Iron stress modulates auxin and ethylene pathways to regulate lateral root formation in Arabidopsis.
Abstract
A stunted root system is a significant symptom of iron (Fe) toxicity, yet little is known about the effects of excess Fe on lateral root (LR) development. In this work, we show that excess Fe has different effects on LR development in different portions of the Arabidopsis (Arabidopsis thaliana) root system and that inhibitory effects on the LR initiation are only seen in roots newly formed during excess Fe exposure. We show that root tip contact with Fe is both necessary and sufficient for LR inhibition and that the auxin, but not abscisic acid, pathway is engaged centrally in the initial stages of excess Fe exposure. Furthermore, Fe stress significantly reduced PIN-FORMED2 (PIN2)-green fluorescent protein (GFP) expression in root tips, and pin2-1 mutants exhibited significantly fewer LR initiation events under excess Fe than the wild type. Exogenous application of both Fe and glutathione together increased PIN2-GFP expression and the number of LR initiation events compared with Fe treatment alone. The ethylene inhibitor aminoethoxyvinyl-glycine intensified Fe-dependent inhibition of LR formation in the wild type, and this inhibition was significantly reduced in the ethylene overproduction mutant ethylene overproducer1-1. We show that Auxin Resistant1 (AUX1) is a critical component in the mediation of endogenous ethylene effects on LR formation under excess Fe stress. Our findings demonstrate the relationship between excess Fe-dependent PIN2 expression and LR formation and the potential role of AUX1 in ethylene-mediated LR tolerance and suggest that AUX1 and PIN2 protect LR formation in Arabidopsis during the early stages of Fe stress.
Iron (Fe) is an essential trace element for plants (Pilon et al., 2009), and species differ greatly in how much Fe they require for optimal growth (Wheeler and Power, 1995; Batty and Younger, 2003). As Fe is frequently limiting, Fe deficiency is more commonly studied than toxicity arising from excess Fe exposure (Lei et al., 2014; Bashir et al., 2015; Briat et al., 2015). Fe is also a major focus for efforts in biofortification by targeting Fe transporters (Zhai et al., 2014; Pinto and Ferreira, 2015). However, the excessive presence of Fe in soils is equally common, in particular in soils characterized by low pH and hypoxic or anoxic conditions (Connolly and Guerinot, 2002). Toxicity arising from excess Fe exposure is recognized as one of the major plant diseases attributable to abiotic factors that impact the development and yield potential in the world’s leading cereal crops, rice (Oryza sativa) and wheat (Triticum aestivum; Becker and Asch, 2005; Khabaz-Saberi et al., 2012). Understanding the mechanisms underlying excess Fe toxicity is therefore essential.
Plastic responses in the plant’s root system architecture are known to constitute a major mechanism by which plants cope with fluctuating environments. Lateral roots (LRs), which typically comprise the majority of the root system, contribute pivotally to nutrient acquisition from soil, and modulating LR development is a very important avoidance strategy for plants when confronted with unfavorable edaphic conditions, such as high salinity or heavy metals (Ivanov et al., 2003). In the case of excess exposure to Fe, stunting of the root system is among the chief symptoms of toxicity (Becker and Asch, 2005). However, while some information has been emerging on the primary root axis (Li et al., 2015), the specific role of the plant’s LR apparatus remains poorly studied. Yamauchi and Peng (1995) reported retardation of root growth and a reduction in LR length and number under excess Fe conditions. Recently, Reyt et al. (2015) showed that excess Fe had no significant effect on LR initiation in the LR branching zone and that ferritins play an important role in LR emergence under excess Fe in this portion of the root, although the authors had not investigated LR development in the root portions near the growing tip of the primary root. Because LR initiation is restricted to specific pericycle cell files adjacent to a xylem pole in the basal region of the meristem (De Smet et al., 2007; Fukaki and Tasaka, 2009), and LR formation in this new growing root portion may be more susceptible to stress stimuli, such as observed with exposure to high NH4+ and salt (Duan et al., 2013; Li et al., 2013), it is reasonable to suggest that modulation of LR formation near the growing tip of the primary root is critical to the response to excess Fe stress.
In Arabidopsis (Arabidopsis thaliana), the development of LRs proceeds through the following stages: lateral root primordia (LRP) initiation, establishment, emergence, activation into mature LRs, and final maintenance of LR elongation (Fukaki and Tasaka, 2009; Péret et al., 2009). The hormones abscisic acid (ABA) and auxin are important internal negative and positive regulators during LR development, respectively (Fukaki and Tasaka, 2009). ABA has been implicated in LRP emergence and meristem activation independent of auxin (De Smet et al., 2003). Auxin is an important internal positive regulator during LR development (Fukaki and Tasaka, 2009), and auxin transport is critical (Blilou et al., 2005). Mutants in auxin efflux carriers such as PIN-FORMED (PIN) and P-Glycoprotein show significant defects in LR formation (Fukaki and Tasaka, 2009; Péret et al., 2009). For example, LR initiation frequency was significantly reduced in pin2 and pin3 mutants (Dubrovsky et al., 2009), and PIN2 was also shown to be involved in exogenous and endogenous signal-mediated LR development (by brassinosteroid, jasmonate, and fungal challenge; Li et al., 2005; Felten et al., 2009; Sun et al., 2009). Similarly, Auxin Resistant1 (AUX1), an auxin influx carrier, also regulates LRP positioning and initiation (De Smet et al., 2007). While both AUX1 and PIN2 are required specifically for the basipetal transport of auxin through the outer root cell layers (Fukaki and Tasaka, 2009), PIN1 localized at the basal end of vascular cells is responsible for direct acropetal auxin flow in the root stele (Blilou et al., 2005). Recently, the roles of ethylene on LR development have also been highlighted, and the ethylene-mediated LR formation is dependent on the auxin pathway (Ivanchenko et al., 2008; Lewis et al., 2011). Ethylene treatment could mediate fluorescence of AUX1 and PIN2 fluorescent protein fusions at the root tip (Růzicka et al., 2007; Lewis et al., 2011). Although ABA, auxin, and ethylene signals have been implicated as important for LR development, it is not known whether and how the three hormones are involved in the response of LR formation to Fe stress.
The previously described phenotypes and physiological processes related to Fe toxicity do not clarify the effect of excess Fe on LR formation. In this study, we employed the Arabidopsis wild type and ABA-, auxin-, and ethylene-related mutants to explore the LR formation response to Fe toxicity and to elucidate the roles of ABA, auxin, and ethylene. Potential mechanisms involved in the early stress response to Fe stress are discussed.
RESULTS
Effect of Excess Fe on LR Development
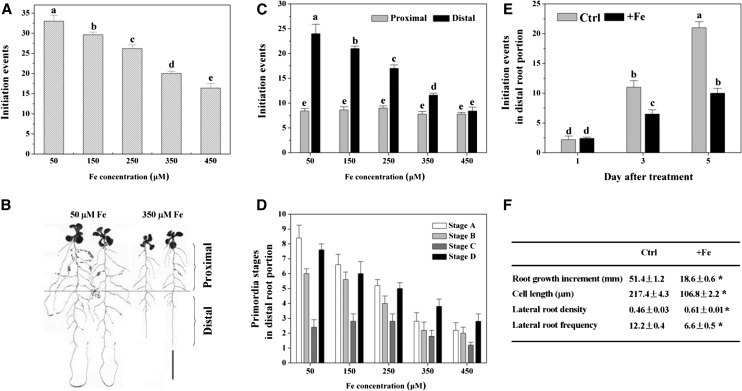
To investigate the effects of excess Fe toxicity on LR development, Arabidopsis seedlings were grown on standard growth medium for 5 d and then transferred either to standard medium or medium supplemented with varying concentrations of Fe-EDTA for periods of up to 5 d. The total LR initiation events (including LRPs and emerged LRs) were significantly reduced by exposure to excess Fe stress, and the reduction of LR initiation events correlated positively with Fe concentrations (Fig. 1A). To further explore the effect of excess Fe on LR formation, two root portions were analyzed separately: the proximal root portion, representing the root that formed prior to the onset of the treatment with excess Fe, and the distal root portion, representing the root that formed following the transfer to media containing excess Fe, (Fig. 1B; following the definition by Dubrovsky and Forde [2012]). Following treatment of Arabidopsis seedlings over a wide range of Fe concentrations, we observed no significant changes in the total LR initiation events in the proximal portion (Fig. 1C), similar to the results of Reyt et al. (2015), who found no effect of excess Fe on LR initiation in the LR branching zone. Furthermore, we did not observe any strong effects on the distribution of the developmental stages for preemergent LR primordia (Supplemental Fig. S1). However, the total LR initiation events in the distal portion displayed dose-dependent inhibition (Fig. 1C). Although the number of LRP at the four developmental stages also decreased with increasing exogenous Fe concentrations, the proportion in the four stages was not significantly modified in the distal portion (Fig. 1D). To ascertain that the observed LR phenotypes were specifically due to high Fe concentrations and not to the organic chelate, similar experiments were performed using Fe-citrate instead of Fe-EDTA (Supplemental Fig. S2). The LR development parameters were affected similarly by Fe regardless of the chelation ligands used (Supplemental Fig. S2). Furthermore, the response of LR development to excess Fe was also observed under hypoxic hydroponic conditions (Supplemental Fig. S3). Thus, excess Fe affected LR initiation strictly in the distal primary root portion that elongated during the treatment. Based on these and previous results (Reyt et al., 2015), we focused on LR development in the distal portion in further experiments. To analyze LR initiation in the distal portion under excess Fe in more detail, LR initiation events were also quantified at 1, 3, and 5 d after transfer. There were no significant changes in total LR initiation events between control and excess Fe treatments at 1 d, but a clear suppression was found in excess Fe treatments growing on days 3 to 5 (Fig. 1E).
Figure 1.
Effect of excess Fe on LR formation in Arabidopsis (Col-0). Seedlings (DR5:GUS lines) at 5 d after germination were transferred to varying concentrations of Fe (supplied as Fe-EDTA) treatment medium and grown for an additional 5 d. A, Inhibition of LR initiation events by varying concentrations of Fe. B, Photograph of representative seedlings after 5 d of vertical growth in medium lacking or supplemented with, 350 μm Fe. C, The number of LR initiation events in the proximal and distal root portions. D, The number of LR initiation events at three developmental stages, as indicated, was determined in the distal root portions. E, Effects of excess Fe on LR initiation in the wild type. Total LR initiation at 1, 3, and 5 d in control (Ctrl, 50 μm) and under excess Fe (+Fe, 350 μm) conditions. F, Effect of excess Fe (+Fe, 350 μm) on primary root growth, cortical cell length, and LR initiation. LR density refers to number of initiation events per mm, and LR frequency refers to number of initiation events per 100 cortical cells. Values are means of 10 plants ± se. Different letters represent means statistically different at the 0.05 level (one-way ANOVA with Duncan post hoc test). Asterisks indicate statistical differences between control and excess Fe treatment (+Fe) conditions (independent samples, Student’s t test, *P < 0.05). Bars = 1cm.
Our results show that cortical cell length was reduced by excess Fe in the distal root portion (Fig. 1F). To better understand the effect, we normalized for cell length. For this, a parameter called the LR initiation frequency, which defines how many LR primordia are formed along a parent root portion corresponding to 100 cortical cells in a file, was used. The LR initiation frequency reveals how LR initiation is affected in a genotype or by a treatment (Ivanchenko et al., 2008, 2010; Dubrovsky and Forde, 2012). Under Fe treatment, cell length and overall root length significantly decreased, and, therefore, LRP density increased compared with untreated roots (Fig. 1F). However, estimating the LRP frequency per 100 cells, we found that excess Fe can modify the LR initiation frequency. After excess Fe treatment (at 350 μm), the LR frequency decreased approximately 50% compared with control roots (Fig. 1F).
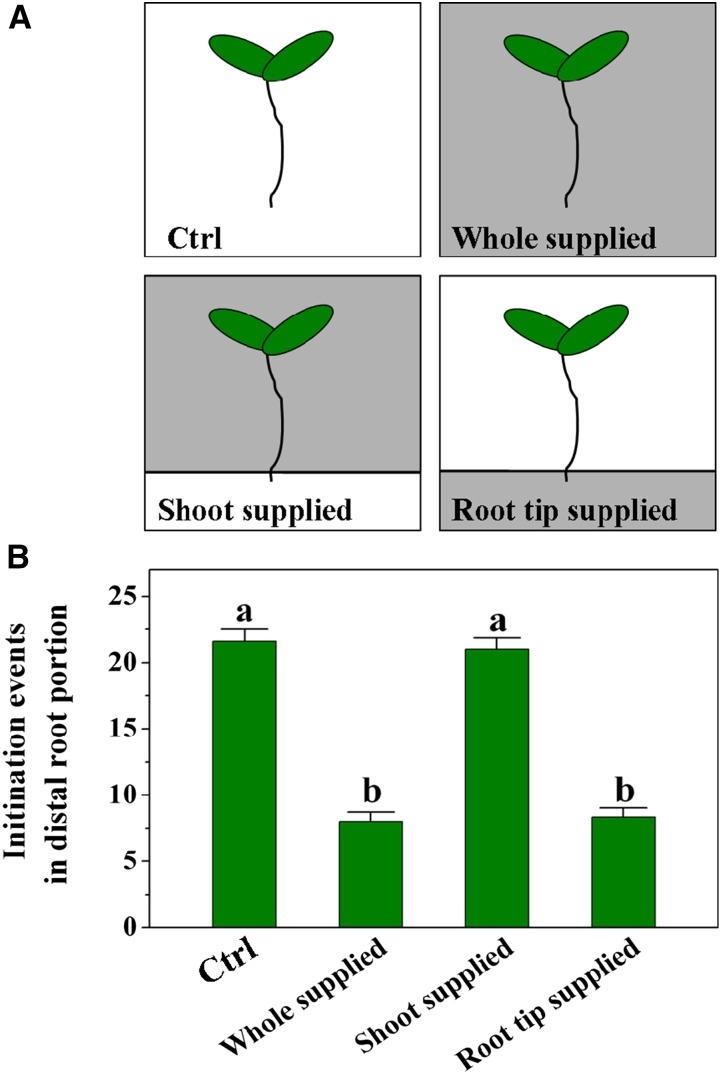
Given the strong impact of excess Fe exposure on LR initiation, we proceeded to determine if this was a localized or systemic response. We first tested the growth response of roots to localized Fe supply (Fig. 2A) and found that the inhibitory effect was identical whether Fe was applied to the whole root or only the tip (Fig. 2B). In a further experiment, no growth inhibition occurred when the shoots and root body were in contact with Fe, but the root tip did not touch the Fe-containing medium (Fig. 2B). These observations demonstrate a highly localized response to excess Fe in the inhibition of LR initiation phenotype.
Figure 2.
Effect of local supply of Fe on LR initiation in Arabidopsis. A, Schematic diagram of the experimental setup for applying excess Fe to the whole root versus the root tip and the shoot-mature root continuum. White sections indicate the basal growth medium with control medium, and gray sections indicate the Fe-enriched medium (350 μm). B, The effect of locally supplied Fe on LR initiation at 5 d. Values are means of 10 plants ± se. Different letters represent means statistically different at the 0.05 level (one-way ANOVA with Duncan post hoc test). Ctrl, Control.
Inhibitory Action of Excess Fe on LR Initiation Does Not Involve the ABA-Mediated Pathway
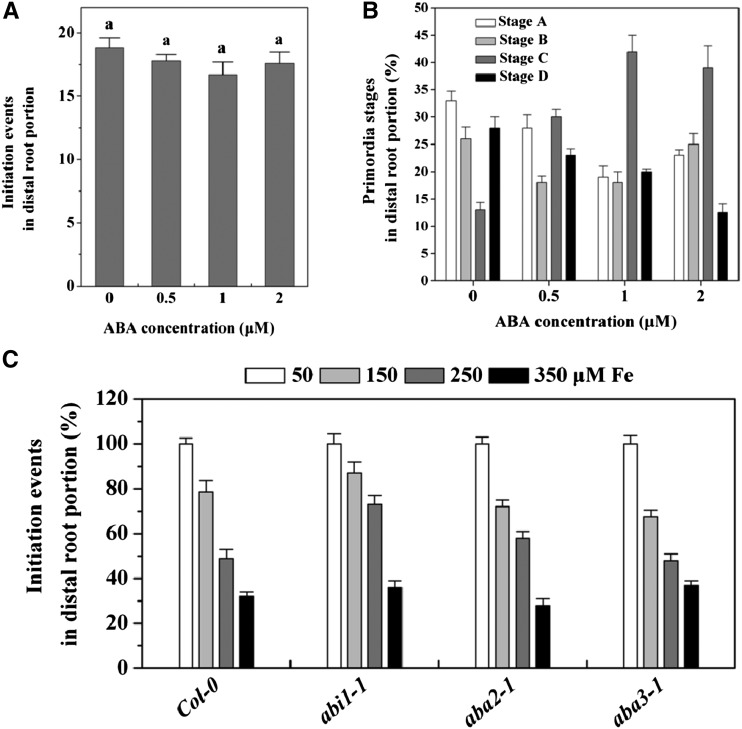
ABA is a negative regulator of LR development (De Smet et al., 2003), and has been suggested to mediate inhibitory effects of several stresses (Deak and Malamy, 2005; Xiong et al., 2006), and, thus, was examined here. Specifically, the involvement of ABA in the regulation of LR development under excess Fe has not been investigated. For this purpose, we first treated roots with varying concentrations of ABA to determine if this hormone treatment had an effect similar to excess Fe. As previously shown (De Smet et al., 2003), ABA treatment arrested meristem activation of LRP without affecting LR initiation in the distal portion of the root (Fig. 3, A and B), different from the inhibitory effect of excess Fe on LR development (Fig. 1, C and D). Further, ABA-insensitive mutant abi1-1 and ABA-deficient mutants aba3-1 and aba2-1 were used to test whether inhibition of LR initiation under Fe stress involves ABA mediation. The mutants and wild-type seedlings were exposed to varying concentrations of Fe continuously for 5 d, and the LR initiation events were analyzed (Fig. 3C). Fe had a similar impact on inhibition of LR initiation events in abi1-1, aba3-1, aba2-1, and wild-type plants.
Figure 3.
ABA-mediated signaling is not required for the inhibitory action of Fe on LR initiation in Arabidopsis. Five-day-old wild-type, abi1-1, aba3-1, and aba2-1 seedlings were transferred to medium, and roots were supplemented with varying concentrations of Fe (supplied as Fe-EDTA) or in combination with varying concentrations of ABA for 5 d, after which LR initiation events were quantified. A, Effect of ABA on LR initiation in DR5:GUS lines in seedlings supplied with varying concentrations of ABA for 5 d. B, The number of LR initiation events at each indicated developmental stage was determined in distal root portions. Values are means of 10 plants ± se. Different letters represent means statistically different at the 0.05 level (one-way ANOVA with Duncan post hoc test). C, Effect of varying concentrations of Fe on LR initiation in wild-type and ABA mutant seedlings. Total LR initiation events in the wild type, abi1-1, aba2-1, and aba3-1 in control were 19.7 ± 0.5, 18.2 ± 0.8, 19.2 ± 0.6, and 22.8 ± 0.9, respectively. Values are the means ± se (n ≥ 5).
Involvement of Auxin in the Response of LR Formation to Excess Fe
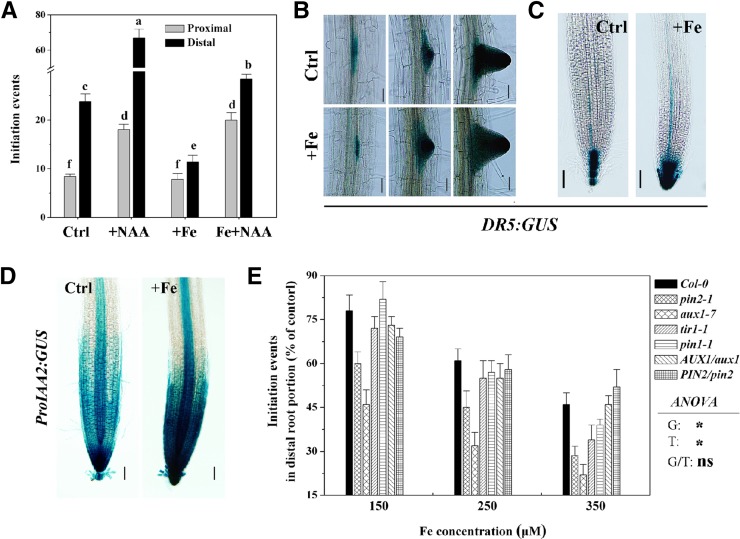
Given the established role of auxin in LR formation (Casimiro et al., 2003; Péret et al., 2009), we examined whether auxin is involved in excess Fe inhibition of LR development. As shown in Figure 4A, application of naphthylacetic acid (NAA) markedly promoted LR initiation events in both proximal and distal portions and significantly alleviated the excess Fe-mediated inhibition of LR formation in the distal portion. Furthermore, applications of indole-3-acetic acid (IAA) significantly promoted LR initiation frequency in the distal root portion under Fe stress (Table I; Supplemental Table S1). We first examined the spatial expression of the Direct Repeat5 (DR5):GUS reporter gene at several stages of LR development to determine whether excess Fe affects the auxin response in LR primordia. Compared with controls, DR5:GUS expression in LRP was not significantly modified in seedlings treated with excess Fe (Fig. 4B), and this might account for the above-described LRP proportion phenotype (Fig. 1D). However, the levels of DR5:GUS in the primary root apex were significantly enhanced under excess Fe stress compared with control (Fig. 4C). Furthermore, the fluorescence intensity in Fe-treated DR5:GFP roots was also significantly higher than that in untreated roots (Supplemental Fig. S4A). Similarly, the expression of ProIAA2:GUS was higher in the primary root apex under excess-Fe treatment than under control condition (Fig. 4D). In this study, we observed uniform activation of DR5:GFP expression in xylem-adjacent pericycle cells after treatment with IAA in both controls and excess Fe treatments, suggesting that the sensitivity of root cells to auxin may be not altered by Fe stress (Supplemental Fig. S5). It was previously shown that transport inhibitor response1 (TIR1) acts as an auxin receptor (Dharmasiri et al., 2005) and that the tir1-1 mutant shows reduced sensitivity to auxin as well as reduced LR formation (Ruegger et al., 1998). Here, we found that the response of LR initiation events to Fe stress in the tir1-1 mutant was similar to the wild type (although slight sensitivity was observed at 350 μm; Fig. 4E), suggesting that excess Fe may not affect the sensitivity of root cells to auxin.
Figure 4.
Effect of excess Fe on auxin response (DR5:GUS and ProIAA2:GUS) and LR initiation in the Arabidopsis wild type and auxin mutants. A, The effect of NAA (0.05 μm) on the number of LR initiation events in the proximal and distal root portions under control (Ctrl, 50 μm) and excess Fe conditions (+Fe, 350 μm) after 5 d. Values are the means ± se, n ≥ 5. Different letters represent means statistically different at the 0.05 level (one-way ANOVA with Duncan post hoc test). B and C, The expression of DR5::GUS in LRP (B) and the primary root tip (C) with and without excess Fe (350 μm) for 3 d. D, The expression of ProIAA2:GUS with and without excess Fe (350 μm) for 3 d. E, Effect of varying concentrations of Fe, applied for 5 d, on LR initiation in auxin response and auxin transport mutants. Total LR initiation events in the wild type, pin2-1, aux1-7, tir1-1, pin1-1, AUX1/aux1, and PIN2/pin2 in control were 21 ± 1.04, 18.8 ± 1.1, 12.5 ± 1.2, 16.4 ± 0.45, 23.4 ± 1.6, 21.8 ± 0.8, and 21.2 ± 1.2, respectively. Values are the means ± se (n ≥ 5). A two-way ANOVA was used to detect the significance of interaction between genotype and environment. NS and asterisk indicate nonsignificant or significant differences at P ≤ 0.05, respectively. G, Genotype (i.e., the wild type and aux1-7); T, treatment (i.e., control and +Fe). Bars = 50 μm.
Table I. Comparison of the effects of IAA, ACC, and AVG on primary root growth, cortex cell length, and LR initiation in Arabidopsis.
Seedlings were treated with 250 μm Fe (+Fe) with or without 0.01 μm IAA, 0.05 μm ACC, and 1 μm AVG for 5 d. The means ± se are reported with n ≥ 5 (P ≤ 0.05). Different letters represent means statistically different at the 0.05 level (one-way ANOVA with Duncan's post hoc test).
| Background | Root Growth Increment | Cell Length | LR Densitya | LR Frequencyb |
|---|---|---|---|---|
| mm | μm | |||
| Control | 51.8 ± 0.67 b | 226.3 ± 5.8 ab | 0.41 ± 0.01 e | 12.8 ± 0.73 c |
| +Fe | 21.1 ± 0.44 e | 156.8 ± 3.8 d | 0.64 ± 0.03 c | 9.02 ± 0.79 d |
| +IAA | 51.2 ± 0.66 b | 212.3 ± 3.7 bc | 0.49 ± 0.02 d | 14.8 ± 0.37 b |
| Fe + IAA | 20.8 ± 0.22 e | 154.4 ± 3.8 d | 1.26 ± 0.02 a | 17.7 ± 0.39 a |
| +ACC | 35.8 ± 0.37 c | 196.2 ± 4.8 c | 0.56 ± 0.03 d | 11.4 ± 1.15 c |
| Fe + ACC | 26.8 ± 0.28 d | 163.7 ± 2.1 d | 1.03 ± 0.01 b | 16.2 ± 0.85 a |
| +AVG | 54.8 ± 0.37 a | 233.5 ± 12.1a | 0.38 ± 0.03 e | 12.2 ± 0.37 c |
| Fe + AVG | 10.0 ± 0.34 f | 103.4 ± 2.2 e | 0.56 ± 0.02 d | 3.50 ± 0.24 e |
No. of initiation events per millimeter.
No. of initiation events per 100 cortical cells.
To further evaluate the role of auxin in LR formation under excess Fe conditions, we examined the response of pin1-1, pin2-1, pin3-5, and aux1-7 mutants defective in auxin transport to excess Fe. Total LR initiation events were decreased in the pin1-1 and pin3-5 mutants to a similar extent as in wild-type plants. However, pin2-1 and aux1-7 plants were found to be significantly more sensitive to excess Fe than the wild type under all tested elevated Fe concentrations (Fig. 4E; Supplemental Fig. S6A; Supplemental Table S2). Furthermore, the wild type and AUX1-complemented aux1 and PIN2-complemented pin2 mutants showed similar LR initiation sensitivity to excess Fe as controls (Fig. 4E). To analyze LR formation in the aux1-7 and pin2-1 mutants in more detail, LR initiation events in the distal portion were counted at 1, 3, and 5 d after transfer. Under control conditions, aux1-7 and pin2-1 mutants showed a reduction in total LRP formation compared with the wild type (Supplemental Fig. S7). Although excess Fe conditions decreased the LR initiation events in the distal portion in both pin2-1 and aux1-7 when compared with control, pin2-1 and, in particular, aux1-7 always showed lower LR initiation events than the wild type at the indicated treatment times of growth under excess Fe conditions (Supplemental Fig. S7). Furthermore, the LR frequency in the mutants slightly decreased under our growth conditions, compared with the wild type, and was approximately 83% of the wild type in pin2-1 and approximately 73% in aux1-7 (Table II). When the mutants were grown on media supplemented with excess Fe, the LR frequency in pin2-1 and aux1-7 plants decreased more significantly than the wild type, with LR frequency being reduced by approximately 35%, 58%, and 81% in wild-type, pin2-1, and aux1-7 plants, respectively (Table II).
Table II. Comparison of the effects of excess Fe on primary root growth, cortex cell length, and LR initiation in the wild type (Col-0) and mutants in Arabidopsis.
For growth conditions and treatments (+Fe, 250 μm Fe), see Fig. 8. The means ± se are reported with n ≥ 5 (P ≤ 0.05). Different letters represent means statistically different at the 0.05 level (one-way ANOVA with Duncan's post hoc test).
| Background | Root Growth Increment | Cell Length | LR Density | LR Frequency |
|---|---|---|---|---|
| mm | μm | |||
| Col-0 | ||||
| Control | 50.4 ± 0.24 a | 219.1 ± 6.1 a | 0.43 ± 0.02 cd | 13.4 ± 0.59 a |
| +Fe | 23.0 ± 1.1 d | 144.2. ± 3.9 d | 0.53 ± 0.02 b | 8.6 ± 0.24 cd |
| aux1-7 | ||||
| Control | 45.2 ± 0.58 b | 189.1 ± 4.1 b | 0.31 ± 0.01 e | 9.8 ± 0.48 bc |
| +Fe | 12.1 ± 0.77 g | 97.6 ± 2.7 g | 0.35 ± 0.03 de | 1.8 ± 0.37 f |
| pin2-1 | ||||
| Control | 44.6 ± 1.2 b | 190.8 ± 5.6 b | 0.38 ± 0.01 de | 11.1 ± 0.31 b |
| +Fe | 16.0 ± 0.31 f | 116.1 ± 2.3 f | 0.53 ± 0.04 b | 4.6 ± 0.39 e |
| eto1-1 | ||||
| Control | 28.2 ± 0.91 c | 157.9 ± 2.2 c | 0.51 ± 0.02 bc | 7.2 ± 1.3 d |
| +Fe | 19.2 ± 0.58 e | 128.5 ± 2.9 e | 0.78 ± 0.02 a | 11.3 ± 0.44 b |
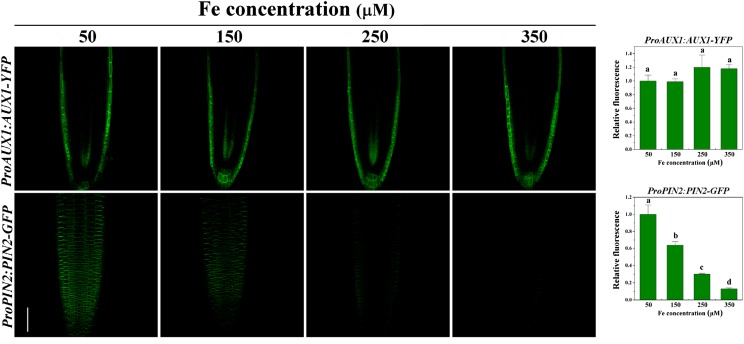
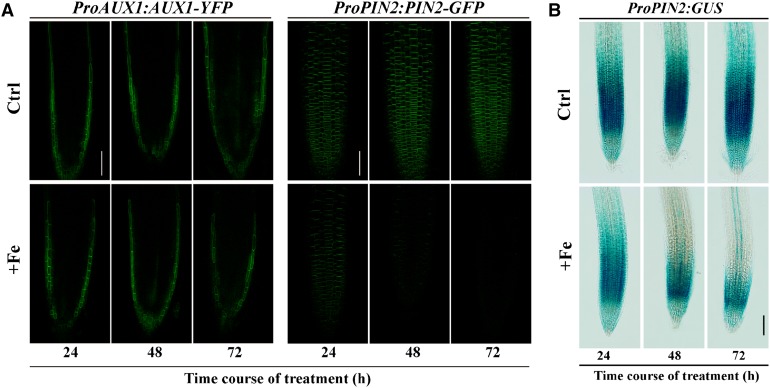
The appropriate expression and location of the AUX1 and PIN2 proteins in root tip epidermal cells are required for normal LR formation (Péret et al., 2009). Thus, we first examined whether AUX1 protein expression was altered when plants were treated with excess Fe, using a ProAUX1:AUX1-Yellow Fluorescent Protein (YFP) line. Intriguingly, the expression and localization patterns of ProAUX1:AUX1-YFP in roots was not affected by varying concentrations of Fe compared with those in untreated ProAUX1:AUX1-YFP roots at different treatment times, although an overall reduction in root length was observed (Figs. 5 and 6). Furthermore, Fe treatment did not change the PIN1-GFP expression profile in ProPIN1:PIN1-GFP plants, although a slight decrease was observed in the expression of ProPIN3:PIN3-GFP in roots (Supplemental Figs. S4B and S6B). By contrast, the fluorescence intensity of ProPIN2:PIN2-GFP in root apices decreased gradually with concentration and time compared with that in untreated roots, indicating the involvement of PIN2 during excess-Fe-modulated LR formation (Figs. 5 and 6). Using transgenic lines containing the ProPIN2:GUS reporter, we observed that the expression of the PIN2 gene was also influenced at the transcriptional level (Fig. 6B). However, ProPIN2:GUS expression was reduced relative slowly; typically, 48 h of exposure were required until significant inhibitory effects were observed (Fig. 6B).
Figure 5.
Effects of varying concentrations of Fe on expression of AUX1 and PIN2 in Arabidopsis. Five-day-old wild-type seedlings, containing ProAUX1:AUX1-YFP or ProPIN2:PIN2-GFP constructs, were transferred onto medium supplemented with varying concentrations of Fe-EDTA for 2 d. The fluorescence signal was detected using a Zeiss LSM710 confocal laser-scanning microscope at the specified time points. One representative image for each experiment is shown. The images were captured using the same confocal setting and are representative of at least 10 roots obtained from at least three independent experiments. Fluorescence is expressed relative to that of the control. Error bars represent se (n ≥ 5). Different letters represent means statistically different at the 0.05 level (one-way ANOVA with Duncan post hoc test). Bars = 50 μm.
Figure 6.
Effects of excess Fe on expression of AUX1 and PIN2 in Arabidopsis. Five-day-old wild-type seedlings, containing ProAUX1:AUX1-YFP, ProPIN2:PIN2-GFP, or ProPIN2:GUS constructs, were transferred onto control medium (Ctrl) or medium supplemented with 250 μm Fe (+Fe) at varying treatment times. The fluorescence signal was detected using a Zeiss LSM710 confocal laser-scanning microscope at the specified time points. One representative image for each experiment is shown. A, Effects of excess Fe on the expression of ProAUX1:AUX1-YFP and ProPIN2:PIN2-GFP in roots. The images were captured using the same confocal setting and are representative of at least 20 roots obtained from at least three independent experiments. B, The expression of ProPIN2:GUS with and without excess Fe for the indicated time periods. Bars = 50 μm (A) and 100 μm (B).
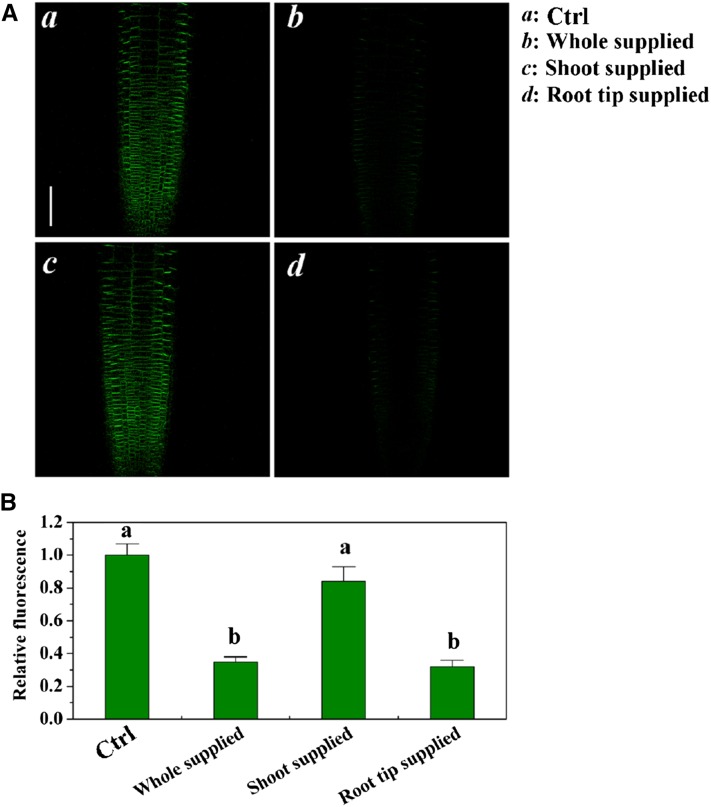
To establish whether PIN2 expression in root tips is regulated by local Fe, we monitored PIN2 expression in root tips by employing ProPIN2:PIN2-GFP lines. Excess Fe localized to the root tip markedly decreased PIN2-GFP in roots (Fig. 7A). Quantification of PIN2-derived fluorescence confined to the root revealed that the localized provision of excess Fe significantly decreased the fluorescence intensity, similar to provision to the whole root (Fig. 7B). However, provision of excess Fe to the shoot-mature root continuum had no impact on the fluorescence intensity of PIN2-GFP compared with control (Fig. 7B).
Figure 7.
Effect of locally supplied Fe on PIN2-GFP expression in the Arabidopsis root tip. A, Locally supplied Fe (250 μm) on PIN2-GFP expression in root tips at 2 d. B, The fluorescence of PIN2-GFP in A. Fluorescence is expressed relative to that of the control. Error bars represent se (n ≥ 5). Different letters represent means statistically different at the 0.05 level (one-way ANOVA with Duncan post hoc test). Bars = 50 μm.
Further considering the involvement of PIN2-dependent basipetal auxin transport in the root tip (from the root tip toward the base of the root) in LR initiation (Luschnig et al., 1998; Ivanchenko et al., 2010), we assayed basipetal auxin transport by examining auxin induction of DR5:GUS expression (Lewis and Muday, 2009). Consistently, auxin-induced DR5:GUS expression was shown within a much shorter region of the root under excess Fe treatment (Supplemental Fig. S8).
Iron excess has been documented to be associated with enhanced reactive oxygen species (ROS) production and oxidative stress in general. The possible role of oxidative stress in Fe inhibition of PIN2 expression and LR development was investigated. We first examined the response of the LR phenotype to paraquat (N,N′-dimethyl-4,4′-bipyridinium dichloride), which is often used to stimulate ROS production in plant cells (Chu et al., 2010). Paraquat significantly inhibited both PIN2-GFP expression and the number of LR initiation events in the distal portion of the root (Supplemental Figs. S9B and S10A). The effects of glutathione, a powerful antioxidant (Chen et al., 2012), on PIN2-GFP expression and LR initiation events in the presence of excess Fe were also investigated. Our results show that exogenous application of both Fe and glutathione together could increase PIN2-GFP expression in Fe-treated seedlings (Supplemental Fig. S10B) and resulted in less inhibition of LR initiation events compared with excess Fe treatment alone (Supplemental Fig. S9C).
Involvement of Ethylene in the Response of LR Formation to Excess Fe
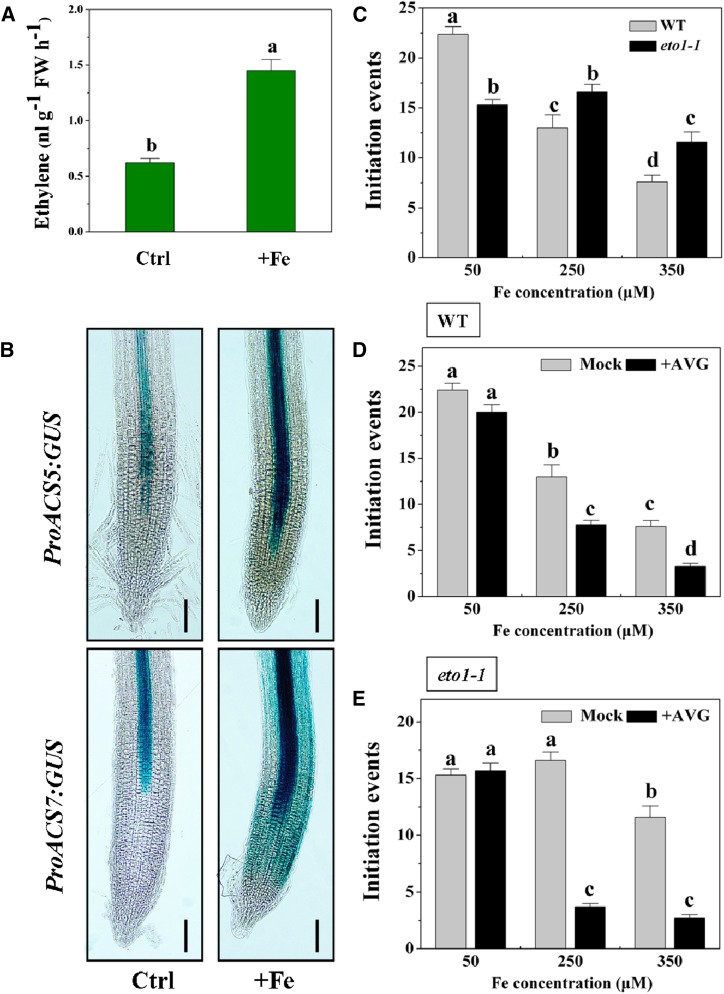
Ethylene has been shown to be involved in LR development (Ivanchenko et al., 2008; Lewis et al., 2011) and has been suggested to alleviate Fe toxicity in some cases (Becker and Asch, 2005; Li et al., 2015). To elucidate the relationship between ethylene accumulation and Fe-mediated LR development, we first measured ethylene production under Fe stress by use of gas chromatography. Excess Fe significantly increased ethylene levels in roots (Fig. 8A). The effect of excess Fe on ethylene production was further investigated by monitoring the AtACS5:GUS and AtACS7:GUS activity (two genes encoding 1-aminocyclopropane-1-carboxylic acid [ACC] synthase, the pivotal enzyme in the ethylene biosynthetic pathway of plants), in which the GUS reporter gene is driven by the AtACS5 and AtACS7 promoter. Excess Fe induced a marked increase of AtACS5:GUS and AtACS7:GUS expression in the root tip compared with control (Fig. 8B). The stimulatory effect of excess Fe on the expression of the AtACS5 and AtACS7 was also further confirmed by using quantitative reverse transcription-PCR (Supplemental Fig. S11), with results consistent with a previous report from our laboratories (Li et al., 2015).
Figure 8.
Effect of ethylene on the LR formation under excess Fe stress in Arabidopsis. A, Ethylene evolution in the root. Seedlings at 4 d after germination were exposed to 350 μm Fe in the roots for 4 d, and then ethylene evolution was determined. Values are the means ± se of three replicates. B, Activity of AtACS5:GUS and AtACS7:GUS in the root tip. Seedlings at 5 d after germination were exposed to 350 μm Fe in the roots for 2 d, and then GUS activity was determined. One representative sample from each treatment (10 plants) is shown. C, Effect of excess Fe on LR initiation in the wild type and ethylene-overproducing mutant eto1-1. Data are from one of three experiments. Seedlings at 5 d after germination were exposed to varying concentrations of Fe for 5 d. Values are the means ± se (n ≥ 7). D, Effect of the ethylene inhibitor AVG on LR initiation of wild-type seedlings grown in varying concentrations of Fe. Values are the means ± se (n ≥ 7). E, Effect of the ethylene inhibitor AVG on LR initiation of eto1-1 seedlings grown in varying concentrations of Fe. Values are the means ± se (n ≥ 7). Different letters represent means statistically different at the 0.05 level (one-way ANOVA with Duncan post hoc test). Ctrl, Control; WT, wild type; FW, fresh weight. Bars = 50 μm.
To investigate the possible role of ethylene in Fe-induced inhibition of LR formation, a genetic approach was adopted, using the ethylene overproduction mutant ethylene overproducer1-1 (eto1-1). Exposure of the eto1-1 mutant to varying Fe concentrations led to less inhibition of LR initiation than in the wild type, with LR initiation events being reduced by approximately 66% and 24% in wild-type and eto1-1 plants, respectively, upon exposure to 350 μm Fe (Fig. 8C). We further investigated the effects of aminoethoxyvinyl-glycine (AVG; an inhibitor of ethylene biosynthesis) on LR formation under varying Fe concentrations. Supplementation with AVG significantly enhanced the Fe-induced inhibition of the total LR initiation event in wild-type plants (Fig. 8D). We next determined if application of the ethylene biosynthesis inhibitor AVG would suppress the LR formation tolerance phenotype in the eto1-1 mutant. We found that supplementation with AVG prevented LR formation tolerance in association with elevated ethylene levels in eto1-1 seedlings exposed to excess Fe (Fig. 8E). Furthermore, supplementation with ACC (an ethylene precursor) effectively reversed the decreased rate of total LR initiation events and the reduced LR frequency seen under excess Fe, compared with the mock condition (Table I; Supplemental Table S1). Consistently, exposure of the eto1-1 mutant to excess Fe stress also led to increased LR frequency compared with the wild type (Table II; Supplemental Table S2). However, AVG enhanced the Fe-induced reduction of LR frequency (Table I).
Involvement of Auxin in Ethylene-Regulated LR Formation during Fe Stress
The data above indicate that excess Fe represses PIN2 expression and up-regulates ethylene production, while increasing the tolerance of the LR formation. To investigate whether increased ethylene could influence the Fe-modulated expression of PIN2, PIN2 expression was analyzed in the roots of ProPIN2:PIN2-GFP seedlings treated with excess Fe plus AVG or with Fe alone. Similar inhibition of PIN2-GFP abundance in the root apex was found in Fe-treated seedlings with or without AVG (Fig. 9, A and B). However, the localization of the PIN2 protein appeared to be altered only slightly (Figs. 7A and 9A). After extending the master gain of the GFP channel from 679 to 1,017, it was found that PIN2-GFP was diffusely distributed in the root tips in seedlings treated with excess Fe plus AVG (Fig. 7A, b; Fig. 9A, b). Surprisingly, excess Fe plus AVG markedly decreased AUX1-dependent fluorescence at the root apex compared with Fe alone (Fig. 9C). Quantification of AUX1-derived fluorescence confined to the root apex revealed that provision of excess Fe plus AVG decreased the AUX1 signal intensity by approximately 40% relative to excess Fe alone (Fig. 9D). In addition, the localization of the AUX1 protein appears altered in the root tip of seedlings grown on excess Fe plus AVG and becomes dispersed near the root tip (Fig. 9C, a–d). To further explore the role of AUX1 in the ethylene-induced LR formation tolerance phenotype, the LR initiation events of the distal portion were analyzed in the wild type, the eto1-1 mutant, the aux1-7 mutant, and the double mutant (eto1/aux1) under excess Fe (Fig. 9E). Our results show that aux1-7 significantly weakens the LR formation tolerance characteristic of eto1-1.
Figure 9.
Effects of AVG on the expression of AUX1 and PIN2 in Arabidopsis. Five-day-old wild-type seedlings, containing ProAUX1:AUX1-YFP or ProPIN2:PIN2-GFP constructs, were transferred onto control medium (Ctrl) or medium supplemented with 250 μm Fe (+Fe) with or without 1 μm AVG for 48 h. A, The expression of ProPIN2:PIN2-GFP with and without excess Fe plus AVG or Fe alone treatment. The treatments were maintained under the same confocal setting, and the inserts (a and b) show roots under Fe treatment, extending the master gain of the GFP channel from 679 to 1,017. B, The fluorescence of PIN2-GFP in A. Fluorescence is expressed relative to that of the control without AVG. Error bars represent se (n ≥ 5). C, The expression of ProAUX1:AUX1-YFP with and without excess Fe plus AVG or Fe alone treatment. The treatments were maintained under the same confocal setting, and the inserts (a–d) show images of partial enlargement, as indicated by white arrows. D, The fluorescence of AUX1-YFP in C. Fluorescence is expressed relative to that of the control without AVG. Bars represent se (n ≥ 5). E, Effect of excess Fe (250 μm) on LR initiation events in wild-type (Col-0), eto1-1, aux1-7, and eto1/aux1 seedlings after 5 d. Values are the means ± se (n ≥ 7). Bars = 50 μm.
DISCUSSION
Excess Fe Regulates LR Initiation Acting at the Tip of the Growing Primary Root
Varying initiation of LRP, frequency of LR emergence, and growth rate of both primary root and LR provide significant combinatorial potential for modifying root system architecture to respond to changing nutrient availability (Liao et al., 2006; Fujiwara and Matoh, 2009; Gruber et al., 2013). Fe is both an essential micronutrient and is highly toxic to root growth when in excess (Becker and Asch, 2005; Kobayashi and Nishizawa, 2012), but the toxicity mechanisms in terms of root development remain largely unknown. Recently, Reyt et al. (2015) found that root in Arabidopsis, rather than LR initiation within the LR branching zone, represented the principal target of inhibition. Whether, and how, LR formation near the growing tip of the primary root responds has not been examined to date. We conclude from the evidence presented here that excess Fe treatment in Arabidopsis not only directly impairs primary root growth, but also strongly arrests LR initiation by acting at the tip of the growing primary root. We show that excess Fe inhibits total LR initiation but not subsequent LR development (Fig. 1A; Supplemental Fig. S1B) and that these effects are only seen in newly grown roots that elongated during exposure to excess Fe (Fig. 1, A and B). Similar results were found in the arrest of salt-mediated LR initiation events, which was, as well, confined to newly grown roots (Duan et al., 2013). Decrease in overall LR number with increasing Fe concentrations could simply reflect the shorter length of the primary root. Excess Fe significantly inhibited both primary root growth and the total LR initiation events, consistent with this simple explanation. However, IAA in our study did not affect primary root growth compared with mock conditions but significantly increased the number of LR initiation events in both control and excess-Fe conditions (Table I; Supplemental Table S1), consistent with results in a previous report (Ivanchenko et al., 2010). Furthermore, ACC, at the concentration applied in our study, significantly reduced primary root growth but had no effect on LR initiation events (Table I; Supplemental Table S1), similar to the results of Ivanchenko et al. (2008) and results obtained with the eto1-1 mutant under excess Fe stress (Table II; Supplemental Table S2). Thus, both our present and previous results suggest a more complex interconnection between root length and LR initiation frequency under excess Fe conditions than a simple correlation between length and number. LR development is initiated by asymmetric divisions in pairs of founder cells within xylem pole pericycle cells close to the root tip (De Smet et al., 2007; Overvoorde et al., 2010). Excess Fe may inhibit xylem-adjacent pericycle cell activation during primordium morphogenesis, because the frequency of LR initiation, estimated as the number of initiation events per 100 cortical cells, is decreased under Fe toxicity (Fig. 1C). This also provides an explanation for the absence of an arrest of initiation events in previously formed roots (proximal portion), because LR patterning in the proximal portion occurred before treatment of roots with excess Fe. No information is as yet available to suggest how Fe stress may inhibit the commitment, or activation, of xylem-adjacent pericycle cells. Our data show that LR initiation frequency is associated with auxin (Table I). However, the sensitivity of root cells to auxin may not be altered by excess Fe (Fig. 4E; Supplemental Fig. S5). Furthermore, aux1-7 and pin2-1 mutants exhibited a higher degree of sensitivity in LR frequency to excess Fe (Table II), suggesting that the effect may, at least partly, be mediated by AUX1 and/or PIN2. However, the issue of how AUX1 and/or PIN2 are involved in Fe-mediated modulation of LR frequency still needs to be explored.
Moreover, we show that the impact of Fe toxicity on LR formation in Arabidopsis is not a systemic response. Split-plate results reported here provide strong demonstration that physical contact of the primary root tip with excess Fe is necessary, and sufficient, for LR formation inhibition in newly grown roots (Fig. 2). Previously, we found that the root tip was also the primary sensing site for the root elongation response to excess Fe stress (Li et al., 2015). In a previous study on NH4+ toxicity in Arabidopsis (Li et al., 2010), it was similarly observed that contact with the root tip was both essential and sufficient to explain the development of the NH4+ toxicity syndrome and, more specifically, the inhibition of primary and LR growth. Several studies have indicated that nutrients, such as N and P, with heterogeneous distribution in the soil and rhizosphere (Hodge, 2004; Britto and Kronzucker, 2013; Hufnagel et al., 2014), are sensed by root-localized mechanisms (Forde and Lorenzo, 2001; Forde and Walch-Liu, 2009). Iron toxicity as a syndrome in plants is typically associated with an excessive amount of the ferrous form (the Fe2+ ion) in the soil (Vigani, 2012). The presence of the Fe2+ ion is increased by low pH and hypoxic or anoxic conditions, and there is an increasing presence in vertically lower strata (Ratering and Schnell, 2000; Becker and Asch, 2005). Thus, we propose that, when the primary root tip reaches the excess Fe zone, plants sense the stress stimulus and trigger a developmental switch in both the primary root and the LR apparatus from indeterminate to determinate growth, the reduction of cell elongation and the LR initiation acting at the tip of the growing primary root. Such a change may adjust root system architecture to restrict excessive Fe absorption, which also occurs predominantly in the Fe2+ form (Vigani, 2012; Li et al., 2015), and prevent serious Fe toxicity. In addition, relatively stable LR number and length in the branching zone, or the proximal root portions, helped by Fe sequestration in the vacuole and by components such as ferritins (Vigani, 2012; Reyt et al., 2015), may play a role in maintaining the absorption of other nutrients in the less stressed areas of the root system.
The Role of AUX1 and PIN2 in LR Formation under Excess Fe Conditions
The inhibitory effects of excess Fe on LR initiation and xylem differentiation resemble the phenotypes of seedlings defective in auxin transport or perception (Casimiro et al., 2003; Fukaki and Tasaka, 2009; Péret et al., 2009). Analysis of the effect of excess Fe on auxin response and auxin transport in roots suggests that an important part of inhibition on LR formation is mediated by the auxin pathway (Fig. 4; Table I). However, we found that DR5:GUS expression in LRP was not significantly modified in seedlings treated with excess Fe (Fig. 4B). This, together with an account of ferritin gene expression reported previously (Reyt et al., 2015), might account for the above-described LRP proportion phenotype (Fig. 1D). Further screening of auxin-related mutants for excess Fe-responsive LR formation showed that LR initiation was inhibited by excess Fe to a greater extent in aux1-7 and pin2-1 mutants than in wild-type plants, at varying concentrations of Fe and with varying treatment time (Fig. 4E; Supplemental Fig. S7), indicating that disruptions in either AUX1 or PIN2 enhance the sensitivity of LR formation under Fe stress. By contrast, wild-type and pin1-1, pin3-5, and tir1-1 mutants showed similar LR initiation sensitivity to excess Fe as controls (Fig. 4E; Supplemental Fig. S6A). Although the expression and localization of AUX1 were unaffected, PIN2 transcript and protein were decreased gradually under early Fe stress (Figs. 5 and 6). The alterations in PIN2 gene expression and PIN2 abundance may be expected to interfere with the distribution of auxin in root tips and with basipetal auxin transport (Abas et al., 2006; Wisniewska et al., 2006). This is supported by the observed reduction in basipetal auxin transport and the increase in auxin accumulation in the root apex under excess Fe stress (Fig. 4, C and D; Supplemental Fig. S8). This suggests a tight relationship for PIN2 in the excess-Fe-induced inhibition of LR formation. Moreover, our analysis of locally supplied Fe (Fig. 7) indicates that Fe supply to only the root tip, but not the shoot-mature root continuum, decreased PIN2:GFP expression in the root tip, consistent with the split-plate results (Fig. 2), and highlights the correlation between Fe-dependent PIN2 expression and LR initiation. The LR development of the pin2-1 mutant was also reported to be more sensitive to NH4+ toxicity (Li et al., 2011). Furthermore, auxin distribution within the root under Fe stress responded to alterations in PIN2 gene expression and PIN2 abundance, and this may modify the direction of root growth away from the stress stimulus (Supplemental Fig. S12; Wisniewska et al., 2006). In addition to participating in AUX1-mediated basipetal auxin transport, PIN2 is seen as a general stress target on account of its pronounced sensitivity to environmental stresses, such as cold, dark, salt, and aluminum and cadmium toxicity, and underlies root avoidance of stress (Supplemental Fig. S10A; Baluska et al., 2010). Iron excess has been linked to oxidative stress, and ROS homeostasis is emerging as a parameter controlling root system architecture more generally (Manzano et al., 2014). However, although antioxidant partially increased PIN2-GFP expression and LR initiation events, it could not rescue Fe inhibition of LR formation completely (Supplemental Figs. S9 and S10). Cadmium, which also induces oxidative stress via Fenton reactions, could inhibit root system development (Supplemental Fig. S9A; Jonak et al., 2004), with various hormones (nitric oxide, jasmonic acid, and salicylic acid) reported to be involved in tolerance (Rodríguez-Serrano et al., 2006; Lin et al., 2012). Our present data suggest that Fe-induced oxidative stress is partly involved in the suppression of PIN2-GFP expression and of LR formation in the distal portion of the root.
Using both pharmacological and genetic approaches, we further demonstrate that ethylene plays an important role in LR formation under excess Fe exposure. Ethylene evolution was enhanced in the root upon exposure of seedlings to excess Fe (Fig. 8A), similar to previous reports (Majerus et al., 2007; Li et al., 2015). Here, we identify ACS5 and ACS7 as two key genes responsible for the observed Fe-induced ethylene production in root tips (Fig. 8B; Supplemental Fig. S11). Remarkably, the excess-Fe-induced inhibition of LR formation was aggravated in the presence of the ethylene synthesis inhibitor AVG in the wild type (Fig. 8D). The finding that the ethylene overproduction mutant eto1-1 showed increased LR formation compared with the wild type under Fe stress (Fig. 8C) supports this notion. Also supportive is the observation that the externally applied ethylene inhibitor AVG prevented tolerance by elevated ethylene levels in eto1-1 exposed to high Fe (Fig. 8E). Moreover, the inhibition of LR frequency was alleviated in the presence of ACC as well as in the ethylene overproduction mutant, while an inhibitor of ethylene biosynthesis (AVG) aggravated it (Tables I and II).
Together with PIN2, AUX1 is well known to form the basic machinery for basipetal auxin transport, which is critical for LR initiation (De Smet et al., 2007). Furthermore, in aux1-7, total LR initiation events and LR frequency were more sensitive to excess Fe than in the wild type (Fig. 4E; Table II; Supplemental Fig. S7), suggesting AUX1 is essential for the maintenance of LR initiation under excess Fe. Furthermore, Giehl et al. (2012) suggested that localized supply of iron regulated LR development in Arabidopsis by altering AUX1-mediated auxin distribution. Based on the present and previous findings, the hypothesis that ethylene induces tolerance in LR formation in the wild type partly through AUX1 mediation was borne out. The findings are also consistent with previous reports showing that ethylene enhances the expression of AUX1 reporters (Růzicka et al., 2007; Lewis et al., 2011). Strong support for this can be seen in the finding that excess Fe and AVG cotreatment markedly reduce AUX1 protein abundance in the root apex of wild-type seedlings compared with excess Fe alone (Fig. 9, C and D). More importantly, the eto1-1 mutant, also lacking AUX1 (double mutant eto1-1/aux1-7), lost its previously documented higher LR tolerance characteristic under excess Fe stress (Fig. 9E), further supporting the hypothesis that AUX1 is involved in ethylene-mediated signaling under excess Fe stress. However, excess Fe and AVG cotreatment had no effect on PIN2 protein abundance (Fig. 9, A and B), although a small alteration in localization was noted after extending the master gain of the GFP channel (Fig. 9A). Together, we suggest that AUX1 is at least partially required for the tolerance effect of endogenous ethylene in Fe-dependent LR formation.
ABA is also often associated with LR development. ABA signaling pathways are recognized as essential for LR responses to salt and osmotic stresses (Deak and Malamy, 2005; Xiong et al., 2006). However, excess Fe does not exert its inhibition of LR number through these pathways. Firstly, excess Fe stress arrests LRP initiation without affecting subsequent LRP development, different from the inhibitory effect of ABA on meristem activation of LRP (Fig. 2, A and B; Signora et al., 2001; De Smet et al., 2003). Secondly, ABA response and biosynthesis mutants, such as abi1-1, aba2-1, and aba3-1, still display LR growth sensitivity to excess Fe, similar to the wild type.
In conclusion, we propose the following model for LR formation under excess Fe stress in the distal portion of the root (Fig. 10). Our results show that the expression of PIN2 protein in root tips is dramatically reduced and involved in arresting LR initiation near the growing tip of the primary root in the early response to excess Fe. These modulations in hormone homeostasis can help root system architecture adjust rapidly to restrict excessive Fe absorption and avoid serious Fe toxicity. Meanwhile, the induced-ethylene and ethylene-related AUX1 functions, as a compensator modulating the process, can partially antagonize the reduction in excess Fe-mediated LR formation in the wild type. These compensatory effects of ethylene may prevent the excessive decline in LR number and maintain the absorption of other nutrients to maintain rigorously controlled plant acclimation to nutritional stress (Heil and Baldwin, 2002). Although the possible involvement of other pathways in Fe inhibition LR formation still needs to be explored, our results provide unique insight into how LR formation is regulated in response to early Fe toxicity. Further research into the interplay of excess Fe supply and auxin will enable a fuller understanding of how plants respond to Fe toxicity by regulating hormonal signaling and will be instrumental in the development of strategies to improve Fe toxicity tolerance in crops.
Figure 10.

A proposed model for LR formation in Arabidopsis under excess Fe stress in the distal root portion. The expression of PIN2 protein in root tips is dramatically reduced and involved in arresting LR initiation near the growing tip of the primary root in the early response to excess Fe stress. Fe-induced endogenous ethylene enhances the tolerance of LR formation to Fe stress, and AUX1 plays a role in ethylene-mediated LR formation.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seedlings of the following lines were used in this study: Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 (Col-0); the mutants aba3-1, aba2-1, abi1-1, eto1-1, aux1-7, pin2-1, pin3-5, tir1-1, pin1-1, and eto1/aux1 (Strader et al., 2010) in the Col-0 background; the transgenic lines DR5:GUS and DR5:GUS in aux1-7 and pin2-1; and ProACS5:GUS and ProACS7:GUS (Wang et al., 2005), ProIAA2:GUS, ProPIN1:PIN1-GFP, ProPIN2:PIN2-GFP, ProPIN3:PIN3-GFP, ProAUX1:AUX1-YFP (Swarup et al., 2004), and the DR5:GFP lines. Seeds were surface sterilized and cold treated at 4°C for 48 h prior to being sown on standard growth medium. The standard growth medium was as described previously (Li et al., 2013) and was composed of 2 mm KH2PO4, 5 mm NaNO3, 2 mm MgSO4, 1 mm CaCl2, 50 μm Fe-EDTA, 50 μm H3BO3, 12 μm MnSO4, 1 μm ZnC12, 1 μm CuSO4, 0.2 μm Na2MoO4, 1% (w/v) Suc, 0.5 g L–1 MES, and 0.8% (w/v) agar (adjusted to pH 5.7 with 1 m NaOH). The day of sowing was considered day 0. Seedlings were grown, oriented vertically on the surface of the culture plates in a growth chamber, and set to a 16-h-light/8-h-dark photoperiod, an irradiance of 100 μmol m–2 s–1, and a temperature of 23°C ± 1°C. Excess Fe was supplied as Fe-EDTA (FeSO4⋅7H2O plus EDTA [1:1 m ratio]). Fe availability and toxicity, and root growth itself, are strongly pH dependent (Koyama et al., 2001; Becker and Asch, 2005), and we thus set pH to 5.3, based on our previous report (Li et al., 2015), for further treatments. To study the effect of exogenous ABA, NAA, IAA, ACC, and AVG, seedlings were supplemented with varying concentrations of Fe-EDTA plus ABA (as indicated), 0.05 μm NAA, 0.01 μm IAA, 0.05 μm ACC, or 1 μm AVG. All chemicals were obtained from Sigma-Aldrich. For hydroponic culture, gravity stimulation, and qRT-PCR experiments, see Supplemental Materials and Methods S1.
Localized Fe Supply Experiments
Localized excess-Fe treatments were shown in Figure 2A, as described in our previous report (Li et al., 2015). It is briefly described here: For control or whole-supplied plants, seedlings were grown in the normal growth medium with or without excess Fe following seedling transfer. For root tip-supplied plants, segmented agar plates were separated into upper and bottom parts with a 3-mm air gap using movable glass strips 3 mm in width. Normal growth medium was poured into the upper part, and control medium containing excess Fe-EDTA was poured into the bottom part. Plates on which only the primary root tip of the seedlings (approximately 2 mm) was in contact with the bottom of the medium are referred to as root tip-supplied Fe plants. For shoot-supplied Fe plants, normal growth medium was poured into the bottom part, and control medium containing excess Fe-EDTA was poured into the upper part. Plates on which only the shoot and mature primary root zone of the seedlings were in contact with the upper of the medium are referred to as shoot-supplied Fe plants, and treatment was for the times indicated.
Root Measurements
Root portions that were formed before or during the treatments were harvested separately for analysis. All initiation events, including emerged LRs and LRP, were quantified in cleared roots according to Ivanchenko et al. (2008) or using DR5:GUS lines. The developmental stage of each LRP was classified according to Zhang et al. (1999): stage A, up to three cell layers; stage B, unemerged, more than three cell layers; stage C, emerged LRs, less than 0.5 mm in length; and stage D, LRs, greater than 0.5 mm. The emerged but not activated LRP are still referred to as LRP, and only mature LRs (exceeding 0.5 mm in length) are denoted as LRs. The lengths of at least 10 cortical cells were measured for each root along the entire root growth increment so as to exclude the possibility of differential response to the treatment. The average root length corresponding to 100 cortical cells in a file was estimated for each root based on the elongation increment and average cortex cell length of the root (Ivanchenko et al., 2008, 2010). The lengths of primary roots of individual seedlings were measured directly using Image J software from digital images captured with a Canon G7 camera.
Image Analysis
Histochemical analysis of GUS reporter enzyme activity was performed as described elsewhere (Weigel and Glazebrook, 2002). GUS staining patterns in roots were analyzed using an Olympus BX51 microscope with differential interference contrast optics. The DR5:GFP, ProPIN1:PIN1-GFP, ProPIN2:PIN2-GFP, ProPIN3:PIN3-GFP, and ProAUX1:AUX1-YFP reporters were analyzed using a Zeiss LSM710 confocal microscope, and image analysis was performed using Zeiss 2009 software. All staining and image analysis procedures were repeated at least twice. All the images and graphs were arranged in Adobe Photoshop.
Ethylene Measurements
After seedling exposure to various concentrations of Fe-EDTA for 4 d, roots were weighed separately and put into 5-mL gas-tight vials containing 1 mL of agar medium (0.7% agar). Headspace samples (1 mL) were withdrawn and analyzed using a GC-6850 gas chromatograph (Agilent Technologies Japan), which was equipped with a flame ionization detector.
Statistical and Graphical Analyses
For all experiments, data were statistically analyzed using the SPSS 13.0 program. Details are as presented in figure legends. Graphs were produced using Origin 8.0. All graphs and images were prepared using Adobe Photoshop 7.0.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: ABA3 (At1g16540), ABA2 (At1g52340), ABI1 (At4g26080), ACS5 (At5g65800), ACS7 (At4g26200), AUX1 (At2g38120), ETO1 (At3g51770), IAA2 (At3g23030), PIN1 (At1g73590), PIN2 (At5g57090), PIN3 (At1g70940), and TIR1 (At3g62980).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Effects of excess Fe on LR primordium proportion in Arabidopsis.
Supplemental Figure S2. Effects of Fe-citrate and K-citrate on LR initiation events in Arabidopsis.
Supplemental Figure S3. Effects of Fe excess on Arabidopsis LR initiation events in hydroponic condition.
Supplemental Figure S4. Effects of excess Fe on expression of DR5:GFP and ProPIN1:PIN1-GFP.
Supplemental Figure S5. Auxin activation of the DR5 response in xylem-adjacent pericycle cells.
Supplemental Figure S6. Effects of excess Fe on the LR initiation of pin3-5 and expression of ProPIN3:PIN3-GFP in Arabidopsis.
Supplemental Figure S7. Effects of excess Fe on the wild-type (Col-0), pin2-1, and aux1-7 LR initiation.
Supplemental Figure S8. Auxin induction of DR5:GUS expression in Col-0, with or without excess Fe treatment.
Supplemental Figure S9. Effects of cadmium, paraquat, and glutathione on LR initiation in Arabidopsis.
Supplemental Figure S10. Effects of cadmium, paraquat, and glutathione on the expression of ProPIN2:PIN2-GFP in Arabidopsis.
Supplemental Figure S11. Effects of excess Fe treatment on AtACS5 and AtACS7 transcript levels in Arabidopsis.
Supplemental Figure S12. Experimental system for examining agravitropic response in response to excess Fe.
Supplemental Table S1. Comparison of the effects of IAA, ACC, and AVG on total LR initiation events in Arabidopsis.
Supplemental Table S2. Comparison of the effects of excess Fe on total LR initiation events in the wild type and mutants in Arabidopsis.
Supplemental Materials and Methods S1. Hydroponic culture, gravity measurement, and qRT-PCR and auxin transport analysis.
Supplementary Material
Acknowledgments
We thank Drs. Malcolm Bennett (University of Nottingham), Ben Scheres (Utrecht University), Tom Guilfoyle (University of Missouri), Chuanyou Li (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences), Ningning Wang (Nankai University), Zhizhong Gong and Zhizhong Chen (China Agricultural University), Bonnie Bartel (Rice University), and Jiri Friml (Ghent University) for the mutants and transgenic lines of Arabidopsis and the Arabidopsis Biological Resource Center for the mutant seeds.
Glossary
- LR
lateral root
- LRP
lateral root primordia
- ABA
abscisic acid
- AVG
aminoethoxyvinyl-glycine
- Col-0
ecotype Columbia-0
- NAA
naphthylacetic acid
- IAA
indole-3-acetic acid
- ROS
reactive oxygen species
- ACC
1-aminocyclopropane-1-carboxylic acid
Footnotes
This work was supported by the National Natural Science Foundation of China (31300210 and 41171234) and the Natural Sciences and Engineering Research Council of Canada (Discovery Grant no. 217277–2009).
Articles can be viewed without a subscription.
References
- Abas L, Benjamins R, Malenica N, Paciorek T, Wiśniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C (2006) Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8: 249–256 [DOI] [PubMed] [Google Scholar]
- Baluska F, Mancuso S, Volkmann D, Barlow PW (2010) Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci 15: 402–408 [DOI] [PubMed] [Google Scholar]
- Bashir K, Ishimaru Y, Itai RN, Senoura T, Takahashi M, An G, Oikawa T, Ueda M, Sato A, Uozumi N, et al. (2015) Iron deficiency regulated OsOPT7 is essential for iron homeostasis in rice. Plant Mol Biol 88: 165–176 [DOI] [PubMed] [Google Scholar]
- Batty LC, Younger PL (2003) Effects of external iron concentration upon seedling growth and uptake of Fe and phosphate by the common reed, Phragmites australis (Cav.) Trin ex. Steudel. Ann Bot (Lond) 92: 801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M, Asch F (2005) Iron toxicity in rice-conditions and management concepts. J Plant Nutr Soil Sci 168: 558–573 [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Briat JF, Dubos C, Gaymard F (2015) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci 20: 33–40 [DOI] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ (2013) Ecological significance and complexity of N-source preference in plants. Ann Bot (Lond) 112: 957–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Chen JH, Jiang HW, Hsieh EJ, Chen HY, Chien CT, Hsieh HL, Lin TP (2012) Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol 158: 340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SH, Noh HN, Kim S, Kim KH, Hong SW, Lee H (2010) Enhanced drought tolerance in Arabidopsis via genetic manipulation aimed at the reduction of glucosamine-induced ROS generation. Plant Mol Biol 74: 493–502 [DOI] [PubMed] [Google Scholar]
- Connolly EL, Guerinot M (2002) Iron stress in plants. Genome Biol 3: S1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Signora L, Beeckman T, Inzé D, Foyer CH, Zhang H (2003) An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J 33: 543–555 [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, Frei dit Frey N, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, et al. (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690 [DOI] [PubMed] [Google Scholar]
- Deak KI, Malamy J (2005) Osmotic regulation of root system architecture. Plant J 43: 17–28 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M (2005) The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Duan L, Dietrich D, Ng CH, Chan PMY, Bhalerao R, Bennett MJ, Dinneny JR (2013) Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 25: 324–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Forde BG (2012) Quantitative analysis of lateral root development: pitfalls and how to avoid them. Plant Cell 24: 4–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Soukup A, Napsucialy-Mendivil S, Jeknic Z, Ivanchenko MG (2009) The lateral root initiation index: an integrative measure of primordium formation. Ann Bot (Lond) 103: 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felten J, Kohler A, Morin E, Bhalerao RP, Palme K, Martin F, Ditengou FA, Legué V (2009) The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol 151: 1991–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B, Lorenzo H (2001) The nutritional control of root development. Plant Soil 232: 51–68 [Google Scholar]
- Forde BG, Walch-Liu P (2009) Nitrate and glutamate as environmental cues for behavioural responses in plant roots. Plant Cell Environ 32: 682–693 [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Matoh T (2009) Plant nutrition: roots of life for fundamental biology and better crop production. Plant Cell Physiol 50: 2–4 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M (2009) Hormone interactions during lateral root formation. Plant Mol Biol 69: 437–449 [DOI] [PubMed] [Google Scholar]
- Giehl RF, Lima JE, von Wirén N (2012) Localized iron supply triggers lateral root elongation in Arabidopsis by altering the AUX1-mediated auxin distribution. Plant Cell 24: 33–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber BD, Giehl RF, Friedel S, von Wirén N (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol 163: 161–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Baldwin IT (2002) Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci 7: 61–67 [DOI] [PubMed] [Google Scholar]
- Hodge A. (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162: 9–24 [Google Scholar]
- Hufnagel B, de Sousa SM, Assis L, Guimaraes CT, Leiser W, Azevedo GC, Negri B, Larson BG, Shaff JE, Pastina MM, et al. (2014) Duplicate and conquer: multiple homologs of PHOSPHORUS-STARVATION TOLERANCE1 enhance phosphorus acquisition and sorghum performance on low-phosphorus soils. Plant Physiol 166: 659–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanchenko MG, Muday GK, Dubrovsky JG (2008) Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 55: 335–347 [DOI] [PubMed] [Google Scholar]
- Ivanchenko MG, Napsucialy-Mendivil S, Dubrovsky JG (2010) Auxin-induced inhibition of lateral root initiation contributes to root system shaping in Arabidopsis thaliana. Plant J 64: 740–752 [DOI] [PubMed] [Google Scholar]
- Ivanov V, Bystrova E, Seregin I (2003) Comparative impacts of heavy metals on root growth as related to their specificity and selectivity. Russ J Plant Physiol 50: 398–406 [Google Scholar]
- Jonak C, Nakagami H, Hirt H (2004) Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol 136: 3276–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabaz-Saberi H, Barker SJ, Rengel Z (2012) Tolerance to ion toxicities enhances wheat (Triticum aestivum L.) grain yield in waterlogged acidic soils. Plant Soil 354: 371–381 [Google Scholar]
- Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63: 131–152 [DOI] [PubMed] [Google Scholar]
- Koyama H, Toda T, Hara T (2001) Brief exposure to low-pH stress causes irreversible damage to the growing root in Arabidopsis thaliana: Pectin-Ca interaction may play an important role in proton rhizotoxicity. J Exp Bot 52: 361–368 [PubMed] [Google Scholar]
- Lei GJ, Zhu XF, Wang ZW, Dong F, Dong NY, Zheng SJ (2014) Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in Arabidopsis. Plant Cell Environ 37: 852–863 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Muday GK (2009) Measurement of auxin transport in Arabidopsis thaliana. Nat Protoc 4: 437–451 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Negi S, Sukumar P, Muday GK (2011) Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 138: 3485–3495 [DOI] [PubMed] [Google Scholar]
- Li B, Li Q, Su Y, Chen H, Xiong L, Mi G, Kronzucker HJ, Shi W (2011) Shoot-supplied ammonium targets the root auxin influx carrier AUX1 and inhibits lateral root emergence in Arabidopsis. Plant Cell Environ 34: 933–946 [DOI] [PubMed] [Google Scholar]
- Li G, Li B, Dong G, Feng X, Kronzucker HJ, Shi W (2013) Ammonium-induced shoot ethylene production is associated with the inhibition of lateral root formation in Arabidopsis. J Exp Bot 64: 1413–1425 [DOI] [PubMed] [Google Scholar]
- Li G, Xu W, Kronzucker HJ, Shi W (2015) Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis. J Exp Bot 66: 2041–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xu J, Xu ZH, Xue HW (2005) Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in Brassica and Arabidopsis. Plant Cell 17: 2738–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li BH, Kronzucker HJ, Shi WM (2010) Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity. Plant Cell Environ 33: 1529–1542 [DOI] [PubMed] [Google Scholar]
- Liao H, Wan H, Shaff J, Wang X, Yan X, Kochian LV (2006) Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system. Plant Physiol 141: 674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Wang Y, Tang J, Xue P, Li C, Liu L, Hu B, Yang F, Loake GJ, Chu C (2012) Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol 158: 451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus V, Bertin P, Swenden V, Fortemps A, Lobréaux S, Lutts S (2007) Organ-dependent responses of the African rice to short-term iron toxicity: ferritin regulation and antioxidative responses. Biol Plant 51: 303–331 [Google Scholar]
- Manzano C, Pallero-Baena M, Casimiro I, De Rybel B, OrmanLigeza B, Van Isterdael G, Beeckman T, Draye X, Casero P, del Pozo JC (2014) The emerging role of ROS signaling during lateral root development. Plant Physiol 165: 1105–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvoorde P, Fukaki H, Beeckman T (2010) Auxin control of root development. Cold Spring Harb Perspect Biol 2: a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ (2009) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14: 399–408 [DOI] [PubMed] [Google Scholar]
- Pilon M, Cohu CM, Ravet K, Abdel-Ghany SE, Gaymard F (2009) Essential transition metal homeostasis in plants. Curr Opin Plant Biol 12: 347–357 [DOI] [PubMed] [Google Scholar]
- Pinto E, Ferreira IM (2015) Cation transporters/channels in plants: tools for nutrient biofortification. J Plant Physiol 179: 64–82 [DOI] [PubMed] [Google Scholar]
- Ratering S, Schnell S (2000) Localization of iron-reducing activity in paddy soil by profile studies. Biochem 48: 341–365 [Google Scholar]
- Reyt G, Boudouf S, Boucherez J, Gaymard F, Briat JF (2015) Iron and ferritin dependent ROS distribution impact Arabidopsis root system architecture. Mol Plant 8: 439–453 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, Del Río LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29: 1532–1544 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M (1998) The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast grr1p. Genes Dev 12: 198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růzicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19: 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signora L, De Smet I, Foyer CH, Zhang H (2001) ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. Plant J 28: 655–662 [DOI] [PubMed] [Google Scholar]
- Strader LC, Chen GL, Bartel B (2010) Ethylene directs auxin to control root cell expansion. Plant J 64: 874–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Xu Y, Ye S, Jiang H, Chen Q, Liu F, Zhou W, Chen R, Li X, Tietz O, et al. (2009) Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 21: 1495–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Kargul J, Marchant A, Zadik D, Rahman A, Mills R, Yemm A, May S, Williams L, Millner P, et al. (2004) Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16: 3069–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigani G. (2012) Discovering the role of mitochondria in the iron deficiency-induced metabolic responses of plants. J Plant Physiol 169: 1–11 [DOI] [PubMed] [Google Scholar]
- Wang NN, Shih MC, Li N (2005) The GUS reporter-aided analysis of the promoter activities of Arabidopsis ACC synthase genes AtACS4, AtACS5, and AtACS7 induced by hormones and stresses. J Exp Bot 56: 909–920 [DOI] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J (2002) Arabidopsis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York, pp 243–245 [Google Scholar]
- Wheeler DM, Power IL (1995) Comparison of plant uptake and plant toxicity of various ions in wheat. Plant Soil 172: 167–173 [Google Scholar]
- Wisniewska J, Xu J, Seifertová D, Brewer PB, Ruzicka K, Blilou I, Rouquié D, Benková E, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312: 883. [DOI] [PubMed] [Google Scholar]
- Xiong L, Wang RG, Mao G, Koczan JM (2006) Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol 142: 1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Peng XX (1995) Iron toxicity and stress-induced ethylene production in rice leaves. Plant Soil 173: 21–28 [Google Scholar]
- Zhai Z, Gayomba SR, Jung HI, Vimalakumari NK, Piñeros M, Craft E, Rutzke MA, Danku J, Lahner B, Punshon T, et al. (2014) OPT3 is a phloem-specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in Arabidopsis. Plant Cell 26: 2249–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96: 6529–6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.