Abstract
Pegaspargase is a chemotherapy drug used in the treatment of acute lymphoblastic leukemia (ALL). One of the adverse effects of pegaspargase is hepatotoxicity, which can rapidly lead to liver failure and death. We report a patient with ALL who developed pegaspargase-induced severe hepatotoxicity that was rescued by treatment with vitamin B complex and L-carnitine. Our patient had a quicker response than prior reported cases, suggesting this treatment might be a better regimen.
Acute lymphoblastic leukemia (ALL) in young adults is frequently treated with pediatric-inspired multiagent chemotherapy. Some of these treatments include asparaginase as an integral part of the regimen. Asparaginase has a significant adverse effect profile, including risk of thrombosis, bleeding, pancreatic hypersensitivity, and hepatotoxicity. Asparaginase toxicity on the liver can be quite severe and may produce a rapid functional decline and even lead to death (1). Typical therapy for this adverse event is supportive care. We report a patient with ALL who developed pegaspargase-induced severe hepatotoxicity that dramatically recovered after treatment with a regimen of vitamin B complex and L-carnitine.
CASE REPORT
A 23-year-old man with precursor B-cell ALL was treated with an induction chemotherapy regimen of augmented Berlin-Frankfurt-Münster. This pediatric regimen includes weekly doxorubicin at 25 mg/m2, weekly vincristine at 2 mg for three doses, one dose of pegaspargase at 2500 unit/m2 (intravenous, on day 4), and prednisone at 60 mg twice daily for 1 month. The patient also received prednisone and palonosetron. On day 8, his total bilirubin level started to increase from a baseline of 0.8 mg/dL, gradually reaching 13.5 mg/dL on day 22. Over the same time period, his international normalized ratio also increased from 1.1 to 1.9. Subsequently, his alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase rose to 495 U/L, 150 U/L, and 416 U/L, respectively, on days 27 to 29. He did not develop encephalopathy or ascites. He had no history of smoking, drinking alcohol, or using recreational drugs. His body mass index and his fasting cholesterol level were within normal limits. Testing for viral hepatitis A, B, and C, herpes simplex virus, human immunodeficiency virus, Epstein-Barr virus, cytomegalovirus, parvovirus B19, mononucleosis screen, and bacterial infections was negative. Abdominal ultrasound with Doppler revealed no venoocclusive disease. An abdominal computed tomography (CT) scan revealed an enlarged steatotic liver with normal gallbladder.
Vitamin B complex (Dialyvite; one tablet containing vitamin C 100 mg, thiamine 1.5 mg, riboflavin B2 1.7 mg, niacin 20 mg, pantothenic acid 10 mg, vitamin B6 10 mg, folic acid 1 mg, vitamin B12 6 mcg, and biotin 300 mcg), one tablet twice daily, and intravenous levocarnitine 75 mg/kg every 4 hours were started on day 20 and continued for 11 days. The patient's total bilirubin level started to decrease 3 days after treatment. Within 1 week, his total bilirubin level was 4.6 mg/dL, and it normalized on day 40. Simultaneously, his transaminase and prothrombin time also normalized, as shown in Figure 1. A CT scan 2 months later showed a resolution of fatty liver (Figure 2).
Figure 1.
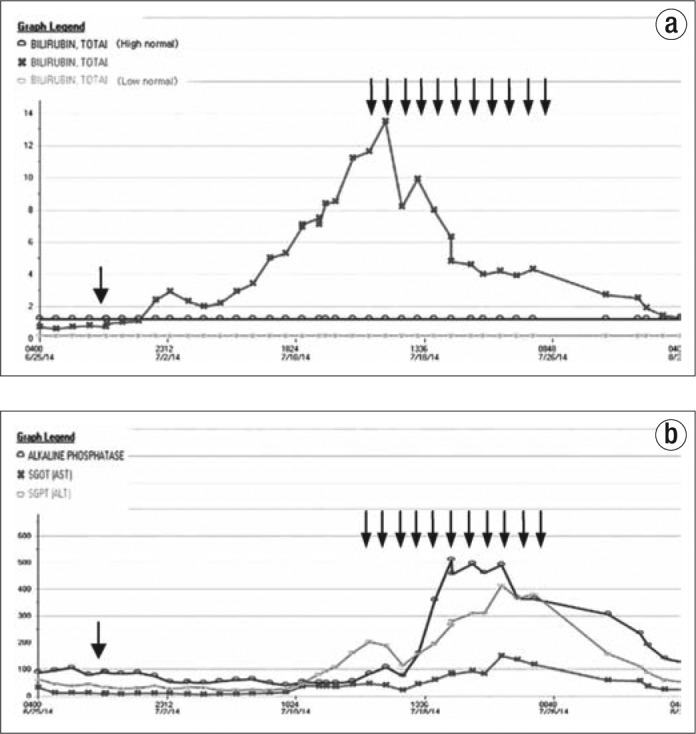
(a) Total bilirubin and (b) alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase values after treatment. The arrow on day 4 indicates one dose of pegaspargase, and the 11 arrows on days 20 to 31 indicate administration of vitamin B complex and L-carnitine.
Figure 2.
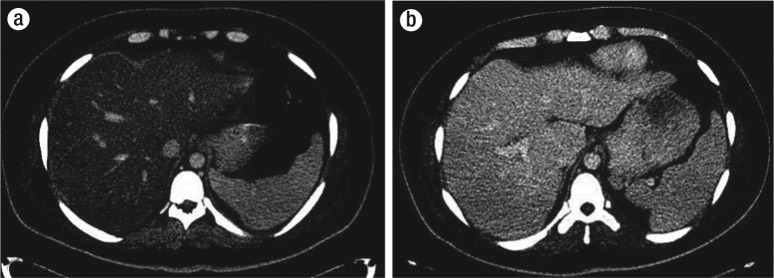
Liver CT, under liver window, showing fatty liver, induced by aspargase, (a) at day 14 and (b) resolved 2 months later.
DISCUSSION
Asparaginase, a major drug component in the therapy for ALL, depletes the circulating asparagine pool, thereby inhibiting protein synthesis and producing apoptosis (2). Administration of asparaginase in all dosages has been associated with disturbance of hepatic function and lipid metabolism (3). An asparaginase dosing strategy based on its pharmacokinetic characteristics in adults has been reported (4), but 20% of patients with this regimen have to discontinue their treatment due to severe hepatic toxicities. L-carnitine and vitamin B complex have been used in the treatment of nucleoside reverse transcriptase inhibitor–induced mitochondrial toxicities with success (5). The underlying mechanisms of hepatotoxicity induced by these two classes of medications are similar and involve drug-related mitochondrial toxicities. L-carnitine and vitamin B complex are mitochondrial cofactors. By supplementing these cofactors, the mitochondrial toxicities can be corrected (5, 6). Only one case report describes how asparaginase-related liver dysfunction can be managed by a combination of L-carnitine and vitamin B complex (6). Overall, we believe that the vitamin B complex and levocarnitine regimen may be an effective and safe therapy for pegaspargase-induced hepatotoxicity.
References
- 1.Bodmer M, Sulz M, Stadlmann S, Droll A, Terracciano L, Krähenbühl S. Fatal liver failure in an adult patient with acute lymphoblastic leukemia following treatment with L-asparaginase. Digestion. 2006;74(1):28–32. doi: 10.1159/000095827. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 3.Whitecar JP, Jr, Bodey GP, Harris JE, Freireich EJ. L-asparaginase. N Engl J Med. 1970;282(13):732–734. doi: 10.1056/NEJM197003262821307. [DOI] [PubMed] [Google Scholar]
- 4.Douer D, Aldoss I, Lunning MA, Burke PW, Ramezani L, Mark L, Vrona J, Park JH, Tallman MS, Avramis VI, Pullarkat V, Mohrbacher AM. Pharmacokinetics-based integration of multiple doses of intravenous pegaspargase in a pediatric regimen for adults with newly diagnosed acute lymphoblastic leukemia. J Clin Oncol. 2014;32(9):905–911. doi: 10.1200/JCO.2013.50.2708. [DOI] [PubMed] [Google Scholar]
- 5.Brinkman K, Vrouenraets S, Kauffmann R, Weigel H, Frissen J. Treatment of nucleoside reverse transcriptase inhibitor-induced lactic acidosis. AIDS. 2000;14(17):2801–2802. doi: 10.1097/00002030-200012010-00027. [DOI] [PubMed] [Google Scholar]
- 6.Al-Nawakil C1, Willems L, Mauprivez C, Laffy B, Benm'rad M, Tamburini J, Fontaine H, Sogni P, Terris B, Bouscary D, Moachon L. Successful treatment of L-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors. Leuk Lymphoma. 2014;55(7):1670–1674. doi: 10.3109/10428194.2013.845886. [DOI] [PubMed] [Google Scholar]