Abstract
Objective
To examine the potential for rejuvenation of aged periosteum by local injection of transforming growth factor-beta1 (TGF-β1) and insulin-like growth factor-1 (IGF-1) alone or in combination to induce cambium cell proliferation and enhance in vitro periosteal cartilage formation.
Methods
A total of 367 New Zealand white rabbits (6, 12, and 24+ month-old) received subperiosteal injections of TGF-β1 and/or IGF-1 percutaneously. After 1, 3, 5, or 7 days, the rabbits were sacrificed and cambium cellularity or in vitro cartilage forming capacity was determined.
Results
A significant increase in cambium cellularity and thickness, and in vitro cartilage formation was observed after injection of TGF-β1 alone or in combination with IGF-1. In 12 month-old rabbits, mean cambium cellularity increased 5-fold from 49 to 237 cells/mm and in vitro cartilage production increased 12-fold from 0.8 to 9.7 mg seven days after TGF-β1 (200 ng) injection compared to vehicle controls (p<0.0001). A correlation was observed between cambium cellularity and in vitro cartilage production (R2=0.98). An added benefit of IGF-1 plus TGF-β1 on in vitro cartilage production compared to TGF-β1 alone was observed in the 2 year old rabbits. IGF-1 alone generally had no effect on either cambium cellularity or in vitro cartilage production in any of the age groups.
Conclusions
These results clearly demonstrate that it is possible to increase cambium cellularity and in vitro cartilage production in aged rabbit periosteum, to levels comparable to younger rabbits, using local injection of TGF-β1 alone or in combination with IGF-1, thereby rejuvenating aged periosteum.
Keywords: Cartilage, Chondrogenesis, Insulin-Like Growth Factor-1, Periosteum, Transforming Growth factor-beta
Introduction
Periosteum, the connective tissue that surrounds bones, can be used for tissue engineering or regeneration of bone and cartilage1-12. However, the principal limitation to the use of periosteum is the age-related decrease in cambium cellularity and thickness of the cambium where the precursor cells reside13, 14. This decline in precursor cells, and cambium thinning is consistent with the observed decrease in the capacity of aged periosteum to form bone and cartilage5, 13. Previously, we documented that cambium cellularity and thickness were significantly lower in 6, 12 and 24 month old rabbits compared to 2 month-old rabbits13. Likewise, the in vitro cartilage forming capacity was also reduced by 50% in 6 month old rabbits and reached steady state minimal levels by 12 months of age compared to 1.5-2 month-old rabbits13. Therefore, similar to observations in humans5, a marked decrease in the biological activity of rabbit periosteum occurs after completion of skeletal growth (approximately 6 months of age)1, 13. Thus, the rabbit is an excellent model to study the effect of age on periosteum and potential approaches to rejuvenate aged periosteum.
Previously, we demonstrated that in vitro periosteal chondrogenesis could be induced with a brief exposure (30 to 60 minutes) of high-dose (50 or 100 ng/mL) TGF-β115. In addition, we observed that the combination of IGF-1 and TGF-β1 resulted in a significant increase in in vitro cartilage yield as early as 2 weeks compared to either growth factor alone16. Importantly, the combined treatment also stimulated significantly more proliferation of cambium layer cells during the first week of culture compared to either growth factor alone. These results suggest that the combined treatment may be advantageous for rapid stimulation of periosteum.
Joyce et al. demonstrated that periosteum can be stimulated to grow and differentiate into cartilage and bone in situ after subperiosteal injection of TGF-β in newborn rats17. Similar results were also reported in rabbits18. In these studies, stimulation of cambium layer cell proliferation by subperiosteal injection of TGF-β2 was observed in rabbits 3 days, 3 months and 2-years old18. However, a quantitative analysis of the effects of a single subperiosteal injection of TGF-β or IGF-1 on cambium cellularity and thickness, and in vitro cartilage formation has not been previously reported.
In this study, we examined the potential for rejuvenation of aged periosteum by a single local injection of TGF-β1 and IGF-1 alone or in combination to induce cambium cell proliferation and enhance in vitro periosteal cartilage formation in rabbits. This study was also designed to determine the optimal duration between injection and periosteal graft harvest, and growth factor concentration and composition for in vivo cartilage repair studies in this model of aged periosteum.
Methods and Materials
STUDY DESIGN
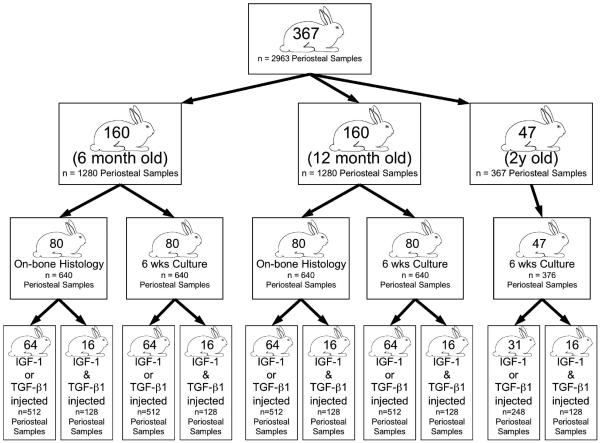
All procedures were conducted with the approval of our Institutional Animal Care and Use Committee. A total of 367 New Zealand white rabbits (6, 12, and 24+ month-old) received subperiosteal injections of growth factors or vehicle in the medial side of the proximal tibia (see Figure 1 for distribution of rabbits). After 1, 3, 5, or 7 days, the rabbits were sacrificed and periosteum containing the injection sites (eight samples per rabbit) was harvested together with the underlying bone as an intact osteoperiosteal specimen for histology (to determine cambium cellularity and thickness)13, or periosteal explants were elevated from the bone at the injection sites and cultured for 6 weeks (to determine in vitro cartilage forming capacity)6. Due to limited availability of the older animals, only in vitro cartilage formation was analyzed in the 24+ month-old rabbits. The rabbit ages were selected based on previous studies demonstrating significantly decreased cambium cellularity and thickness, and in vitro cartilage forming capacity in periosteum from rabbits 6-24 months old compared to 2 month-old13.
Fig. 1.
Flow diagram illustrating the distribution of rabbits and periosteal tissue samples used in this study. A total of 367 New Zealand white rabbits (6, 12, and 24+ month-old) received subperiosteal injections of growth factors or vehicle in the medial side of the proximal tibia. After 1, 3, 5, or 7 days, the rabbits were sacrificed and periosteum containing the injection sites (eight samples per rabbit) was harvested together with the underlying bone as an intact osteoperiosteal specimen for histology (to determine cambium cellularity and thickness)13, or periosteal explants were elevated from the bone at the injection sites and cultured for 6 weeks (to determine in vitro cartilage forming capacity)6.
SUBPERIOSTEAL INJECTIONS
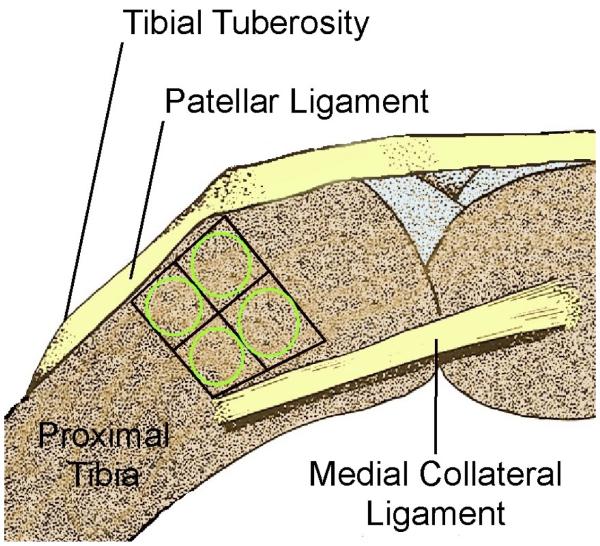
Under general anesthesia (IM Ketamine/Xylazine/Acepromazine at 50/5.0/0.75 mg/Kg), subperiosteal injections of growth factor or vehicle were performed percutaneously using a Hamilton syringe with a 30-gauge needle. As illustrated in Figure 2, a single injection (10 μl) of the same vehicle or growth factor was made 5 mm proximal to the distal edge of the tibial tuberosity in each of four approximately 3.5 × 3.5 mm2 regions on each tibia (corresponding to the area that is normally used for periosteal harvesting in this model)6. One limb received the growth factor injection and the contralateral limb received vehicle, alternating sides to eliminate potential bias (thus eight tissue samples were generated per rabbit). The 10 μl injections contained recombinant human TGF-β1 (20 or 200 ng) or vehicle (4 mM HCl, 1 mg/ml BSA), recombinant human IGF-1 (0.2 or 2.0 μg) or vehicle (10 mM acetic acid, 0.1 % BSA), or a combination of 200 ng TGF-β1 and 2.0 μg IGF-1 or vehicle (5 mM acetic acid, 2 mM HCL, 0.1 % BSA). The recombinant human TGF-β1 and recombinant human IGF-1 were purchased from R&D Systems, Inc. (Minneapolis, MN). Prior to initiation of the experiment, dye was injected in rabbit carcasses in order to establish the injection technique.
Fig. 2.
Schematic drawing of the medial proximal rabbit tibia showing the region bounded by the patellar ligament, growth plate, and medial collateral ligament that is used for harvesting 3.5 × 3.5 mm2 periosteal explants (grid). The four percutaneous injections were targeted in these regions (circles).
CAMBIUM CELLULARITY, THICKNESS, AND CELL DENSITY
Using a bone saw, periosteum containing the injection sites was harvested together with the underlying bone as an intact osteoperiosteal specimen from the medial proximal tibiae region. The samples were decalcified using EDTA. After decalcification, the individual injection sites were separated and individually paraffin embedded. Each sample was oriented “on edge” during the embedding process to yield cross-sections of the cambium layer and underlying bone. Samples were then sectioned at 5 μm thick and stained with haematoxylin and eosin (H & E). Cambium maximum thickness (μm), cellularity (cells/mm), and cell density (cells/cm2) were determined by a blinded technician using a previously published technique 19, 20 that was adapted for the KS300 computerized image analysis system (Carl Zeiss Vision GmbH, Hallbergmoos, Germany). Briefly, three sections were obtained from each tissue sample and scanned at the injection site using the Zeiss AuxioCam MRc at the same magnification with the periosteal layer line horizontal to the bottom edge of the scan’s frame of reference. Each cambium layer was outlined by hand. Maximum thickness was determined by comparing thicknesses in the outlined area from top to bottom. For determining cellularity, a custom macro was used to detect, mark and calculate the number of nuclei in the outlined areas based on color. The computer program determined the total area occupied by nuclei within the traced cambium layer and divided that by average nuclear size (determined separately). The total number of cells within the cambium layer was then divided by the length of the cambium layer. The result was the average number of cambium cells per mm. Cell density was determined by dividing the total number of cells within the cambium layer by the area of the outlined section (cells/cm2). Values obtain from sections made from the same tissue sample were then averaged to give overall mean values for cambium maximum thickness, cellularity and cell density for each tissue sample.
IN VITRO CARTILAGE FORMATION
Periosteum was elevated as four 3.5 × 3.5 mm2 sections from the medial side of the proximal tibia of 6, 12, or 24+ month-old New Zealand white rabbits using sharp dissection in the regions of the injection sites (Figure 2). All periosteal explants were obtained within 15 minutes of death to control for post-mortem effects on chondrogenic potential. The explants were then cultured as previously reported6. Briefly, immediately after surgical harvesting, the periosteal explants were placed in Dulbecco's Modified Eagle Media (DMEM), 10% FBS with penicillin/streptomycin and 1 mM proline at 4°C for no more than 1.5 hours prior to transfer to 1% agarose culture. The medium was supplemented with 10 ng/mL TGF-β1 for the first two days of culture and 50 μg/mL Vitamin C through the duration of the culture period. The medium above the gel layer was replaced every second day. Cultures were maintained for 6 weeks at 37°C and 5% CO2 and 95% air. The explants were then weighed, embedded in paraffin, sectioned and stained with Safranin O/fast green. Computerized histomorphometry was then used by a blinded technician to determine the cartilage yield (% area) and total cartilage index (% area × wet weight = mg total cartilage) in each specimen21, 22.
STATISTICAL ANALYSIS
Data are expressed as mean ± S.D (tables) or S.E.M. (bar graphs). Statistical differences between each treatment group and corresponding vehicle control were evaluated using one-way Analysis of Variance (ANOVA). Statistical differences between treatment groups were evaluated using the Least Squares Means Differences Student’s t-Test (p = 0.05).
RESULTS
CAMBIUM CELLULARITY, THICKNESS AND CELL DENSITY
Injection of TGF-β1 with or without IGF-1 increased cambium cellularity and maximum cambium thickness in both the 6 and 12 month-old rabbits (Figure 3 and Table 1). In the 12 month-old rabbits, cambium cellularity and maximum cambium thickness values were dependent on the duration of the treatment (p<0.0001) and the dose (p≤0.0001). Peak cambium cellularity (252 ± 121 cells/mm, n=13) and maximum cambium thickness (252 ± 120 μm, n=13), were observed in the 200 ng TGF-β1 group 7 days post-injection (Figure 3 and Table 1). In the 12 month-old 200 ng TGF-β1 plus 2.0 μg IGF-1-injected rabbits, the peak cambium cellularity (211 ± 154 cells/mm, n=11) and maximum cambium thickness (260 ± 177 μm, n=14), also occurred at 7 days post-injection (Figure 3 and Table 1). Cambium cell density was generally consistent in the 12 month-old rabbits with the exception being a significant increase observed in the 200 ng TGF-β1 group on day 3 post-injection compared to vehicle (Figure 3 and Table 1).
Fig. 3.
Cambium cellularity (A), maximum cambium thickness (B) and cambium cell density (C) of periosteum from 12-month old rabbit tibia measured 1, 3, 5, or 7 days post injection of vehicle, TGF-β1 (20, or 200 ng), or TGF-β1 (200 ng) plus IGF-1 (2 μg). Lowercase letters indicate the results of post-hoc testing using least squares means differences Student’s t-test (p<0.05). Columns with letters in common are not statistically different from one another.
Table 1.
Cambium Cellularity, Thickness and Cell Density
| Days Post-Injection | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | ||||||||||
| Age (Months) |
Vehicle | 20 ng TGF-β1 |
200 ng TGF-β1 |
Vehicle | 20 ng TGF-β1 |
200 ng TGF-β1 |
Vehicle | 20 ng TGF-β1 |
200 ng TGF-β1 |
Vehicle | 20 ng TGF-β1 |
200 ng TGF-β1 |
|
| Cellularity (cells/mm) |
6 | 72±51a-c | 36±39a | 129±52b-g | 87±52a-c | 163±79f-i | 145±76d-h | 86±92a-c | 175±116g-j | 128±83c-g | 93±65a-d | 194±131h-i | 150±149e-h |
| 12 | 41±45a,b | 54±68a,b | 22±14a | 34±41a,b | 62±39a,b | 80±57b | 64±70a,b | 137±100c | 184±83c,d | 49±46a,b | 136±117c | 237±130e | |
| Thickness (μM) |
6 | 88±53a,b | 60±21a | 157±73c-g | 98±85a-c | 132±42b-e | 162±84d-g | 113±85a-d | 200±77e-g | 208±88g | 108±77a-c | 279±100h | 185±134f,g |
| 12 | 36±43a,b | 28±16a,b | 21±12a | 24±21a | 74±75b,c | 43±36a,b | 33±26a,b | 104±63c,d | 115±63c,d | 40±27a,b | 149±91d | 252±120e | |
| Cell Density (cell/cm2) |
6 | 5±2.6a-d | 3±1.9a,b | 5±2.1a-f | 5±2.4c-f | 7±1.7f | 5±1.8a-f | 4±3.3a-c | 5±2.4a-f | 8±3.0b | 5±2.4a-d | 5±1.5a-e | 4±1.8a-c |
| 12 | 7±4.6b-e | 8±2.0c-e | 5±3.7a-c | 5±3.2a,b | 8±2.0b-e | 9±5.4e | 8±5.0d-e | 7±4.7b-e | 5±1.7d,e | 6±4.1a-c | 5±2.7a-c | 5±2.0a-c | |
| Age (Months) |
Vehicle | 200 ng TGF-β1 & 2 μg IGF-1 |
Vehicle | 200 ng TGF-β1 & 2 μg IGF-1 |
Vehicle | 200 ng TGF-β1 & 2μg IGF-1 |
Vehicle | 200 ng TGF-β1 & 2μg IGF-1 |
|
|---|---|---|---|---|---|---|---|---|---|
| Cellularity (cells/mm) |
6 | 66±31a-c | 136±113c-g | 98±52a-f | 160±75f-i | 55±17a,b | 230±111j | 86±47a-e | 226±126i,j |
| 12 | 17±5.9a | 25±11a,b | 48±37a,b | 163±90c,d | 46±47a,b | 150±74c | 50±57a,b | 211±154d,e | |
| Thickness (μM) |
6 | 116±78a-d | 147±92c-f | 126±78a-d | 125±52a-d | 77±45a,b | 198±66f,g | 103±64a-c | 206±77g |
| 12 | 46±73a,b | 34±20a,b | 47±33a,b | 124±106c,d | 40±30a,b | 145±71d | 35±27a,b | 260±177e | |
| Cell Density (cell/cm2) |
6 | 5±1.8a-f | 5±2.1a-f | 5±2.6c-f | 6±2.8e-f | 5±2.4c-f | 6±1.6d-f | 5±1.9a-f | 6±2.4c-f |
| 12 | 4±1.2a | 5±3.3a,b | 6±3.0a-e | 8±3.5c-e | 6±3.7a-e | 6±1.8a-d | 5±2.4a,b | 5±1.1a-c |
The data are presented as means ± S.D. Lowercase letters indicate the results of post-hoc testing using Student’s test (p<0.05). Values in each row with letters in common are not statistically different from one another. The rows from the TGF-β1 experiment (top) can also be compared to the corresponding TGF-β1 & IGF-1 combined experiment (bottom).
In the 6 month-old TGF-β1-injected rabbits, cambium cellularity and maximum cambium thickness values were dependent on the duration of the treatment (p<0.0001) and the dose (p<0.02). The peak cambium cellularity (194 ± 131 cells/mm, n=13) and maximum cambium thickness (279 ± 100 μm, n=13), were found in the 20 ng TGF-β1 group 7 days post-injection group (Table 1). In the 6 month-old 200 ng TGF-β1 plus 2.0 μg IGF-1-injected rabbits, cambium cellularity and maximum cambium thickness values were only dependent on treatment (p<0.0001) not duration. In the combined treatment, peak cambium cellularity (230 ± 111 cells/mm, n=15) occurred at 5 days post-injection while peak maximum cambium thickness (206 ± 77 μm, n=15) was observed at 7 days post-injection (Table 1). In the 6 month-old rabbits, no significant differences in cell density compared to vehicle controls were observed (Table 1).
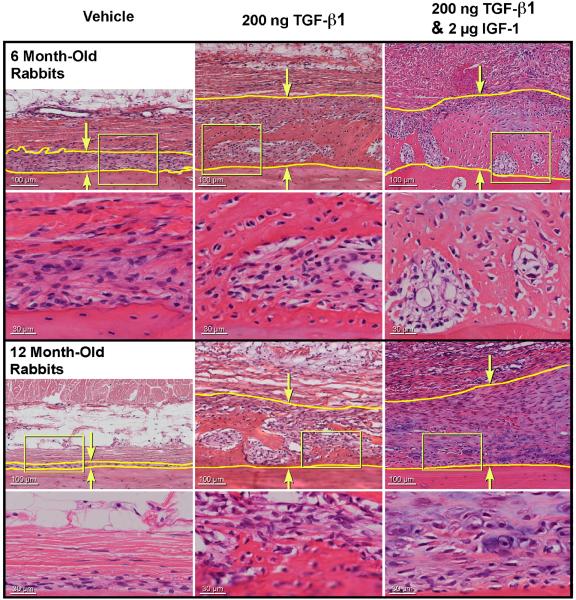
Figure 4 shows examples of typical (representative of the means) H & E stained histological sections of periosteum on bone from the 200 ng TGF-β1 and the 200 ng TGF-β1 plus 2.0 μg IGF-1 combined treatment groups compared to vehicle controls at 7 days post-injection. In the vehicle control group, the periosteum in the 6 month-old rabbits is clearly thicker than in the 12 month-old rabbits. The mean maximum cambium thickness of the vehicle-injected limbs was significantly higher in the 6 month-old rabbits (117 ± 84 μm, n=304 histological samples) compared to the 12-month–old rabbits (33 ± 30 μm, n=287 histological samples), p<0.0001 (data pooled from all time points) demonstrating the age-related thinning of the periosteum in the experimental rabbits. Thickening of the periosteum is apparent in both the 200 ng TGF-β1, and the 2.0 μg IGF-1 plus 200 ng TGF-β1 groups compared to the vehicle controls. In addition, increased extracellular matrix is visible within the cambium of both growth factor treated groups. Figure 5 illustrates the effect of 200 ng TGF-β1, and the 2.0 μg IGF-1 plus 200 ng TGF-β1 injection in the 12 month-old rabbits over time. In the combined treatment group, thickening of the cambium was observed by day 3 post-injection, is apparent in both treatment groups by day 5, and increased further by day 7 post-injection. Increased extracellular matrix in the cambium was noted in both treatment groups by day 5 and increased by day 7 post-injection.
Fig. 4.
Typical (representative of the means) H & E stained histological specimens of periosteum on-bone, from 6 and 12 month-old rabbits 7 days after injection with 200 ng TGF-β1 or 200 ng TGF-β1 plus 2 μg IGF-1. The yellow lines and arrows indicate the cambium.
Fig. 5.
Typical (representative of the means) H & E stained histological specimens of periosteum on-bone, from 12 month-old rabbits 1, 3, 5, or 7 days after injection with 200 ng TGF-β1 or 200 ng TGF-β1 plus 2 μg IGF-1. The yellow lines and arrows indicate the cambium.
Subperiosteal injection of IGF-1 (0.2 or 2.0 μg) had no significant effect on cambium layer thickness compared to vehicle controls in the 6 month-old rabbits at any of the time points (data not shown). In the 12 month-old rabbits, a small, but statistically significant increase in the maximum cambium thickness was observed 7 days post-injection in both the 0.2 μg (44 ± 69 μm, n=16) and 2.0 μg (36 ± 22 μm, n=16) IGF-1 doses compared to vehicle controls (30 ± 14 μm, n=32), p<0.05.
IN VITRO PERIOSTEAL CARTILAGE FORMATION
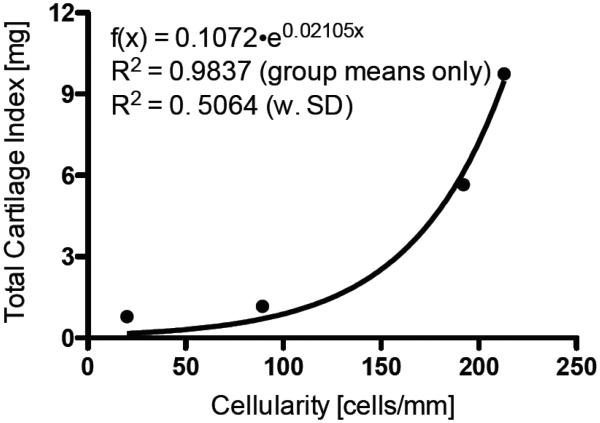
Injection of 200 ng TGF-β1 with or without 2.0 μg IGF-1 increased in vitro periosteal cartilage yield, explant wet weight, and total cartilage index in all age groups after six weeks of culture (Figure 6-8 and Table 2). In the 12 month-old TGF-β1-treated rabbits, cartilage yield, explant wet weight, and total cartilage index values were dependent on dose and duration of the treatment (p<0.0001). Injection of 200 ng TGF-β1 significantly increased in vitro periosteal cartilage yield and total cartilage index when harvested 5 and 7 days post-injection (Figure 6 and Table 2). Peak cartilage yield (36 ± 14 %, n=16) and explant wet weight (27 ± 6.3 mg, n=16) in the 12 month-old rabbits were observed in the 200 ng TGF-β1 7-day post-injection group, resulting in a 12-fold increase in total cartilage index in the 200 ng TGF-β1 (9.7 ± 4.7 mg, n=16) injected rabbits (p<0.0001) compared to vehicle controls (0.8 ± 1.0 mg, n=32). As shown in Figure 8, a strong correlation between the mean total cartilage index and cambium cellularity was observed in the 200 ng TGF-β1 injected rabbits. In the 12 month-old rabbits, wet weight values were dependent on both duration and treatment, whereas cartilage yield and total cartilage index were dependent on treatment only (p<0.0001). Injection of 200 ng TGF-β1 plus 2.0 μg IGF-1 significantly increased cartilage yield when the periosteum was explanted 1, 5, or 7 days post-injection (Figure 6 and Table 2). The peak cartilage yield (17 ± 17 %, n=16) and peak total cartilage index (3.4 ± 3.9 mg, n=16) values in the combined treatment groups were lower than the 200 ng TGF-β1 in the 12 month-old rabbits. However, when compared to vehicle-injected controls (0.2 ± 0.3 mg, n=16) a comparable 17-fold increase in total cartilage index was observed.
Fig. 6.
Cartilage Yield (A), explant wet weight (B) and total cartilage index (C) of periosteum from 12-month old rabbit tibia measured 1, 3, 5, or 7 days post injection of vehicle, TGF-β1 (20, or 200 ng), or TGF-β1 (200 ng) plus IGF-1 (2 μg). Lowercase letters indicate the results of post-hoc testing using least squares means differences Student’s t-test (p<0.05). Columns with letters in common are not statistically different from one another.
Fig. 8.
Correlation between the total cartilage index and cambium cellularity from 12-month old rabbit periosteum injected with vehicle or TGF-β1 (200 ng) and harvested 1, 3, 5, or 7 days post injection.
Table 2.
Cartilage Yield, Wet Weight, and Total Cartilage Index
| Days Post-Injection | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | ||||||||||
| Age (Months) |
Vehicle | 20 ng TGF-β1 |
200 ng TGF-β1 |
Vehicle | 20 ng TGF-β1 |
200 ng TGF-β1 |
Vehicle | 20 ng TGF-β1 |
200 ng TGF-β1 |
Vehicle | 20 ng TGF-β1 |
200 ng TGF-β1 |
|
| Cartilage Yield (%) |
6 | 20±17c-f | 13±14a-c | 33±19g | 26±20e-g | 17±12b-e | 27±21e-g | 10±12a,b | 13±14a-c | 29±21f,g | 6±8a | 9±11a,b | 18±13b-e |
| 12 | 9±13b,c | 14±16c-f | 6±9a,b | 5±7a,b | 9±12b-e | 7±9a-c | 8±9b,c | 6±5a,b | 25±19h | 15±14d-f | 25±13g,h | 36±14i | |
| 24 | 0.1±0.3a | NA | 6±13a-c | 0.4±1a | NA | 6±10a-c | 0.3±0.7a | NA | 5±9a-c | 0.9±2a | NA | 12±10c,d | |
| Wet Weight (mg) |
6 | 11±4.4a,b | 12±4.2a,b | 19±6.4e-g | 14±4.7b-d | 18±6.9d-g | 15±8.5b-f | 10±4.6a | 15±8.2b-f | 19±6.9g,h | 11±6.4a,b | 15±5.0b-f | 22±7.7g |
| 12 | 12±6.0c,e | 11±3.7b-e | 12±5.1c-f | 8±3.2a,b,d | 10±2.9b-e | 14±8.8e-g | 8±3.4a,b | 20±6.6h,i | 21±5.8i | 5±2.2a | 17±6.2g,h | 27±6.3j | |
| 24 | 6±3.8a | NA | 6±3.8a | 6±4.3a | NA | 8±2.5a,b | 9±5.7a,b | NA | 10±4.7b,c | 6±2.8a | NA | 17±6.4d | |
| Total Cartilage Index (mg) |
6 | 2.3±0.4a-d | 1.5±0.4a-d | 6.8±1.2j,k | 4.0±0.6e-h | 3.1±0.6b-h | 4.6±0.94g-j | 1.1±0.3a,b | 2.2±0.7a-f | 5.5±1.3h-k | 0.9±0.3a | 1.2±0.3a-c | 4.3±1.1e-i |
| 12 | 0.9±1.4a-c | 1.7±2.0a-c | 0.8±1.1b,c | 0.4±0.6a | 0.9±1.1a-c | 1.2±2.5a-c | 0.6±0.7a,b | 1.1±1.0a-c | 5.7±4.9f | 0.8±1.0a,b | 4.4±2.8e,f | 9.7±4.7g | |
| 24 | .01±0.02a | NA | 0.6±1.6a | 0.02±0.06a | NA | 0.6±1.1a | 0.02±0.05a | NA | 0.7±1.4a | 0.06±0.2a | NA | 2.4±2.2b | |
| Age (Months) |
Vehicle | 200 ng TGF-β1 & 2 μg IGF-1 |
Vehicle | 200 ng TGF-β1 & 2 μg IGF-1 |
Vehicle | 200 ng TGF-β1 & 2μg IGF-1 |
Vehicle | 200 ng TGF-β1 & 2μg IGF-1 |
|
|---|---|---|---|---|---|---|---|---|---|
| Cartilage Yield (%) |
6 | 11±13a-c | 16±17b-d | 20±20c-f | 24±20d-g | 9±4a,b | 24±4d-g | 15±13a-d | 9±10a,b |
| 12 | 2±6a,b | 16±18e,f | 8±9a-d | 8±9a-d | 2±3a,b | 17±17f,g | 0.4±1a | 10±10b-f | |
| 24 | 2±2a,b | 4±7a,b | 8±9b,c | 24±22f | 6±9a-c | 22±17e,f | 2±2a,b | 16±14d,e | |
| Wet Weight (mg) |
6 | 15±4.6b-f | 14±4.3a-d | 17±5.8c-f | 22±6.2g | 12±7.5a-c | 30±21h | 14±5.7a-d | 30±6.8h |
| 12 | 8±3.9a,b,d | 12±4.3c-f | 7±2.3a,b | 16±8.0f-h | 8±2.8a,b | 20±6.8h,i | 8±4.2a-d | 29±19j | |
| 24 | 6±2.9a | 9±3.5a-c | 8±3.2a,b | 13±7.1c | 9±3.1a-c | 20±6.8d | 7±3.8a | 30±18e | |
| Total Cartilage Index (mg) |
6 | 1.8±0.7a-d | 3.0±0.7a-g | 4.5±5.0f-j | 6.3±4.8i-k | 2.0±3.1a-e | 7.1±7.1k | 3.5±3.4c-h | 3.7±3.7d-h |
| 12 | 0.3±1.1a,b | 2.0±2.6c,d | 0.6±0.8a,b | 1.1±1.3a-c | 0.2±0.3a | 3.4±3.9d,e | 0.04±0.2a | 2.1±2.3c,d | |
| 24 | 0.08±0.09a | 0.4±0.7a | 0.7±1.0a | 3.7±4.6b,c | 0.6±1.0a | 5.1±4.4c | 0.1±0.1a | 4.6±4.8c |
The data are presented as means± S.D. Lowercase letters indicate the results of post-hoc testing using Student’s test (p<0.05). Values in each row with letters in common are not statistically different from one another. The rows from the TGF-β1 experiment (top) can also be compared to the corresponding TGF-β1 & IGF-1 combined experiment (bottom).
In the 2 year-old rabbits, 200 ng TGF-β1 injections significantly increased in vitro periosteal cartilage yield at all time points, and 200 ng TGF-β1 plus 2.0 μg IGF-1 injections increased cartilage yield from periosteum explanted 3, 5, and 7 days post-injection (Figure 7 and Table 2). In the 2 year-old 200 ng TGF-β1-injected rabbits, explant wet weights (p<0.0001) and total cartilage index (p<0.004) were treatment and duration-dependent, whereas cartilage yield was only treatment dependent (p<0.0001). In the 2 year-old 200 ng TGF-β1 plus 2.0 μg IGF-1 combined treatment group, cartilage yield (p<0.0001), explant wet weight (p<0.0001) and total cartilage index (p<0.003) were duration and treatment dependent. In contrast to the 12 month-old rabbits peak cartilage production was observed in the 200 ng TGF-β1 plus 2.0 μg IGF-1 combined treatment group. Peak cartilage yield (24 ± 22 %, n=16) in the 2 year-old rabbits was observed in periosteum explanted 3-days post-injection and peak total cartilage index (5.1 ± 4.4 mg, n=16) was found in periosteum explanted 5-day post-injection.
Fig. 7.
Cartilage Yield (A), explant wet weight (B) and total cartilage index (C) of periosteum from 2 year-old rabbit tibia measured 1, 3, 5, or 7 days post injection of vehicle, TGF-β1 (200 ng), or TGF-β1 (200 ng) plus IGF-1 (2 μg). Lowercase letters indicate the results of post-hoc testing using least squares means differences Student’s t-test (p<0.05). Columns with letters in common are not statistically different from one another.
In the 6 month-old TGF-β1-treated rabbits, cartilage yield (p<0.0001), and total cartilage index (p<0.04) values were dose and duration-dependent and explant wet weights (p<0.0001) were only dose dependent. Injection of 200 ng TGF-β1 significantly increased cartilage yield from periosteum explanted 1, 5 and 7 days post-injection in 6 month-old rabbits. Peak cartilage yield (33 ± 19 %, n=16) and total cartilage index (6.8 ± 1.2, n=16) in the 6 month-old rabbits were observed in periosteum explanted 1-day after injection of 200 ng TGF-β1. In the combined 200 ng TGF-β1 plus 2.0 μg IGF-1 treated rabbits, total cartilage index (p<0.05) and explant wet weights (<0.02) were duration and treatment dependent. The combined treatment significantly increased cartilage yield from periosteum explanted 5 days post-injection (Table 2).
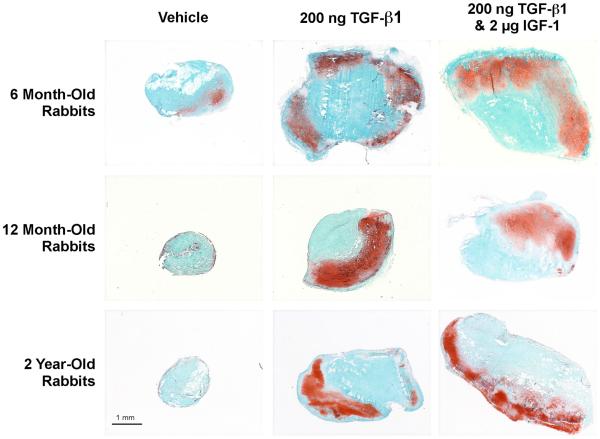
Figure 9 contains typical (representative of the means) examples of safranin O/fast green stained sections of periosteal explants after in situ vehicle or growth factor injection followed by six weeks in culture. The mean cartilage yield produced by periosteal explants after six weeks of culture in the vehicle-injected periosteum was significantly higher in the 6 month-old rabbits (17.6 ± 17.2 %, n=319 periosteal explants) compared to the 12-month–old rabbits (7.4 ± 11.1 %, n=318 periosteal explants) and the 2 year-old rabbits (1.9 ± 4.6 %, n=188 periosteal explants), p<0.0001 (data pooled from all time points) demonstrating an age-related decline in periosteal chondrogenesis in the experimental animals. Figure 9 clearly illustrates the increase in cartilage formation, as detected by safranin O/fast green staining, observed after injection of 200 ng TGF-β1 alone or in combination with 2.0 μg IGF-1 followed by six weeks of culture. It is obvious from these examples that periosteal explant size was also increased. This observation is further illustrated by comparing the wet weights of the periosteal explants after the culture period (Figure 6 and 7 and Table 2). Subperiosteal injection of IGF-1 (0.2 or 2.0 μg) did not significantly increase cartilage yield, explant wet weights or total cartilage index compared to vehicle controls at any of the post-injection explant times in any age group (data not shown). However, injection 200 ng TGF-β1 alone or in combination with 2 μg IGF-1 significantly increased wet weight in all age groups.
Fig. 9.
Typical (representative of the means) Safranin O/fast green stained histological specimens of cultured periosteum harvested from 6, 12 and 24-month old rabbits 7 days after injection of vehicle, TGF-β1 (200 ng), or TGF-β1 (200 ng) plus IGF-1 (2 μg).
DISCUSSION
Previously, we demonstrated in rabbits that the chondrogenic potential of periosteum declines with age and that this decline is closely related to the decrease in the number of cambium layer cells and cambium thickness observed with age13. From a technical standpoint, this biological limitation makes it more difficult to surgically harvest an intact periosteal graft containing sufficient cambium cells for cartilage tissue engineering or regeneration. Thus, in order to extend the utility of periosteum for tissue regeneration to patients past skeletal maturity, it is necessary to develop methods to rejuvenate periosteum by increasing the precursor cell pool in the cambium layer to levels similar to those observed during skeletal growth. In the present study, we demonstrate that it is possible to reverse these age related effects on periosteum, at least temporarily, in rabbits with a single local injection of TGF-β1 alone or in combination with IGF-1.
Based on the samples from the vehicle-injected controls in this study, the periosteum was thicker and contained more cambium cells, and produced more cartilage in vitro in the 6 month-old rabbits compared to the 12 and 24 month-old rabbits, confirming our previous findings regarding the effects of age on periosteum in the rabbit model13.
Injection of periosteum with IGF-1 alone had little or no effect on either cambium thickness or in vitro cartilage formation in any of the ages studied. These results are not entirely surprising since we previously observed that stimulation of periosteum in vitro by IGF-1 alone requires continuous exposure to IGF-116. Thus, it is possible that multiple daily injections of IGF-1 alone would produce better results. However, this approach was outside the scope of this study.
In contrast, a single subperiosteal injection of 200 ng TGF-β1 resulted in both thickening of the cambium, increased cambium cell numbers, and increased in vitro cartilage production in all age groups examined. The cambium cellularity and thickness findings are consistent with the results from multiple TGF-β injections in both newborn rats17 and rabbits18 reported previously. Whereas, the in vitro cartilage production results are consistent with our previous findings demonstrating that a brief exposure (30 to 60 minutes) of high-dose (50 or 100 ng/mL) TGF-β1 is sufficient to induce periosteal chondrogenesis in vitro 15.
In the present study, subperiosteal injections of TGF-β1 plus IGF-1 produced inconsistent results between the different age groups, compared to TGF-β1 alone. An added benefit of IGF-1 on cartilage production was only observed in the 2 year old rabbits. Whereas a potential inhibitory effect of IGF-1 on total cartilage production was observed in the 12 month old rabbits. However, the fold increases compared to the vehicle controls were similar in the combined treatment compared to TGF-β1 only. Thus, it is not clear if these results represent an age associated response difference or simply variability between the rabbits, sample procurement, or culture conditions. In order to resolve this issue, the TGF-β1 vs. TGF-β1 plus IGF-1 treatments may need to be compared in the same rabbits.
There are many potential autologous cell sources for cartilage regeneration including cartilage, bone marrow, perichondrium, periosteum, adipose, and synovium 23, 24. Each cell source has advantages and disadvantages and the most efficacious cell source for durable cartilage repair has yet to be determined. Periosteum has been used clinically for biological resurfacing arthroplasty with the majority of the patients receiving excellent to good results 25-34. One major advantage of the use of a periosteal graft for cartilage regeneration is the fact that an in vitro cell expansion step is not required, thus, specialized cell culture facilities are not needed. As such, this approach should be more globally accessible than those requiring such facilities. Recent studies demonstrate that periosteal cells from adult and even elderly humans retain their proliferative and multilineage capacity35, 36. Specifically, these isolated human periosteal cells can form cartilage in vitro in response to TGF-β135, 36. Based on these findings35, 36 and the present study, it is reasonable to predict that local periosteal injection of TGF-β1 in humans could be used to increase cambium cellularity and periosteal chondrogenesis for cartilage tissue engineering or regeneration regardless of age.
Results from the present studies clearly demonstrate that local injection of TGF-β1 alone or in combination with IGF-1 is a feasible approach to increase cambium layer thickness and in vitro cartilage production. The cambium cellularity, thickness and cartilage formation values in the growth factor treated 6-24 month old rabbits in these studies are also comparable to our previous observations in younger rabbits suggesting that periosteum in older rabbits can be rejuvenated for tissue engineering13. This finding is important because the age related decline in periosteal chondrogenesis is considered the primary limitation to the use of periosteum for cartilage tissue regeneration or engineering13, 14. The results presented herein also support the observation made by Kolodny eighty-five years ago that “periosteum of an adult animal plus reactive stimulation are equal to periosteum of a young animal”, referring to the bone forming capacity of periosteum37. However, it is important to note that there are likely to be differences between the rejuvenated periosteum and periosteum from younger animals that are yet to be defined. In vivo cartilage regeneration experiments in animal models are now needed to test the efficacy of periosteal transplantation to regenerate articular cartilage in adult animals after in situ TGF-β1 pretreatment. If this simple approach can be translated to the clinic, the number of patients who could possibly benefit from the use of periosteum for tissue engineering or regeneration of cartilage would be greatly increased. In addition, it is plausible that the potential benefits of this rejuvenation approach may also extend to bone regeneration.
ACKNOWLEDGMENTS
Funding for this project was provided by NIH/NIAMS through grant AR52115. The authors would also like to thank Dr. James J. Stone and Mr. Yaping Huang for their technical assistance as well as James E. Tarara for writing the KS300 macro.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
Mayo Clinic has filed a patent application related to this study on behalf of authors GGR, JSF and SWO.
REFERENCES
- 1.O'Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg. 1986;68A:1017–35. [PubMed] [Google Scholar]
- 2.O'Driscoll SW. Articular cartilage regeneration using periosteum. Clin Orthop. 1999;367(Suppl):186–203. doi: 10.1097/00003086-199910001-00020. [DOI] [PubMed] [Google Scholar]
- 3.Nakahara H, Bruder SP, Haynesworth SE, Holecek JJ, Baber MA, Goldberg VM, et al. Bone and cartilage formation in diffusion chambers by subcultured cells derived from the periosteum. Bone. 1990;11:181–8. doi: 10.1016/8756-3282(90)90212-h. [DOI] [PubMed] [Google Scholar]
- 4.O'Driscoll SW, Fitzsimmons JS. The role of periosteum in cartilage repair. Clin Orthop. 2001;391(Suppl):S190–207. doi: 10.1097/00003086-200110001-00019. [DOI] [PubMed] [Google Scholar]
- 5.Nakahara H, Goldberg VM, Caplan AI. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Orthop Res. 1991 Jul;9(4):465–76. doi: 10.1002/jor.1100090402. [DOI] [PubMed] [Google Scholar]
- 6.O'Driscoll SW, Recklies AD, Poole AR. Chondrogenesis in periosteal explants. An organ culture model for in vitro study. J Bone Joint Surg Am. 1994 Jul;76(7):1042–51. doi: 10.2106/00004623-199407000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Hutmacher DW, Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003;9(Suppl 1):S45–64. doi: 10.1089/10763270360696978. [DOI] [PubMed] [Google Scholar]
- 8.Stevens MM, Marini RP, Schaefer D, Aronson J, Langer R, Shastri VP. In vivo engineering of organs: the bone bioreactor. Proc Natl Acad Sci U S A. 2005 Aug;102(32):9, 11450–5. doi: 10.1073/pnas.0504705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg. 1988;70A:595–606. [PubMed] [Google Scholar]
- 10.O'Driscoll SW, Salter RB. The repair of major osteochondral defects in joint surfaces by neochondrogenesis with autogenous osteoperiosteal grafts stimulated by continuous passive motion. An experimental investigation in the rabbit. Clin Orthop Relat Res. 1986 Jul;(208):131–40. [PubMed] [Google Scholar]
- 11.Mardones RM, Reinholz GG, Fitzsimmons JS, Zobitz ME, An KN, Lewallen DG, et al. Development of a biologic prosthetic composite for cartilage repair. Tissue Eng. 2005 Sep-Oct;11(9-10):1368–78. doi: 10.1089/ten.2005.11.1368. [DOI] [PubMed] [Google Scholar]
- 12.Knothe UR, Springfield DS. A novel surgical procedure for bridging of massive bone defects. World J Surg Oncol. 2005 Feb;3(1):3, 7. doi: 10.1186/1477-7819-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Driscoll SW, Saris DB, Ito Y, Fitzimmons JS. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001 Jan;19(1):95–103. doi: 10.1016/S0736-0266(00)00014-0. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Fitzsimmons JS, Sanyal A, Mello MA, Mukherjee N, O'Driscoll SW. Localization of chondrocyte precursors in periosteum. Osteoarthritis Cartilage. 2001;9:215–23. doi: 10.1053/joca.2000.0378. [DOI] [PubMed] [Google Scholar]
- 15.Miura Y, Parvizi J, Fitzsimmons JS, O'Driscoll SW. Brief exposure to high-dose transforming growth factor-beta1 enhances periosteal chondrogenesis in vitro: a preliminary report. J Bone Joint Surg Am. 2002 May;84-A(5):793–9. doi: 10.2106/00004623-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003 Jan;11(1):55–64. doi: 10.1053/joca.2002.0869. [DOI] [PubMed] [Google Scholar]
- 17.Joyce ME, Roberts AB, Sporn MB, Bolander ME. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110:2195–207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Critchlow MA, Bland YS, Ashhurst DE. The effects of age on the response of rabbit periosteal osteoprogenitor cells to exogenous transforming growth factor-ß2. J Cell Sci. 1994;107:499–516. doi: 10.1242/jcs.107.2.499. [DOI] [PubMed] [Google Scholar]
- 19.Gallay SH, Miura Y, Commisso CN, Fitzsimmons JS, O'Driscoll SW. Relationship of donor site to chondrogenic potential of periosteum in vitro. J Orthop Res. 1994;12(4):515–25. doi: 10.1002/jor.1100120408. [DOI] [PubMed] [Google Scholar]
- 20.O'Driscoll SW, Saris DBF, Ito Y, Fitzsimmons JS. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001;19(1):95–103. doi: 10.1016/S0736-0266(00)00014-0. [DOI] [PubMed] [Google Scholar]
- 21.O'Driscoll SW, Marx RG, Fitzsimmons JS, Beaton DE. A method for automated cartilage histomorphometry. Tissue Eng. 1999;5:13–23. doi: 10.1089/ten.1999.5.13. [DOI] [PubMed] [Google Scholar]
- 22.Fitzsimmons JS, Sanyal A, Gonzalez C, Fukumoto T, Clemens VR, O'Driscoll SW, et al. Serum-free media for periosteal chondrogenesis in vitro. J Orthop Res. 2004 Jul;22(4):716–25. doi: 10.1016/j.orthres.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432–63. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005 Aug;52(8):2521–9. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 25.Alfredson H, Lorentzon R. Superior results with continuous passive motion compared to active motion after periosteal transplantation. A retrospective study of human patella cartilage defect treatment. Knee Surg Sports Traumatol Arthrosc. 1999;7(4):232–8. doi: 10.1007/s001670050154. [DOI] [PubMed] [Google Scholar]
- 26.Alfredson H, Thorsen K, Lorentzon R. Treatment of tear of the anterior cruciate ligament combined with localised deep cartilage defects in the knee with ligament reconstruction and autologous periosteum transplantation. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):69–74. doi: 10.1007/s001670050124. [DOI] [PubMed] [Google Scholar]
- 27.Fujii K, Sai S, Tanak T, Tsuji M, Mori M, Murota K. Biological resurfacing of full-thickness defects in patellar cartilage utilizing autogenous periosteal grafts. Combined Meeting of the Orthopaedic Research Socities of USA, Japan, and Canada; 1991; Alberta; 1991. :144. [Google Scholar]
- 28.Hoikka VEJ, Jaroma HJ, Ritsilä VA. Reconstruction of the patellar articulation with periosteal grafts. 4-year follow-up of 13 cases. Acta Orthop Scand. 1990;61:36–9. doi: 10.3109/17453679008993062. [DOI] [PubMed] [Google Scholar]
- 29.Korkala OL. Periosteal primary resurfacing of joint surface defects of the patella due to injury. Injury. 1988;19:216–8. doi: 10.1016/0020-1383(88)90021-6. [DOI] [PubMed] [Google Scholar]
- 30.Korkala O, Kuokkanen H. Autogenous osteoperiosteal grafts in the reconstruction of full-thickness joint surface defects. Int Orthop. 1991;15(3):233–7. doi: 10.1007/BF00192300. [DOI] [PubMed] [Google Scholar]
- 31.Korkala OL, Kuokkanen HO. Autoarthroplasty of knee cartilage defects by osteoperiosteal grafts. Arch Orthop Trauma Surg. 1995;114(5):253–6. doi: 10.1007/BF00452081. [DOI] [PubMed] [Google Scholar]
- 32.Lorentzon R, Hildingsson C, Alfredson H. Treatment of deep cartilage defects in the knee with periosteum transplantation. Acta Orthop Scand. 1997;68(Suppl 274):1. [Google Scholar]
- 33.Lorentzon R, Alfredson H, Hildingsson C. Treatment of deep cartilage defects of the patella with periosteal transplantation. Knee Surg Sports Traumatol Arthrosc. 1998;6(4):202–8. doi: 10.1007/s001670050100. [DOI] [PubMed] [Google Scholar]
- 34.Niedermann B, Boe S, Lauritzen J, Rubak JM. Glued periosteal grafts in the knee. Acta Orthop Scand. 1985;56:457–60. doi: 10.3109/17453678508993034. [DOI] [PubMed] [Google Scholar]
- 35.De Bari C, Dell'Accio F, Vanlauwe J, Eyckmans J, Khan IM, Archer CW, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006 Apr;54(4):1209–21. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 36.Jansen EJ, Emans PJ, Guldemond NA, van Rhijn LW, Welting TJ, Bulstra SK, et al. Human periosteum-derived cells from elderly patients as a source for cartilage tissue engineering? J Tissue Eng Regen Med. 2008 Aug;2(6):331–9. doi: 10.1002/term.100. [DOI] [PubMed] [Google Scholar]
- 37.Kolodny A. The periosteal blood supply and healing of fractures: experimental study. The Journal of Bone and Joint Surgery. 1923;5:698–711. [Google Scholar]