Novel monolignol p-coumarate conjugates in eudicot lignin result from the introduction of a monocot acyltransferase.
Abstract
Lignin acylation, the decoration of hydroxyls on lignin structural units with acyl groups, is common in many plant species. Monocot lignins are decorated with p-coumarates by the polymerization of monolignol p-coumarate conjugates. The acyltransferase involved in the formation of these conjugates has been identified in a number of model monocot species, but the effect of monolignol p-coumarate conjugates on lignification and plant growth and development has not yet been examined in plants that do not inherently possess p-coumarates on their lignins. The rice (Oryza sativa) p-COUMAROYL-Coenzyme A MONOLIGNOL TRANSFERASE gene was introduced into two eudicots, Arabidopsis (Arabidopsis thaliana) and poplar (Populus alba × grandidentata), and a series of analytical methods was used to show the incorporation of the ensuing monolignol p-coumarate conjugates into the lignin of these plants. In poplar, specifically, the addition of these conjugates did not occur at the expense of the naturally incorporated monolignol p-hydroxybenzoates. Plants expressing the p-COUMAROYL-Coenzyme A MONOLIGNOL TRANSFERASE transgene can therefore produce monolignol p-coumarate conjugates essentially without competing with the formation of other acylated monolignols and without drastically impacting normal monolignol production.
Lignification of plant cell walls prototypically involves the polymerization of the monolignols (MLs), p-coumaryl alcohol, coniferyl alcohol (CA), and sinapyl alcohol (SA), predominantly by stepwise radical coupling of each monomer to the phenolic end of the growing polymer (Sarkanen and Ludwig, 1971; Boerjan et al., 2003; Ralph et al., 2004). The contribution of various MLs to the lignins depends on plant species, cell type, plant tissue, and tissue age. Although the majority of the lignin polymer is derived from these three MLs, the lignification process has a high degree of metabolic plasticity (Boerjan et al., 2003; Ralph et al., 2004; Ralph, 2007; Vanholme et al., 2012). Of particular interest are ML conjugates in which the ester group can be acetate (Ac; Sarkanen et al., 1967; Ralph, 1996; Ralph and Lu, 1998; Del Río et al., 2007; del Río et al., 2008; Martínez et al., 2008), p-hydroxybenzoate (pBz; Venverloo, 1971; Monties and Lapierre, 1981; Landucci et al., 1992; Tomimura, 1992a, 1992b; Hibino et al., 1994; Sun et al., 1999; Kuroda et al., 2001; Lu et al., 2004, 2015; Morreel et al., 2004; Rencoret et al., 2013), p-coumarate (pCA; Monties and Lapierre, 1981; Ralph et al., 1994; Crestini and Argyropoulos, 1997; del Río et al., 2008, 2012a, 2012b; Withers et al., 2012; Rencoret et al., 2013; Petrik et al., 2014), or ferulate (FA; Grabber et al., 2008; Ralph, 2010; Wilkerson et al., 2014). In all cases, the MLs are acylated before polymerization as proven by the presence in the lignins of unique β-β coupling products that only arise when one or both of the MLs are acylated, preventing the formation of the typical resinols from internal trapping of the quinone methide intermediates by the γ-OH (Lu and Ralph, 2002, 2008; Del Río et al., 2007; Lu et al., 2015).
The BAHD acyltransferase, FERULOYL-CoA MONOLIGNOL TRANSFERASE (FMT), was recently identified in Angelica sinensis and transformed into poplar (Populus alba × grandidentata), which naturally incorporates other acylated MLs, namely ML-pBz conjugates, into its lignin (Wilkerson et al., 2014). Plants that incorporate ML-FAs into their lignins have the potential to be particularly important economically, because their lignin backbones are permeated with readily cleavable ester bonds, facilitating lignin breakdown and removal under alkaline pretreatment conditions. Determining the extent to which ML-FAs are incorporated into the lignin polymer is, however, extremely difficult because of the diversity of products generated during the polymerization events, which is described in the supplemental information in Wilkerson et al., 2014.
There is currently only one technique, derivatization followed by reductive cleavage (DFRC), that can release diagnostic chemical marker compounds from lignins containing ML-FAs (Lu and Ralph, 2014; Wilkerson et al., 2014). The DFRC method selectively cleaves β-ethers while leaving ester linkages intact. This technique was recently used to show that ML-FA conjugates are fully incorporated into the lignin of the FMT poplar (Wilkerson et al., 2014), but the extent of incorporation, the spatial distribution, the exact mechanism of delivery to the developing cell wall, and the efficiency of incorporation remain largely unknown.
The biological role of pCA in lignin has been highly speculative. It is hypothesized that the pCA moieties may function as a radical sensitizer (Takahama and Oniki, 1996, 1997; Takahama et al., 1996; Ralph et al., 2004; Hatfield et al., 2008; Ralph, 2010). Peroxidases and/or laccases readily oxidize pCA to a radical but are poor oxidizers for SA. Free radicals of pCA readily undergo radical transfer to SA, which in turn, forms a homodimer or couples to the end of a growing polymer chain. Conjugating pCA to an ML, like SA, to form SA-pCA, the most prevalent ML-pCA conjugate in grasses, creates a compound with a built-in radical sensitizer that can participate in the polymerization event. The prevalence of these conjugates in potential biofuel crops and the impact that these ester-linked conjugates have on the lignin polymer during pretreatment and downstream fermentation processes have driven the search to find the genes and their enzymes responsible for acylating MLs in monocots (Withers et al., 2012; Marita et al., 2014; Petrik et al., 2014; Wilkerson et al., 2014).
In rice (Oryza sativa), enzymes have been characterized that function specifically in the addition of pCA onto hemicelluloses (Bartley et al., 2013) or lignin (Withers et al., 2012; Petrik et al., 2014). The p-COUMAROYL-CoA MONOLIGNOL TRANSFERASE (PMT) was identified as one of many grass-specific BAHD acyltransferases produced by rice and found to coexpress with many ML biosynthetic enzymes (Withers et al., 2012). The enzyme preferentially forms a γ-ester through its specificity toward p-coumaroyl-CoA and an ML, and has kinetic efficiency with p-coumaryl alcohol > SA > CA. In most grasses, the PMT enzyme predominantly produces SA-pCA conjugates that are then incorporated into the lignin polymer (Petrik et al., 2014).
To test the role of PMT during cell wall lignification, genetic manipulation of PMT genes has been performed in Brachypodium distachyon and maize (Zea mays), two model monocots. The suppression and overexpression of a BdPMT revealed the PMT to be involved only in the acylation of MLs before polymerization and not in the acylation of hemicelluloses (Petrik et al., 2014). RNA interference-mediated suppression of BdPMT resulted in decreased incorporation of ML-pCA conjugates into the cell wall without adversely affecting growth, height, or digestibility of the mature plants. Even deleterious mutations in the BdPMT gene, which resulted in a complete absence of pCA-acylating B. distachyon lignins, did not affect plant growth or development (Petrik et al., 2014). The arabinose-bound FA and pCA levels remained virtually unchanged in the PMT-misregulated plants, illustrating the specificity of the PMT enzyme for the p-coumaroyl-CoA substrate and its ML acylation. The PMT enzyme identified in maize (pCAT = ZmPMT) also displayed the highest catalytic efficiency with p-coumaroyl-CoA and SA as substrates (Marita et al., 2014). RNAi-mediated suppression of ZmPMT also resulted in decreased production of the ML conjugates. The effect on the lignin polymer when introducing PMT into plants that do not normally express a homologous enzyme is, however, unknown.
pCAs, because they favor radical transfer over radical coupling, are overwhelmingly seen as free-phenolic pendant entities on the lignin polymer (Ralph et al., 1994; Ralph, 2010). As a result, the pCA itself can be completely quantified by simple saponification. The units to which the pCA is attached are, like their normal ML-derived counterparts, not fully releasable from lignin as identifiable monomers (during degradative reactions), but the pCA’s terminal location makes p-coumaroylated units more readily releasable and detectable than if they participated in lignification (as FAs do). Examining the effect of PMT and its resulting conjugates on lignification in plants that do not naturally produce such conjugates will contribute to our understanding of the role of PMT in lignification in general.
In this study, we aimed to assess the ability of the model eudicot plants Arabidopsis (Arabidopsis thaliana) and poplar, neither of which naturally produces ML-pCA conjugates, to express a PMT gene and incorporate these novel conjugates into their cell wall lignins. We also investigated the effect that the introduction of PMT has on the native levels of ML-pBz conjugates in poplar lignin. Various analytical techniques were optimized and used to examine the cell walls of the transgenic plants for pCA conjugates and determine whether they were specifically incorporated into the lignin polymer in the cell wall.
RESULTS AND DISCUSSION
Plant Transformations
In an attempt to maximize the number of cells producing and incorporating the ML conjugates into the eudicot lignins, the PMT transgenes were expressed universally in poplar under the control of the CAULIFLOWER MOSAIC VIRUS promoter (pro35S) and specifically, in lignifying cells of Arabidopsis under the control of the CELLULOSE SYNTHASE7 promoter (proCESA7). In poplar stem, the 35S promoter targets PMT to all cell types (Wilkerson et al., 2014), whereas the Arabidopsis proCESA7 is specific to secondary cell wall cellulose synthesis and therefore, targets PMT to all tracheary elements and fibers in the xylem tissue (Smith et al., 2013). The generation of OsPMT transgenic Arabidopsis and poplar did not manifest in altered growth, architecture, or developmental timing, regardless of promoter used (data not shown).
NMR Examination of Cell Walls for the Presence of pCA
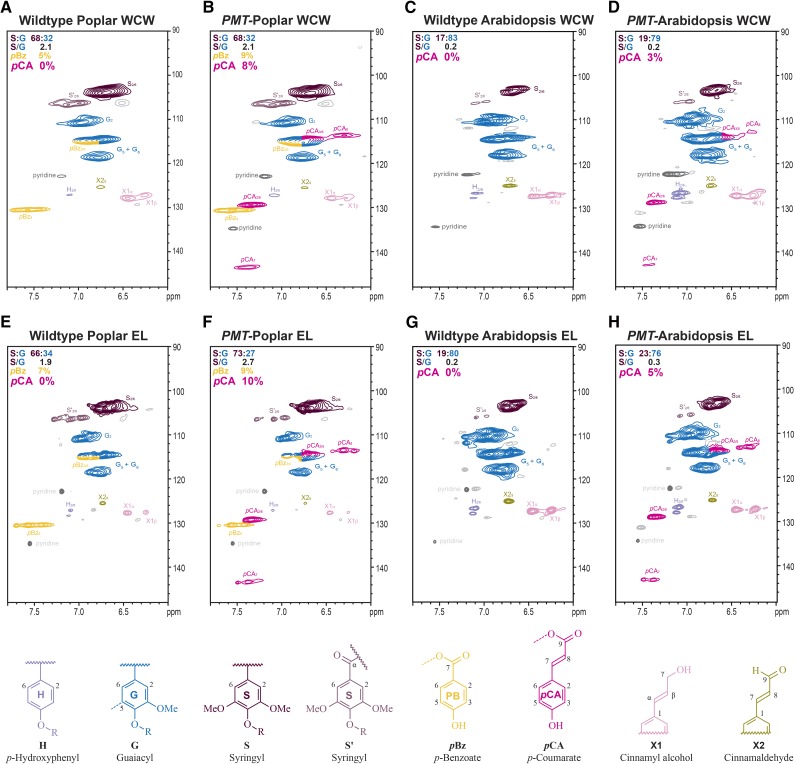
Heteronuclear single-quantum coherence (HSQC) NMR spectroscopy was used to determine the extent of variation in extract-free whole-cell wall (WCW) samples of wild-type and transgenic plants; specifically, we assessed whether there were changes in the relative abundance of syringyl (S) and guaiacyl (G) units derived from the dominant MLs (CA and SA) and if novel cell wall acylation by pCA could be detected. In poplar and Arabidopsis, wild-type plants did not contain any detectable pCA esters, indicating that acylation is not inherent to their lignification (Fig. 1, A and C). As expected, the wild-type poplar samples did show a significant amount of pBz, accounting for 5% of the total peak volume of the total S and G lignin units (Fig. 1A). The WCW samples of the pro35S::PMT transgenic poplar and the proCESA7::PMT transgenic Arabidopsis plants (hereafter referred to as PMT-Poplar and PMT-Arabidopsis) clearly contained pCAs (Fig. 1, B and D). These esters in poplar represent 2% to 8% of the total S and G lignins (by volume integrals), whereas in Arabidopsis, these same conjugates represent about 1% to 3% of the total lignin. (Note that the relative percentage of pCA is overestimated by the HSQC experiment, because it is a mobile pendant component with a longer relaxation time than the polymer backbone [Mansfield et al., 2012].) Interestingly, the biosynthesis of the PMT products, and their incorporation into lignin did not negatively impact the production of the traditional MLs, because the S:G ratio and the acetyl bromide (AcBr) lignin content in both poplar and Arabidopsis and the Klason lignin content in poplar remained unchanged (Table I). Empirically, as determined by wet chemistry (mild base hydrolysis), the pBz level was 1.2% of the cell wall of wild-type poplar trees and ranged from 1.1% to 1.8% of the cell wall in the PMT-poplar trees (Table I). The production of pBz was, therefore, not significantly altered in the PMT-poplar transgenics, suggesting that not only is the biosynthesis of ML-pCA conjugate a unique pathway introduced into poplar and Arabidopsis, but it essentially does not occur at the expense of MLs or naturally occurring acylated MLs.
Figure 1.
pCA is incorporated into transgenic PMT-poplar and PMT-Arabidopsis lignins. 2D-HSQC-NMR spectra of WCW samples (A–D) and the cellulase-digested EL for each sample (E–H). The pCA (magenta) is only present in the PMT-expressing lines and not wild-type control samples. The amount of pBz (orange) in the poplar samples does not seem to change between the wild type and PMT-expressing poplar. Note that the total lignin is the sum of all S, G, and H units (100%); therefore, when the sum of S + G does not add up to 100%, the remaining fraction should be ascribed to H units.
Table I. Analytical data on the Arabidopsis and poplar samples.
The AcBr and Klason lignin values represent the averages of two technical replicates ± sem. The ranges indicate the ranges of conjugate levels released from three independent lines, representing three biological replicates, each with two technical replicates. N/D, Not detected; —, assay was not performed.
| Method/Component | Wild-Type Arabidopsis | PMT Arabidopsis | Wild-Type Poplar | PMT Poplar | Maize (Rind) |
|---|---|---|---|---|---|
| AcBr lignin (wt% WCW) | 9.7 ± 0.8 | 9.7 ± 0.7 | 15.7 ± 1.7 | 15.0 ± 0.5 | 21.4 ± 0.5 |
| Klason lignin (wt% WCW) | — | — | 21.3 ± 0.7 | 21.5 ± 1.1 | — |
| Saponification | |||||
| p-Coumaric acid (mg g−1 WCW) | N/D | 1.0–2.0 | N/D | 1.2–3.5 | 39.6a |
| AcBr lignin (mg g−1 calculated) | — | 9.9–21 | — | 8.1–23 | 185 |
| Klason lignin (mg g−1 calculated) | — | — | — | 5.6–16 | — |
| p-Hydroxybenzoic acid (mg g−1 WCW) | N/D | N/D | 1.2 | 1.1–1.8 | N/D |
| AcBr lignin (mg g−1 calculated) | — | — | 7.6 | 7.5–12 | — |
| Klason lignin (mg g−1 calculated) | — | — | 5.6 | 5.1–8.4 | — |
| DFRC | |||||
| pCA (mg g−1 sample) | N/D | 1.1–1.7 | N/D | 0.9–2.0 | 4.8 |
| AcBr lignin (mg g−1) | — | 11–18 | — | 6–13 | 36.9 |
| Klason lignin (mg g−1 calculated) | — | — | — | 4–8 | — |
Maize has a large amount of arabinose-bound pCA that is released during saponification.
Because the pCA detected in the WCW could be conjugated to either the polysaccharide or lignin component of the wall, extract-free WCW samples were treated with cellulase to remove the cell wall polysaccharides (Fig. 1, E–H). In the resulting isolated enzyme lignin (EL) samples, the pCA signatures remained in both poplar and Arabidopsis at levels (on a lignin basis) similar to those in the WCW samples (Fig. 1, F and H), suggesting that the ML-pCA conjugates synthesized are being incorporated into the lignin. Additional evidence for this contention is provided below.
Plants with 50% to 80% acylation typically have S to G ratios ranging from 4 to 9 and, consequently, primarily linear, unbranched lignins (del Río et al., 2008). In contrast, the S to G ratio of poplar and especially, Arabidopsis is lower compared with many plants that naturally produce ML-Ac or ML-pCA conjugates (del Río et al., 2008). However, lignin acylation is not correlated to the S to G ratio (del Río et al., 2008), and therefore, the relatively low levels of S lignin do not impact the ability of either Arabidopsis or poplar to produce and incorporate acylated MLs.
Confirming the Presence of pCA by Tetramethyl-Ammonium Hydroxide Pyrolysis-Gas Chromatography-Mass Spectrometry
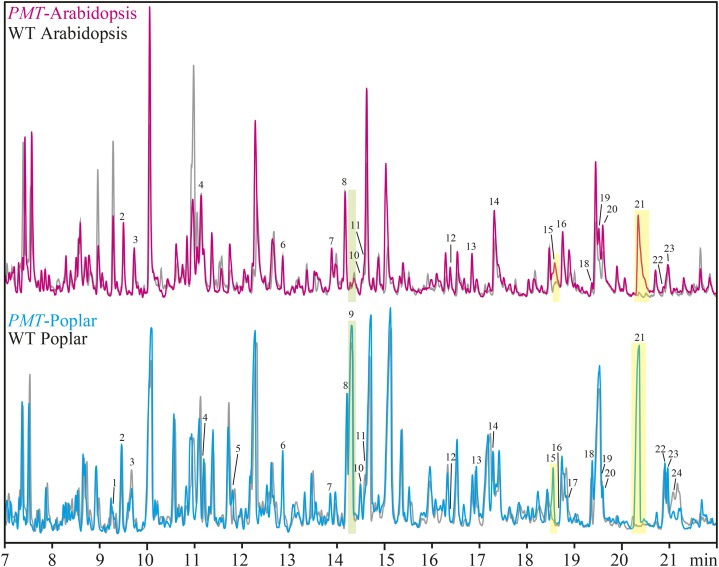
Pyrolysis of plant biomass converts plant material into a complex mixture of low Mr compounds that can be analyzed by gas chromatography (GC)-mass spectrometry (MS). When tetramethyl-ammonium hydroxide (TMAH) is used in the pyrolysis reaction, carboxylic acids/esters are efficiently converted into methyl esters (and in the case of phenols and phenolic ether products, the phenol is also methylated). When pCA or its esters are pyrolyzed with TMAH, they are efficiently converted to methyl 4-methoxycinnamate, which is easily identified among the other pyrolysis products by GC-MS (Fig. 2; Table II).
Figure 2.
TMAH-pyrolysis-GC-MS confirms the presence of pCA in the cell walls of the PMT-expressing plants. TMAH-pyrolysis-GC-MS chromatograms of wild-type (WT) Arabidopsis (gray) overlaid with PMT Arabidopsis (magenta) and wild-type poplar (gray) overlaid with PMT poplar (cyan). The peaks arising from pCA (15 and 21) are indicated with yellow highlighting, and that from pBz (9) has green highlighting. The peak labels refer to the compound numbers defined in Table II.
Table II. TMAH-pyrolysis-GC-MS products list.
LG, Lignin guaiacyl type; LH, lignin p-hydroxyphenyl type; LS, lignin syringyl type; PS, 1,4-linked polysaccharides.
| Peak | Product Name | Formula | Mr | Origin |
|---|---|---|---|---|
| 1 | 4-vinylanisole | C9H10O | 134 | LH |
| 2 | 1,2-dimethoxybenzene | C8H10O2 | 138 | LG |
| 3 | 4-methoxyanisole | C8H10O2 | 138 | PS |
| 4 | 3,4-dimethoxytoluene | C9H12O2 | 152 | LG |
| 5 | 1-(4-methoxyphenyl)prop-2-ene | C10H12O | 148 | LH |
| 6 | 1,2,3-trimethoxybenzene | C9H12O3 | 168 | LS |
| 7 | 3,4-dimethoxystyrene | C10H12O2 | 164 | LG |
| 8 | 1,2,4-trimethoxybenzene | C9H12O3 | 168 | PS |
| 9 | Methyl 4-methoxybenzoate | C9H10O3 | 166 | pBz |
| 10 | Syringol | C8H10O3 | 154 | LS |
| 11 | 3,4,5-trimethoxytoluene | C10H14O3 | 182 | LS |
| 12 | 3-(3,4-dimethoxyphenyl)prop-1-ene | C11H14O2 | 178 | LG |
| 13 | 3,4,5-trimethoxystyrene | C11H14O3 | 194 | LS |
| 14 | 3,4-dimethoxybenzaldehyde | C9H10O3 | 166 | LG |
| 15 | Methyl cis-4-methoxycinnamate | C11H12O3 | 192 | pCA |
| 16 | Methyl 3,4-dimethoxybenzoate | C10H12O3 | 196 | LG |
| 17 | 3-(3,4,5-trimethoxyphenyl)prop-1-ene | C12H16O3 | 208 | LS |
| 18 | cis-1-(3,4-dimethoxyphenyl)-2-methoxyethene | C11H14O3 | 194 | LG |
| 19 | 3,4,5-trimethoxybenzaldehyde | C10H12O4 | 196 | LS |
| 20 | trans-1-(3,4-dimethoxyphenyl)-2-methoxyethene | C11H14O3 | 194 | LG |
| 21 | Methyl trans-4-methoxycinnamate | C11H12O3 | 192 | pCA |
| 22 | 3,4,5-trimethoxyacetophenone | C11H14O4 | 210 | LS |
| 23 | Methyl 3,4,5-trimethoxybenzoate | C11H14O5 | 226 | LS |
| 24 | 4-allylguaiacol | C11H14O3 | 194 | LG |
By examining the pyrolysis GC-MS traces of WCW wild-type Arabidopsis and PMT-Arabidopsis samples and normalizing the signal intensity for the polysaccharide-derived 1,2,4-trimethoxybenzene, very little change was observed in most of the pyrolysis products. The PMT-Arabidopsis sample, however, had a strong signal for the pCA-derived methyl 4-methoxycinnamate (Fig. 2, magenta, peaks 15 and 21 with yellow highlighting). Performing a similar comparison between the wild-type and PMT-poplar samples showed only minor changes in most of the pyrolysis products and again, the presence of methyl 4-methoxycinnamate (pCA) only in the transgenic plants (Fig. 2, cyan, peaks 15 and 21). In the poplar samples, the lignin-bound pBz is converted to methyl 4-methoxybenzoate, and consistent with the wet chemical estimation, there is little change in the amount of pBz (Fig. 2, peak 9 with green highlighting) compared with the other products. This again indicates that the formation and subsequent lignification of ML-pCA conjugates do not affect the production and subsequent incorporation of ML-pBz conjugates into lignin.
Measurement of Total pCA by Saponification
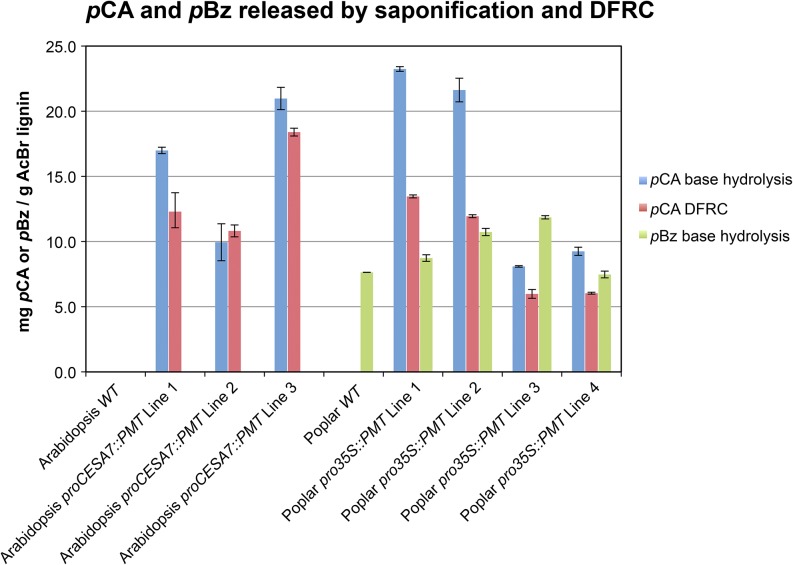
If it is assumed (as is reasonable) that all of the pCA incorporation in the transgenic plants is the result of the introduced PMT, that pCA is only on the lignin (not on polysaccharides), and that, as in grasses, pCA is always a terminal free-phenolic unit, then the pCA level on lignin should be easily quantified by simply measuring the pCA released by saponification. As a corollary, this method provides a measure of the total amount of pCA introduced by expression of the PMT enzyme in these transgenic plants, regardless of whether it is on the lignin or the wall polysaccharides. As in the two-dimensional (2D)-HSQC-NMR and pyrolysis-GC-MS analyses, pCA was absent in both wild-type Arabidopsis and poplar samples when analyzed by mild base hydrolysis GC-flame ionization detection analysis (Fig. 3; Table I). Base hydrolysis of PMT-Arabidopsis transgenic plants showed the presence of 9.9 to 21.0 mg pCA g−1 AcBr lignin. Similar levels of pCA, 8.1 to 23.2 mg g−1 AcBr lignin (5.6–16 mg g−1 Klason lignin), were found in the PMT-poplar transgenic plants (Fig. 3). For comparison, the rind from a mature maize stem released 36.9 mg pCA g−1 AcBr lignin. The PMT-poplar plants had no significant difference in pBz production (7.5−11.9 mg pBz g−1 AcBr lignin and 5.1–8.4 mg g−1 Klason lignin) compared with wild-type plants (7.6 mg g−1 AcBr lignin and 5.6 mg g−1 Klason lignin; Fig. 3), again supporting the hypothesis that there is little to no competition between the natural production of ML-pBz conjugates and the newly introduced ML-pCA conjugates.
Figure 3.
The amount of pCA and pBz released by mild base hydrolysis or DFRC. Wild-type (WT) Arabidopsis and poplar plants do not produce pCA, but the PMT plants produce pCA detectable in the WCW preparations (blue bars). DFRC release of ML-pCA conjugates (i.e. pCA on the lignin; red bars) is necessarily at a lower level than total pCA, even if all of the pCA is on the lignin. Biosynthesis and incorporation of ML-pCA conjugates do not greatly affect the level of pBz in poplar cell walls (green bars). Bars represent the average of two technical replicates, and error bars are the sem.
DFRC Analysis of Cell Walls for the Presence of pCA Conjugates
Base hydrolysis can provide a quantifiable estimate of the amount of pCA introduced into the transgenic plants, but it gives no indication of which cell wall component is acylated by pCA. The DFRC method is invaluable for showing that the pCA is on lignin but yields no information on pCA bound to the polysaccharides. DFRC specifically cleaves the β-ether linkages between ML-derived units while retaining the ester linkages coupling pCA to the lignin unit (i.e. releasing a diagnostic ML-dihydro-p-coumarate [DHpCA] conjugate from free-phenolic or etherified β-ether units bearing pCA moieties). The pCA yield (calculated from the conjugate yield) from DFRC analysis is expected to be lower than that of base hydrolysis, because the method only detects the ML-DHpCA conjugates that are released after the cleavage of β-ether linkages. (Note that we will dispense with the dihydro descriptor here and refer to them simply as pCA.) Conjugates associated with any of the so-called condensed units (i.e. those in 4-O-5, 5-5, β-β, or β-5 units) will not be released/detected by DFRC, whereas such pCA units would still be released and detected by base hydrolysis. In PMT Arabidopsis, ML-bound pCA comprised approximately 11 to 18 mg pCA g−1 AcBr lignin, whereas transgenic poplar trees had 6 to 13 mg pCA g−1 AcBr lignin (4–8 mg pCA g−1 Klason lignin; Table I). Again, in the rind from mature maize stem, 36.9 mg g−1 AcBr lignin was measured. Various lines of our transgenic dicots, which inherently do not incorporate pCA in wild-type plants, therefore have up to one-half the amount of pCA found in a monocot naturally permeated with p-coumaroylated lignins.
The relative ratios of ML-pCA conjugates released from our PMT-Arabidopsis and PMT-poplar transgenics differed from those previously observed in B. distachyon and other grasses. In the grasses, an approximately 90:10 ratio of SA-pCA:CA-pCA is consistently observed (Grabber et al., 1996; Lu and Ralph, 1998a; Withers et al., 2012). In both the PMT Arabidopsis and the PMT poplar, CA-pCA is the most prevalent form, despite the biochemical report that the activity of PMT was lower with CA as a substrate, instead preferring either SA or p-coumaryl alcohol (Withers et al., 2012). Based on the cell wall analysis, it seems that the enzyme uses CA, even in poplar, with its relatively high levels of S units in its lignins. What drives the production of SA-pCA versus CA-pCA in model eudicots is unknown. It may be the greater availability of CA as a substrate at the time that p-coumaroyl-CoA is available for the PMT.
The amount of ML-pCA conjugates released by DFRC from PMT poplar (0.4%–0.8% Klason lignin) is higher than the amount of ML-FA conjugates released from FMT-poplar trees (0.15%–0.52% Klason lignin; Wilkerson et al., 2014). This is fully anticipated, even at the same level of activity, because the pCAs are entirely free-phenolic (and therefore, DFRC releases the conjugates in the same way as it releases the MLs themselves), whereas the FA moiety also undergoes particularly diverse radical coupling of its own, releasing the conjugate from only a fraction of those products (see supplemental figure S2 in Wilkerson et al., 2014).
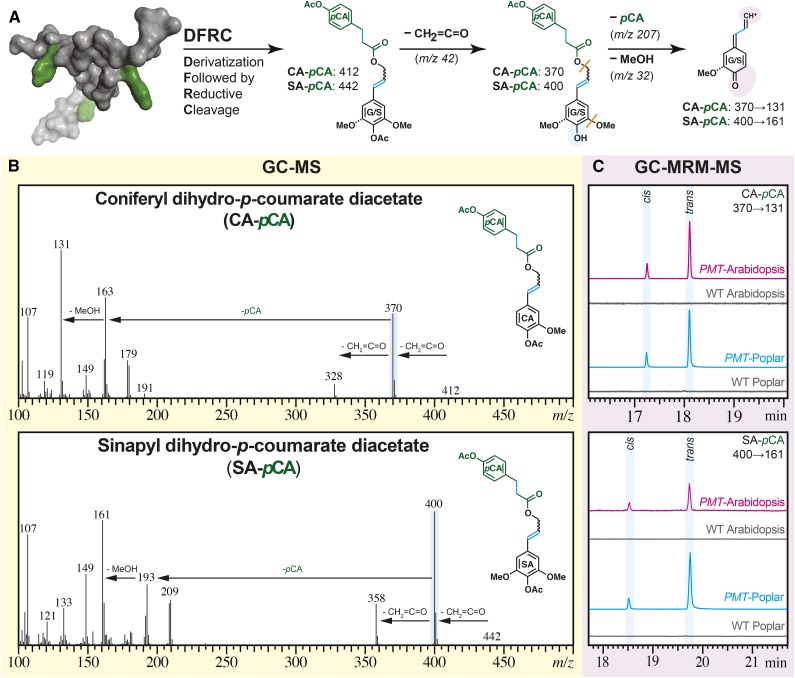
Overall, all of the methods clearly illustrate the successful incorporation of pCA esters into plant cell walls using this transgenic approach in Arabidopsis and poplar. The NMR results on the isolated lignins but particularly, the DFRC results prove that this pCA is on lignin and that, as in grasses, it acylates the γ-OH on lignin side chains (Fig. 4).
Figure 4.
MS evidence for the incorporation of ML-pCA conjugates into transgenic PMT Arabidopsis and PMT poplar. A, The DFRC assay releases a fraction of the pCA as diagnostic ML-pCA-derived structural signatures as indicated with cyan shading; both CA-DHpCA (generally just denoted as CA-pCA here) and SA-DHpCA (SA-pCA) are evidenced. The shaded ovals highlight the structural changes caused by electron ionization (blue) and MRM fragmentation (purple). The orange lines indicate bonds that cleave during the MRM transitions to the detected fragment. B, The mass spectra for synthetic model compounds CA-pCA and SA-pCA from a Q3 scan on a GC-triple-quadrupole MS: molecular ion (mass-to-charge ratio [m/z] = 412 and 442) loss of ketene (m/z = 42) to yield the base peak (m/z = 370 and 400). C, A stacked plot of the triple-quadrupole MRM chromatograms of DFRC-derived parent ions (m/z = 370 and 400) to one of the diagnostic product ions (m/z = 131) from WCW of PMT Arabidopsis (magenta), wild-type (WT) Arabidopsis (gray), PMT poplar (cyan), and wild-type poplar (gray).
CONCLUSION
Various chemical and analytical techniques conclusively showed that the introduction of PMT from rice into Arabidopsis and poplar results in the production of ML-pCA conjugates at levels approaching those in monocots. Results from DFRC and 2D-HSQC-NMR of isolated lignins showed that the pCA specifically acylates the lignin polymer and is therefore logically derived from the ML-pCAs biosynthesized only in the presence of the introduced PMT enzyme. In poplar, which naturally produces acylated MLs in the form of pBzs, the production of pCA conjugates was essentially independent of the pBz biosynthesis. Together, these data show the ability of Arabidopsis and poplar to incorporate unique ML conjugates into their cell walls and also further highlight the plasticity of the lignification process in planta. However, this apparent plasticity raises some questions that require further examination. In vitro, the preferred substrate for the PMT enzyme is SA, but in the eudicots examined in this study, CA-pCA was the predominant product, despite the abundance of SA in Arabidopsis and especially, poplar. The reason behind this discrepancy remains unclear. Furthermore, the incorporation of a diversity of monomers into lignin, beyond the three traditional monomers, begs the question of how the monomers are exported into the cell wall. If the monomers are actively transported out of the cells, which has been hypothesized (Miao and Liu, 2010; Alejandro et al., 2012), the ML transporter(s) would have to be particularly accommodating, or there may be many more transporters involved than previously considered.
MATERIALS AND METHODS
Generating the PMT Constructs and Transgenic Plants
PMT Construct Development
The PMT gene (LOC_Os01g19744) was identified from a list of candidate BAHD acyltransferases (Withers et al., 2012). The open reading frame of the gene was cloned into the pDONR221 vector. Cloning to generate the poplar (Populus alba × grandidentata) yellow fluorescent protein-tagged PMT gene (pro35S::YFP::PMT construct) was performed as described in Wilkerson et al., 2014. Briefly, the open reading frame for PMT was cloned into pDONR221 and subsequently transferred into the pH7WGY2 destination vector. In Arabidopsis (Arabidopsis thaliana), the promoter region, represented by a 1,127-bp sequence upstream of the transcriptional start codon for CESA7, was amplified by PCR using the primers 5′-AAAAAGCAGGCTGGCTCCAACGTTTTCAGTTT-3′ (forward) and 5′-AGAAAGCTGGGTCGGTGATCAATGAGAGACGA-3′ (reverse), which have adaptor sites for Gateway cloning (underlined). The CESA7 promoter was cloned into pDONR221 and then further transferred into the pKGW destination vector using LR Gateway cloning. The PMT gene was amplified by PCR using the primers 5′-ATGGGATTTGCTGTTGTCC-3′ (forward) and 5′-GGTATCACTTATCGAAGGC-3′ (reverse) and placed into the pKGW vector by blunt end cloning following the promoter.
Plant Transformations and Growth Conditions
For generation of poplar transgenics, Agrobacterium tumefaciens strain EHA105 containing the pro35S::YFP::PMT vector was used to transform a poplar hybrid (P39; Wilkerson et al., 2014). The Arabidopsis PMT expression construct was introduced into A. tumefaciens strain GV3101 and transformed into Arabidopsis Columbia-0 using the floral dip method (Clough and Bent, 1998). Seeds from the dipped plants were plated on one-half-strength Murashige and Skoog media (Sigma) supplemented with 50 mg mL−1 kanamycin to select for transformants. Two-week-old seedlings were transferred to soil (Sunshine Mix 4; Sungrow Horticulture) and grown in a growth chamber at 21°C and in 16-h-light/8-h-dark cycles until mature (approximately 2 months old). Rosette leaves from mature plants were subjected to genotyping using the PMT primers listed above to confirm that the transformation was effective. First and second generation transformants were used for the chemical analyses.
Analyses
2D-HSQC-NMR Analysis of Cell Wall Components
The preparation of the extract-free WCWs and the EL was performed as described previously (Lu and Ralph, 2003; Wagner et al., 2007; Kim and Ralph, 2010). The ball-milled extract-free Arabidopsis and poplar samples were prepared in 5-mm NMR tubes and swelled/suspended in dimethyl sulfoxide (DMSO)-d6:pyridine-d5 (4:1; 500 μL) for NMR (Kim and Ralph, 2010; Mansfield et al., 2012).
EL samples were prepared following the procedure described previously (Chang et al., 1975; Wagner et al., 2007). Briefly, the material (200 mg) was digested at room temperature with 20 mg of crude cellulases (Cellulysin; Calbiochem) in 40 mL of Ac buffer (pH 5.0) on a shaker for 3 d. The samples were then centrifuged (Sorval Biofuge Primo; 8,500 rpm or 10,016g for 10 min) and decanted. The cellulase digestion was then repeated. After decanting the second hydrolysate, the sample was rinsed with reverse osmosis water (3 × 40 mL) and lyophilized. From the resulting brown powder (40 mg), 10 to 15 mg were transferred to a 5-mm NMR tube and dissolved/swollen in DMSO-d6:pyridine-d5 100% (4:1; 500 μL).
The gel was characterized by HSQC spectroscopy under the conditions previously reported (Kim and Ralph, 2010; Mansfield et al., 2012). The spectroscopic data were acquired on a Bruker Biospin Avance 700-MHz NMR spectrometer equipped with an inverse gradient 5-mm TXI 1H-13C-15N Cryoprobe. The central DMSO solvent peak was used as internal reference (δc = 39.5 ppm and δH = 2.49 ppm). Peak assignments for S, G, and p-hydroxyphenyl (H) lignin units as well as pCA and pBz were made by comparison with previously assigned spectra (Lu and Ralph, 2003; Marita et al., 2003; Kim et al., 2008; Kim and Ralph, 2010; Ralph and Landucci, 2010; del Río et al., 2012a, 2012b; Mansfield et al., 2012; Rencoret et al., 2013).
Analytical Pyrolysis
To confirm the formation of pCA by the PMT transgene, the pyrolysis GC-MS spectra of the transgenic plant lines were compared with those of the corresponding wild-type plants. The sample (approximately 300 μg) was treated with approximately 50 μL of TMAH (25%, w/w in methanol) and air dried to remove excess methanol. The pyrolysis GC-MS was performed on a Py-2020iD Microfurnace Pyrolyzer (Frontier Laboratories Ltd.) connected to a Shimadzu 2010 GC equipped with an Agilent DB-1701 Fused-Silica Capillary Column (30 m × 0.25 mm i.d. × 0.25-μm film thickness) and a Shimadzu QP2010 Plus Mass Spectrometer (electron impact at 70 eV). The helium carrier gas was held at a constant linear velocity (36.3 cm s−1). The pyrolysis was performed in helium atmosphere by lowering the sample into the 500°C furnace, where the injection port was operating with a split ratio of 10:1. The GC oven program was a 50-min temperature gradient as follows: 50°C hold for 2 min, ramp at 20°C per minute to 100°C, ramp at 6°C per minute to 300°C, and hold at 300°C for 12 min. Compound identification was performed by comparison of the detected mass spectra with those in the National Institute of Standards and Technology library and comparison with spectra reported in the literature from pyrolysis of plant tissue in the presence of TMAH (Clifford et al., 1995; Kuroda, 2002; del Río et al., 2012a, 2012b).
AcBr Lignin
To estimate the amount of lignin in each Arabidopsis and poplar sample to express the amount of pCA as a proportion of lignin in the sample, AcBr lignin analysis was performed as previously described (Hatfield et al., 1999; Fukushima and Hatfield, 2004; Chang et al., 2008) with the absorbance at λ = 280 nm and extinction coefficients of e280 = 20.0 for poplar and e280 = 23.3 for Arabidopsis. Briefly, the samples (2–3 mg) were treated with 0.5 mL of 20% (v/v) AcBr in acetic acid for 2 h at 50°C. The solution was then quantitatively transferred to a 10-mL volumetric flask containing 2 m NaOH (2 mL) and 0.5 m hydroxylamine (0.35 mL) and adjusted to a final volume of 10 mL using acetic acid. Samples were then transferred to quartz cuvettes, and the absorbance was measured at 280 nm. The average weight of lignin (percentage) from two technical replicates was used to estimate the amount of lignin in the samples, and this was used to express the amount of pCA in terms of the amount of lignin for base hydrolysis and DFRC experiments.
Mild Alkaline Hydrolysis to Determine Releasable pCA and pBz
The determination of ester-linked pCA and pBz was by mild alkaline hydrolysis following previously published procedures (Ralph et al., 1994).
DFRC Method for Lignin-Bound pCA
The quantitation of lignin-bound pCA was performed using the DFRC method that cleaves lignin β-ethers while retaining esters (Lu and Ralph, 1997, 1998b, 1998c, 1999; Petrik et al., 2014). The same dry extract-free WCWs used for pyrolysis and base hydrolysis (20–40 mg) were treated with a solution of AcBr:acetic acid (1:4, v/v) at 50°C for 3 h. The acetylated and benzylic-brominated lignin solution was quantitatively transferred to a 50-mL round-bottomed flask using glacial acetic acid, and the solvent was evaporated to dryness on a rotary evaporator (water bath heated to 50°C). The dry film was treated with absolute ethanol (2 mL), which was then removed on a rotary evaporator. The dry sample was then immediately dissolved in a mixture of 1,4-dioxane:acetic acid:water (5:4:1, v/v/v; 5 mL), and zinc nanopowder (150 mg) was added to the flask in four portions at 15-min intervals. The reaction was stirred for 1 h (16 h for Arabidopsis samples) at room temperature and then quenched with saturated ammonium chloride. The quenched reaction was spiked with an internal standard (diethyl 5,5′-di-FA). The organics were extracted with dichloromethane (DCM; 4 × 15 mL), and the combined organic fractions were dried over sodium sulfate. The DCM was removed under vacuum, and the free hydroxyl groups were acetylated overnight using a mixture of acetic anhydride:pyridine (1:1, v/v). The excess acetic anhydride and pyridine were removed on a rotary evaporator, after which the crude product was loaded on a Supelco Supelclean LC-SI SPE Tube (Sigma-Aldrich) with the aid of DCM (3 mL) for poplar or ethyl Ac:hexane (1:1; approximately 1 mL) for Arabidopsis. The purified product was then eluted using a mixture of 1,4-dioxane:ethyl Ac (1:1; 5 mL) for poplar and ethyl Ac:hexane (1:1; 8 mL) for Arabidopsis and concentrated to dryness. The dry film was dissolved in DCM (1 mL) and injected into a Shimadzu GCMS-TQ8030 Triple-Quadrupole GC-MS-MS operating in multiple reaction monitoring (MRM) mode for quantitative analysis (using calibration curves derived from synthetic standards). Each line represents an independent biological replicate composed of two technical replicates. The error bars represent the sem between the technical replicates.
Received May 29, 2015; accepted October 27, 2015; published October 28, 2015.
Glossary
- Ac
acetate
- AcBr
acetyl bromide
- CA
coniferyl alcohol
- 2D
two dimensional
- DFRC
derivatization followed by reductive cleavage
- DHpCA
dihydro-p-coumarate
- DMSO
dimethyl sulfoxide
- EL
enzyme lignin
- FA
ferulate
- G
guaiacyl
- GC
gas chromatography
- H
p-hydroxyphenyl
- HSQC
heteronuclear single-quantum coherence
- ML
monolignol
- MRM
multiple reaction monitoring
- MS
mass spectrometry
- pBz
p-hydroxybenzoate
- pCA
p-coumarate
- S
syringyl
- SA
sinapyl alcohol
- TMAH
tetramethyl-ammonium hydroxide
- WCW
whole-cell wall
Footnotes
This work was supported by the Department of Energy’s Great Lakes Bioenergy Research Center, Department of Energy, Biological and Environmental Research, Office of Science (grant no. DE–FC02–07ER64494).
References
- Alejandro S, Lee Y, Tohge T, Sudre D, Osorio S, Park J, Bovet L, Lee Y, Geldner N, Fernie AR, et al. (2012) AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Curr Biol 22: 1207–1212 [DOI] [PubMed] [Google Scholar]
- Bartley LE, Peck ML, Kim SR, Ebert B, Manisseri C, Chiniquy DM, Sykes R, Gao L, Rautengarten C, Vega-Sánchez ME, et al. (2013) Overexpression of a BAHD acyltransferase, OsAt10, alters rice cell wall hydroxycinnamic acid content and saccharification. Plant Physiol 161: 1615–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54: 519–546 [DOI] [PubMed] [Google Scholar]
- Chang HM, Cowling EB, Brown W, Adler E, Miksche G (1975) Comparative studies on cellulolytic enzyme lignin and milled wood lignin of sweetgum and spruce. Holzforschung 29: 153–159 [Google Scholar]
- Chang XF, Chandra R, Berleth T, Beatson RP (2008) Rapid, microscale, acetyl bromide-based method for high-throughput determination of lignin content in Arabidopsis thaliana. J Agric Food Chem 56: 6825–6834 [DOI] [PubMed] [Google Scholar]
- Clifford DJ, Carson DM, Mckinney DE, Bortiatynski JM, Hatcher PG (1995) A new rapid technique for the characterization of lignin in vascular plants - thermochemolysis with tetramethylammonium hydroxide (TMAH). Org Geochem 23: 169–175 [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Crestini C, Argyropoulos DS (1997) Structural analysis of wheat straw lignin by quantitative 31P and 2D NMR spectroscopy. The occurrence of ester bonds and α-O-4 substructures. J Agric Food Chem 45: 1212–1219 [Google Scholar]
- Del Río JC, Marques G, Rencoret J, Martínez AT, Gutiérrez A (2007) Occurrence of naturally acetylated lignin units. J Agric Food Chem 55: 5461–5468 [DOI] [PubMed] [Google Scholar]
- del Río JC, Prinsen P, Rencoret J, Nieto L, Jiménez-Barbero J, Ralph J, Martínez ÁT, Gutiérrez A (2012a) Structural characterization of the lignin in the cortex and pith of elephant grass (Pennisetum purpureum) stems. J Agric Food Chem 60: 3619–3634 [DOI] [PubMed] [Google Scholar]
- del Río JC, Rencoret J, Marques G, Gutiérrez A, Ibarra D, Santos JI, Jiménez-Barbero J, Zhang L, Martínez AT (2008) Highly acylated (acetylated and/or p-coumaroylated) native lignins from diverse herbaceous plants. J Agric Food Chem 56: 9525–9534 [DOI] [PubMed] [Google Scholar]
- del Río JC, Rencoret J, Prinsen P, Martínez ÁT, Ralph J, Gutiérrez A (2012b) Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J Agric Food Chem 60: 5922–5935 [DOI] [PubMed] [Google Scholar]
- Fukushima RS, Hatfield RD (2004) Comparison of the acetyl bromide spectrophotometric method with other analytical lignin methods for determining lignin concentration in forage samples. J Agric Food Chem 52: 3713–3720 [DOI] [PubMed] [Google Scholar]
- Grabber JH, Hatfield RD, Lu F, Ralph J (2008) Coniferyl ferulate incorporation into lignin enhances the alkaline delignification and enzymatic degradation of cell walls. Biomacromolecules 9: 2510–2516 [DOI] [PubMed] [Google Scholar]
- Grabber JH, Quideau S, Ralph J (1996) p-Coumaroylated syringyl units in maize lignin; implications for β-ether cleavage by thioacidolysis. Phytochemistry 43: 1189–1194 [Google Scholar]
- Hatfield R, Ralph J, Grabber JH (2008) A potential role for sinapyl p-coumarate as a radical transfer mechanism in grass lignin formation. Planta 228: 919–928 [DOI] [PubMed] [Google Scholar]
- Hatfield RD, Grabber J, Ralph J, Brei K (1999) Using the acetyl bromide assay to determine lignin concentrations in herbaceous plants: some cautionary notes. J Agric Food Chem 47: 628–632 [DOI] [PubMed] [Google Scholar]
- Hibino T, Shibata D, Ito T, Tsuchiya D, Higuchi T, Pollet B, Lapierre C (1994) Chemical properties of lignin from Aralia cordata. Phytochemistry 37: 445–448 [Google Scholar]
- Kim H, Ralph J (2010) Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d(6)/pyridine-d(5). Org Biomol Chem 8: 576–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ralph J, Akiyama T (2008) Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6. Bioenergy Res 1: 56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K. (2002) Electron-impact (ET) mass spectra of 1,2-dimethoxybenzenes related to the pyrolysis products of guaiacyl lignin in the presence of tetramethylammonium hydroxide (TMAH). J Anal Appl Pyrolysis 64: 433–451 [Google Scholar]
- Kuroda K, Ozawa T, Ueno T (2001) Characterization of sago palm (Metroxylon sagu) lignin by analytical pyrolysis. J Agric Food Chem 49: 1840–1847 [DOI] [PubMed] [Google Scholar]
- Landucci LL, Deka GC, Roy DN (1992) A 13C NMR study of milled wood lignins from hybrid Salix Clones. Holzforschung 46: 505–511 [Google Scholar]
- Lu F, Karlen SD, Regner M, Kim H, Ralph SA, Sun Rc, Kuroda Ki, Augustin MA, Mawson R, Sabarez H, et al. (2015) Naturally p-hydroxybenzoylated lignins in palms. Bioenergy Res 8: 934–952 [Google Scholar]
- Lu F, Ralph J (1997) The DFRC method for lignin analysis. Part 1. A new method for β-aryl ether cleavage: lignin model studies. J Agric Food Chem 45: 4655–4660 [Google Scholar]
- Lu F, Ralph J (1998a) Facile synthesis of 4-hydroxycinnamyl p-coumarates. J Agric Food Chem 46: 2911–2913 [Google Scholar]
- Lu F, Ralph J (1998b) The DFRC method for lignin analysis. 2. Monomers from isolated lignins. J Agric Food Chem 46: 547–552 [DOI] [PubMed] [Google Scholar]
- Lu F, Ralph J (1998c) The DFRC method for lignin analysis. Part 3. NMR studies. J Wood Chem Technol 18: 219–233 [Google Scholar]
- Lu F, Ralph J (1999) Detection and determination of p-coumaroylated units in lignins. J Agric Food Chem 47: 1988–1992 [DOI] [PubMed] [Google Scholar]
- Lu F, Ralph J (2002) Preliminary evidence for sinapyl acetate as a lignin monomer in Kenaf. Chem Commun (Camb) (1): 90–91 [DOI] [PubMed] [Google Scholar]
- Lu F, Ralph J (2003) Non-degradative dissolution and acetylation of ball-milled plant cell walls: high-resolution solution-state NMR. Plant J 35: 535–544 [DOI] [PubMed] [Google Scholar]
- Lu F, Ralph J (2008) Novel tetrahydrofuran structures derived from β-β-coupling reactions involving sinapyl acetate in Kenaf lignins. Org Biomol Chem 6: 3681–3694 [DOI] [PubMed] [Google Scholar]
- Lu F, Ralph J (2014) The DFRC (Derivatization Followed by Reductive Cleavage) method and its applications for lignin characterization. In Lu F, ed, Lignin: Structural Analysis, Applications in Biomaterials, and Ecological Significance. Nova Science Publishers, Inc., Hauppauge, NY, pp 27–65 [Google Scholar]
- Lu F, Ralph J, Morreel K, Messens E, Boerjan W (2004) Preparation and relevance of a cross-coupling product between sinapyl alcohol and sinapyl p-hydroxybenzoate. Org Biomol Chem 2: 2888–2890 [DOI] [PubMed] [Google Scholar]
- Mansfield SD, Kim H, Lu F, Ralph J (2012) Whole plant cell wall characterization using solution-state 2D NMR. Nat Protoc 7: 1579–1589 [DOI] [PubMed] [Google Scholar]
- Marita JM, Hatfield RD, Rancour DM, Frost KE (2014) Identification and suppression of the p-coumaroyl CoA:hydroxycinnamyl alcohol transferase in Zea mays L. Plant J 78: 850–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marita JM, Vermerris W, Ralph J, Hatfield RD (2003) Variations in the cell wall composition of maize brown midrib mutants. J Agric Food Chem 51: 1313–1321 [DOI] [PubMed] [Google Scholar]
- Martínez AT, Rencoret J, Marques G, Gutiérrez A, Ibarra D, Jiménez-Barbero J, del Río JC (2008) Monolignol acylation and lignin structure in some nonwoody plants: a 2D NMR study. Phytochemistry 69: 2831–2843 [DOI] [PubMed] [Google Scholar]
- Miao YC, Liu CJ (2010) ATP-binding cassette-like transporters are involved in the transport of lignin precursors across plasma and vacuolar membranes. Proc Natl Acad Sci USA 107: 22728–22733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monties B, Lapierre C (1981) Donnés récentes sur l’hétérogénéite de la lignine. Physiol Veg 19: 327–348 [Google Scholar]
- Morreel K, Ralph J, Kim H, Lu F, Goeminne G, Ralph S, Messens E, Boerjan W (2004) Profiling of oligolignols reveals monolignol coupling conditions in lignifying poplar xylem. Plant Physiol 136: 3537–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik DL, Karlen SD, Cass CL, Padmakshan D, Lu F, Liu S, Le Bris P, Antelme S, Santoro N, Wilkerson CG, et al. (2014) p-Coumaroyl-CoA:monolignol transferase (PMT) acts specifically in the lignin biosynthetic pathway in Brachypodium distachyon. Plant J 77: 713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph J. (1996) An unusual lignin from Kenaf. J Nat Prod 59: 341–342 [Google Scholar]
- Ralph J. (2007) Perturbing lignification. In Entwistle K, Harris PJ, Walker J, eds, The Compromised Wood Workshop 2007. Wood Technology Research Centre, University of Canterbury, Canterbury, New Zealand, pp 85–112 [Google Scholar]
- Ralph J. (2010) Hydroxycinnamates in lignification. Phytochem Rev 9: 65–83 [Google Scholar]
- Ralph J, Hatfield RD, Quideau S, Helm RF, Grabber JH, Jung HJG (1994) Pathway of p-coumaric acid incorporation into maize lignin as revealed by NMR. J Am Chem Soc 116: 9448–9456 [Google Scholar]
- Ralph J, Landucci LL (2010) NMR of Lignins. In Heitner C, Dimmel DR, Schmidt JA, eds, Lignin and Lignans; Advances in Chemistry. CRC Press, Taylor & Francis Group, Boca Raton, FL, pp 137–234 [Google Scholar]
- Ralph J, Lu F (1998) The DFRC method for lignin analysis. Part 6. A modified method to determine acetate regiochemistry on native and isolated lignins. J Agric Food Chem 46: 4616–4619 [DOI] [PubMed] [Google Scholar]
- Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH, et al. (2004) Lignins: natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem Rev 3: 29–60 [Google Scholar]
- Rencoret J, Ralph J, Marques G, Gutiérrez A, Martínez Á, del Río JC (2013) Structural characterization of lignin isolated from coconut (Cocos nucifera) coir fibers. J Agric Food Chem 61: 2434–2445 [DOI] [PubMed] [Google Scholar]
- Sarkanen KV, Chang HM, Allan GG (1967) Species variation in lignins. III. Hardwood lignins. Tappi 50: 587–590 [Google Scholar]
- Sarkanen KV, Ludwig CH (1971) Lignins, Occurrence, Formation, Structure and Reactions. Wiley-Interscience, New York [Google Scholar]
- Smith RA, Schuetz M, Roach M, Mansfield SD, Ellis B, Samuels L (2013) Neighboring parenchyma cells contribute to Arabidopsis xylem lignification, while lignification of interfascicular fibers is cell autonomous. Plant Cell 25: 3988–3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RC, Fang JM, Tomkinson J (1999) Fractional isolation and structural characterization of lignins from oil palm trunk and empty fruit bunch fibres. J Wood Chem Technol 19: 335–356 [DOI] [PubMed] [Google Scholar]
- Takahama U, Oniki T (1996) Enhancement of peroxidase-dependent oxidation of sinapyl alcohol by esters of 4-coumaric and ferulic acid. In Obinger C, Burner U, Ebermann R, Penel C, Greppin H, eds, Plant Peroxidases, Biochemistry and Physiology. Université de Genève, Geneva, pp 118–123 [Google Scholar]
- Takahama U, Oniki T (1997) Enhancement of peroxidase-dependent oxidation of sinapyl alcohol by an apoplastic component, 4-coumaric acid ester isolated from epicotyls of Vigna angularis L. Plant Cell Physiol 38: 456–462 [Google Scholar]
- Takahama U, Oniki T, Shimokawa H (1996) A possible mechanism for the oxidation of sinapyl alcohol by peroxidase-dependent reactions in the apoplast: enhancement of the oxidation by hydroxycinnamic acids and components of the apoplast. Plant Cell Physiol 37: 499–504 [Google Scholar]
- Tomimura Y. (1992a) Chemical characteristics and utilization of oil palm trunks. Jpn Agric Res Q 25: 283–288 [Google Scholar]
- Tomimura Y. (1992b) Chemical characteristics of oil palm trunk. Bull Forestry Forest Prod Res 0: 133–142 [Google Scholar]
- Vanholme R, Morreel K, Darrah C, Oyarce P, Grabber JH, Ralph J, Boerjan W (2012) Metabolic engineering of novel lignin in biomass crops. New Phytol 196: 978–1000 [DOI] [PubMed] [Google Scholar]
- Venverloo CJ. (1971) Lignin of Populus nigra and some other Salicaceae. Holzforschung 25: 18–24 [Google Scholar]
- Wagner A, Ralph J, Akiyama T, Flint H, Phillips L, Torr K, Nanayakkara B, Te Kiri L (2007) Exploring lignification in conifers by silencing hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyltransferase in Pinus radiata. Proc Natl Acad Sci USA 104: 11856–11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson CG, Mansfield SD, Lu F, Withers S, Park JY, Karlen SD, Gonzales-Vigil E, Padmakshan D, Unda F, Rencoret J, et al. (2014) Monolignol ferulate transferase introduces chemically labile linkages into the lignin backbone. Science 344: 90–93 [DOI] [PubMed] [Google Scholar]
- Withers S, Lu F, Kim H, Zhu Y, Ralph J, Wilkerson CG (2012) Identification of grass-specific enzyme that acylates monolignols with p-coumarate. J Biol Chem 287: 8347–8355 [DOI] [PMC free article] [PubMed] [Google Scholar]