):330–7. doi: 10.1097/MPA.0b013e31819436e6. [DOI] [PubMed] [Google Scholar]