Abstract
The Notch signaling pathway regulates specification of zebrafish liver progenitor cells towards a biliary cell fate. Here, using staged administration of a pharmacological inhibitor of Notch receptor processing, we show that activation of the Notch pathway is also important for growth and expansion of the intrahepatic biliary network in zebrafish larvae. Biliary expansion is accompanied by extensive cell proliferation and active remodeling of the nascent ductal network, as revealed by time lapse imaging of living zebrafish larvae that express a Notch responsive fluorescent reporter transgene. Together, these data support a model in which the Notch signal functions reiteratively during biliary development; first to specific biliary cells and then to direct remodeling of the nascent biliary network. As the Notch pathway plays a comparable role during mammalian biliary development, including humans, these studies also indicate broad conservation of the molecular mechanisms directing biliary development in vertebrates.
Keywords: Notch, bile ducts, cholangiocytes, DAPT
Introduction
The biliary system drains bile produced by hepatocytes to the gallbladder and intestine. Ducts within the liver, the intrahepatic bile ducts, arise from primitive tubules that undergo extensive remodeling in late embryogenesis and the early post-natal period. The cells that comprise these ducts are derived from a bipotential progenitor that also gives rise to hepatocytes. Heritable disorders that disrupt development of the intrahepatic bile ducts are well described in humans. Based on liver biopsy, these disorders are often divided into two distinct categories: in patients with ductopenic disorders, such as Alagille syndrome, the number of interlobular bile ducts in the portal tracts is reduced, whereas in conditions associated with ductal plate malformation, such as congenital hepatic fibrosis and autosomal dominant polycystic kidney disease, the portal tracts contain excessive numbers of immature biliary tubules (1). It is generally believed that ductal plate malformation arises as a result of defective remodeling of nascent biliary ducts whereas ductopenic disorders arise from a primary defect of biliary development or the loss of differentiated biliary cells.
Identification of dominant JAGGED1 mutations in ductopenic patients with Alagille syndrome initially pointed to a role for the Notch signal in the specification of liver progenitors towards a biliary fate (1–3). Studies in mice however have questioned this model and instead argue that the primary function of the Notch pathway is to direct biliary remodeling (4–6). This model of Notch function is compatible with the clinical features of the Alagille syndrome, such as the frequent onset of cholestasis (reduced bile flow) in childhood rather than in the post-natal period, and normal intrahepatic biliary morphology in infants with extra-hepatic features of the Alagille syndrome that subsequently develop ductopenia in childhood. These findings suggest that Notch signaling may be required for expansion of the biliary network rather than specification of biliary epithelial cells during early development.
Notch activity persists in the developing liver of Alagille syndrome patients (because of autosomal dominant inheritance), and in the livers of mice carrying non-lethal heterozygous or conditional mutations in Notch pathway genes that phenocopy Alagille syndrome (4–6). The relatively late onset bile duct paucity in children with Alagille syndrome and in related animal models therefore does not exclude the possibility that the original specification of biliary progenitors is dependent on the Notch signal. Indeed, it is conceivable that the Notch pathway is used reiteratively during biliary development, first to direct an early cell fate decision and later to direct morphogenetic events and or biliary cell survival. Indeed, recent experiments conducted in mice support this model: targeted disruption in hepatoblasts of Rbpj, the gene encoding the DNA binding component of the Notch transcription complex led to a 50% reduction in the number of biliary epithelial cells and a comparable reduction in the number of bile ducts (7). Consistent with a role for Notch in biliary specification, constitutive activation of the Notch signal in developing hepatoblasts and committed hepatocytes led to the formation of ectopic biliary tubules in the hepatic lobule (7, 8).
In previous work we defined a role for jagged-mediated Notch signaling during the specification of intrahepatic biliary cells of zebrafish (9). Zebrafish larvae rendered deficient in Jagged proteins by morpholino antisense knockdown do not form biliary cells and their hepatocytes expressed a keratin protein normally restricted to the biliary lineage. These data argue that Notch regulates the development of a bipotential progenitor towards a biliary fate. We extend these observations in the present study by analyzing the role of the Notch signal during later stages of zebrafish liver development using staged administration and withdrawal of the gamma-secretase inhibitor DAPT, an established pharmacological inhibitor of Notch signaling (10). Using a recently engineered Notch reporter line, we also visualize ductal expansion in vivo, and show that Notch activity functions autonomously during zebrafish intrahepatic biliary development.
Results
Zebrafish intrahepatic biliary development
The intrahepatic biliary system transports bile from hepatocytes into the large extrahepatic bile ducts that join the liver to the gallbladder and intestine. In previous work we visualized the biliary system using confocal whole mount projections of embryos and larvae immunostained with an antibody directed against the human Keratin-18 protein (9, 11–13). These studies showed that the intrahepatic system consists of a lattice-like network of long ducts joined by short interconnecting ducts along with small ducts that directly link long ducts to the hepatocyte canalicular membrane, through which bile is secreted (Figure 1). The hepatocytes of teleost larvae are arranged in an acinar pattern and the intrahepatic ducts reside within tubules and muralia formed by contiguous hepatocytes. This architectural pattern of biliary cells and hepatocytes has been described in other teleosts and differs from the lobular architecture of the mammalian liver, although recent studies have identified some similarities (9, 14, 15).
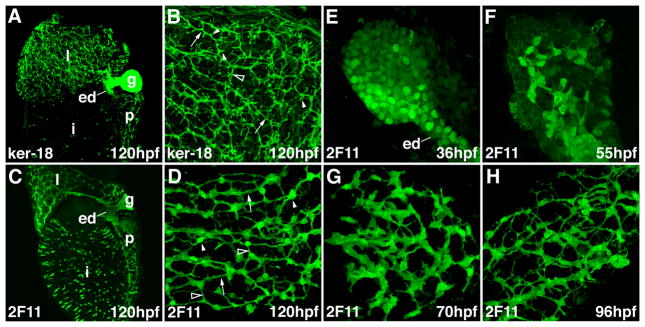
Figure 1. Zebrafish intrahepatic biliary development.
(A, B) Confocal projections through the liver of a 120 hpf larvae stained with the anti-Keratin 18 antibody. The low power image (A) shows the intrahepatic biliary network (l), extraehepatic ducts (ed) and gallbladder (g). The high power image (B) shows that the keratin-18 protein detects 3 classes of intrahepatic ducts: long ducts (arrow), interconnecting ducts (open arrowhead) and terminal ductules (arrowhead). (C, D) Confocal projections through the liver of a 120 hpf larvae stained with the 2F11 antibody. The low power image (C) shows that the 2F11 epitope is presented within the intrahepatic (l) and extrahepatic (ed) ductal systems as well as the gallbladder (g). The high power image (D) shows that the 2F11 epitope is present in the nucleus of the biliary epithelial cell (arrowhead). This epitope is also detected on the long ducts (arrow) and interconnecting ducts (open arrowhead) but not the terminal ductules. (E–H) Developmental pattern of the 2F11 epitope in the intrahepatic biliary system between 36 hpf and 96 hpf.
Although Keratin-18 immunostains do a good job delineating intrahepatic architecture at late stages of larval development (4 – 5 days post-fertilization; dpf), the resolution of this marker at embryonic stages is poor. To further characterize the anatomy and development of the intrahepatic biliary system in zebrafish embryos, we compared the staining pattern of the Keratin-18 epitope with an undefined epitope present in biliary epithelia that is recognized by the monoclonal antibody 2F11 (16). We first compared the two staining patterns at the end of larval development (5 days post-fertilization; dpf). Confocal projections through the liver of these larvae revealed Keratin-18 and 2F11 to be complementary markers of biliary epithelial cells; 2F11 decorated the biliary cell body and long ducts and some interconnecting ducts whereas Keratin-18 was evident in all ductal segments, including the small ducts that join the hepatocyte canaliculus, but not the biliary cell body (Figure 1A–D). Faint 2F11 reactivity was seen in the hepatocyte membrane with some but not all fixatives, and thus was considered to be non-specific (data not shown). Weak Keratin-18 staining was frequently detected in endothelial cells of the liver sinusoids, consistent with published data showing that teleost Keratin proteins are detected in both mesodermally and endodermally derived cell types (9, 17, 18)
Having established the 2F11 staining pattern in mature larvae, we next examined the distribution of this epitope in cells of the developing liver. Whole mount projections from 36 hpf embryos revealed discrete round 2F11-positive cells within the liver anlage and the anlage of the extrahepatic duct (Figure 1E). Low level 2F11 immunoreactivity was evident in most liver cells at this stage whereas a minority of cells that were associated with the developing extrahepatic duct (ed in Figure 1E) had higher levels. At 55 hpf, the 2F11 epitope was detected in this latter group of cells and contiguous cells that extended into the liver periphery (Figure 1F). The 2F11-positive cells were arranged in a network at this stage and they had begun to adopt a stellate morphology. At 70 hpf the 2F11-positive cells had a clear stellate morphology and were arranged in a well defined network that was contiguous with the extrahepatic ducts and gallbladder (Figure 1G and Supplementary Figure 1). The developing pancreatic ductal network was evident at this stage as were secretory cells within the intestinal epithelium (Supplementary Figure 1). Between 80 and 96 hpf the 2F11-positive cells underwent significant morphological alterations; the contiguous cells apparent at 70 hpf separated from one another during this period and there was progressive narrowing of the diameter of the contiguous segments. This led to the appearance of well-defined ducts. Low power images showed progressive liver enlargement throughout these stages and continued maturation of the extrahepatic biliary system and the gallbladder and the pancreatic ducts (Supplementary Figure 1).
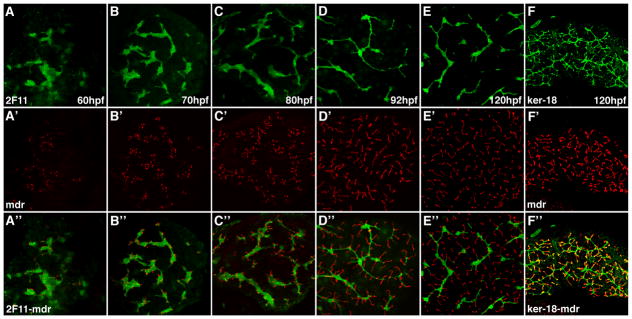
To complement these studies, we assessed the maturation of the hepatocyte secretory apparatus in relation to the development of the biliary network (Figure 2). Double immunostains with the 2F11 antibody and an antibody that recognizes the Multidrug resistant transporter-1 (Mdr-1), a canalicular protein (9, 19), showed juxtaposition of the apical hepatocyte membrane with adjacent biliary cells beginning at 60 hpf, thus suggesting coordination of biliary and canalicular development, as previously reported (19). The canaliculi, which in zebrafish and other teleost fish are invaginations of the apical membrane of single hepatocytes (9, 10, 21, 22), showed progressive enlargement during development. Double immunostains revealed strong and consistent overlap of the Mdr-1 epitope in the canaliculi with terminal ducts recognized by the Keratin-18 antibody at 5 dpf whereas at earlier stages there was only partial overlap (Figure 2F and Supplementary Figure 2). We interpret these latter data as evidence that the smallest projections of the biliary cell lumen “insert” into the canalicular channel and that prior to insertion, the canaliculi and terminal ductules develop independently of one another.
Figure 2. Development of hepatocyte canaliculi and intrahepatic biliary network.
Developmental pattern of the 2F11 epitope (A–E) and Mdr epitope, a canalicular transporter (A′–E′) with overlap of the two markers (A″–E″). The length and number of the canaliculi increases between 60 hpf and 120 hpf. The overlap of the two markers shows that each canaliculus develops in close association with the 2F11 positive biliary epithelia. Each canaliculus drains into a single intrahepatic bile duct. The ducts associated with many of the canaliculi in the 120 hpf sample are out of the plane of focus. (F, F′ and F″) Comparable confocal projections through the liver of a larva immunostained with the keratin-18 and Mdr antibodies. Note overlap (yellow) of the keratin-18 epitope in the terminal ductules with the Mdr protein in the canalicular membrane.
Notch signaling is required for morphogenesis and proliferative expansion of the zebrafish biliary network
To understand the role of Notch signaling following the stage when biliary epithelial cells are first specified, we treated embryos and larvae with the γ-secretase inhibitor DAPT, an established inhibitor of the Notch pathway in zebrafish (10) and then analyzed biliary morphology using the Keratin-18 antibody (Figure 3). DAPT treatment initiated at 24 hpf caused severe developmental defects and delays that precluded analyses of liver morphology in these embryos (data not shown). Embryos treated with DAPT beginning at later stages however could be analyzed, even when these treatments were extended to 120 hpf. The continuous DAPT treatments had a profound effect on biliary system morphogenesis. Biliary development appeared to arrest close to the stage when DAPT was added to these larvae (Figure 3). For example, when examined at 120 hpf, the biliary system of embryos that received DAPT continuously beginning at 50 hpf resembled 55 hpf wild type larvae (Figure 3B). Few intrahepatic ducts were present in these larvae besides the largest ducts that were contiguous with the extrahepatic system. Consistent with this, biliary excretion of ingested fluorescent lipids was significantly reduced by DAPT treatment at these stages (Supplementary Figure 3). A comparable stage-dependent arrest of biliary development was detected in larvae treated with DAPT beginning at 70 hpf and 92 hpf (Figure 3C, D). At all time points examined biliary development reinitiated when DAPT was removed, thus indicating that biliary epithelia remain responsive to the Notch signal throughout development (Figure 4A–C; 4D, E). In contrast to its strong effect on intrahepatic biliary development, DAPT had little effect on development of the extrahepatic biliary duct and gallbladder (Figure 3 and 4 and data not shown). In addition, DAPT had only a small effect on overall liver growth during these stages (Figures 3 and 4). Comparable effects of DAPT treatment on biliary development were detected in larvae immunostained with the 2F11 antibody (Supplementary Figure 4).
Figure 3. Notch signaling is required for expansion of the intrahepatic biliary ductal network.
(A–D) Confocal projections through the liver of a control 120 hpf larva and 120 hpf larvae treated with DAPT beginning at 50 hpf (B), 70 hpf (C) and 96 hpf (D) that were stained with the keratin-18 antibody. These images show that at all stages examined expansion of the biliary network is disrupted when Notch signaling is inhibited by DAPT. Development of the extrahepatic duct (arrows) and gallbladder (g) are not affected by Notch inhibition. These structures are out of the plane of focus in the 96 hpf larva.
Figure 4. Liver cells respond to the Notch signal throughout larval development.
(A–F) Biliary expansion resumes upon reinitiation of Notch signaling associated with DAPT withdrawal. There is progressive increase in the complexity of the biliary network in 125 hpf larvae treated with DAPT for shorter intervals beginning at 53 hpf. All images are derived from larvae immunostained with the anti keratin-18 antibody. The gallbladder (g) is out of the plane of focus in panels D and E. Pancreas – p.
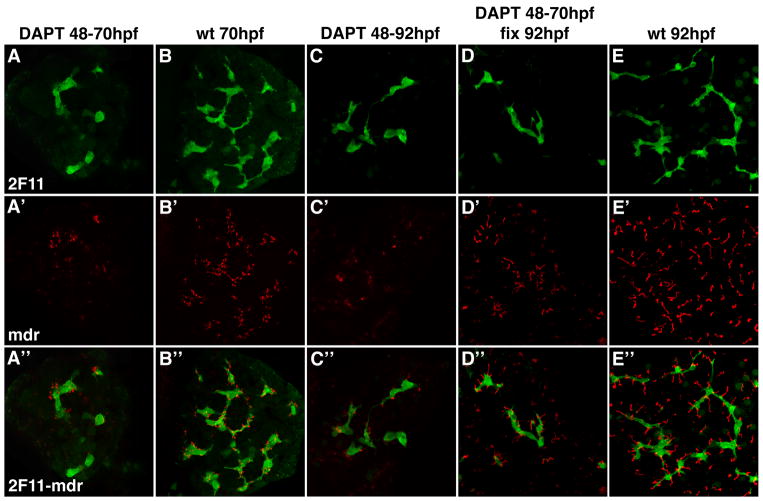
Mdr-1 immunostains revealed that canalicular development was markedly altered by DAPT treatment and that this too was reversed upon DAPT withdrawal (Figure 5). Since overall liver morphology and liver size were only modestly reduced in DAPT treated larvae (Figures 3 and 4), as well as Jagged deficient larvae (9), we speculate that the biliary defect associated with Notch inhibition secondarily disrupts canalicular development in hepatocytes, the principal liver cell type.
Figure 5. Notch signaling is required for canalicular development.
Confocal projections (5 μm) through the liver of a 70 hpf larva (A) and two 92 hpf (C, D) larvae treated with DAPT from 48 – 70 hpf (A); DAPT from 48 – 92 hpf (C) and DAPT from 48 – 70 hpf (D). A 70 hpf (B) and 92 hpf wild type larva (E) are also shown. All larvae were immunostained with Mdr (red) and 2F11 (green) antibodies. DAPT treatment arrests canalicular development (red) and biliary development (green). Canalicular development reinitiates with DAPT withdrawal (D). Note that the depth of these confocal projections is too small to detect reinitiation of duct development upon DAPT withdrawal.
Hepatocytes in Jagged-2/-3 deficient larvae are arranged in spherical rosettes rather than the elongated tubules present in the wild type liver (9). These cells express Keratin-18, a biliary marker and thus were considered to be hybrid hepatocyte/biliary cells. We considered the rosettes to be an indirect marker of failed biliary specification because biliary cells did not develop in Jagged-deficient larvae. Rosettes were detected in 120 hpf larvae that were treated with DAPT beginning at 31 hpf, but only rarely when Notch inhibition was instituted after the onset of bile duct morphogenesis (> 48 hpf) even though bile duct formation was markedly abnormal in these larvae (data not shown). This suggested to us that biliary growth was likely to be driven, at least in part, by the proliferation of existing biliary epithelial cells rather than the development of new cells from undifferentiated progenitors. To explore this hypothesis we measured liver cell proliferation in embryos and larvae pulse labeled with the nucleoside analogue EdU (23). For these assays, biliary cells, hepatocytes and sinusoidal endothelial cells were distinguished by 2F11 staining and nuclear morphology defined by DAPI using confocal microscopy. Based on these criteria, biliary epithelia comprised between 14.5 % and 16.6% of total liver cells at 3 dpf and 9.2% and 11.1% of total liver cells at 4 dpf, whereas hepatocytes comprised 70.7% – 75.9% and 61.7% – 63.8% of total cells at 3dpf and 4 dpf, respectively (Table 1). The remainder of the cells were predicted to be a combination of sinusoidal endothelial cells, stromal cells and nucleated erythrocytes within the sinusoids. Based on EdU detection, we recorded strong biliary proliferation in 80 hpf and 100 hpf larvae, stages when there is significant biliary growth, but little proliferation at 125 hpf (Table 1). By contrast, there was less proliferation of hepatocytes and other cell types (not shown) at these developmental stages.
Table 1.
| Larval Age | Total Liver Cells | Total / % Biliary Cells | Total / % Hepatocytes | Total /% Edu + Biliary Cells | Total / % Edu + Hepatocytes |
|---|---|---|---|---|---|
| 3 dpf | 1874 | 300 / 16.0% | 1326 / 70.7% | 55 / 18.3% | 124 / 9.3% |
| 3 dpf | 1387 | 231 / 16.6% | 997 / 71.8% | 53 / 22.9% | 104 / 10.4% |
| 3 dpf | 2093 | 303 / 14.5% | 1576 / 75.3% | 57 / 18.8% | 142 / 9.0% |
| 4 dpf | 1710 | 158 / 9.2% | 1043 / 61% | 21 / 13.2 % | 49 / 4.6% |
| 4 dpf | 1794 | 199 / 11% | 1145 / 63.8% | 33 / 16.5% | 74 / 6.4% |
Total Dapi; Total Biliary/Biliary; Biliary Edu/%Biliary Edu; Total Hep/%Hep; Hep Edu/% Hep Edu
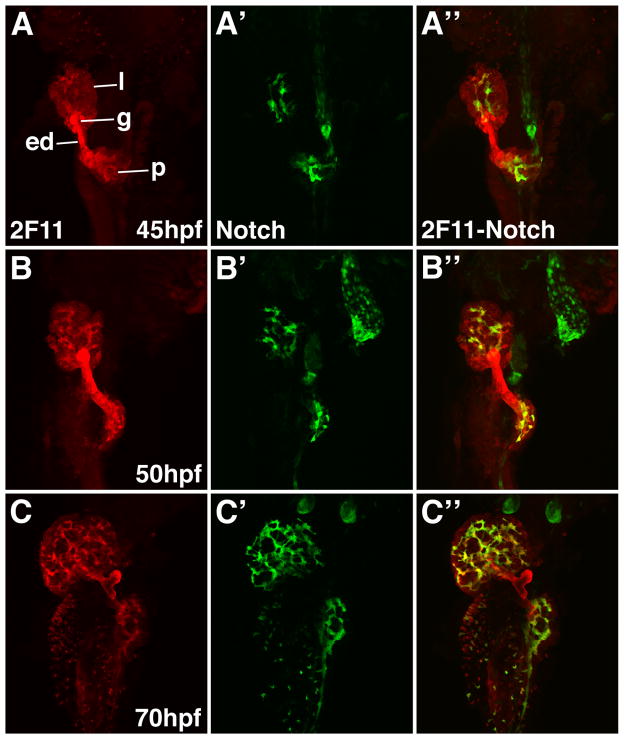
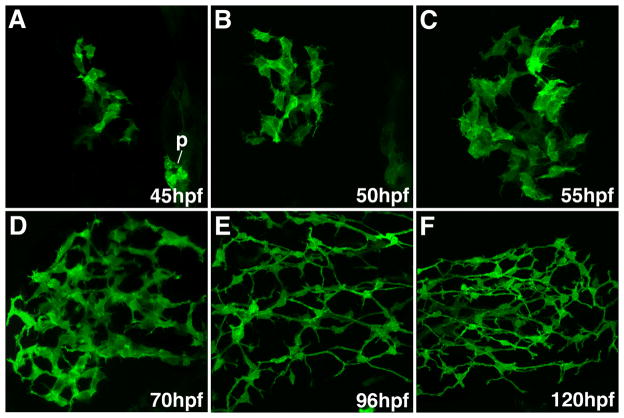
During the stages when biliary cells proliferate, their morphology and the morphology of the developing network changes (Figures 1 and 2). To better understand the underlying mechanism that regulates these morphogenetic processes, we took advantage of a recently described line of bigenic zebrafish that are hemizygous for a Notch responsive fluorescent reporter gene (GFP) and a cDNA encoding the DsRed fluorescent protein under the control of the endothelial cell promoter fli-1 (24). Confocal analyses showed that the pattern of liver GFP expression in the transgenic zebrafish larvae was nearly identical to the distribution of the 2F11 epitope (Figure 7). Double immunostains with anti-GFP and 2F11 antibodies showed nearly complete overlap of the GFP and 2F11 epitope in intrahepatic bilary epithelia at 60 hpf and 70 hpf (Figure 7C, D and Supplementary Figure 3) and later stages (data not shown) but was not detected in the gallbladder or extrahepatic ducts that were all 2F11-positive. At earlier stages the pattern of these 2 markers began to diverge (Figure 7A, B and Supplementary Figure 5). At 50 hpf, all of GFP-positive cells were 2F11-positive, however isolated cells that were 2F11-positive but GFP-negative could be identified. A cluster of these cells was located adjacent to the extrahepatic duct, whereas small groups containing 2–3 cells were present in the periphery in the liver. At 45 hpf a smaller number of GFP-positive cells were positive for the 2F11 epitope. While most of the 2F11-positive cells were confined to the region surrounding the extrahepatic duct, isolated 2F11-positive cells in the liver periphery were also detected at this stage. At 36 hpf there were no GFP-positive cells detected within the liver anlage (data not shown). All of the hepatic cells were 2F11-positive at this stage but the cells closest to the extrahepatic duct had the strongest expression (Figure 1E).
Figure 7. Notch reporter expression overlaps with the 2F11 epitope in developing intrahepatic biliary cells.
Whole mount confocal images through the liver of developing Notch reporter larvae stained with the 2F11 antibody (A–D) and a GFP antibody (A′–D′) with overlap of the two markers (A″–D″). GFP positive biliary epithelia are first detected at 45 hpf (A′). At this stage there is strong 2F11 expression in the gallbladder (g), extrahepatic duct (ed) and in a few liver parenchymal cells (l). Only partial overlap between GFP and 2F11 is seen in the liver at this stage. GFP positive cells are detected in the pancreas. From 50 hpf – 70 hpf there is progressive increase in the number of GFP positive cells. At 50 hpf there is significant overlap between the GFP and 2F11 epitopes in the biliary cells. At 60 hpf and 70 hpf there is nearly complete overlap between these markers in the biliary cells. The gallbladder and extrahepatic duct remain GPF negative at all stages. The pancreatic ductal network is also GFP positive (p).
We next used the Notch reporter line to follow the morphogenetic movement of developing biliary epithelia. For these studies, confocal timelapse imaging was performed after the liver and adherent digestive organs of 76hpf larvae were dissected from the embryo and yolk. During the 5 hr recordings the dissected organs were maintained in media that supported hepatic and intestinal growth. Recordings could not be obtained at earlier developmental stages because of difficulty in dissecting the liver from the yolk. As shown in Figure 8 and the accompanying timelapse movies (Supplementary Movie 1), there was significant movement and rearrangement of the GFP-positive biliary cells between 76 hpf and 81 hpf. A dynamic pattern of filopodia extension and retraction was evident during this period. The biliary cells appear to use the filopodia to sense their neighbors, thereby enabling them to establish intercellular connections that lead to elaboration of the developing ductal network. Cytoplasmic vesicles or vacuoles were evident in many filopodia and in more proximal regions of the biliary cells. Over time, the vesicles appeared to fuse, thus generating intracellular lumens within unicellular ducts. This model of lumen formation was also supported by 1 μM confocal projections derived from 2F11 immunostains and transmission electron micrographs (data not shown). Recordings obtained from 76 hpf larvae pretreated with DAPT for 24 hrs showed a dramatic reduction in biliary cell number, cell movement and filopodia formation (Supplementary movie 2).
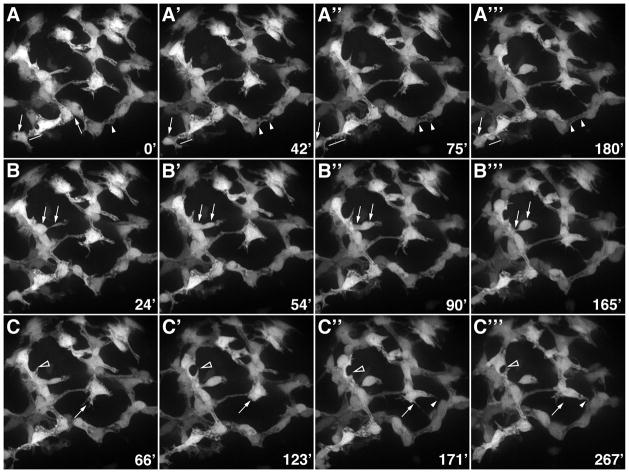
Figure 8. Morphogenesis of the intrahepatic biliary network.
Confocal projections through the liver that was dissected from a 76 hpf Notch reporter larvae and cultured for 4.5 hours. Numbers in lower right corner indicate duration of culture in minutes. (A) These images show progressive lengthening and then shortening of the cytoplasmic bridge between two adjacent biliary cells (arrows). Fusion of cytoplasmic vesicles leading to the formation of a ductal lumen within two adjacent cells is also evident (arrowheads). (B) These images show the migration of a single biliary cell via a cytoplasmic extension (arrows). (C) These images show the dynamic pattern of filopodia extension and retraction from two biliary cells (arrows and arrowheads).
Simultaneous time lapse recordings of DsRed-positive endothelial cells were similar to the biliary recordings in that cell proliferation, cell movement and filopodia formation were all evident, albeit each was less pronounced than in the GFP-positive biliary cells (Supplementary movie 3). Reduced cell movement or remodeling of DsRed-positive endothelia was detected as a result of the DAPT treatment (Supplementary Movie 4). In addition, the endothelial cells had a larger number of intracytoplasmic vesicles, suggesting delayed lumen formation.
Discussion
The data presented in this study concern the formation and growth of the intrahepatic biliary system in zebrafish larvae. Analyses of the expression pattern of the 2F11 epitope in the liver of Notch reporter larvae provide a more detailed picture of biliary development than previously reported with Keratin-18 immunostains (9, 12 – 14). The 2F11 and GFP markers allow visualization of the body of the biliary cell, and not just its periphery, the current study enabled us to track individual cells during embryonic and larval development. Using these markers we show that biliary cells are detected during the second day post-fertilization (~45 hpf) in the vicinity of the extrahepatic duct. Taking into account prior studies concerning the role of Jagged mediated Notch signaling in biliary cell specification (9), and the current data showing the restricted pattern of the Notch reporter in the liver, it is likely that these cells represent a founder population of biliary cells whose development is dependent upon the cell-autonomous activation of the Notch signal.
As development proceeds, the nascent biliary cells align to form a contiguous network that undergoes proliferative expansion and extensive remodeling. This second stage of intrahepatic biliary development is also dependent on Notch signaling, as DAPT treatment reversibly arrested biliary growth. These data show that the Notch signal is used reiteratively during biliary development: first to drive formation of the biliary lineage; second, to drive growth and morphogenesis of cells within the ductal network. Although our data argue that biliary expansion is driven by proliferation of committed biliary epithelial cells that already reside within the developing ductal network, the possibility that some of the new cells arise from an undifferentiated progenitor cannot be excluded in the absence of a formal lineage study.
Combined 2F11 and Mdr-1 immunostains strongly suggest that the development of the hepatocyte secretory apparatus and the biliary system are coordinated. These studies show close association of the canaliculus and biliary epithelia beginning at around 60 hpf, the stage when the ductal network is beginning to form and canalicular markers such as Mdr-1 and Bsep (KL and MP, unpublished data) are first detected. Bile acids, phospholipids and other constituents of bile are secreted through the canaliculus into the biliary system. Transmission electron micrographs show fusion of the canalicular membrane with the intracellular membrane that forms the wall of the biliary lumen (9). Thus, it is not surprising that their development is linked. Indeed, we have observed that canalicular development is altered when biliary development is disrupted (9, 25). Consistent with this, a recent study that identified an important role for sinusoidal endothelium in the polarization of developing hepatocytes also reported a link between polarization and biliary development (19). Neither the 2F11 epitope nor GFP protein in Notch reporter fish decorated the smallest biliary channels identified by the Keratin-18 antibody that overlap the canaliculus (Figure 2). Although it is conceivable that neither the 2F11 epitope nor GFP protein resides within this portion of the biliary cell, the current data cannot exclude the possibility that the Keratin-18 protein is also expressed by hepatocytes but localized exclusively to the canaliculus. Indeed, mammalian keratin proteins reside in a pericanalicular sheath of intermediate filaments, however they arise from cortical intermediate filaments that envelope the entire hepatocyte cytoplasm (26). In the future, immuno-EM studies will be utilized to more precisely determine the morphology and cellular origin of the small ducts we refer to as biliary ductules.
Combined 2F11 and anti-GFP immunostains in the Notch reporter fish showed nearly complete overlap of the two markers during larval development (55 hpf – 120 hpf). A small number of 2F11 positive cells were negative for GFP at 45 hpf. The majority of these cells were located in the vicinity of the extrahepatic duct. Thus, it is conceivable that these cells will contribute to this lineage, which remains GFP-negative throughout development. The lack of GFP in the extrahepatic duct and gallbladder of Notch reporter larvae is consistent with prior studies showing normal development of the extrahepatic bile ducts and gallbladder in Jagged-2/-3 deficient larvae that lack intrahepatic biliary cells (8) and Alagille syndrome patients with intrahepatic bile duct paucity (1). GFP expression in the intrapancreatic ductal epithelia of Notch reporter larvae is also consistent with prior reports of altered pancreatic ductal development in the Jagged-2/-3 deficient larvae (9, 27) and a related recently published zebrafish study (24). Pancreatic ductal defects were also reported following disruption of Jag-1 in mouse pancreatic progenitor cells (28)
Gene targeting experiments have identified a cell autonomous role for Notch signaling in the remodeling phase of mammalian biliary development. The Notch target gene Hes-1 is expressed in biliary epithelia but not adjacent cell types. Hes-1 disruption arrests development of nascent interlobular bile ducts located adjacent to portal vein radicles in what it referred to as the ductal plate. These immature biliary tubules normally undergo remodeling to form the one or two mature interlobular bile ducts that are typically present in the portal tract (29). Similar ductal plate defects are also detected in mice in which Notch signaling is disrupted by hypomorphic mutations in Jagged-1 and Notch-2 (4–6). Analyses of the DAPT treated Notch reporter larvae argue that Notch signaling plays a related morphogenetic role during biliary development in zebrafish, following an early role in biliary specification (9). We found that GFP was strongly expressed in biliary epithelia and was not detected in other cell types, thus confirming that Notch functions in a cell autonomous fashion. Timelapse imaging showed that expansion of the biliary network involves active remodeling of intercellular connections as well as cell migration. These similarities are noteworthy because they indicate that the role of Notch signaling in directing development of the biliary system has been conserved in vertebrates, despite the significant variation in biliary anatomy.
A role for Notch signaling during mammalian billary specification was only recently confirmed in experiments that used promoters capable of activating Cre recombinase in hepatoblasts during early liver development (7). This role for Notch in the developing mammalian liver is analogous to the role played by Notch in developing zebrafish, as shown by our previous work using knockdown technology to disrupt Jagged gene function (8). Given the architectural differences of the mammalian and teleost liver, it is interesting to speculate on the way in which the Notch signal drives commitment of hepatic progenitors to the biliary lineage. In mammals, Jagged-1 protein is detected in portal vein endothelia that are in close proximity to hepatoblasts that express the Notch-2 receptor. This suggests that a direct inductive signal might drive specification of a hepatic progenitor cell towards the biliary lineage (4). We speculate that a related inductive mechanism drives zebrafish biliary specification based on the cell autonomous role of Notch signaling during this process.
Reiterative use of the Notch signal, as reported here during zebrafish biliary development, has also been observed during vascular development in zebrafish (30, 31). During early embryonic stages, Notch directs artery-vein cell fate decisions, whereas at later time points, Notch controls cell fate specification and vessel morphogenesis. Other common aspects of zebrafish vascular and biliary development include the use of filopodia to guide cell migration and the formation of unicellular lumens by fusion of intracellular and intercellular vacuoles (29, 30). Whether all of these phenomena in biliary cells are directly controlled by the Notch signal is not known but can be addressed in future studies. Given the similarities between vascular and biliary development it will also be interesting to examine whether the molecular signals used to guide vascular patterning play a related role during morphogenesis of the biliary ductal network (32).
Experimental Procedures
Fish maintenance and breeding
Fish maintenance and matings were performed as described (Pack et al., 1996). Fish strains used were tupfel long fin (http://zfin.org/cgi-bin/webdriver?MIval=aa-genotypeview.apg&OID=ZDB-GENO-990623-2) wild type fish or a recently described transgenic line (Tg(tp1bglob:egfp) hemizygous for a notch responsive fluorescent reporter gene (GFP) and a cDNA encoding the DsRed fluorescent protein under the control of the endothelial cell promoter fli-1 (24).
Immunohistochemistry
Primary antibodies used: mouse monoclonal anti-human cytokeratin 18 antibody (Ks 18.04), 1:500 (Maine Biotechnology Services), rabbit anti-human mdr (P-glycoprotein, Ab-1), 1:200 (Oncogene), mouse monoclonal 2F11, 1:1000 (gift from Julian Lewis), and rabbit anti-GFP, 1:500 (Invitrogen). Secondary antibodies used: Alexa Fluor 488-conjugated goat anti-mouse IgG, Alexa Fluor 488-conjugated goat anti-rabbit IgG, Alexa Fluor 568-conjugated goat anti-mouse IgG, Alexa Fluor 568-conjugated goat anti-rabbit IgG (Invitrogen). All secondary antibodies were used at a 1:600 dilution.
For mdr, 2F11, and anti-GFP immunostaining, embryos and larvae were pre-treated with 0.1% collagenase (Sigma-Aldrich) in PBS for 10 to 35 minutes at room temperature followed by washes in PBS supplemented with 0.1% Tween (PBST). Incubation in primary antibody was overnight at 4°C. Secondary antibody incubations were for 4 hours at room temperature, or overnight at 4°C. A Zeiss LSM 510 confocal microscope was used for all analyses.
DAPT treatment
Larvae were incubated in 50μM DAPT or N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (Sigma-Aldrich) (stock solution of 100mM in DMSO) at different time points for 24h to 89h. The DAPT solution was replaced daily. Some 5dpf larvae were soaked in 0.3μg/ml fluorescent lipid bodipy-C16 (Invitrogen) for 3 hours to visualize lipid processing.
EdU injection and visualization
The Click-iT EdU Alexa Fluor 488 Assay (Invitrogen) was performed following the manufacturers protocol. Briefly, approximately 100 nl of 10mM EdU was injected into the yolk of 3dpf – 5dpf larvae. After a 30–60 min incubation, the larvae were fixed in 4% paraformaldehyde (Polysciences), washed in PBST, dehydrated in a graded methanol series, and stored at −20°C in methanol. For the detection assay larvae were rehydrated in PBST and the skin was manually peeled using watchmaker forceps (Fine Science Tools). The peeled larvae were treated with 0.1% collagenase for 20–35 minutes, washed in PBST and then incubated in Click-iT reaction cocktail for 2 hours at room temperature followed by rinses in PBST. Following EdU detection larvae were incubated overnight at 4°C in 2F11 antibody as previously described. Livers were mounted in Vectashield containing DAPI (Vector Laboratories). A Zeiss LSM 510 confocal microscope was used for analysis. Seven to eight 5μm Z-stacks were taken throughout each liver of three 3dpf and two 4dpf larvae and EdU, 2F11, and DAPI positive cells were counted.
Live imaging
The intestine, liver and pancreas of transgenic notch reporter larvae were dissected and cultured in Medium 199 (Gibco) in MatTek glass bottom Culture dishes (# P35-G1.5-10-C; 35mm dish, 10mm well). The glass bottom was coated with BD Cell-Tak (BD Biosciences) 24 hours prior to incubation to improve tissue adherence. Live imaging was performed on a spinning disc confocal microscope (Olympus IX71 inverted microscope) using IPLab acquisition software. Images were taken every 3 minutes. Individual frames were selected using Image J software. Three wt and two DAPT treated livers were analyzed.
Supplementary Material
Confocal projections through the liver of embryos and larvae immunostained with the 2F11 antibody. These images outline liver morphogenesis and development of the gallbladder (g) and extrahepatic ducts (ed) in relation to the intrahepatic ductal network. The 2F11 epitope is also detected in secretory cells on the intestinal epithelium (i), the ductal network of the pancreas (p).
(A, B) Confocal projections through the liver of a 80 hpf (A) and 96 hpf (B) larva immunostained with Keratin-18 (green) and Mdr (red) antibodies. Compared with double immunostains of 5 dpf larvae (Figure 2F″) there is much less overlap of the Mdr-1 and Keratin-18 epitopes at these developmental stages (open arrowheads). (C–F) Transmission electron micrographs showing the canalicular-terminal duct junction (arrows) in 71 hpf (C), 96 hpf (D) and 120 hpf (E, F) larvae. bd – bile duct, c- canaliculus, arrowhead – tight junctions. Bar is 500nm in C–E, 100nm in F
(A–D) Fluorescence microscopy images (lateral view) of live 6 dpf larvae (144 hpf) following ingestion of the fluorescent lipid bodipy-C16. In wild types (wt) strong fluorescence is detected in the intestine (i) and gallbladder (g). Fluorescence emission from both tissues is reduced in larvae treated with DAPT between 50 – 120 hpf. Each panel shows a unique larva. These data are representative of 10 wild type and 10 DAPT treated larvae.
(A–C) Confocal projections through the liver of wild type larvae (70 hpf, 92 hpf and 120 hpf) and larvae treated with DAPT from 48 hpf – 70 hpf (E), 48 – 92 hpf (F), 31 – 120 hpf (D) and 48 – 120 hpf (H), fixed immediately after the treatment, followed by 2F11 immunostainings. A larva treated with DAPT from 48 – 70 hpf and then fixed at 92 hpf after DAPT withdrawal at 70 hpf is also shown (G). DAPT treatment arrests intrahepatic biliary development (compare A with E; B with F; C with D and H). There is expansion of the ductal network after DAPT withdrawal (compare G with F).
Whole mount confocal images (63X) through the liver of developing Notch reporter larvae stained with the 2F11 antibody (A–D) and a GFP antibody (A′–D′) with overlap of the two markers (A″–D″). GFP positive biliary epithelia are first detected at 45 hpf (A′). At this stage scattered 2F11 positive cells are present in the liver with only partial overlap with GPF. From 50 hpf – 70 hpf there is progressive increase in the number of GFP positive cells. AT 50 hpf there is significant overlap between the GFP and 2F11 epitope in the biliary cells. There is nearly complete overlap between these markers at 60 hpf and 70 hpf. The gallbladder (g) and extrahepatic duct (ed) remain GPF negative.
Figure 6. Notch reporter expression in developing intrahepatic biliary cells.
Confocal projections through the liver of 45 hpf – 120 hpf Notch reporter larvae following anti-GFP immunostains. The GFP expression pattern closely resembles the distribution of the 2F11 epitope within intrahepatic biliary cells.
Acknowledgments
Grant sponsor: NIH
Grant number: DK54942
We thank Andrea Stout for assistance with live spinning disc confocal imaging. The work was also supported by the Fred and Suzanne Biesecker Pediatric Liver Center at The Children’s Hospital of Philadelphia.
References
- 1.Kamath BM, Piccoli DA. Heritable disorders of the bile ducts. Gastroenterol Clin North Am. 2003;32:857–875. doi: 10.1016/s0889-8553(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, Costa T, Pierpont ME, Rand EB, Piccoli DA, Hood L, Spinner NB. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 3.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 4.McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- 5.Lozier J, McCright B, Gridley T. Notch signaling regulates bile duct morphogenesis in mice. PLoS One. 2008;3:e1851. doi: 10.1371/journal.pone.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisler F, Nagl F, Mazur PK, Lee M, Zimber-Strobl U, Strobl LJ, Radtke F, Schmid RM, Siveke JT. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48(2):607–16. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- 7.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger BZ. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchorz JS, Kinter J, Müller M, Tornillo L, Heim MH, Bettler B. Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology 2009. 2009 Apr 27; doi: 10.1002/hep.23048. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Lorent K, Yeo SY, Oda T, Chandrasekharappa S, Chitnis A, Matthews RP, Pack M. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development. 2004;131:5753–5766. doi: 10.1242/dev.01411. [DOI] [PubMed] [Google Scholar]
- 10.Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–689. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews RP, Lorent K, Russo P, Pack M. The zebrafish onecut gene hnf-6 functions in an evolutionarily conserved genetic pathway that regulates vertebrate biliary development. Dev Biol. 2004;274:245–259. doi: 10.1016/j.ydbio.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Matthews RP, Lorent K, Pack M. Transcription factor onecut3 regulates intrahepatic biliary development in zebrafish. Dev Dyn. 2008;237:124–131. doi: 10.1002/dvdy.21407. [DOI] [PubMed] [Google Scholar]
- 13.Matthews RP, Plumb-Rudewiez N, Lorent K, Gissen P, Johnson CA, Lemaigre F, Pack M. Zebrafish vps33b, an ortholog of the gene responsible for human arthrogryposis-renal dysfunction-cholestasis syndrome, regulates biliary development downstream of the onecut transcription factor hnf6. Development. 2005;132:5295–5306. doi: 10.1242/dev.02140. [DOI] [PubMed] [Google Scholar]
- 14.Hampton JA, Lantz RC, Goldblatt PJ, Lauren DJ, Hinton DE. Functional units in rainbow trout (Salmo gairdneri, Richardson) liver: II. The biliary system. Anat Rec. 1988;221:619–634. doi: 10.1002/ar.1092210208. [DOI] [PubMed] [Google Scholar]
- 15.Hardman RC, Volz DC, Kullman SW, Hinton DE. An in vivo look at vertebrate liver architecture: three-dimensional reconstructions from medaka (Oryzias latipes) Anat Rec (Hoboken) 2007;290:770–82. doi: 10.1002/ar.20524. [DOI] [PubMed] [Google Scholar]
- 16.Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, Lewis J. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development. 2005;32:1093–1104. doi: 10.1242/dev.01644. [DOI] [PubMed] [Google Scholar]
- 17.Schaffeld M, Haberkamp M, Braziulis E, Lieb B, Markl J. Type II keratin cDNAs from the rainbow trout: implications for keratin evolution. Differentiation. 2002;70:292–299. doi: 10.1046/j.1432-0436.2002.700607.x. [DOI] [PubMed] [Google Scholar]
- 18.Schaffeld M, Höffling S, Haberkamp M, Conrad M, Markl J. Type I keratin cDNAs from the rainbow trout: independent radiation of keratins in fish. Differentiation. 2002;70:282–291. doi: 10.1046/j.1432-0436.2002.700606.x. [DOI] [PubMed] [Google Scholar]
- 19.Arias IM, Gatmaitan Z, Mazzanti R, Shu H, Kumamoto Y. Structure and function of P-glycoprotein in the normal liver and intestine. Princess Takamatsu Symp. 1990;21:229–239. Review. [PubMed] [Google Scholar]
- 20.Sakaguchi TF, Sadler KC, Crosnier C, Stainier DY. Endothelial signals modulate hepatocyte apicobasal polarization in zebrafish. Curr Biol. 2008;18:1565–1571. doi: 10.1016/j.cub.2008.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalashnikova MM, Kazanskaia NI. Unusual structure of the biliary system in the liver of the grass carp and the silver carp. Biull Eksp Biol Med. 1986;102:485–488. [PubMed] [Google Scholar]
- 22.Tanuma Y. Electron microscope observations on the intrahepatocytic bile canalicules and sequent bile ductules in the crucian, Carassius carassius. Arch Histol Jpn. 1980;43:1–21. doi: 10.1679/aohc1950.43.1. [DOI] [PubMed] [Google Scholar]
- 23.Warren M, Puskarczyk K, Chapman SC. Chick embryo proliferation studies using EdU labeling. Dev Dyn. 2009;238:944–949. doi: 10.1002/dvdy.21895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N, Leach SD. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho SY, Lorent K, Pack M, Farber SA. Zebrafish fat-free is required for intestinal lipid absorption and Golgi apparatus structure. Cell Metab. 2006;3:289–300. doi: 10.1016/j.cmet.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsuma Y, Marceau N, Ohta M, French SW. Cytokeratin intermediate filaments of rat hepatocytes: different cytoskeletal domains and their three-dimensional structure. Hepatology. 1988;8:559–568. doi: 10.1002/hep.1840080321. [DOI] [PubMed] [Google Scholar]
- 27.Yee NS, Lorent K, Pack M. Exocrine pancreas development in zebrafish. Dev Biol. 2005;284:84–101. doi: 10.1016/j.ydbio.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 28.Golson ML, Loomes KM, Oakey R, Kaestner KH. Ductal malformation and pancreatitis in mice caused by conditional Jag1 deletion. Gastroenterology. 2009;136(5):1761–71. doi: 10.1053/j.gastro.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 29.Kodama Y, Hijikata M, Kageyama R, Shimotohno K, Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127:1775–1786. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 31.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 32.Larrivée B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circ Res. 2009;104:428–441. doi: 10.1161/CIRCRESAHA.108.188144. Review. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Confocal projections through the liver of embryos and larvae immunostained with the 2F11 antibody. These images outline liver morphogenesis and development of the gallbladder (g) and extrahepatic ducts (ed) in relation to the intrahepatic ductal network. The 2F11 epitope is also detected in secretory cells on the intestinal epithelium (i), the ductal network of the pancreas (p).
(A, B) Confocal projections through the liver of a 80 hpf (A) and 96 hpf (B) larva immunostained with Keratin-18 (green) and Mdr (red) antibodies. Compared with double immunostains of 5 dpf larvae (Figure 2F″) there is much less overlap of the Mdr-1 and Keratin-18 epitopes at these developmental stages (open arrowheads). (C–F) Transmission electron micrographs showing the canalicular-terminal duct junction (arrows) in 71 hpf (C), 96 hpf (D) and 120 hpf (E, F) larvae. bd – bile duct, c- canaliculus, arrowhead – tight junctions. Bar is 500nm in C–E, 100nm in F
(A–D) Fluorescence microscopy images (lateral view) of live 6 dpf larvae (144 hpf) following ingestion of the fluorescent lipid bodipy-C16. In wild types (wt) strong fluorescence is detected in the intestine (i) and gallbladder (g). Fluorescence emission from both tissues is reduced in larvae treated with DAPT between 50 – 120 hpf. Each panel shows a unique larva. These data are representative of 10 wild type and 10 DAPT treated larvae.
(A–C) Confocal projections through the liver of wild type larvae (70 hpf, 92 hpf and 120 hpf) and larvae treated with DAPT from 48 hpf – 70 hpf (E), 48 – 92 hpf (F), 31 – 120 hpf (D) and 48 – 120 hpf (H), fixed immediately after the treatment, followed by 2F11 immunostainings. A larva treated with DAPT from 48 – 70 hpf and then fixed at 92 hpf after DAPT withdrawal at 70 hpf is also shown (G). DAPT treatment arrests intrahepatic biliary development (compare A with E; B with F; C with D and H). There is expansion of the ductal network after DAPT withdrawal (compare G with F).
Whole mount confocal images (63X) through the liver of developing Notch reporter larvae stained with the 2F11 antibody (A–D) and a GFP antibody (A′–D′) with overlap of the two markers (A″–D″). GFP positive biliary epithelia are first detected at 45 hpf (A′). At this stage scattered 2F11 positive cells are present in the liver with only partial overlap with GPF. From 50 hpf – 70 hpf there is progressive increase in the number of GFP positive cells. AT 50 hpf there is significant overlap between the GFP and 2F11 epitope in the biliary cells. There is nearly complete overlap between these markers at 60 hpf and 70 hpf. The gallbladder (g) and extrahepatic duct (ed) remain GPF negative.