Summary
Our published research has focused upon the role of Goa1p, an apparent regulator of the Candida albicans mitochondrial complex I (CI). Lack of Goa1p effects optimum cell growth, CI activity, and virulence. Eukaryotic CI is composed of a core of 14 alpha-proteobacterial subunit proteins and a variable number of supernumerary subunit proteins. Of the latter group of proteins, one (NUZM) is fungal-specific, and a second (NUXM) is found in fungi, algae and plants but is not a mammalian CI subunit protein. We have established that NUXM is orf19.6607 and NUZM is orf19.287 in C. albicans. Herein, we validate both subunit proteins as NADH:ubiquinone oxidoreductases (NUO) and annotate their gene functions. To accomplish these objectives, we compared null mutants of each with WT and gene-reconstituted strains. Genetic mutants of genes NUO1 (19.6607) and NUO2 (19.287), not surprisingly, each had reduced oxygen consumption, decreased mitochondrial redox potential, decreased CI activity, increased reactive oxidant species (ROS), and a decrease in chronological aging in vitro. Loss of either gene results in a disassembly of CI. Transcriptional profiling of both mutants indicated significant down regulation of genes of carbon metabolism, as well as upregulation of mitochondrial-associated gene families which may occur to compensate for the loss of CI activity. Profiling of both mutants also demonstrated a loss of cell wall β-mannosylation but not in a conserved CI subunit (ndh51Δ). The profiling data may indicate specific functions driven by the enzymatic activity of Nuo1p and Nuo2p. Of importance, each mutant is also avirulent in a murine blood-borne, invasive model of candidiasis associated with their reduced colonization of tissues. Based upon their fungal-specificity and roles in virulence, we suggest both as drug targets for antifungal drug discovery.
Keywords: virulence, mitochondria, tissue colonization, Candida albicans
Introduction
Candida albicans is one of the most common opportunistic fungal pathogens of humans. This species as well as other Candida species are part of the mucosal and skin microbiome; from these sites superficial or invasive candidiasis can occur in patients with risk factors such as HIV/AIDS (oral candidiasis), or nosocomial, blood-borne infections following surgery, in premature infants, and neutropenic patients undergoing cancer chemotherapy or allogeneic bone marrow transplantation (Calderone et al.,2014; Calderone et al., 2012).
Survival of C. albicans within anatomically distinct host niches highlights the fact that the organism must have unique strategies for adaptation to a range of physiological extremes (Bambach et al., 2009; Barelle, et al., 2006; Brown et al., 2014a; Brown et al., 2014b; Ramirez and Lorenz, 2007; Enie et al., 2014; Enie et al., 2012; Fradin, et al., 2003; Askew et al., 2000; Prieto, et al., 2014). For example, on mucosal surfaces, nutrient limitation and oxidative stress cause selective pressures which impact survival of pathogens and commensals (Brown et al., 2014b; Fradin et al., 2003). Signal transduction pathways support adaptation to stress conditions, illustrating the importance of cell communication in the survival of C. albicans (Prieto et al., 2014).
Underlying these complexities, it is also clear that energy production of pathogens like C. albicans is critically important to survival (Enie et al., 2014, 2012). The concept of “flexible metabolism” in C. albicans has been coined to reflect two levels of regulation (Enie et al., 2014): 1) flexiblility to utilize diverse carbon sources such as fatty acids and amino acids in the absence of sustainable glucose; 2) switching from anaerobic to aerobic (mitochondrial oxidation) to provide energy for growth at specific host niches. Flexible metabolism also depends on proper energy-yielding metabolic events. In this regard, the mitochondrial impact on cell growth and energy production of Candida spp in vitro has been studied but less so in the context of virulence and pathogenesis. Without question, the lack of energy production should be a major weakness for most pathogens.
Our criteria for the study of Complex I (CI) subunit proteins is that they must be conserved in fungi and be required for virulence. To this end, in the current study, we have identified CI proteins that fulfill these criteria. We also note our published data on the Goa1p and its role in CI activities in C. albicans (Bambach et al., 2009; Sun et al., 2013; Li et al., 2011; Chen et al., 2012; She et al., 2013; Khamooshi et al., 2014). Goa1p, is required for CI enzyme activity but is not a CI subunit (Bambach et al., 2009). GOA1 is only found in the CTG clade of Candida spp, which includes most of the pathogenic Candida species except C. glabrata (Bambach et al., 2009). Because of its importance in growth and virulence described above, Goa1p might be considered a potential target for antifungal drug therapy. Its highly conserved nature among only species of the CTG clade suggests that an inhibitor may be of narrow specificity but still a potentially useful therapeutic.
Other virulence factors such as phase transition are also regulated by cell respiratory pathways (McDonough et al., 2002; Velluci et al., 2007). Recently, cell levels of glutathione were deemed critical to the yeast → hyphae transition as a reduction in glutathione, CI activity, and ATP, as well as an increase in the alternative oxidant pathway (AOX) accompanied the loss of phase transition (Gredouari, et al., 2014). Loss of other genes results in mitochondrial deficiencies that likewise affect drug resistance, antifungal susceptibility, drug tolerance, and virulence in C. albicans (Shingu-Vazquez and Traven, 2011; Dagley et al., 2011). Others have suggested that the common denominator of these effects on cells is a reduction of membrane phospholipid homeostasis (Dagley, et al., 2011). With respect to cell wall synthesis, the C. albicans Ccr4-Pop2 mRNA deadenylase is a regulator of mRNA stability and translation. Mutants lacking this gene are compromised in cell wall β-glucan synthesis, resulting in an increased sensitivity to caspofungin, a decrease in filamentation, and a decrease in virulence (Shingu-Vazquez and Traven, 2011). Underlying these cell wall changes are dysfunctional mitochondria and a phospholipid imbalance. In this regard, a mitochondrial outer membrane complex protein (Sam37) of the Sorting and Assembly Machinery complex is critical to phospholipid synthesis and other properties of mitochondria. The sam37Δ is phenotypically similar to that described for the Ccr4-Pop2 mRNA deadenylase (Dagley et al., 2011; Qu et al., 2012). Thus, as the major source of energy, mitochondria are crucial for growth, response to the environment, reproduction, and decreased virulence.
The supposed underrepresentation of mitochondrial studies in the field of fungal pathogens may be due to the presumed conserved nature of the mitochondria-encoded and imported nuclear proteins. To address this presumption in this study, we focus on two non-mammalian subunits of C. albicans mitochondrial CI to confirm their importance in cell growth and virulence in a murine model. Both subunits were first denoted as NUXM and NUZM (Gabaldon et al., 2005). NUXM is broadly conserved in C. albicans, Neurospora crassa, Yarrowia lipolytica, Arabidopis thalina, and Chlamydomonas reinhardtii. On the other hand, NUZM is fungal-specific (Gabaldon et al., 2005; Abdrakhmanova et al., 2004; Bridges, Feamley and Hirst, 2010; Bridges et al., 2009; Pereira et al., 2013; Videira and Duarte, 2002; Videira, 1998). We have identified these as orf19.6607 (NUXM) and orf19.287 (NUZM), and now report the phenotypes of gene-deleted strains in comparison to parental and gene-reconstituted controls. Based upon their non-conservation in mammals and the loss of important activities in mutants (such as virulence), we suggest that both may be attractive targets for therapeutic intervention.
Following their now established functions as NADH:Ubiquinone Oxidoreductases, we named orf19.6607 (NUO1) and orf19.287 (NUO2) and use this gene nomenclature throughout the manuscript.
Results
Identification of the C. albicans NUXM and NUZM
The gene sequences of NUXM and NUZM were first reported for Neurospora crassa (Azevedo et al., 1992; Videira et al., 1990). Those sequences were identified as orf19.6607 and orf19.287 from the Candida Genome Database by protein BLAST analysis of protein sequences (http://www.candidagenome.org).
Growth on non-glucose carbon sources and oxygen consumption are reduced in the CI mutants
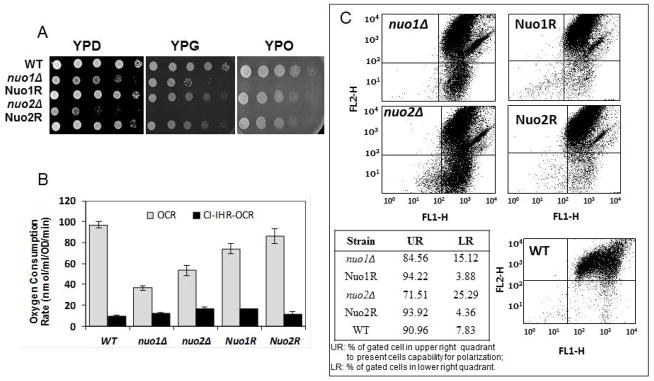
The generation times (GT) in YPD broth of both mutants were 109 min (nuo1Δ) and 133 min (nuo2Δ) compared to 87 min in WT cells (Noble et al., 2010). Since NUO1 (19.6607) and NUO2 (orf19.287) are putative NADH:Ubiquinone oxidoreductases, we determined if null mutants of each (nuo1Δ and nuo2Δ) have phenotypes suggestive of typical defects of other CI mutants. Our assays included growth on mitochondrial substrates such as glycerol or oleic acid, oxygen consumption, mitochondrial membrane potential, reactive oxidant production (ROS), chronological life span (CLS), and CI enzyme activity. Both the nuo1Δ and nuo2Δ mutants had reduced growth on YPD agar (Fig. 1A, left panel) as well as on mitochondrial substrates compared to WT and gene reconstituted strains (Nuo1R and Nuo2R) (Fig. 1A, middle and right panels). nuo2Δ displayed a greater growth defect than nuo1Δ on YP-glycerol. Both mutants were equally unable to grow on oleic acid (Fig. 1A, right panel).
Figure 1.
A. Growth defects on glucose and non-glucose carbon media. C. albicans strains were grown overnight in YPD medium, washed and serially diluted in PBS. 3 μL of each serial dilution was plated on the YP medium supplemented with 2% glucose (YPD), 2% glycerol-YP (YPG), or 3% oleic acid YP-agar plates. All cultures were incubated for 2 days at 30°C. Both nuo1Δ and nuo2Δ grew less than control strains on YPD and had additionally reduced growth on YPG and YPO. The Nuo1R and Nuo2R gene-reconstituted strains were intermediate in growth compared to WT and null strains. B. The oxygen consumption rates (OCR) of nuo1Δ, nuo2Δ, Nuo1R, and Nuo2R and WT are shown. Both mutants consume less oxygen than control strains. Oxygen consumption was also measured in the presence of rotenone, a CI inhibitor (CI-IHR-OCR) demonstrating that respiration is mainly due to CI activity. C. Mitochondria membrane potential (ΔψM) for all strains is shown. Flow cytometric analysis of strains stained with JC-1 indicates a greater proportion of cells with non-polarized mitochondria from nuo1Δ and nuo2Δ mutants (upper left), reconstituted strains (upper right) and WT cells (lower right). The WT strain and reconstituted strains Nuo1R and Nuo2R display a typical fluorescent profile of high staining in the upper right quadrants (UR). Non-polarized cells fluoresce approximately 3–5 times more in both mutant cell populations than control strains. The relative proportions of mutant cells (UR, LR) are indicated in the table insert.
Likewise, nuo1Δ and nuo2Δ had reduced oxygen consumption rates (OCR) when compared to WT or to each gene-reconstituted strain (Fig. 1B). A statistically significant lower oxygen consumption rate (OCR) was noted with the nuo1Δ versus nuo2Δ (p<0.05). The OCR is ~ 36% and 53% for nuo1Δ and nuo2Δ mutants, respectively, compared to WT cells. The OCRs of the reconstituted strains Nuo1R and Nuo2R are 75% and 86% of WT levels. OCR was lowered substantially in all strains following treatment with the CI-inhibitor rotenone (Fig. 1B, CI-IHR-OCR), demonstrating that the reduction in oxygen consumption is associated with CI. The remaining oxygen consumption is probably due to other alternative NADH-Q oxidoreductases that are rotenone- insensitive.
Mitochondrial membrane potential is decreased in nuo1Δ and nuo2Δ
The measurement of mitochondrial membrane potential (Δψm) is a valuable indicator of the energy status of cells. Using the JC-1 fluorescent dye, we found higher numbers of nonpolarized cells in both nuo1Δ and nuo2Δ mutants, respectively (Fig. 1C, compare the null and gene reconstituted strains, upper 2 panels with WT cells, lower panel). The reduction in membrane potential (Δψm) in nuo2Δ was greater than in nuo1Δ (Fig. 1C, Table insert). The membrane potential of the gene reconstituted strains was higher than the null mutants. These data support a correlation of reduced growth with a reduction in membrane potential (ATP synthesis) and respiration.
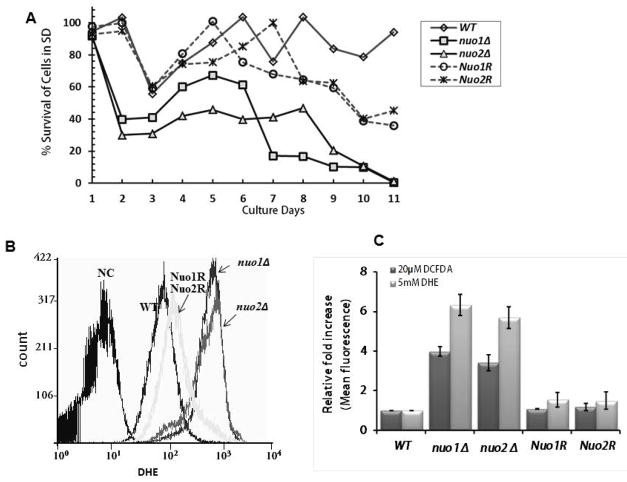
Chronological life span (CLS) is shorter in both mutants
To determine if CLS is affected in the null strains (nuo1Δ and nuo2Δ), growth in minimal medium plus amino acids and glucose was compared to WT and gene reconstituted strains. CLS is a measure of the length of time cells remain in stationary phase. Cells of each strain were collected daily for 9 days from SD medium supplemented with 2% glucose, standardized to equal cell numbers, and plated on YPD agar for 48 h to determine % survival. CLS was affected in both null strains, while the gene reconstituted strains were intermediate in CLS compared to WT cells (Fig. 2A). In comparing WT and reconstituted strains, both mutants had nearly similar percentages of surviving cells by the day 9. At day 2, % survival of nuo1Δ and nuo2Δ decreased to 30~40%. Following this sharp decrease, the survival rate of nuo1Δ increased to 60% from days 4–6 much like WT and reconstituted strains. But, survival decreased again to about 10% by day 9. On the other hand, the decrease in survival of nuo2Δ in % was was more gradual than nuo1Δ from days 4–8 (~ 42% –~ 46%). The slower decrease in viability of nuo2Δ may suggest that a difference of mitochondrial quality control or retrograde response between two null mutants. Likely, Nuo1p is engaged in such compensatory pathway due to imperfect of ETC and damaging ROS level.
Figure 2.
The chronological life span (CLS) of each strain measures the length of time when cells are viable in stationary phase. (A). C. albicans strains (OD600 of 0.2) were grown at 30°C in 100 ml of SD containing 2% glucose. From 1 to 9 day-old cultures, 1-ml of culture was removed, serially diluted, and spread on YPD agar to determine viability. The % survival are shown (CFU/primary inoculum) as: WT (SN250); nuo1Δ; nuo2Δ; Nuo1R; Nuo2R. (B) DHE staining was done to determine superoxide content. Both null mutants are strongly stained with DHE compared to WT cells; the corresponding reconstituted strains have slightly higher levels of superoxide than WT. (C) cellular peroxide of each strain is also estimated with DC-FDA. The relative fold-increase of fluorescence with both DHE and DC-FDA stains in the null mutants nuo1Δ and nuo2Δ was 6-fold higher in superoxide and 3-times higher in peroxide compared to control strains.
The longer generation times and decline in cell survival (CLS) of nuo1Δ and nuo2Δ are likely due to decreased CI activity and a loss in mitochondrial membrane potential so that cells produce less total ATP. Also, a consequence of reduced CI enzyme activity (see section below) is an increase in reactive oxidant species (ROS) that is known to contribute to a shortened CLS and a decrease in cell viability (Chen et al., 2012). As we expected, the reduction in CLS in both mutants is accompanied by an increase in ROS (Fig. 2B). Mitochondria are potent producers of cellular superoxide then was detected by DHE in this study. Meanwhile the cellular peroxide (H2O2) was measured by DC-FDA fluorescent dye. As Figure 2B,C shown, both superoxide and peroxide are increased significantly in null mutants when compared to each control strain. We found the superoxide levels in nuo1Δ and nuo2Δ are 6.9-fold and 6.1-fold higher than WT, indicating that electron leakage occurred in the null mutants. At same time, the cellular peroxide levels were > 3-fold higher in both mutants compared to reconstituted strains Nuo1R, Nuo2R and WT with DC-FDA measurement. The ROS levels of nuo1Δ and nuo2Δ mutants in fact are lower than goa1Δ described previously (Li et al, 2011), in which 7-fold higher ROS was observed by DC-FDA fluorescent that corresponded prior to death of cells at day 7. Nevertheless, these data correlate with a greater loss in CLS with ETC mutants.
CI enzyme activity is reduced in both nuo1Δ and nuo2Δ
Mitochondrial NADH dehydrogenase activity was also evaluated in both CI mutant strains and ndh51Δ. As shown in Table 1, CI activity in nuo1Δ and nuo2Δ mutants was reduced by 62 and 55%, respectively, while CI enzyme activity was reduced by 87% in the ndh51Δ compared to WT. The large loss of CI activity in ndh51Δ is probably related to the fact that the Ndh51p subunit is a flavoprotein and responsible for NADH binding to CI (McDonough et al., 2002).
Table 1.
Mitochondrial ETC CI, CII, and CIV enzyme activities
| Enzyme activity (nM/min/mg protein) | |||
|---|---|---|---|
| CI | CII | CIV | |
| WT | 828.68 ± 24.68 | 516.15 ± 55.84 | 301.80 ± 30.45 |
| nuo1Δ | 317.63 ± 15.97* | 420.31 ± 21.49 | 70.80 ± 35.97* |
| nuo2Δ | 375.78 ± 27.41* | 522.84 ± 19.50 | 243.47 ± 26.49 |
| ndh51Δ | 109.15 ± 5.05* | 127.43 ± 49.67* | 155.96 ± 22.95* |
: p<0.001, mutant versus the WT
By contrast, CII activities of nuo1Δ and nuo2Δ mutants were similar to that of WT cells (Table 1), but higher than ndh51Δ cells (succinate oxidation), suggesting a major role for Ndh51p in CII activity. These data indicate that NUO1, NUO2 and NDH51 are all critical for CI activity and that CII enzyme activity may require Ndh51p as well. CIV activity is much lower in nuo1Δ (23% of WT cells) but higher in nuo2Δ (80% of WT cells, while about 50% of control in the ndh51Δ. Likely, NUO1 is also associated with maintenance of CIV enzymatic activity.
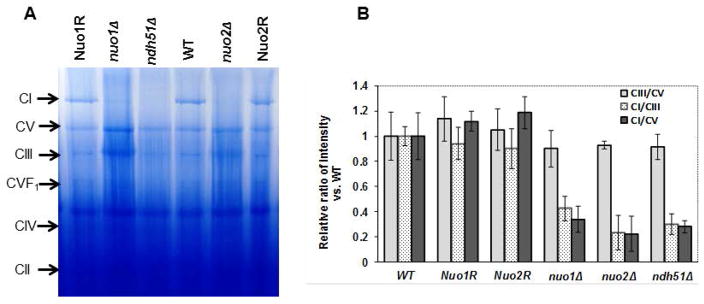
Defects in CI assembly in the absence of CI subunits and Goa1
In order to understand how NUO1 and NUO2 affect CI activity, the ETC complexes of mitochondrial extracts were electrophoresed in blue native polyacrylamide gels (BN-PAGE). The CI protein (~1MD molecular mass) is absent or minimally apparent in nuo1Δ, nuo2Δ, and ndh51Δ compared to WT, Nuo1R, and Nuo2R (Fig. 3A). CIII and CV are largely unaffected in each mutant. This observation may point to a role of both genes in stability or assembly of the CI complex.
Figure 3.
(A) BN-PAGE electrophoresis of C. albicans mitochondrial OXPHOS complexes in WT, nuo1Δ, nuo2Δ, and ndh51Δ, as well as the gene reconstituted strains. Equal concentrations of total mitochondrial proteins from all strains were separated by BN-PAGE. The CI band is markedly reduced in each of the three CI mutants compared to levels in the matched set of control strains, WT and reconstituted strains. (B) The stainable amount of CI from each strain is expressed as relative ratios of the intensity of CI/CIII and CI/CV in each strain versus to WT. Levels of CIII/ CV are minimally changed in all mutants but CI/CIII and CI/CIV are considerably reduced (intensities) suggesting less CI in all null strains.
To quantify the loss of CI in each mutant, we also measured the gel density of CI for each strain by using ratios of CI/CIII and CI/CV since CIII and CV are largely unaffected in each mutant (Fig. 3B). We found that the CI of nuo1Δ is reduced by ~60 to 70% compared to WT or Nuo1R cells when data are expressed as gel density ratios of CI/CIII and CI/CV. Similar to nuo1Δ, a reduction of CI subunit is also found in nuo2Δ. An ~80% reduction of CI in this mutant is seen using both CI/CIII and CI/CV ratios, and a 75% reduction of CI staining is apparent in the ndh51Δ. The Ndh51p is one of core members of CI that is responsible NADH binding and oxidation as stated above. These results suggest that loss of CI enzyme activity in all mutants may reflect a disassembly or instability of CI, similar to published data with GOA1 (Li et al., 2011).
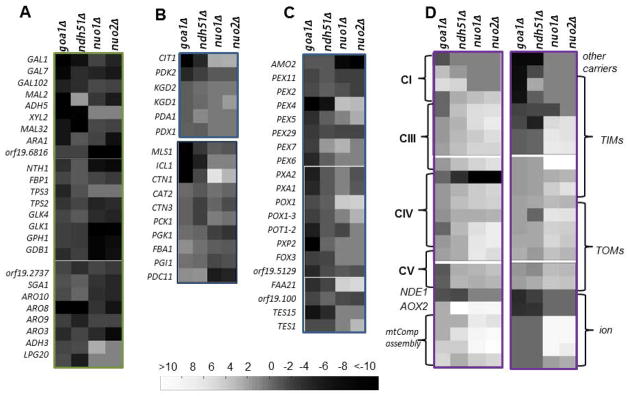
Transcriptional changes in nuo1Δ, nuo2Δ, ndh51Δ, and goa1Δ
Transcriptional profiles of each mutant were compared to the corresponding reconstituted and WT strains. A total of 586 genes are down-regulated, and 808 genes are up-regulated in both nuo1Δ and nuo2Δ mutants. The overall transcriptional changes are similar for nuo1Δ and nuo2Δ in regard to metabolic pathways, ribosomal biogenesis, signal transduction, oxidative responses, and DNA repair mechanisms. The similarities of nuo1Δ and nuo2Δ may suggest redundant roles for both genes. Transcriptional profiling of genes with changes 3-fold greater or less that are involved in carbon metabolic pathways is indicated in Fig. 4. For broad comparisons, we have also included the transcription profiles of ndh51Δ and goa1Δ. Since Nuo1p, Nuo2p, and Ndp51p are CI subunits, we focused on genes, among others, that are related to carbon metabolism and mitochondria functions (Fig. 4).
Figure 4.
Transcriptional profiling of carbon metabolism and mitochondria in mutants goa1Δ, ndh51Δ, nuo1Δ, and nuo2Δ. Up and down regulated genes of mutants are compared to WT cells. Down regulated and up regulated genes are coded from black to white as indicated. Four functional gene groups are shown: A. glycolysis, glycogen metabolism, non-glucose metabolism, and the Ehrlich Pathway of which amino acids are used as carbon sources; B. Mitochondrial TCA and peroxisomal glyoxylate cycle and gluconeogenesis; C. Peroxisomal transporters, β-oxidation; D. Mitochondria CI-CV complexes, transporters of inner membranes (TIMs) and transporters of outer membrane (TOMs) of mitochondria; mitochondrial ETC assembly, and ion channels (magnesium, phosphate). General observations are that: 1) each of the 4 mutants down regulate numerous genes of carbon metabolism (A), 2) goa1Δ is especially affected in genes in categories B, C and D, that includes the glyoxylate cycle (carbon conservation), peroxisomal transporters (PEX genes), and TIM/TOM genes, 3) nuo1Δ and nuo2Δ exhibit many ETC Complex and TIM/TOM genes that are upregulated (D), and 4) peroxisomal genes are less associated with nuo1Δ and nuo2Δ (C).
In regard to the carbon metabolism, a large number of hexose transporters including glucose (HGTs) and other sugar transporters (HXTs) as well as genes of the major facilitator superfamily are significantly down-regulated in both nuo1Δ and nuo2Δ when compared to WT (-3.4 to -45.8-fold) (Supplementary Table S2 and Fig. 4A). Transcriptional levels of glucokinase GLK1 and GLK4 decreased by −7.0 to −9.2-fold; genes of alternative carbon pathways are also down regulated, for example, GALs (galactose utilization, −3.5 to −5.8- fold) and glycogen catabolic processes (GPH1, −6.3 to −8.3-fold, Fig. 4A,C and Supplementary Table S2). Gene changes associated with carbon metabolism are similar to those of goa1Δ. In addition, several ARO genes (ARO3, ARO8, ARO10) are also decreased significantly in both CI nuo mutants, similar to goa1Δ and ndh51Δ. These genes encode aromatic decarboxylases of the Ehrlich fusel oil pathway which leads to the formation of aromatic alcohol and products of central carbon metabolism. These results suggest that NUO1 and NUO2 may be associated directly or indirectly with non-glucose carbon metabolism.
In goa1Δ, genes encoding β-oxidation of fatty acids and other peroxisomal activities are significantly down regulated (Fig. 4,B,C). For example, genes of the TCA cycle (CIT1, PDK2), fatty acid transporters of peroxisomes (PEXs) for fatty acid metabolism (POX, POT, PXP), and the glyoxylate cycle (ICL1 and MLS1) are down regulated. However, these genes are not changed or have minor changes in transcriptional levels in both nuo1Δ and nuo2Δ (Figure 4,B,C). Instead, gluconeogenesis is decreased in both NUO mutants more than goa1Δ, as demonstrated by PDC11 (−5.8 fold), PGI11 (−7.11 to −7.55-fold) and FBA1 (−2.79 to −2.92-fold) but not in goa1Δ and ndh51Δ. The products of these genes are the key enzymes to direct three irreversible steps of gluconeogenesis. As NUO1 and NUO2 have less influence on peroxisomal functions, GOA1 may provide functions related to the integrity of both mitochondria and peroxisomes.
In regard to mitochondrial function, we found that the gene changes are very different in nuo1Δ and nuo2Δ from goa1Δ except for mitochondrial rRNA biogenesis and protein translation (Fig. 4D and Supplementary Table S2). In the absence of NUO1 and NUO2, the genes encoding mitochondrial ETC proteins of CI, CIII, CIV and CV subunit proteins, ETC protein complex assembly, transporters of several intermembrane (TIMs) proteins are up-regulated, opposite that of goa1Δ. Upregulation of mitochondrial rRNA biogenesis is consistence with cytoplasmic rRNA machinery shown in all four mutants and suggests a likely universal regulatory network to govern the ribosome biogenesis system based on cell energetic status (data not shown). Down regulation of several CI encoding genes occurred in the goa1Δ only (Fig. 4D).
Genes encoding cell wall synthases and cell surface proteins in nuo1Δ and nuo2Δ differ from the ndh51Δ
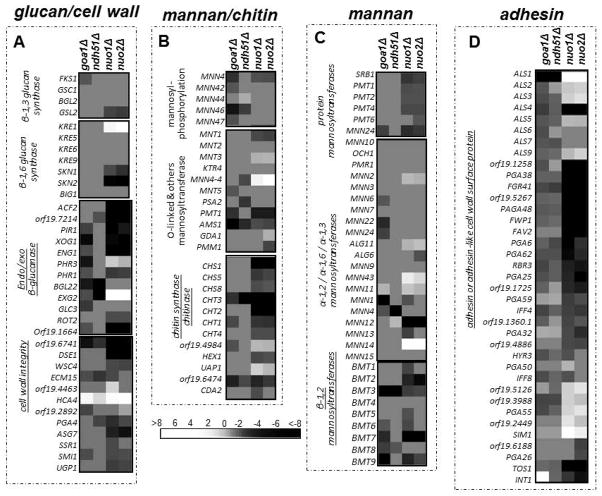
We note that gene changes in several categories of cell wall mannosyltransferases, chitin syntheses and chitinases, exo/endo glucanases, and adhesins/cell surface proteins are much more highly represented in nuo1Δ and nuo2Δ than in the ndh51Δ subunit mutant (Fig. 5, A–D). As many as 15 adhesin-encoding genes are down regulated in nuo1Δ and nuo2Δ (Fig. 5D). Especially down regulated genes among nuo1Δ and nuo2Δ are adhesins and other cell wall surface proteins. These changes are not seen in ndh51Δ.
Figure 5.
Transcriptional profiling of genes encoding cell wall functions in mutants. Functional annotation for each gene set is indicated (A–D) and displays of upregulated versus down regulated genes are coded in white (up) to black (down). Differences are observed especially among the gene groups that are underlined. Gene changes are similar in the nuo1Δ and nuo2Δ but strikingly different compared to ndh51Δ.
Deletion of NUO1 and NUO2 results in avirulence and reduced fungal growth in a mouse model of systemic candidiasis
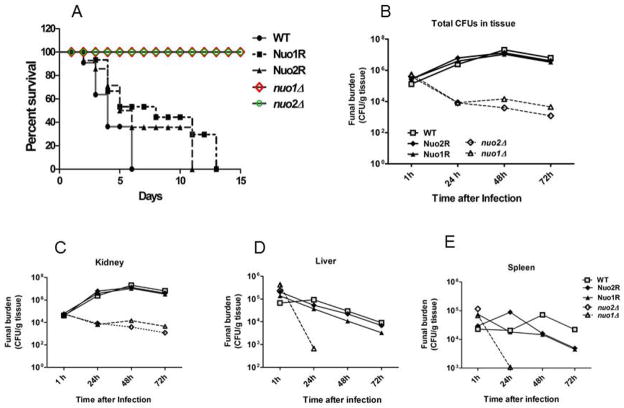
C. albicans mutants with slow growth in vitro usually have defects in virulence but, not always (Noble et al., 2010). To address the role of these genes in causing candidiasis, we used a murine, blood-borne invasive candidiasis model. Experiments were done to measure survival of mice and tissue loads of each strain. Data represent two biological replicates. Both nuo1Δ and nuo2Δ are avirulent (Fig. 6A). A decrease in survival in mice infected with WT cells of C. albicans began on day 2; all mice were moribund by day 6. For the gene reconstituted strains, partial virulence was seen by day 6 (40–55 % mortality) and all mice infected with Nuo1R and Nuo2R inoculation were moribund by day11 and 13, respectively. On the other hand, mice survived infection when infected with nuo1Δ and nuo2Δ (Fig. 6A). These data indicate that both genes are required for C. albicans virulence in vivo.
Figure 6.
A. Mouse survival (female BALB/c, 20 g, 10 mice/group) following infection with 1 × 106 yeast cells of the C. albicans. WT (solid line), the one-allele reconstituted strain Nuo1R and Nuo2R) (dashed lines). The colored lines indicate each mutant strain, Δnuo1 (red); Δnuo2 (green). Both nuo1Δ and nuo2Δ are avirulent, while the Nuo1R and Nuo2R are intermediate in virulence compared to the fully virulent WT cells. B–E. Tissue counts (CFU/g tissue) of mice infected with null mutants as well as WT and gene reconstituted strains. Following infection, at 1, 24, 48 and 72 h, kidney, liver and spleens were removed, weighed, homogenized and samples plated on YPD agar medium. The total CFUs in tissue for each strain are shown over time. Data are from two experiments with 3 mice per time point for each strain (mean ± SD). The CFU/gram of tissue of nuo1Δ and nuo2Δ mutants is 2–3 logs less than control strains from infected kidneys. Both mutants were also readily cleared from liver and spleen; no fungi were recovered from both of organs by 24–48h. Both of mutants are indicated as dashed lines. B–E. WT strain and reconstituted strains Nuo1R and Nuo2R tissue counts are represented as CFUs/g tissue. B. all tissues; C, Kidney; D. Liver; E. Spleen.
To correlate the avirulence of null mutants with their growth in tissues, the fungal load (CFU/g tissue) was measured from kidney, spleen, and liver of infected mice at 1,24,48, and 72 h post-infection. After 1 h, tissue loads were the same for all strains (Fig. 6, B–E). Compared to the high tissue load (3-log10) in WT, Nuo1R and Nuo2R at 48 h post infection, colonization of both mutants was reduced by at least 2 logs10 in all tissues (Fig. 6B). The difference in colonization among organs is seen in all strains during disease, but fungal clearance is more affected in nuo1Δ and nuo2Δ infected mice. CFU in kidneys decreased 2-logs by 48 h and 3-logs by 72 h and the rapid clearance is even more apparent in spleen and liver. By 24 h, mutant strains are cleared totally or have scant growth (Fig. 6). These results imply that each CI I subunit is required by C. albicans for colonization and tissue invasion.
Discussion
Complex I (CI) of eukaryotes
CI of the mitochondrial electron complex chain is a slightly open L-shaped protein, with a hydrophobic “arm” that functions as a proton pump. This arm is located in the mitochondrial inner membrane (P module), and a hydrophilic arm extends into the mitochondrial matrix and is composed of an N module for binding and oxidation of NADH and a Q module which transfers electrons to ubiquinone (Pereira et al., 2013). The mammalian CI contains hydrophobic core subunits of prokaryotic origin that are conserved in all species. This core consists of 14 subunit proteins which include seven hydrophobic subunits (encoded by the mitochondrial genome for proton translocation through the inner membrane arm) and seven other subunits that ligate either FMN to catalyze NADH oxidation or iron-sulfur clusters to transfer electrons from the flavin to the ubiquinone. Besides these 14 core subunits, eukaryotes possess a number of supernumerary protein subunits which are encoded by nuclear or mitochondrial genes. For example, 31 proteins have been described in human CI, of which 21 are thought to be ancestral eukaryotic core subunits (Gabaldon et al., 2015; Kuffner et al., 1998). The majority of subunits in the eukaryotic core group are conserved in fungi such as C. albicans and N. crassa.
The number of accessory subunits varies among fungi, plants and animals. For example, CAI84 is found in C. parapsilosis but not in C. albicans. In comparison to the human mitochondrial CI, C. albicans lacks 10 subunits (NDUFA4, FA7, FA10, NDUFB1, B2, B5, B6, NDUFC1, FC2 and NDUFV3), but on the other hand has 2 fungal-specific subunits NUXM (NUO1) and NUZM (NUO2) and also may have one additional accessory protein (ACPM) (Gabaldon et al., 2015). As a result, we conclude that the mitochondrial CI of C. albicans is composed of at least 39 subunit proteins (Supplementary Table S3). To date, the function of most of these accessory subunits is not well defined in every species, even though many human diseases have been associated with some CI deficiencies (Mimaki et al., 2012).
The assembly of CI has been investigated in N. crassa (Pereira et al., 2013; Kuffner et al., 1998), and at least two findings of CI assembly are thought to explain the inability of cells to form a complete CI. First, mutations in subunits of the matrix arm (modules N and Q) lead to a disassembly of CI and an accumulation of membrane arm (P module) fragments (Mimaki et al., 2012). Second, accessory assembly factors have been suggested, in which NDUFAF2 appears to connect P and Q modules (Pereira et al., 2013; Kuffner et al., 1998). The assembly of the C. albicans CI has not been studied, but our data suggest that gene deletions causing a loss of CI subunit proteins (Nuo1p, Nuo2p, Ndh51p) result in an almost complete loss of the CI protein (Fig 3). Further, the goa1Δ, an unlikely CI subunit, also does not assemble a CI. Perhaps Goa1p is an accessory protein for CI structural integrity or stability in C. albicans as well (Li et al, 2011).
Mitochondria in model yeast and C. albicans
The model yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe use a single enzyme for mitochondrial CI activity (Gabaldon et al., 2005; Mimaki et al., 2012). In addition to not having a CI, S. cerevisiae lacks an alternative respiratory pathway (AOX) (Helmerhorst et al., 2005). Further, S. cerevisiae is Crabtree negative, fermenting glucose and repressing mitochondrial functions when oxygen is available (Shingu-Vazquez and Traven, 2011). In contrast, C. albicans is Crabtree negative and mitochondrial respiration is active when optimal glucose is present (Shingu-Vazquez and Traven, 2011). The contrast in glucose utilization is also associated with oxidative stress resistance, being higher in C. albicans compared to yeast (Rodacky et al., 2009). New data suggest that C. albicans acts differently from S. cerevisiae in carbon catabolite control (Sandai et al., 2014). The premise of this data is that alternative carbon assimilation in C. albicans continues even after a preferred source (glucose) is available, which allows this pathogen to adapt to a host niche where carbon availability changes over time. This adaptation would require metabolic pathways such as the glyoxylate cycle, gluconeogenesis, and fatty acid degradation, each affected by loss of CI.
Virulence and a functional CI
Our current study supports the idea of others that a loss of mitochondrial function causes reduced virulence or biofilm formation in C. albicans (Table 2) (Bambach et al., 2009; Shingu-Vazquez and Traven, 2011; Qu et al., 2012; Noble et al., 2010; Becker et al., 2010; Marchais et al., 2005). The putative or characterized functional assignments of genes that are essential for virulence encode CI subunits, mitochondrial DNA repair, ribosomal activities, cell integrity, and an apparent regulator of CI (Goa1p) that may be required for complex assembly or stabilization, as described above. The orf/genes listed in Table 2 represent functional annotation derived from phenotypes of knockout (null) mutants, or by strain fitness in repressible conditional mutants. Considering the lack of virulence of these mutants and their fungal-specific origins, we suggest they may have high potential as antifungal drug targets. Inactivating mitochondria, as has been reported in at least mutants of GOA1 and SAM37, has profound crippling effects on fungal cell functions (Bambach et al., 2009; Shingu-Vazquez and Traven, 2011).
Table 2.
C. albicans null or conditional mutants of mitochondrial genes with reported avirulence or decreased virulence phenotypes.
| Orf/gene | Gene functiona | Other functionsb | Reference |
|---|---|---|---|
| Orf19.2019* | Putative Mt- Large ribosomal subunit | Protein maturation & Mt-translation, S. cerevisiae MRPL16 | Becker et al., 2010 |
| Orf19.2956* | Putative Mt – genome maintenance | Recombinational repair | Becker et al., 2010 |
| Orf19.7019 | Mt-ribosomal protein | Induced upon adherence to polystyrene | Becker et al., 2010, Marchais et al, 2005 |
| GOA1** | Putative Regulator of Mt-CI | Cross-talking with peroxisomes, cell wall synthesis, Chronological life span (CLS)*** | Bambach et al., 2009 |
| Orf19.6607* Orf 19.287 |
CI subunit | Optimal growth | Noble et al., 2010; this paper |
| SAM37* | Sorting and Assembly; Machinery, Mt-outer membrane | Avirulent; fitness, cell wall integrity | Shingu-Vazquez et al., 2011; Qu et al., 2012 |
gene function for mitochondria;
functions other than mitochondria.
Fungal-specific;
CTG clade of Saccharomycotina (subphylum of Ascomcetes); includes most Candida species except C. glabrata.
CLS, chronological life span.
A comparison of transcriptional profiling of all CI mutants
We compared all mutants by microarray analysis. All mutants had significant down regulation of carbon metabolism genes (Fig. 4). In most of the transcriptional profiling of gene families, such as mitochondrial membrane transporters (TIM/TOMS) and gluconeogenesis, nuo1Δ and nuo2Δ clustered together, implying some redundancy in direct or indirect functions. In contrast, down regulation of peroxisomal genes of the glyoxylate cycle, β-oxidation, and peroxisomal transporters overlapped substantially in goa1Δ and ndh51Δ. Diversity among mutants was especially prominent among genes encoding cell wall functions. Here, nuo1Δ, nuo2Δ and goa1Δ were more similar in their profiles than ndh51Δ (Fig. 5). These data may imply additional functions (besides CI enzyme activity) associated with NUO1 and NUO2 compared to NDH51. It would seem, therefore, that the fungal-specific subunits of C. albicans are functionally more diverse than the conserved Ndh51p in driving cell activities.
Mitochondrial therapeutics
Mitochondria are at the forefront of therapeutics against cancers and even neurological diseases (Mimaki et al., 2012; Sarosiek et al., 2013; Theodosakis et al., 2014; Sakhrani and Padh, 2013; Swerdlow et al., 2013). Anticancer approaches to target mitochondria include apoptosis induction in tumor cells as well as remodeling cellular energetic status via changes in respiration and metabolic pathways. Cancer cells exhibit a Crabtree-negative, fermentative metabolism (glycolysis) even in the presence of abundant oxygen, like S. cerevisiae (Shingu-Vazquez and Traven, 2011; Mimaki et al., 2012). Reversing this activity is one of several scenarios of metabolic reprogramming in cancer cells. Consequently, therapies are sought against glycolytic enzymes such as PKM2 which is elevated in tumor cells. Other inhibitors of lactate production show promise (Sakhrani and Padh, 2013). Targeting the BCL-2 family of pro-and anti-apoptotic proteins of cancer organelles (like mitochondria) has been developed to induce different levels of mitochondria apoptotic priming in cancers (Phan et al., 2014; Sakhrani and Padh, 2013). Delivery of chemotherapeutics directly to “cancer programmed” cells is underway in drug discovery (Swerdlow et al., 2013). The role of mitochondria in therapy against melanomas is being defined; one approach is the reversal of glycolysis as described above (Theodosakis et al., 2014).
New antifungal therapies against new targets seem to be a useful pursuit due to the limitations of current antifungals. Given the availability of many extensive compound libraries, it would be useful to screen repurposed inhibitors against select reduced fitness mutants, such as those discussed in this paper. Such an approach is ongoing in several labs including ours, summarized in (Calderone et al, 2014). It follows that compounds that target specific mitochondrial proteins should extensively disrupt cell metabolism and growth. In this regard, a recent observation is that cell division is dependent upon mitochondrial protein transport and energy production (Harbauer et al., 2014).
Experimental procedures
Strains and Chemicals
All strains used in this study are listed in Supplemental Table S1. The homozygous mutants of C. albicans mitochondrial null mutants orf19.6607 and orf19.287 were originally described by Noble et al., 2010. The gene reconstituted strains of each mutant Nuo1R (nuo1Δ/NUO1) and Nuo2R (nuo2Δ (nuo2Δ/NUO2) were constructed in our lab by introducing one allele of each gene into the corresponding mutant strain lacking the ARG4. Cassettes for each transformation were constructed in plasmid pSN69 (Arg4+) by insertion of two PCR products flanking ARG4. The primers used to amplify the flanking sequences are listed in Supplemental Table S1. At the 5′-end, fragment A corresponds to the full length gene ORF plus ~1.5kb upstream sequence of the ATG codon. At the 3′-end, fragment B is a ~1.0 kb homologous fragment from the stop codon of each gene. Arg4+ transformants were selected on yeast nitrogen base-minimal medium lacking arginine but containing histidine. The integration of the transforming DNA was confirmed by PCR analysis of 10 transformants. Phenotypes of null and reconstituted strains (Nuo1R and Nuo2R) were compared to the parental strain WT (SN250) (Arg−His−Leu−). In addition, ndh51Δ (JM02) and its reconstituted strain Ndh51R (JM03) were used in some experiments for comparison to the mutants described above (Velluci et al, 2007). Ndh51p is a CI subunit protein but not fungal-specific.
The fluorescent dye JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbo-cyanine iodide) (Perelman et al., 2012; Gallagher, 2010) was obtained from Invitrogen, Inc. The other fluorescent dyes DHE (dihydroethidium) and DCFDA (2′,7′-dichlorofluorescein diacetate) were purchased from Santa Cruz Biotechnology and Sigma-Aldrich respectively. All the reagents for mitochondrial function studies were acquired from Sigma-Aldrich, Inc. Precast BN-PAGE gradient gels (4 to 16%) and a protein ladder were purchased from Invitrogen, Inc.
Growth in non-glucose media and chronological life span (CLS) determinations
All strains were grown at 30°C in YPD with 2% glucose, or YP medium supplemented with 2% glycerol (YPG) or 3% oleic acid as carbon sources. For CLS experiments, minimal medium (SD) with 2% glucose and 0.67% yeast nitrogen base supplemented with amino acids was used. All strains were routinely grown in 5 ml of YPD broth (Difco Laboratories) at 30°C overnight, washed in phosphate buffered saline (PBS) and then -inoculated in 100 ml of SD with an OD600 of 0.2 at day 0. Shake cultures were grown at 30°C (200rpm), and one- ml samples were then removed from each strain daily at 1–9 days. Each aliquot of 400–600 cells was transferred to two YPD agar plates. Colony counts (% survival) were determined after a 48 h incubation. Data are indicated as % survival compared to the original inocula (Chen et al., 2012).
Oxygen consumption, membrane polarization and ROS measurements
Oxygen consumption was measured polarographically using a Hansatech Oxygraph, (Model 910) Cells of each strain were collected from exponential phase growth (3 to 4 h) in YPD broth. The oxygen consumption rate (OCR) was determined at 0–5 min from 2.5 × 108 cells suspended in 1 ml of YPD broth (Li et al., 2011). For determinations of mitochondrial membrane potential, the cyanine dye JC-1 was used (Li et al., 2011; Hao et al 2013; Ding et al, 2014). Two forms of JC-1 (green fluorescence and red fluorescence) are used to discriminate energized and non-energized mitochondria since the red fluorescent aggregation in mitochondrial matrix is depend on the mitochondrial membrane potential. All strains were grown in YPD for 8h at 30°C, washed, and suspended in 1 ml of PBS then incubated with 5 μM JC-1 in 2% DMSO for 30 min at 30°C in the dark. The emission spectra at 488 nm and excitation spectra at 595 nm were determined by FACScan flow cytometer (Becton Dickinson).
Two oxidation-sensitive fluorescent dyes commonly are used for detecting cellular H2O2 (dihydrofluorescein diacetate, DCFDA) or superoxide anion (dihydroethidium, DHE) respectively. All yeast cultures were collected at 24 h after inoculation for ROS measurement of each strain and washed once before staining with PBS (pH 7.2). For determination of superoxide, cells (5 × 106) of each strain in 1-ml of PBS were stained with 5mM DHE in PBS (pH 7.2) at 30°C for 15 min and washed with PBS once prior to measurements by FACScan (excitation at 488 and emission at 567 nm). The same number of yeast cells was stained with 20 μM DCFDA at 30°C for 15 min for measuring intracellular ROS (excitation at 488 and emission at 535 nm). PI (to 10 μg/ml) was added 1 min before flow cytometry (Li et al., 2011). ROS levels were determined from triplicate samples of each strain.
Extraction of mitochondrial protein; Complex I, II and CIV enzyme assays
Extraction of mitochondrial proteins was done as previously described (Li et al., 2011; Barrientos, 2002). In brief, one liter of the overnight grown cells was collected, washed, and converted to spheroplasts with 100 mg of Zymolyase 20T (Seikagaku Biobusiness, Inc.) in 30 ml of 1 M sorbitol-Tris buffer containing 1 mM DTT. Spheroplasts lysis was carried out by sonication, and mitochondria were obtained using a two-step Percol gradient. 30% Percol in buffer PB1 (0.3 M mannitol, 10 mM TES, 0.1% BSA, pH 7.5) was placed at the bottom of a centrifugation tube and an equal volume of 20% Percol in PB2 buffer (0.3 M sucrose, 10 mM TES, 0.1% BSA, pH 7.5) was layered on top of the PB1 buffer. Crude mitochondria were then overlaid at the top of a centrifuge tube before centrifugation at 40,000 × g for 45 min at 4°C. Purified mitochondria (whitish band) were collected and extracted with10% w/v N-dodecyl β-D maltoside.
Enzyme activities of the mitochondrial electron transport chain (ETC) complexes CI, CII and CIV were measured spectrophotometrically in 96-well plates (Li et al., 2011). The oxidation rates of CI or CII, and reduced cytochrome C (CIV) substrates were monitored by plotting changes in OD values per min to determine mitochondrial complex activities (see below).
CI assay
The reduction of the electron acceptor DCIP (2,6-dichloroindophenol) was monitored at OD600 in a 200 μl volume containing 25 mM potassium phosphate (pH 7.4), 0.35% BSA, 70 μM decylubiquinone, 60 μM DCIP and 4 μM antimycin A (Li et al., 2011). 10 ~ 50 μg purified mitochondrial protein was first pre-incubated with 195 μl of the reaction aliquot at 37°C for 10 min. The decrease in absorbance at OD600 was measured at 30 sec intervals for 5 min at 37°C immediately after the addition of 5 μl of 10 mM NADH. Assays were also measured in the presence of 1mM rotenone CI inhibitor following an additional 5 min incubation.
CII assay
DCIP was again used as an electron acceptor, and the substrate for the CII assay was succinate. 200 μl of incubation buffer containing 80 mM potassium phosphate (pH 7.5), 0.1% BSA, 2 mM EDTA, 0.2 mM ATP, 50 μM decylubiquinone, and 80 μM DCIP was used for enzyme reactions. 10 ~ 50 μg of purified mitochondrial protein was added to the reaction buffer at 37°C for 10 min in 96-well plates without substrate. After 10 min, 0.3 mM KCN and 10 mM succinate (5 μl volume) was added to each assay reaction. The absorbance at OD600 was measured at 1 min intervals for 5 min at 37°C. The specificity of CII activity was verified in the presence of 5 mM malonate for an additional 5 min.
Complex IV
Activity was determined at OD550 by measuring a decrease in absorbance during the oxidation of reduced cytochrome C (cytC). In our previous protocol, the reduced form of cytC was obtained by treatment with ascorbic acid followed by a tedious dialysis process (Li et al., 2011). In the current CIV assay, we used 0.1 mM DTT to reduce cytC which is measured as a color change from dark orange-red to pale purple-red after a 15 min treatment at RT. 10 ~ 50 μg of purified mitochondrial protein was dissolved in 190 μl of reaction buffer in 96-well plates containing 10 mM potassium phosphate (pH 6.5), 0.25 M sucrose, 0.1% BSA, 2 mM EDTA at 37°C, 10 min. The reaction was measured after the addition of 5 μl of 1 mM lauryl maltoside and 10 μM of reduced cytC in 5 μl.
Blue native polyacrylamide gel (BN-PAGE) electrophoresis
Mitochondrial protein was prepared as described previously (Li et al., 2011). Prior to loading mitochondrial proteins onto a BN-PAGE gradient gel (4 to 16%), the protein samples derived from mitochondria of each C. albicans strain were concentrated and solubilized in 10% DDM (n-dodecyl-β-D-maltoside). A total of 20 μl of each sample (~100 to 150 μg of protein) was mixed with BN sample buffer (2X). Electrophoresis was performed in an X-Cell SureLock mini-cell system (Invitrogen) with 200 ml of cathode buffer in the upper (inner) buffer chamber and 150 ml of anode buffer in the lower (outer) buffer chamber. The cathode buffer contains 0.02% Serva Blue G-250, supplemented with 0.02% DDM, and the anode buffer consisted of 50 mM bis-Tris, pH 7.0. Electrophoresis was carried out with 65 V for 1 h and then raised to 120 V overnight at 4°C. The blue gel was decolorized using methanol: acetic acid: water (50 : 10 : 40 v/v/v), with shaking, until the appearance of blue bands was noted. Image intensity was measured using ImageJ software (Li et al., 2011). All strains were evaluated for the reduction or loss of ETC complexes.
RNA and microarray analysis
The contribution of mitochondrial CI NUO1 and NUO2 to transcriptional events was determined. RNA isolation and microarray experiments were done as described previously (Li et al, 2011; Sun et al., 13, She et al., 2013; Khamooshi et al., 2014). All strains were grown in 20-ml of 2% SD medium at 30°C for either 5 h for WT and reconstituted strains, or 8 h for both mutants. One-color microarray-based gene expression analysis was done using the Agilent format chips containing 6,101 C. albicans genes including nuclear and 12 mitochondrial-encoded genes all in duplicate. Hybridization was completed in an Agilent SureHyb hybridization chamber, and scans were processed with an Agilent SCAN G2505C Microarray Scanner System. The image files were analyzed by Agilent Feature Extraction Software, and cyanine 3 intensities were then logarithmically transformed and normalized. The fold-change for each gene in duplicate was calculated by comparisons to WT and the corresponding gene reconstituted strains. A fold-change of ≥ 3 was used as a cut off value for determining significant changes.
Murine model of blood-borne disseminated candidiasis
A mouse model of disseminated candidiasis was used in order to measure the virulence of the mitochondrial mutants (Lionakis et al., 2011). Virulence of each null strain was compared to the corresponding gene reconstituted (Nuo1R, Nuo2R) and WT strains in 6–8 week old female BALB/c mice from Yangzhou University (China). Tissue levels of strains were measured as CFU/g in kidney, liver and spleen. The animal experiments were done under the guidance of a protocol approved by the Animal Study Committee of the Institute of Dermatology, CAMS, according to the National Guidelines for Animal Care. Mice were injected via the lateral tail vein with 1 × 106 yeasts of C. albicans WT, mutant strains nuo1Δ and nuo2Δ, and the gene reconstituted strains. All strains were grown in YPD overnight, harvested, washed and suspended in PBS (pH 7.5) prior to injection into mice. For survival experiments, each C. albicans strain was used to inoculate 10 mice. Mice were randomly assigned to groups for each strain (WT, nuo1Δ, nuo2Δ, Nuo1R and Nuo2R) and given food and water ad libitum. We observed mice 2-times per day to determine those that were moribund. Concomitantly, 12 mice were also inoculated with each strain for the determination of fungal burden. Three mice from each group were euthanized after 1, 24, 48, and 72 h post-infection. Kidney, spleen, and liver were removed, weighed, and homogenized in PBS. Following serial dilutions, tissue homogenates were plated in duplicate on YPD agar plates containing penicillin and streptomycin to quantitate the CFU (colony forming units) per gram of tissue after 48 h of incubation at 30°C.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the Georgetown University Medical Center Lombardi Cancer Center for their help with the microarray analysis. The GUMC Biomedical Graduate Research Organization generously provided bridge funds. We are also wish to thank the National Key Basic Research Program of China for funds (2013CB531605). Funds were also provided by the NIH-NIAID, AI090290 to RC. We are indebted to Dr. Suzanne Noble for providing the CI null mutants and Dr. Margaret Hostetter for providing the ndh51Δ and gene reconstituted strains.
Footnotes
Competing interests.
The authors have no conflict of interests.
References
- Abdrakhmanova A, Zickermann V, Bostina M, Radermacher M, Schagger H, Brandt U. Subunit composition of mitochondrial complex I from the yeast Yarrowia lipolytica. Biochim Piophys Acta. 2004;1658:148–156. doi: 10.1016/j.bbabio.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Askew C, Sellam A, Epp E, Hogues H, Mullick A, Nantel A, Whiteway M. Transcriptional regulation of central metabolic metabolism in a fungal pathogen. PLoS Pathogens. 2009;3:e1000329. doi: 10.1371/journal.ppat.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo J, Nehls U, Eckerskorn C, Heinrich H, Rothe H, Weiss H, Werner S. Primary structure and mitochondrial import in vitro of the 20.9 kDa subunit of complex I from Neurospora crassa. Biochem J. 1992;288:29–34. doi: 10.1042/bj2880029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambach A, Fernandes M, Ghosh A, Kruppa M, Alex D, Li D, et al. Goa1p of Candida albicans localizes to the mitochondria during stress and is required for mitochondrial function and virulence. Eukary Cell. 2009;8:1706–1720. doi: 10.1128/EC.00066-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barelle C, Priest CL, Maccallum DM, Gow NA, Odds FC, Brown AJ. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol. 2006;8:961–971. doi: 10.1111/j.1462-5822.2005.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A. In vivo and organelle assessment of OXPHOS activities. Methods. 2002;26:307–316. doi: 10.1016/S1046-2023(02)00036-1. [DOI] [PubMed] [Google Scholar]
- Becker J, Kauffman S, Hauser M, Huang L, Lin M, Sillaots S, et al. Pathway analysis of Candida albicans survival and virulence determinants in a murine infection model. Proc Natl Acad Sci. 2010;107:22044–22049. doi: 10.1073/pnas.1009845107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges HR, Feamley I, Hirst J. The subunit composition of mitochondrial NADH:ubiquinoneoxidoreductase (Complex I) of Pichia pastoris. Mol Cell Proteomics. 2010;9:2318–2326. doi: 10.1074/mcp.M110.001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges HR, Grgic L, Harbour ME, Hirst J. The respiratory complexes I from the mitochondria of two Pichia species. Biochem J. 2009;422:151–159. doi: 10.1042/BJ20090492. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Brown GD, Netea MG, Gow NA. Metabolism impacts upon Candida immunogenicity and pathogenicity at different levels. Trends Microbiol. 2014 doi: 10.1016/j.tim.2014.07.001. doi:10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Budge S, Kalonti D, Tillmann A, Jacobson MD, Yen Z, Enie I, et al. Stress adaptation to a pathogenic fungus. J Exp Biol. 2014;217:144–155. doi: 10.1242/jeb.088930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R, Sun N, Gay-Andrieu F, Groutas Q, Weerawama W, Prasad S, et al. Antifungal drug discovery: the process and outcomes. Future Microbiol. 2014;9:791–805. doi: 10.2217/fmb.14.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R, Clancy CJ. Candida and candidiasis. 2. ASM Press, Inc; DC, USA: 2012. [Google Scholar]
- Chen H, Calderone R, Sun N, Wang Y, Li D. Caloric restriction restores the chronological life span of the goa1Δ null mutant of Candida albicans in spite of high levels of ROS. Fungal Genet Biol. 2012;49:1023–1032. doi: 10.1016/j.fgb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Dagley M, Gentile I, Beilharz T, Pettoloni F, Djordjevic J, Lo T, et al. Cell wall integrity is linked to mitochondrial and phospholipid homeostasis in Candida albicans through the activity of the post-translational regulator Ccr4-Pop2. Mol Microbiol. 2011;79:968–989. doi: 10.1111/j.1365-2958.2010.07503.x. [DOI] [PubMed] [Google Scholar]
- Ene IV, Brunke S, Brown AJ, Hube B. Metabolism in Fungal Pathogenesis. Cold Spring Harbor Perspect Med. 2014 doi: 10.1101/cshperspect.a019695. doi:10.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene IV, Adya AK, Wehmeier S, Brand AC, MacCallum DM, Gow NA, Brown AJ. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol. 2012;14:1319–1335. doi: 10.1111/j.1462-5822.2012.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin C, Kretschmar M, Nichterlein T, Gaillardin C, d’Enfert C, Hube B. Stage-specific gene expression of Candida albicans in blood. Mol Microbiol. 2003;47:1523–1543. doi: 10.1046/j.1365-2958.2003.03396.x. [DOI] [PubMed] [Google Scholar]
- Gabaldon T, Rainey D, Huynen MA. Tracing the evolution of a large protein complex in the eukaryotes, NADH:ubiquinoneoxidoreducatase (Complex I) J Mol Biol. 2005;348:857–870. doi: 10.1016/j.jmb.2005.02.067. [DOI] [PubMed] [Google Scholar]
- Gallagher S. Digital image Processing and analysis with Image J. Current. Protoc Essent Lab Tech. 2010;3:A3C.1–A3c.24. doi: 10.1002/9780470089941. [DOI] [Google Scholar]
- Ding X, et al. The type II Ca2+/calmodulin-dependent protein kinases are involved in the regulation of cell wall integrity and oxidative stress response in Candida albicans. Biochem Biophys Res Commun. 2014;446:1073–1078. doi: 10.1016/j.bbrc.2014.03.059. [DOI] [PubMed] [Google Scholar]
- Hao B, et al. Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob Agents Chemother. 2013;57:326–332. doi: 10.1128/AAC.01366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredouari H, Gergondey R, Bourdais A, Vanparis O, Bulteau A, Camardo J, Auchere F. Changes in glutathione-dependent redox status and mitochondrial energetic strategies are part of the adaptive response during the filamentous process in Candida albicans. Biochim Biophys Acta. 2014;1842:1855–1869. doi: 10.1016/j.bbadis.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Harbauer A, Opalinska M, Gerbeth C, Herman J, Rao S, Schönfisch B, et al. Cell-cycle dependent regulation of mitochondrial preprotein translocase. Science. 2014;346:1109–1113. doi: 10.1126/science.1261253. [DOI] [PubMed] [Google Scholar]
- Helmerhorst EJ, Stan M, Murphy MP, Sherman F, Oppenheim FG. The concomitant expression and availability of conventional and alternative, cyanide-insensitive, respiratory pathways in Candida albicans. Mitochondrion. 2005;5:200–211. doi: 10.1016/j.mito.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hewitt V, Heinz E, Shingu-Vazquez M, Qu Y, Jelicic B, Lo T, et al. A model system for mitochondrial biogenesis reveals evolutionary rewiring of protein import and membrane assembly pathways. Proc Natl Acad Sci USA. 2012;109:3358–3366. doi: 10.1073/pnas.1206345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen AJ, Trijbels FJ, Sengers RC, Smeitink JA, van den Heuvel LP, et al. Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin Chem. 2007;53:729–734. doi: 10.1373/clinchem.2006.078873. [DOI] [PubMed] [Google Scholar]
- Khamooshi K, Sikorski P, Sun N, Calderone R, Li D. The Rbf1, HFl1 and Dpb4 of Candida albicans regulate common as well as transcription factor-specific mitochondrial and other cell activities. BMC Genomics. 2014;15:56. doi: 10.1186/1471-2164-15-56. doi:10.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffner R, Rohr A, Schmiede A, Krull C, Schulte U. Involvement of two novel chaperones in the assembly of mitochondrial NADH:Ubiquinone Oxidoreductase (Complex I) J Mol Biol. 1998;283:409–417. doi: 10.1006/jmbi.1998.2114. [DOI] [PubMed] [Google Scholar]
- Li D, Chen H, Florentino A, Alex D, Sikorski P, Fonzi W, Calderone R. Enzymatic dysfunction of mitochondrial complex I of the Candida albicans goa1 mutant is associated with increased reactive oxidants and cell death. Eukaryot Cell. 2011;10:672–682. doi: 10.1128/EC.00303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS, Lim JK, Lee CC, Murphy PM. Organ-specific Innate immune responses in a mouse model of invasive candidiasis. J Innate Immun. 2011;3:180–199. doi: 10.1159/000321157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchais V, Kempf M, Liczna P, LeFrancois C, Bouchara JP, Robert R, Cottin J. DNA array analysis of Candida albicans gene expression in response to adherence to polystyrene. FEMS Microb Lett. 2005;245:25–37. doi: 10.1016/j.femsle.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McDonough JA, Bhattacherjee V, Sadlon T, Hostetter MK. Involvement of Candida albicans NADH dehydrogenase complex I in filamentation. Fungal Genet Biol. 2002;36:117–127. doi: 10.1016/S1087-1845(02)00007-5. [DOI] [PubMed] [Google Scholar]
- Mimaki M, Wang X, McKenzie M, Thorburn D, Ryan M. Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta. 2012;1817:851–862. doi: 10.1016/j.bbabio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Noble S, French S, Kohn L, Chen V, Johnson A. Systematic screens of Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nature Genetics. 2010;42:590–605. doi: 10.1038/ng.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira B, Videira A, Duarte M. Novel insights into the role of Neurospora crassa NDUFAF2, an evolutionarily conserved mitochondrial complex I assembly factor. Mol Cell Biol. 2013;33:2623–2634. doi: 10.1128/MCB.01476-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelman A, Wachtel C, Cohen M, Haupt S, Shapiro H, Tzur A. JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012;3:e430. doi: 10.1038/cddis.2012.171. doi:10.1038/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan LM, Yeung S-AJ, Lee MH. Cancer reprogramming: importance, main features, and potentials for precise-targeted anti-cancer therapies. Cancer Biol Med. 2014;11:1–19. doi: 10.7497/j.issn.2095-3941.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto D, Roman E, Correla I, Pla J. The HOG pathway is critical for the colonization of the mouse gastrointestinal track by Candida albicans. PLoS One. 2014;9:1.ee87128. doi: 10.1371/journal.pone.0087128. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Jelicic B, Pettoloni F, Perry A, Lo T, Hewitt VL, Bantun F, et al. Mitochondrial sorting and assemble machinery subunit Sam37 in Candida albicans: insight into the roles of mitochondria and fitness, cell wall integrity and virulence. Eukaryot Cell. 2012;11:532–544. doi: 10.1128/EC.05292-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MA, Lorenz MC. Mutations in alternative carbon utilization pathways in Candida albicans attenuate virulence and confer pleiotropic phenotypes. Eukary Cell. 2007;6:280–290. doi: 10.1128/EC.00372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaki A, Bohovych IM, Enjalbert B, Young T, Odds FC, Gow NA, Brown AJ. Glucose promotes stress resistance in the fungal pathogen Candida albicans. Mol Cell Biol. 2009;20:4845–4855. doi: 10.1091/mbc.E09-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhrani NM, Padh H. Organelle targeting: third level of drug targeting. Drug Des Devel Ther. 2013;7:585–599. doi: 10.2147/DDDT.S45614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandai D, Yin Z, Selway L, Stead D, Walker M, Leach MD, et al. The evolutionary rewiring of ubiquitination targets has reprogrammed the regulation of carbon assimilation in the pathogenic yeast Candida albicans. mBio. 2012;3:e00495–12. doi: 10.1128/mBio.00495-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarosiek KA, Chonghaile TN, Letai A. Mitochondria: gatekeepers of response to chemotherapy. Cell. 2013;23:612–619. doi: 10.1016/j.tcb.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She X, Zhang L, Chen H, Calderone R, Li D. Cell changes in the Candida albicans mitochondria mutant goa1Δ are associated with reduced recognition by innate immune cells. Cell Microbiol. 2013;15:1572–1584. doi: 10.1111/cmi.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingu-Vazquez M, Traven A. Mitochondria and fungal pathogenesis: drug tolerance virulence and potential for antifungal therapy. Eukaryot Cell. 2011;10:1376–1383. doi: 10.1128/EC.05184-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Fonzi W, Chen H, She X, Zhang L, Calderone R. Azole susceptibility and transcriptome profiling in the Candida albicans mitochondrial electron transport chain complex I mutants. Antimicrob Agents Chemother. 2013;57:532–542. doi: 10.1128/AAC.01520-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R, Burns J, Khan S. The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2013.09.010. http://doi.org/10.1016. [DOI] [PMC free article] [PubMed]
- Theodosakis N, Micevic G, Kelly D, Bosenberg M. Mitochondrial function in melanoma. 2014 doi: 10.1016/j.abb.2014.06.028. http://dx.doi.org/a0.1016/ [DOI] [PubMed]
- Velluci VF, Gygax SE, Hostetter MK. Involvement of the Candida albicans pyruvate dehydrogenase complex protein (Pdx1) in filamentation. Fungal Genet Biol. 2007;44:974–990. doi: 10.1016/j.fgb.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira A, Duarte M. From NADH to ubiquione in Neurospora mitochondria. Biochim Biophys Acta. 2002;1555:187–191. doi: 10.1016/s0005-2728(02)00276-1. [DOI] [PubMed] [Google Scholar]
- Videira A. Complex I from the fungus Neurospora crassa. Biochem Biophys Acta. 1998;1364:89–100. doi: 10.1016/s0005-2728(98)00020-6. [DOI] [PubMed] [Google Scholar]
- Videira A, Tropschug M, Werner S. Primary structure, in vitro expression and import into mitochondria of a 29-/21 kDa subunit of complex I from Neuropsora crassa. Biochem Biophys Res Commun. 1990;166:280–285. doi: 10.1016/0006-291x(90)91942-l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.