Abstract
Small cell prostate carcinoma (SCPC) has a clinical course and prognosis that is markedly different from that of common adenocarcinoma of the prostate. The patient in this case presented with fever of unknown origin, dyspnea, and near spinal cord compression. He was subsequently found to have widely metastatic high-grade neuroendocrine carcinoma of prostatic origin. This case emphasizes that despite the commonality of prostate cancer, there are rare presentations of this common disease.
Small cell prostate carcinoma (SCPC) is a rare form of extrapulmonary high-grade neuroendocrine carcinoma accounting for <0.5% to 1% of all prostate cancers (1). It is characterized by an aggressive clinical course and portends a poor prognosis. Locally advanced or metastatic disease is common at the time of presentation. SCPC shares many clinical and morphologic features with small cell carcinoma of the lung. Given the rarity of this malignancy, treatment is frequently extrapolated from experience with small cell carcinoma of the lung. Presented is a case of a lung nodule found to be an extrapulmonary high-grade neuroendocrine carcinoma of prostatic origin.
CASE PRESENTATION
A 78-year-old man with chronic obstructive pulmonary disease presented with a 6-month history of fever of unknown origin. Over the preceding months he had nonproductive cough, night sweats, dyspnea, intractable back pain, and a 20 lb unintentional weight loss. Radiographs showed no focal lesions. Despite multiple rounds of antibiotics, the fever continued with no identifiable etiology. Examination revealed crackles in the left lung base and pain to palpation along the thoracic vertebrae. His white blood cell count was 12.7 cells/mcL; C-reactive protein, 13.5 mg/L; erythrocyte sedimentation rate, 40 mm/hr; and carcinoembryonic antigen, 46.5 mcg/L. His body mass index was 31.1 kg/m2.
Computed tomography of the chest and abdomen/pelvis revealed innumerable pulmonary and pleural lesions and a large, necrotic hepatic mass (Figure 1). The prostate gland measured 5.6 × 7.8 cm. Magnetic resonance imaging of the thoracic and lumbar spine also revealed diffuse bone involvement. The patient underwent lung nodule biopsy. Histologic study disclosed a poorly differentiated neuroendocrine carcinoma, small cell variant, with markers positive for synaptophysin, chromogranin, and prostatic acid phosphatase (Figure 2). An MIB-1 fraction (a cellular marker of proliferation) was measured at 40%, consistent with G3 (high-grade) disease. Transrectal ultrasound-guided prostate needle biopsy demonstrated adenocarcinoma of the prostate gland, Gleason 9 with high-grade neuroendocrine differentiation focally noted.
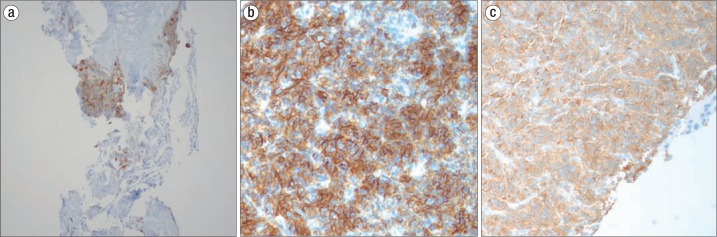
Figure 1.
Lung nodule with positive staining for (a) prostatic acid phosphatase, (b) synaptophysin, and (c) chromogranin.
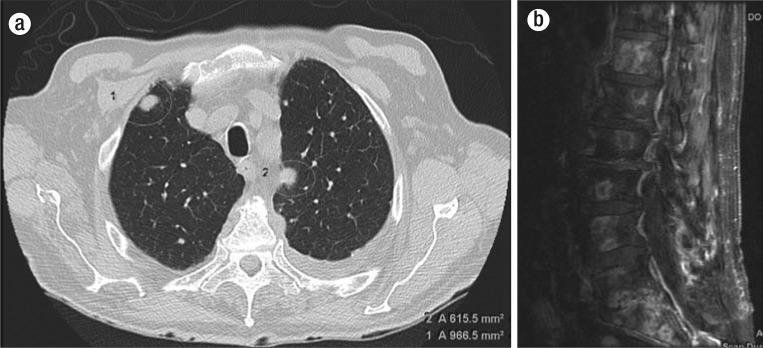
Figure 2.
Imaging. (a) Noncontrast axial CT chest image shows multiple bilateral pulmonary nodules consistent with pulmonary metastatic disease. (b) Postcontrast sagittal T1-weighted MRI of the lumbar spine demonstrates multiple predominantly peripheral enhancing osseous metastases on a background of diffuse marrow signal abnormality.
Systemic chemotherapy, radiation, and hormonal therapy were initiated with a combination of carboplatin and etoposide. Following six cycles of systemic therapy and segmental radiation to the spine, the patient's symptoms significantly improved. Repeat imaging revealed significant reduction of metastatic lesions with diffuse improvement in his widespread bone disease.
DISCUSSION
SCPC was first described by Wenk et al in 1977 (2). It accounts for <1% to 2% of all small cell cancers and occurs in 0.5% to 1% of men with prostate cancer (3). It has an aggressive clinical course. At the time of diagnosis, approximately 75% of patients have advanced stage disease. Common sites of metastasis include the lung, bladder, liver, and bone (4). Patients typically present with symptoms related to enlarged prostate, specifically changes in urine stream. Interestingly, our patient presented only with shortness of breath, fever, and back pain. The diagnosis of SCPC was made only after the lung nodule was biopsied and stained positive for prostate-specific antigen (PSA). The low-grade fevers our patient experienced were ultimately attributed to his underlying malignancy.
SCPC can occur concomitantly with adenocarcinoma or as isolated disease; approximately one-half of patients have mixed tumors (5). Positive staining for neuroendocrine markers including chromogranin, CD-56, synaptophysin, and neuron-specific enolase are frequently noted in the diagnosis of SCPC (6). The presence of at least one such marker occurs in 90% of SCPC cases (3). Although SCPC and prostatic adenocarcinoma can occur concomitantly, serum PSA levels do not correlate with burden of disease (7). Our patient's PSA was mildly elevated at 6.25 ng/mL; however, imaging revealed diffusely metastatic disease. Typically, such elevations of PSA are seen in cases of combined adenocarcinoma and SCPC.
Given the lack of randomized data for any high-grade neuroendocrine carcinomas of extrapulmonary origin, frontline treatments for SCPC derive their origin from commonly accepted therapies for small cell lung cancer. Accepted front-line treatment generally involves a platinum-based therapy plus etoposide, with radiation included as appropriate. In the setting of advanced disease there is currently no curative therapy.
One study by Hindson et al used a treatment regimen of cyclophosphamide, doxorubicin, and vincristine but could only induce a 4-month remission in patients with widely metastatic disease (8). General survival ranges from 9 to 13 months (9). However, there is limited data regarding survival difference in pure SCPC versus combined adenocarcinoma with concomitant SCPC. In a study by Asmis et al, the overall survival time was 9.5 months for combined prostate adenocarcinoma and small cell prostate carcinoma, with similar survival for pure small cell carcinoma (9).
Given widespread metastatic disease, systemic chemotherapy and radiation were pursued in our patient. Hormonal therapy has utility if concomitant prostatic adenocarcinoma is present. The patient in our case received a month-long course of bicalutamide followed by scheduled leuprorelin injections on a 3-month basis. At the time of discharge, he was able to walk out of the hospital without dyspnea. Nine months after discharge, the patient was still alive and in hospice.
References
- 1.Abbas F, Civantos F, Benedetto P, Soloway MS. Small cell carcinoma of the bladder and prostate. Urology. 1995;46(5):617–630. doi: 10.1016/S0090-4295(99)80290-8. [DOI] [PubMed] [Google Scholar]
- 2.Wenk RE, Bhagavan BS, Levy R, Miller D, Weisburger W. Ectopic ACTH, prostatic oat cell carcinoma, and marked hypernatremia. Cancer. 1977;40(2):773–778. doi: 10.1002/1097-0142(197708)40:2<773::aid-cncr2820400226>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Nadal R, Schweizer M, Kryvenko ON, Epstein JI, Eisenberger MA. Small cell carcinoma of the prostate. Nat Rev Urol. 2014;11(4):213–219. doi: 10.1038/nrurol.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubenstein JH, Katin MJ, Mangano MM, Dauphin J, Salenius SA, Dosoretz DE, Blitzer PH. Small cell anaplastic carcinoma of the prostate: seven new cases, review of the literature, and discussion of a therapeutic strategy. Am J Clin Oncol. 1997;20(4):376–380. doi: 10.1097/00000421-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Têtu B, Ro JY, Ayala AG, Johnson DE, Logothetis CJ, Ordonez NG. Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer. 1987;59(10):1803–1809. doi: 10.1002/1097-0142(19870515)59:10<1803::aid-cncr2820591019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32(1):65–71. doi: 10.1097/PAS.0b013e318058a96b. [DOI] [PubMed] [Google Scholar]
- 7.Oesterling JE, Hauzeur CG, Farrow GM. Small cell anaplastic carcinoma of the prostate: a clinical, pathological and immunohistological study of 27 patients. J Urol. 1992;147(3 Pt 2):804–807. doi: 10.1016/s0022-5347(17)37390-1. [DOI] [PubMed] [Google Scholar]
- 8.Hindson DA, Knight LL, Ocker JM. Small-cell carcinoma of prostate. Transient complete remission with chemotherapy. Urology. 1985;26(2):182–184. doi: 10.1016/0090-4295(85)90060-3. [DOI] [PubMed] [Google Scholar]
- 9.Asmis TR, Reaume MN, Dahrouge S, Malone S. Genitourinary small cell carcinoma: a retrospective review of treatment and survival patterns at The Ottawa Hospital Regional Cancer Center. BJU Int. 2006;97(4):711–715. doi: 10.1111/j.1464-410X.2006.06041.x. [DOI] [PubMed] [Google Scholar]