Abstract
This study was designed to assess evidence for an association between the treatment of gastroesophageal reflux disease (GERD) with proton pump inhibitors (PPIs) and improvement in obstructive sleep apnea (OSA). We conducted a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies to evaluate the treatment effect of PPIs on OSA symptoms and indices in patients with GERD. EMBASE, MEDLINE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and ClinicalTrials.gov were reviewed up to October 2014. From 238 articles, two randomized trials and four prospective cohort studies were selected. In four cohort studies there were no differences in the apnea-hypopnea indices before and after treatment with PPIs (standard mean difference, 0.21; 95% confidence interval, −0.11 to 0.54). There was moderate heterogeneity among these studies. Two cohort studies revealed significantly decreased apnea indices after treatment (percent change, 31% and 35%), but one showed no significant difference. A significant improvement in the Epworth Sleepiness Scale was observed in three cohort studies and one trial. The frequency of apnea attacks recorded in diaries was decreased by 73% in one trial. In conclusion, available studies do not provide enough evidence to make firm conclusions about the effects of PPI treatment on OSA symptoms and indices in patients with concomitant GERD. Controlled clinical trials with larger sample sizes are needed to evaluate these associations. We recommend PPIs in OSA patients with concomitant GERD to treat reflux symptoms. This treatment may improve the quality of sleep without any effect on apnea-hypopnea indices.
The prevalence of gastroesophageal reflux disease (GERD) is significantly higher in patients with obstructive sleep apnea (OSA) (1–6), and 54% to 76% of patients with OSA have GERD (2, 3). These two conditions share one common risk factor, namely obesity, which increases the risk for both apnea and reflux (7). This association may be explained by lower esophageal sphincter pressures and prolonged esophageal relaxation following swallowing (8). These changes could increase the frequency and severity of reflux. Nocturnal reflux could cause sleep arousals and sleep fragmentation in OSA patients, and acid exposure could cause edema and inflammation in the upper airway, which increase the frequency of airway occlusions during inspiration (6). Studies supporting this hypothesis have shown that continuous positive airway pressure (CPAP) has antireflux effects in OSA patients (9, 10). However, other studies have reported that the severity of OSA does not correlate with the occurrence of reflux symptoms (11). Given the significant morbidity and mortality of OSA, including left ventricular dysfunction, arrhythmias, myocardial infarction, stroke, systemic hypertension, and risk of motor vehicle accidents, it is important to evaluate all factors associated with OSA and to consider the potential benefit of treatment of factors not directly related to upper airway anatomy (12–15). Several studies have suggested that proton pump inhibitors (PPIs) improve OSA symptoms and reduce some complications. We conducted a systematic review and meta-analysis of the published reports to better understand the treatment effect of GERD on the OSA-hypopnea syndrome.
METHODS
We searched EMBASE, MEDLINE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and ClinicalTrials.gov from database inception to October 2014. We used the following text words as search terms: “sleep apnea syndrome” and “proton pump inhibitor.” Our search included articles published in English and non-English languages. We also scanned the bibliographies of all retrieved articles for additional relevant articles.
Two independent reviewers (S.R. and S.K.) performed article selection, data extraction, and assessment of the risk of bias. Disagreements were resolved through consensus. Studies were included if they met the following criteria: 1) were controlled clinical trials or observational studies that assessed the effect of PPIs on OSA, including symptoms of daytime somnolence, nocturnal symptoms, Epworth Sleepiness Score (ESS), apnea-hypopnea index (AHI), apnea index, hypopnea index, and respiratory disturbance index; 2) reported concomitant GERD in patients with OSA; and 3) provided adequate data to extract the outcomes. We excluded studies that reported only the prevalence of GERD in OSA patients, patients who were treated with CPAP and PPIs, and OSA patients without underlying GERD who were treated with PPIs. If multiple updates of the same data were found, we used the most recent version for analysis. From each study, we abstracted the study design, setting, population characteristics (including sex, age, race or ethnicity, baseline body mass index [BMI], and baseline AHI), patient eligibility and exclusion criteria, number of patients, type of PPIs used, treatment duration, and method of outcome determination.
Two reviewers (S.R. and S.K.) independently assessed the quality of each trial by using a tool developed by the Cochrane Collaboration (16). Each trial was given an overall summary assessment of low, unclear, or high risk of bias. We adapted existing tools to assess the quality of observational studies. The strength of evidence for outcomes was graded as high, moderate, low, or very low according to the approach of the GRADE working group (17).
AHIs in patients before treatment versus after treatment were evaluated. Statistical analysis was conducted with Review Manager (RevMan Version 5.3, The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). The chi-square test and I2 statistic were used to address heterogeneity among studies. The results of the studies were pooled, and an overall standard mean difference with 95% confidence intervals (CIs) was obtained using generic inverse variance weighting and a random effects method.
RESULTS
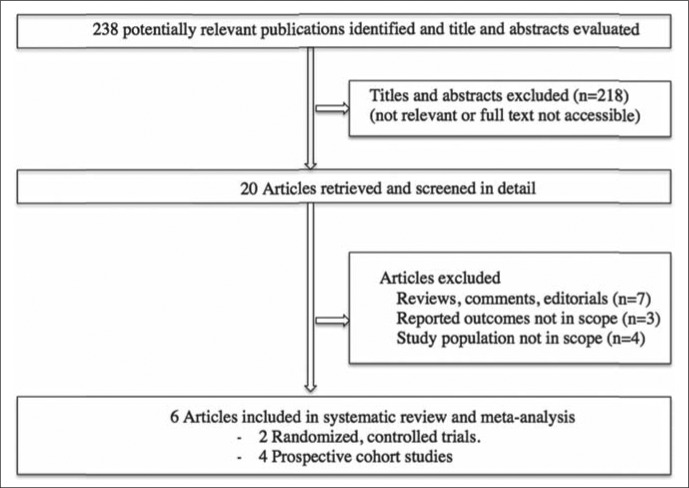
The electronic and manual searches yielded 238 total citations (Figure 1). We identified 20 potentially relevant full-text articles and analyzed six published articles, published as full papers, which met our inclusion criteria. No additional abstracts were identified by hand searches of conference proceedings.
Figure 1.
Literature search strategy.
Two controlled clinical trials compared the effects of PPIs and placebos on OSA (18, 19). The first trial was a double-blind, randomized, placebo-controlled crossover trial (18). This trial recruited 57 patients with ESS > 8 (mean ESS ± SD: 14 ± 3.5), mild to moderate OSA (mean AHI ± SD: 10 ± 8.4), and typical symptoms and finding of GERD between February 2004 and August 2006 and treated them with either placebo or pantoprazole 40 mg once daily followed by a 2-week washout period and then a 2-week crossover treatment period. ESS decreased with pantoprazole (–1.8, 95% CI: −3.0 to −0.5) compared with placebo (–1.5, 95% CI: −2.1 to −0.4, P = 0.04). There were no significant changes in sleep-related quality of life using the Functional Outcomes Sleep Questionnaire or reaction times, tested by having subjects push a button as fast as they could whenever they saw a clock begin counting up from 0000 (18). The trial had a low risk of bias.
The second trial included 20 patients with confirmed OSA (mean AHI: 30.9) by overnight polysomnography and confirmed GERD by 24 h esophageal pH electrode (19). The patients were randomly divided into two groups and treated with omeprazole 20 mg or placebo 30 minutes before breakfast and before dinner (n = 10 each group) for 6 weeks. The number of apnea attacks, which were defined as a symptom of nocturnal choking, gasping, or snoring that awakened the patients, was recorded by patients in diaries. The frequency of apnea attacks decreased 73% in the treatment group compared to their basal period and in the treatment group compared with the placebo group in the sixth week (P < 0.001). This trial had an unclear risk of bias due to unclear reporting of randomization and allocation concealment techniques.
The four prospective cohort studies analyzed in this review included 91 patients who had OSA and GERD. The main characteristics of these studies are summarized in Table 1. Four studies (20–23) reported data on AHI; three studies (20, 21, 23) provided data on apnea index, and three studies (20, 22) reported data on ESS. These trials had methodological limitations that led to an unclear risk of bias since all studies were single-center prospective cohort studies conducted in the United States, used patients' baseline parameters as the control, and had small sample sizes.
Table 1.
Characteristics of obstructive sleep apnea patients using proton pump inhibitors
| First author | Treated cases (n) |
Control cases (n) |
Mean age (years) |
Women (n) |
BMI (kg/m2) |
Mean ESS |
Mean AHI |
Proton pump inhibitor (40 mg/day) |
|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||
| Suurna | 28* | 29 | 51 | 33 | 31 | 14 | 10 | Pantoprazole |
| Bortolotti | 10 | 10 | 55 | 3 | 30 | NR | 30.9 | Omeprazole |
| Prospective trials | ||||||||
| Friedman | 29 | – | 45 | 17 | 33 | 14.2 | 38 | Esomeprazole |
| Steward | 27 | – | 49 | 9 | 33 | 12.9 | 15.4 | Pantoprazole |
| Orr | 25 | – | 43 | 7 | 31 | 12 | 9.3 | Rabeprazole |
| Senior | 10 | – | 18–59 | 0 | NR | NR | 62 | Omeprazole |
Crossover design.
AHI indicates apnea-hypopnea index; ESS, Epworth Sleepiness Scale; NR, not reported; –, not applicable.
Four observational studies compared the effects of PPI use on AHI in patients before treatment versus after treatment (20–23). There was no statistically significant difference between the two groups. The standard mean difference was 0.21 (95% CI: −0.11 to 0.54) (Table 2). Heterogeneity was minimal in the studies (P for heterogeneity = 0.31, I 2 = 17%).
Table 2.
Effect of proton pump inhibitors on apnea and hypopnea indices*
| Study or subgroup | Before treatment |
After treatment |
Weight | Std. mean difference IV, random, 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Total | Mean | SD | Total | |||
| Friedman (2007) | 37.9 | 19.1 | 29 | 28.8 | 11.5 | 29 | 30.3% | 0.57 (0.04, 1.10) |
| Orr (2009) | 9.3 | 4.7 | 25 | 9.1 | 8.7 | 25 | 27.8% | 0.03 (−0.53, 0.58) |
| Steward (2004) | 15.4 | 11.7 | 27 | 16.2 | 8.0 | 27 | 29.6% | −0.08 (−0.61, 0.45) |
| Senior (2001) | 62.0 | 30.5 | 10 | 46.0 | 37.0 | 10 | 12.2% | 0.45 (−0.44, 1.34) |
| Total (95% CI) | 91 | 91 | 100.0% | 0.21 (−0.11, 0.54) | ||||
Heterogeneity: Tau2 = 0.02; Chi2 = 3.62; df = 3 (P = 0.31); F = 17%. Test for overall effect: Z = 1.28 (P = 0.20).
Three observational studies (20, 21, 23) compared the effects of PPI use on the apnea index in patients before treatment versus after treatment. Two studies reported a statistically significant improvement in the apnea index (mean 5.9 ± 7.2 to 3.8 ± 4.7, P = 0.04 in the study by Friedman [20]; mean 45 [range: 10–108] to 31 [range: 1–78], P = 0.04 in the study by Senior [23]). However, the study reported by Steward revealed no statistically significant difference between the two groups (mean change 1.4, 95% CI: −0.1 to 2.9, P = 0.07) (21). No baseline mean apnea index in this study was available.
Three observational studies (20–22) compared the effects of PPI use on ESS in patients before treatment versus after treatment. Two studies reported statistically significant improvement of ESS and provided numerical scores for the ESS before and after treatment (Figure 2). One study reported a statistically significant mean change in ESS (21). Two studies reported decreased snoring as assessed by bed partners (20, 21), and one reported decreased upper airway inflammation based on fiberoptic nasopharyngoscopy examinations before and after treatment (22).
Figure 2.
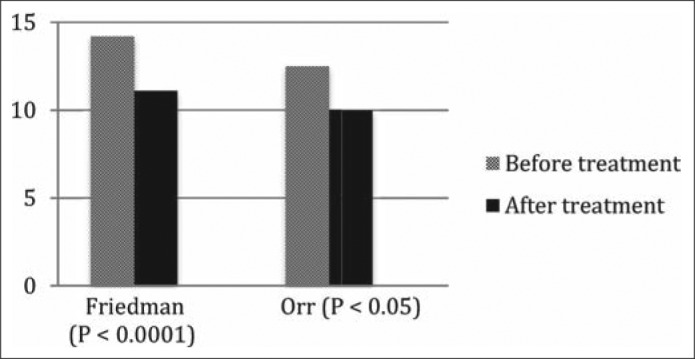
Effect of proton pump inhibitors on Epworth Sleepiness Scale.
DISCUSSION
The articles used in this systematic review and meta-analysis studied the effects of PPIs on OSA indices, daytime sleepiness, and other nocturnal symptoms. This analysis included two randomized controlled trials and four observational prospective studies. The studies identified a modest benefit of PPI therapy on daytime somnolence but did not identify any significant difference in apnea or hypopnea indices. The effect on the ESS was modest and not associated with any change in sleep-related quality of life or reaction times. The other trial reported a reduction in nocturnal symptoms based on diary records. The symptoms are not necessarily unique to OSA and could have other causes during the night. The results with AHI comparisons should be interpreted with caution, due to the clinical heterogeneity among studies and unclear risk of bias. These limitations are discussed more below.
There are several potential explanations for the lack of benefit with PPI therapy in patients with OSA. GERD and OSA are relatively common problems; they could occur coincidentally in some patients and have no causal relationships. Shepherd and coworkers reported detailed studies in eight patients with OSA undergoing polysomnography with esophageal manometry and pH monitoring (8). During the recording phase without CPAP, the patients had 70 ± 39 respiratory events per hour and 2.7 ± 1.8 reflux events per hour. The number of obstructive events in this study appeared to have little or no effect on reflux events. In addition, overlapping symptoms could confuse this situation and an y conclusions about cause and effect relationships. However, very frequent reflux events could influence OSA and sleep quality through central nervous system arousals, chronic lower esophageal inflammation with vagal stimulation, and laryngeal inflammation with changes in the upper airway dynamics.
There might be a threshold in the number of reflux events required before any effect occurs, and the duration of these two syndromes could influence any interaction. The treatment of GERD with PPIs may require more time than most studies have used in their study design. An adequate treatment period for PPIs to completely resolve anatomic changes caused by acid reflux–related injury can take up to 6 months; however, only one study in this review reached that period of time (20, 24, 25). Another possibility is that PPI treatment may truly improve OSA symptoms, but this effect would be important only in patients with very frequent reflux events.
In addition, treatment with CPAP could have several possible effects on GERD which might influence results in studies on GERD in patients with OSA. First, CPAP may have no effect, or CPAP could change intrathoracic pressure dynamics and reduce reflux. This effect most likely would occur at the gastroesophageal junction. CPAP could also change esophageal motility and improve esophageal clearance. Shepherd reported that CPAP increased the nadir pressure in the lower esophagus and reduced the duration of the lower esophageal sphincter relaxation time (8). Again, these effects are important only in patients with frequent reflux events.
Our meta-analysis has several limitations. First, our analysis included only six studies with small numbers of patients. Second, there was heterogeneity across the studies in the analysis of AHI reduction. This can be partly explained by different study designs, types of PPIs, and duration of treatment. Most studies had design limitations, including small sample size and single-center cohort studies, which resulted in a low strength of evidence. Observational studies that used patients' baseline parameters as controls may be compromised by placebo effects in addition to other design limitations. The ESS is a self-assessment scale that represents only a predilection to fall asleep and is not a specific outcome in OSA patients. Other symptoms reported in sleep diaries may not be specific for OSA and may introduce another source of variability in study results. Patients with GERD have reduced health-related quality of life, and this is associated with nocturnal reflux symptoms (26, 27). We used a random effects method for analysis to account for heterogeneity among studies, and we attempted to minimize the risk of missing relevant studies by searching multiple databases, bibliographies, and trial registries.
References
- 1.Ing AJ, Ngu MC, Breslin AB. Obstructive sleep apnea and gastroesophageal reflux. Am J Med. 2000;108(Suppl 4a):120S–125S. doi: 10.1016/s0002-9343(99)00350-2. [DOI] [PubMed] [Google Scholar]
- 2.Green BT, Broughton WA, O'Connor JB. Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea treated with continuous positive airway pressure. Arch Intern Med. 2003;163(1):41–45. doi: 10.1001/archinte.163.1.41. [DOI] [PubMed] [Google Scholar]
- 3.Valipour A, Makker HK, Hardy R, Emegbo S, Toma T, Spiro SG. Symptomatic gastroesophageal reflux in subjects with a breathing sleep disorder. Chest. 2002;121(6):1748–1753. doi: 10.1378/chest.121.6.1748. [DOI] [PubMed] [Google Scholar]
- 4.Guda N, Partington S, Vakil N. Symptomatic gastro-oesophageal reflux, arousals and sleep quality in patients undergoing polysomnography for possible obstructive sleep apnoea. Alimentary Pharm Therapeutics. 2004;20(10):1153–1159. doi: 10.1111/j.1365-2036.2004.02263.x. [DOI] [PubMed] [Google Scholar]
- 5.Graf KI, Karaus M, Heinemann S, Körber S, Dorow P, Hampel KE. Gastroesophageal reflux in patients with sleep apnea syndrome. Z Gastroenterol. 1995;33(12):689–693. [PubMed] [Google Scholar]
- 6.Penzel T, Becker HF, Brandenburg U, Labunski T, Pankow W, Peter JH. Arousal in patients with gastro-oesophageal reflux and sleep apnoea. Eur Respir J. 1999;14(6):1266–1270. doi: 10.1183/09031936.99.14612669. [DOI] [PubMed] [Google Scholar]
- 7.Janson C, Gislason T, De Backer W, Plaschke P, Björnsson E, Hetta J, Kristbjarnason H, Vermeire P, Boman G. Daytime sleepiness, snoring and gastro-oesophageal reflux amongst young adults in three European countries. J Intern Med. 1995;237(3):277–285. doi: 10.1111/j.1365-2796.1995.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd K, Hillman D, Holloway R, Eastwood P. Mechanisms of nocturnal gastroesophageal reflux events in obstructive sleep apnea. Sleep Breath. 2011;15(3):561–570. doi: 10.1007/s11325-010-0404-x. [DOI] [PubMed] [Google Scholar]
- 9.Kerr P, Shoenut JP, Millar T, Buckle P, Kryger MH. Nasal CPAP reduces gastroesophageal reflux in obstructive sleep apnea syndrome. Chest. 1992;101(6):1539–1544. doi: 10.1378/chest.101.6.1539. [DOI] [PubMed] [Google Scholar]
- 10.Zanation AM, Senior BA. The relationship between extraesophageal reflux (EER) and obstructive sleep apnea (OSA) Sleep Med Rev. 2005;9(6):453–458. doi: 10.1016/j.smrv.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Kim HN, Vorona RD, Winn MP, Doviak M, Johnson DA, Ware JC. Symptoms of gastro-oesophageal reflux disease and the severity of obstructive sleep apnoea syndrome are not related in sleep disorders center patients. Aliment Pharmacol Ther. 2005;21(9):1127–1133. doi: 10.1111/j.1365-2036.2005.02447.x. [DOI] [PubMed] [Google Scholar]
- 12.Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J. Cooperative Group Burgos-Santander. The association between sleep apnea and the risk of traffic accidents. N Engl J Med. 1999;340(11):847–851. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 13.Hla KM, Young TB, Bidwell T, Palta M, Skatrud JB, Dempsey J. Sleep apnea and hypertension. A population-based study. Ann Intern Med. 1994;120(5):382–388. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 15.Caples SM, Somers VK. Sleep-disordered breathing and atrial fibrillation. Prog Cardiovasc Dis. 2009;51(5):411–415. doi: 10.1016/j.pcad.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, G⊘tzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Suurna MV, Welge J, Surdulescu V, Kushner J, Steward DL. Randomized placebo-controlled trial of pantoprazole for daytime sleepiness in GERD and obstructive sleep disordered breathing. Otolaryngol Head Neck Surg. 2008;139(2):286–290. doi: 10.1016/j.otohns.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Bortolotti M, Gentilini L, Morselli C, Giovannini M. Obstructive sleep apnoea is improved by a prolonged treatment of gastrooesophageal reflux with omeprazole. Dig Liver Dis. 2006;38(2):78–81. doi: 10.1016/j.dld.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Friedman M, Gurpinar B, Lin HC, Schalch P, Joseph NJ. Impact of treatment of gastroesophageal reflux on obstructive sleep apnea-hypopnea syndrome. Ann Otol Rhinol Laryngol. 2007;116(11):805–811. doi: 10.1177/000348940711601103. [DOI] [PubMed] [Google Scholar]
- 21.Steward DL. Pantoprazole for sleepiness associated with acid reflux and obstructive sleep disordered breathing. Laryngoscope. 2004;114(9):1525–1528. doi: 10.1097/00005537-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Orr WC, Robert JJ, Houck JR, Giddens CL, Tawk MM. The effect of acid suppression on upper airway anatomy and obstruction in patients with sleep apnea and gastroesophageal reflux disease. J Clin Sleep Med. 2009;5(4):330–334. [PMC free article] [PubMed] [Google Scholar]
- 23.Senior BA, Khan M, Schwimmer C, Rosenthal L, Benninger M. Gastroesophageal reflux and obstructive sleep apnea. Laryngoscope. 2001;111(12):2144–2146. doi: 10.1097/00005537-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Belafsky PC, Postma GN, Koufman JA. Laryngopharyngeal reflux symptoms improve before changes in physical findings. Laryngoscope. 2001;111(6):979–981. doi: 10.1097/00005537-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Koufman JA, Aviv JE, Casiano RR, Shaw GY. Laryngopharyngeal reflux: position statement of the Committee on Speech, Voice, and Swallowing Disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127(1):32–35. doi: 10.1067/mhn.2002.125760. [DOI] [PubMed] [Google Scholar]
- 26.Farup C, Kleinman L, Sloan S, Ganoczy D, Chee E, Lee C, Revicki D. The impact of nocturnal symptoms associated with gastroesophageal reflux disease on health-related quality of life. Arch Intern Med. 2001;161(1):45–52. doi: 10.1001/archinte.161.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Revicki DA, Wood M, Maton PN, Sorensen S. The impact of gastroesophageal reflux disease on health-related quality of life. Am J Med. 1998;104(3):252–258. doi: 10.1016/s0002-9343(97)00354-9. [DOI] [PubMed] [Google Scholar]