A plant transcription factor regulates the expression of genes necessary to enable arbuscular mycorrhizal fungi to develop branched hyphae in root cortical cells.
Abstract
During arbuscular mycorrhizal symbiosis, arbuscule development in the root cortical cell and simultaneous deposition of the plant periarbuscular membrane generate the interface for symbiotic nutrient exchange. The transcriptional changes that accompany arbuscule development are extensive and well documented. By contrast, the transcriptional regulators that control these programs are largely unknown. Here, we provide a detailed characterization of an insertion allele of Medicago truncatula Reduced Arbuscular Mycorrhiza1 (RAM1), ram1-3, which reveals that RAM1 is not necessary to enable hyphopodium formation or hyphal entry into the root but is essential to support arbuscule branching. In ram1-3, arbuscules consist only of the arbuscule trunk and in some cases, a few initial thick hyphal branches. ram1-3 is also insensitive to phosphate-mediated regulation of the symbiosis. Transcript analysis of ram1-3 and ectopic expression of RAM1 indicate that RAM1 regulates expression of EXO70I and Stunted Arbuscule, two genes whose loss of function impacts arbuscule branching. Furthermore, RAM1 regulates expression of a transcription factor Required for Arbuscule Development (RAD1). RAD1 is also required for arbuscular mycorrhizal symbiosis, and rad1 mutants show reduced colonization. RAM1 itself is induced in colonized root cortical cells, and expression of RAM1 and RAD1 is modulated by DELLAs. Thus, the data suggest that DELLAs regulate arbuscule development through modulation of RAM1 and RAD1 and that the precise transcriptional control essential to place proteins in the periarbuscular membrane is controlled, at least in part, by RAM1.
The symbiotic association of plants and arbuscular mycorrhizal fungi, known as arbuscular mycorrhiza (AM), is widespread in terrestrial ecosystems. In this association, the fungal symbiont delivers mineral nutrients to the plant in return for carbon, and as a consequence, the symbiosis has a major impact on plant productivity (Smith and Read, 2008). AM is an endosymbiosis, and the fungus lives within the root cortical cells, where it develops differentiated hyphae called arbuscules. To support arbuscule development, the cortical cell undergoes considerable reorganization, including development of a new membrane-bound apoplastic compartment in which the arbuscule resides. This symbiotic interface is the site of nutrient exchange between the symbionts and therefore, critical for symbiotic function (Feddermann et al., 2010; Pumplin et al., 2010; Murray et al., 2011; Smith and Smith, 2011).
Development of AM symbiosis is initiated with signal exchange between the symbionts (Akiyama et al., 2005; Besserer et al., 2006; Maillet et al., 2011; Kretzschmar et al., 2012; Wang et al., 2012; Genre et al., 2013) and the activation of a plant signaling pathway referred to as the common symbiosis signaling pathway. In legumes, this pathway is essential for symbiosis with rhizobia as well as AM fungi, and several proteins that constitute the shared core of the pathway have been identified (Oldroyd, 2013). Among these shared components is a transcription factor (TF) called CYCLOPs (Yano et al., 2008; Singh et al., 2014), which is required for arbuscule development. There are extensive transcriptional changes in the root cortex associated with arbuscule development (Gaude et al., 2012; Hogekamp and Küster, 2013), but currently, genes regulated by CYCLOPS that enable arbuscule development are unknown.
Several plant genes required for arbuscule development and/or function have been identified, including Vapyrin (Feddermann et al., 2010; Pumplin et al., 2010; Murray et al., 2011), two Vesicle-Associated Membrane Proteins (Ivanov et al., 2012), EXO70I (Zhang et al., 2015), proteases (Takeda et al., 2009; Rech et al., 2013), a proton ATPase (Krajinski et al., 2014; Wang et al., 2014), ATP-binding cassette (ABC) transporters, Stunted Arbuscule (STR) and STR2 (Zhang et al., 2010; Gutjahr et al., 2012), and phosphate transporters (Javot et al., 2007; Yang et al., 2012; Xie et al., 2013). Some of these genes are expressed only during AM symbiosis, whereas others have broader expression patterns. In addition, some are expressed exclusively in cells containing arbuscules. The variety of expression patterns points to complex transcriptional and possibly posttranscriptional regulation (Devers et al., 2011). Furthermore, trafficking of the symbiotic phosphate transporters and ABC transporters to the periarbuscular membrane requires gene expression coincident with arbuscule branching (Pumplin et al., 2012). Thus, transcriptional control is not only essential to ensure expression of symbiosis-specific genes, but in the cortical cells, precise timing of gene expression ensures the correct protein composition of the periarbuscular membrane (Pumplin et al., 2012).
So far, transcription factors required for AM symbiosis include CYCLOPS (Yano et al., 2008), DELLAs (Floss et al., 2013; Foo et al., 2013; Yu et al., 2014; Takeda et al., 2015), Reduced Arbuscular Mycorrhiza1 (RAM1; Gobbato et al., 2012; Rich et al., 2015; Xue et al., 2015), Required for Arbuscule Development1 (RAD1; Xue et al., 2015), MtERF1 (Devers et al., 2013), and DELLA-Interacting Protein1 (DIP1; Yu et al., 2014). Among these transcriptional regulators, DELLAs, RAM1, RAD1, and DIP1 belong to the GRAS (for GA3 insensitive [GAI], Repressor of GAI [RGA], and Scarecrow [SCR]) family, a large, plant-specific gene family with members that regulate transcription and influence several aspects of plant development, including root development (Bolle, 2004). DELLAs were identified originally as repressors of GA3 signaling but have emerged as major transcriptional regulators that mediate cross talk between hormone, development, and defense signaling pathways through interactions with a wide range of TFs (Davière and Achard, 2013). Medicago truncatula and pea (Pisum sativum) della1della2 double mutants are unable to support arbuscule development (Floss et al., 2013; Foo et al., 2013) and show a symbiosis phenotype similar to that of cyclops. In contrast, in the rice (Oryza sativa) della mutant, slender (slr), the symbiosis fails before arbuscule development at the epidermal stage (Yu et al., 2014). Rice has only one DELLA gene, which possibly accounts for this difference. Ectopic expression of a dominant DELLA protein in cyclops enables arbuscule development, which suggests that DELLAs modulate symbiosis by acting on the symbiosis signaling pathway downstream of CYCLOPS, likely regulating other, as yet unknown TFs (Floss et al., 2013).
In M. truncatula, RAM1 was reported to be necessary to enable hyphopodium formation and directly regulates RAM2, a glycerol-3-P acyltransferase (Gobbato et al., 2012). RAM2 is required for the biosynthesis of the cutin monomer signal that induces hyphophodium formation (Gobbato et al., 2012; Wang et al., 2012). However, subsequently, it was noted that arbuscules in ram1 were small, suggesting a potential role for RAM1 in arbuscule development (Gobbato et al., 2013). Recent analyses of ram1 mutants in Lotus japonicus and Petunia hybrida show that RAM1 is required to support wild-type arbuscule development (Rich et al., 2015; Xue et al., 2015). In L. japonicus, a second GRAS factor RAD1 interacts with RAM1, and rad1 mutants showed a high proportion of small, degenerated arbuscules, suggesting a requirement for RAD1 in maintenance of the symbiosis (Xue et al., 2015). Rice DIP1, identified as an interactor of SLR, also interacts with RAM1, and DIP1 RNA interference lines showed a quantitative reduction in symbiotic development (Yu et al., 2014). Thus, several aspects of AM symbiosis are influenced by GRAS factors, which likely operate in complexes, potentially with a range of partners, such as has been shown for GRAS factors regulating other developmental processes (Cui and Benfey, 2009; Hirsch et al., 2009; Zhang et al., 2011). In addition to the GRAS factors, many other TFs are induced during symbiosis, including ERF1, an Apetala2 domain factor. Downregulation of ERF1 resulted in a quantitative decrease in symbiosis (Devers et al., 2013). Currently, other than RAM2, the genes regulated by these TFs are unknown.
During a reverse genetic screen of TFs expressed in cells hosting arbuscules, we characterized the AM phenotype of a third allele of RAM1, ram1-3. Our data indicate that RAM1 is essential for a plant cellular program that enables arbuscule branching and modulates expression of EXO70I and STR, two genes essential for the branching phase of arbuscule development.
RESULTS
ram 1-3 Is Unable to Support Arbuscule Branching
From a search for TFs that showed transcriptional induction during AM symbiosis, we identified RAM1 (Gobbato et al., 2012), a member of the GRAS family (Fig. 1A). Analysis of M. truncatula roots expressing two RAM1pro-β-glucuronidase (UidA) fusions revealed that 2 kb or 800 bp of putative RAM1 promoter directed transcription in the cortex in colonized regions of the root (Supplemental Fig. S1, A–C) and to a lesser extent, noncolonized lateral roots of a symbiotic root system. Additionally, young lateral roots of mock-inoculated root systems also showed GUS staining, although RAM1 transcripts were not detectable in mock-inoculated roots by quantitative reverse transcription (qRT)-PCR (Fig. 1A; Supplemental Fig. S1, D and E).
Figure 1.
RAM1 expression and the mycorrhizal phenotype of ram1-3. A, Relative expression of RAM1, G. versiforme α-tubulin (GvTUB), and PT4 in wild-type M. truncatula (A17) roots either mock inoculated (M) or colonized with G. versiforme at 3 and 5 wpp. Transcript levels are relative to elongation factor1α. Error bars show sem (n = 3). *, Significant differences between the mock-inoculated control (M) and colonized (G. versiforme) roots (Student’s t test; P < 0.05). B to F, Confocal microscope images of an M. truncatula wild-type segregant (B and E) and ram1-3 (C, D, and F) roots colonized with G. versiforme. The roots at 8 dpi were stained with Alexa Fluor 488 WGA and propidium iodide. The images in E and F are projections of 30 and 20 optical sections on the z axis, respectively, taken at 2-µm intervals. G, A confocal microscope image of a cortical cell from an ram1-3 root expressing BCP1:MtPT1-GFP. Labeling of the arbuscule trunk indicates that the trunk is surrounded by a membrane. The image is a projection of 15 optical sections on the z axis taken at 1-µm intervals. White arrowheads point to undeveloped arbuscules. White arrows point to arbuscule trunks. Arb, Arbuscule; IH, intercellular hyphae; PM, plasma membrane; UnA, undeveloped arbuscules. Bars = 75 µm (B–D), 25 µm (E and G), and 10 µm (F).
To address the function of RAM1, we obtained a M. truncatula line (NF5445) with a transposable element of Nicotiana tabacum cell type1 (Tnt1) insertion between nucleotides 1,318 and 1,319 of Medtr7g027190, which truncates the protein after amino acid 310 (Supplemental Fig. S2). Previously, two mutant alleles of RAM1 had been reported: ram1-1, which has a 71-kb deletion that includes the RAM1 gene, and ram1-2, which contains a Tnt1 insertion that truncates the protein after amino acid 649 (Gobbato et al., 2012; Supplemental Fig. S2). Consequently, we labeled our insertion allele (NF5445) ram1-3. The mycorrhizal phenotype reported for ram1-1 included a defect in hyphopodium formation and substantially reduced colonization but wild-type arbuscules, whereas ram1-2 showed small, undeveloped arbuscules (Gobbato et al., 2012, 2013). Recent reports of ram1 mutants of L. japonicus and P. hybrida noted low colonization and the presence of malformed arbuscules (Rich et al., 2015; Xue et al., 2015).
We evaluated the mycorrhizal phenotype of ram1-3 at 2 to 8 d postinoculation (dpi) with Glomus versiforme spores in a double-cone inoculation system (Lopez-Meyer and Harrison, 2006) and also, 3 and 5 weeks postplanting (wpp) into substrate-containing spores. A microscopic evaluation of the colonized roots revealed obvious differences in fungal development in the root cortex of ram1-3 relative to the wild type (Fig. 1, B–F). In ram1-3, intracellular hyphae, which we inferred to be arbuscule trunks, were present in the inner cortical cells, but arbuscule branches were either absent or limited to one or two thick branches. The fine branches, typical of mature arbuscules, were absent (Fig. 1, C, D, and F). In contrast, infection units with fully branched arbuscules were present in wild-type roots (Fig. 1, B and E). This phenotype was further verified by imaging ram1-3 roots expressing BCP1pro:PT1-GFP, which labels the plasma membrane and periarbuscular membrane of colonized cortical cells (Pumplin et al., 2012). In ram1-3, intracellular hyphae, sometimes coiled and with occasional bifurcations, were visible in the cells, but highly branched arbuscules were never observed (Fig. 1G).
Fungal structures in ram1-3 were quantified and compared with those in a wild-type segregant from the ram1-3 population and the parental line, R108 (Fig. 2). At 2, 4, and 6 dpi, the total number of infection events in which the fungus had successfully penetrated the roots and the number of hyphopodia that had not penetrated the root did not differ between ram1-3 and wild-type controls (Fig. 2, A–C). In contrast, the number of infection units that contain only intercellular hyphae was slightly higher in ram1-3 relative to the controls. At 4 and 6 dpi, most infection units in wild-type roots contained fully branched arbuscules, whereas infection units in ram1-3 contained thick intracellular hyphae, with minimal branching, designated as arbuscule trunks/undeveloped arbuscules (Fig. 2, B and C). The same phenotype was apparent at 8 dpi (Fig. 2, D and E) and also, 3 and 5 wpp (Fig. 3), indicating that mature arbuscules do not develop, even after extensive cocultivation. At these time points, it was not feasible to count the absolute number of infection units, and so, roots were analyzed by the gridline intersect method to obtain the percentage of colonized root length (McGonigle et al., 1990). At 3 and 5 wpp, 18% to 25% of the ram1-3 root length was colonized. This was significantly lower than the two wild types, where the fungus had proliferated to colonize approximately 70% to 80% of the root length (Fig. 3). Differences in colonized root length in the wild type and ram1-3 were magnified over time, because colonization progressed in the wild type but not ram1-3. This phenotype was seen consistently in independent experiments (Supplemental Figs. S3 and S4).
Figure 2.
Mutation of RAM1 does not alter the initial infection by G. versiforme. A to E, Quantitation of total infection units, hyphopodia, infection units with only intercellular hyphae, infection units with arbuscules, and infection units with arbuscule trunks/undeveloped arbuscules in the wild type (R108), ram1-3, and a wild-type segregant (WT-seg) at 2, 4, 6, and 8 dpi in a double-cone assay system. For each line, the data are the means of three biological replicate cones, each containing two plants. Data are averages ± sem (n = 3). Different letters indicate significant differences between the lines in each group of infection phenotypes (all pairs; Tukey’s HSD mean-separation test; P < 0.01 in B and P < 0.05 in C–E). At 2 to 6 dpi, the data shown are the numbers of infections. At 8 dpi, the data shown are percentage of infected root pieces, because the total number of infections was too high to count.
Figure 3.
AM symbiosis is not maintained in ram1-3, and colonization levels decline over time. A and C show the total percentages of colonized root length, whereas B and D show the percentages of infected root pieces containing hyphopodia, intercellular hyphae only, arbuscules, and arbuscule trunks/undeveloped arbuscules in the wild type (R108), ram1-3, and a wild-type segregant (WT-seg) from the ram1-3 backcrossed population colonized with G. versiforme at 3 and 5 wpp into inoculum. Error bars show sem (n = 3). Different letters indicate significant differences between the M. truncatula lines in each group of infection phenotypes (all pairs; Tukey’s HSD mean-separation test; P < 0.01).
Because the phenotype reported originally for ram1-1 (Gobbato et al., 2012) differed substantially from the one that we observed for ram1-3, we carried out a side by side comparison of ram1-3 and ram1-1 in our growth conditions. The two alleles are in different genetic backgrounds, and consequently, controls included A17, R108, and a wild-type segregant from the ram1-3 population. At 3 wpp into substrate containing G. versiforme spores, ram1-1 and ram1-3 showed similar levels of colonization, and both differed significantly from the wild-type controls (Supplemental Fig. S5A). An analysis of the infection phenotype revealed no significant differences between ram1-1 and ram1-3, and both mutants showed cortical infections with arbuscule trunks and an absence of fully developed arbuscules (Supplemental Fig. S5, B and C). In this experiment, differences in the number of infections with intercellular hyphae only did not differ between the mutants and the wild type.
To ensure that the ram1-3 phenotype that we observed is indeed caused by a defect in Medtr7g027190, we carried out a cosegregation analysis and also complemented the ram1-3 with a wild-type copy of Medtr7g027190. Both analyses indicate that the arbuscule branching defect observed in ram1-3 is the result of loss of function of Medtr7g027190 (Supplemental Fig. S6). Thus, in these conditions, we do not find evidence to support a role for RAM1 in hyphal penetration into cells but instead, conclude that it is essential to enable arbuscule development and more specifically, arbuscule branching.
Fungal Morphology in ram1-3 Roots Is Not Affected by the Nutritional Status of the Plant, But ram1-3 Is Insensitive to Phosphate-Mediated Suppression of Colonization
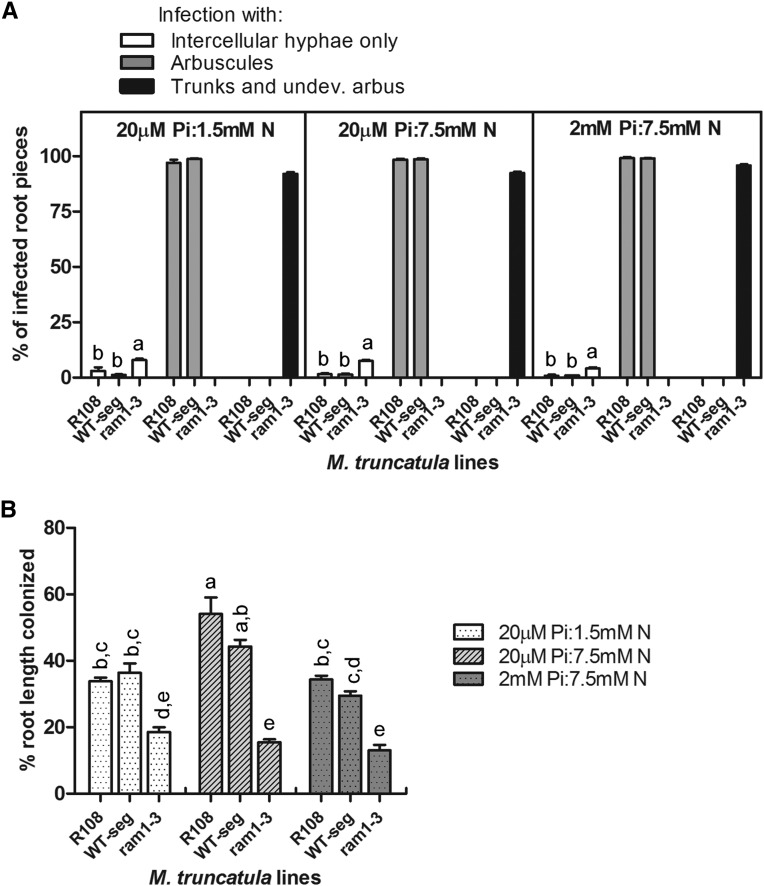
Because a previous study indicated that AM fungi were unable to form hyphopodia on ram1-1 and consequently, impaired in hyphal entry into the root (Gobbato et al., 2012), we questioned whether differences in the nutrient conditions of our experiments might alter the mycorrhizal phenotype and reveal a defect in hyphopodium development. It is well documented that the nutritional status of the plant influences fungal development within the roots (Schwab et al., 1983; Amijee et al., 1989; Baath and Spokes, 1989; Koide and Li, 1990), and in some mutants, nutrient status causes a major phenotypic change (Javot et al., 2011). Consequently, we assessed the mycorrhizal phenotype of ram1-3 during growth in three different phosphorus (P) and nitrogen (N) regimes. These treatments did not alter the morphology of colonization in ram1-3 or the wild type (Fig. 4A). However, fertilization with 2 mm P and 7.5 mm N resulted in a decrease in colonization in R108 and the wild-type segregant but did not alter colonization levels in ram1-3 (Fig. 4B). Currently, the mechanisms by which plant nutrient status regulates colonization levels are unknown. These data suggest that RAM1 may be involved in this process possibly by influencing expression of genes that regulate colonization. Alternatively, it is possible that nutrient-mediated regulation of the symbiosis occurs only on a fully developed mycorrhiza with intact arbuscules and therefore, does not occur in ram1-3.
Figure 4.
Nutrient treatments do not alter the morphology or levels of colonization in ram1-3. The percentages of infected root pieces containing hyphopodia, intercellular hyphae only, arbuscules, and arbuscule trunks/undeveloped arbuscules (A) and the percentages of colonized root length in the wild type (R108), ram1-3, and a wild-type segregant (WT-seg) from the ram1-3 backcrossed population colonized with G. versiforme at 3 wpp in three different nutrient regimes. Data are the means of three biological replicates, and error bars indicate sem. Different letters indicate significant differences between the lines (ANOVA and Tukey’s HSD mean-separation test; P < 0.05 in 20 µm Pi:1.5 mm N treatment in A; P < 0.01 in the two other treatments in A; and P < 0.05 in B).
Gene Expression in ram1-3 Is Consistent with the Failure to Develop Arbuscules
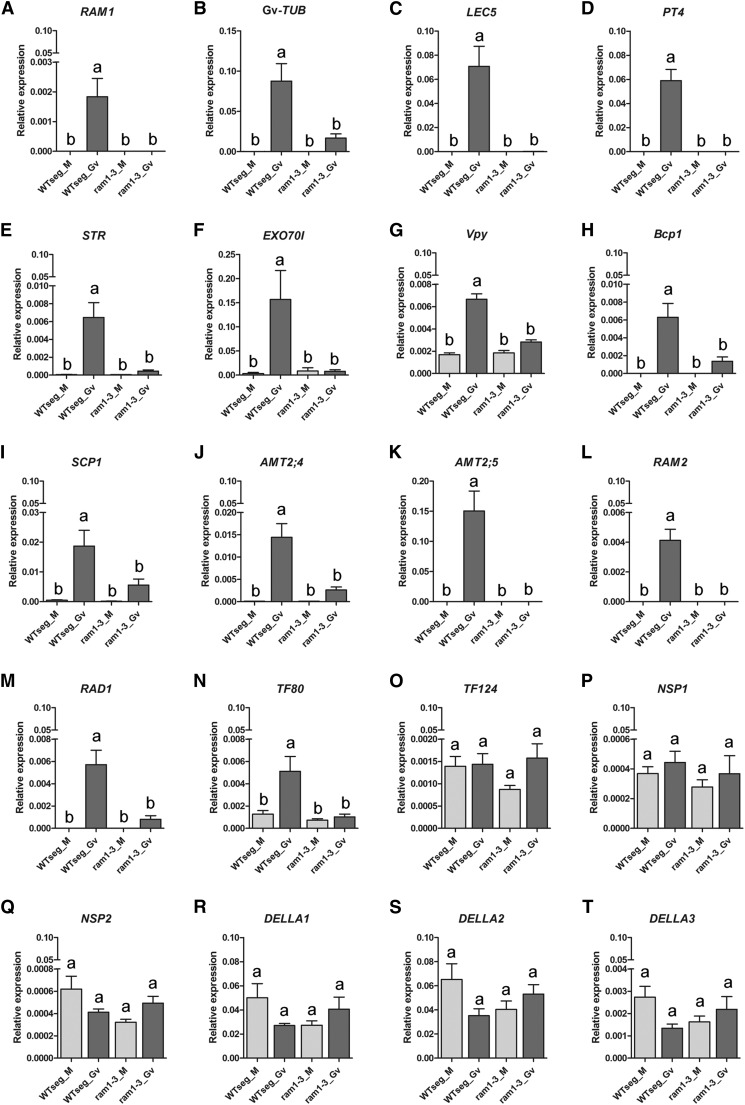
Gene expression in noncolonized ram1-3 roots and ram1-3 colonized with G. versiforme was compared with those of a wild-type segregant control. G. versiforme α-tubulin transcript levels were 4-fold lower in ram1-3 relative to the wild-type segregant, which correlates with lower colonization in ram1-3 (Fig. 5, A and B). Consistent with the severe arbuscule development phenotype, transcripts of genes whose expression is associated with arbuscule development or function were reduced substantially in ram1-3 relative to the wild-type segregant control. Lectin5 (Frenzel et al., 2005) and MtPT4 (Harrison et al., 2002) transcripts were undetected (Fig. 5, C and D), whereas STR (Zhang et al., 2010), EXO70I (Zhang et al., 2015), and Vapyrin (Pumplin et al., 2010; Murray et al., 2011), each of which is required for arbuscule development, showed basal levels of expression in ram1-3 colonized roots (Fig. 5, E–G). Likewise, Blue Copper Binding Protein1 (BCP1; Hohnjec et al., 2005; Pumplin and Harrison, 2009), Serine carboxypeptidase1 (Liu et al., 2003), Ammonium transporter2;4 (AMT2;4), and AMT2;5 (Breuillin-Sessoms et al., 2015) transcripts also remained at basal levels in ram1-3 (Fig. 5, H–K). RAM2, a verified downstream target of RAM1 (Wang et al., 2012), was not expressed in ram1-3 plants (Fig. 5L). We also examined the expression of several other members of the GRAS TF family, including an AM-induced GRAS factor Medtr3g022830 (TF80) and the two closest homologs of RAM1, Medtr4g104020 (RAD1) and Medtr8g442410 (TF124; Supplemental Fig. S7; Supplemental Data Set S1). After colonization, RAD1 and TF80 showed transcriptional induction in the wild-type segregant but not in ram1-3 (Fig. 5, M and N). In contrast, TF124 is expressed constitutively in both ram1-3 and the wild-type segregant (Fig. 5O). Nodulation Signaling Pathway1 (NSP1) and NSP2, two GRAS factors that regulate strigolactone biosynthesis (Liu et al., 2011) and influence development of AM in a quantitative manner (Delaux et al., 2013; Hofferek et al., 2014), were likewise not differentially expressed in ram1-3 (Fig. 5, P and Q). As reported previously, DELLA transcripts were not induced during AM symbiosis (Floss et al., 2013), and this was also the case in ram1-3 (Fig. 5, R–T). Gene expression in control and colonized ram1-1 roots followed the same pattern as observed in ram1-3 (Supplemental Fig. S8).
Figure 5.
Gene expression in ram1-3. Gene expression in ram1-3 and a wild-type segregant (WTseg) from the ram1-3 population mock inoculated (M) or colonized with G. versiforme at 3 wpp. Transcripts levels are relative to M. truncatula elongation factor1α. Different letters indicate significant differences between the lines (all pairs; Tukey’s HSD mean-separation test). Error bars are sem (n = 4; P < 0.01 in A–F, H, and J–M; and P < 0.05 in G, I, and N–T).
Overexpression of RAM1 Results in Increases in RAM2, EXO70I, STR, and RAD1 Transcripts
The differential gene expression observed in ram1-3 could be a secondary consequence of incomplete arbuscule development, or alternatively, these genes might be regulated by RAM1. To identify genes potentially regulated by RAM1, we generated transgenic roots overexpressing RAM1 to levels 100-fold higher than the vector controls and assayed expression of possible RAM1 target genes in the absence of symbiosis (Fig. 6). Transcript levels of four genes, STR, EXO70I, RAD1, and RAM2, showed small but significant increases in mock-inoculated roots overexpressing RAM1 relative to a vector control, whereas TF80 and Vapyrin transcripts showed no change; TF124 transcripts decreased. RAM2 gene expression is regulated by RAM1 (Gobbato et al., 2012), whereas regulation of the other genes has not been reported previously. STR and EXO70I are both required to enable arbuscule development, and in str and exo70i mutants, arbuscule branching fails very early in the developmental process (Zhang et al., 2010, 2015). Their phenotypes are consistent with the ram1-3 phenotype and the conclusion that STR and EXO70I are regulated by RAM1.
Figure 6.
Ectopic overexpression of RAM1 increases transcript levels of several genes involved in AM symbiosis. A to H, Relative expression of a selection of genes in wild-type (A17) roots expressing 35Spro:RAM1 (35S:RAM1) or a control construct 35Spro:NLS-GFP-GUS (35S:NLS). The data are the means of three independent experiments, each with three to five biological replicates. The data were analyzed using a mixed effects model (Fit model on JMP Pro-10) with a fixed treatment effect and a random experiment effect on JMP Pro-10 (“Materials and Methods”). Error bars indicate sem (n ≥ 10). Asterisks indicate significant differences in transcript levels between RAM1 overexpression plants and vector control plants. Expression is relative to M. truncatula elongation factor1α. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Yeast Two-Hybrid Analysis Detects Interactions between RAM1 and Several GRAS TFs
Previous studies indicate that some GRAS factors bind to DNA (Ma et al., 2014), whereas others are engaged with the promoter through a complex with other protein(s) (Fode et al., 2008; Hirsch et al., 2009). We failed to find evidence of direct binding of RAM1 to the STR and EXO70I promoters in either a yeast (Saccharomyces cerevisiae) one-hybrid assay or a Nicotiana benthamiana transactivation assay, and therefore, we used a targeted yeast two-hybrid analysis to identify additional potential interaction partners for RAM1 (Supplemental Fig. S9). As reported previously, we found that RAM1 interacts with the GRAS factor NSP2 (Gobbato et al., 2012) and also, GRAS factors RAD1 and TF80 (Medtr3g022830). The RAM1-RAD1 interaction is consistent with recent data from L. japonicas, where the LjRAD1-LjRAM1 interaction was shown in yeast and in planta (Xue et al., 2015). However, the interaction of RAM1 with TF80 has not been reported previously. Interactions between RAM1 and Medtr8g442410 (TF124), the third homolog in the RAM1 clade, could not be assayed because of autoactivation.
An M. truncatula rad1 Mutant Shows Reduced Colonization
Because TF80 and RAD1 are potential interaction partners of RAM1, we searched for insertion mutants with which to further evaluate the roles of these proteins in symbiosis. A potential TF80 insertion line is reported in the FST database (http://bioinfo4.noble.org/mutant/; NF14469), but the seed batch did not contain an insertion in TF80. However, we were able to obtain M. truncatula line NF9554 and verify a Tnt1 insertion in RAD1 located 75 nucleotides downstream of the start codon. The line was backcrossed, and colonization of the rad1 mutant was compared with either R108 or a wild-type segregant from the backcrossed population. At 2 and 6 dpi, quantitative analysis of infection structures indicated no differences in the morphology of colonization in rad1 relative to the wild-type segregant (Fig. 7A). However, at 3 wpp, colonization levels in rad1 were slightly lower than those in the wild type, and this difference increased at 5 wpp (Fig. 7B). In addition, we examined colonization levels in rad1 grown with slightly higher levels of P fertilization to determine whether the effects were robust and visible in more than one condition. In this experiment, differences in the levels of colonization were apparent only at 5 and 7 wpp but in both experiments, colonization levels in the wild type and wild-type segregant increased more rapidly than in rad1 (Fig. 7, B and C). Based on these data, we suggest that intraradical colonization proceeds more slowly in rad1 than in the wild type. Consistent with the colonization data at 3 wpp, G. versiforme α-tubulin, PT4, and RAM1 transcript levels were similar in rad1 and the wild type; however, STR transcripts were slightly lower in rad1 relative to the wild type, suggesting that RAD1 might also contribute to the regulation of expression of this gene (Supplemental Fig. S10). However, in contrast to RAM1, overexpression of RAD1 did not result in an increase in STR transcripts. Additionally, overexpression of TF80 or TF124 did not result in increases in STR transcripts (data not shown). Based on the phenotypes and gene expression data, it is possible that RAM1 interacts with RAD1 for activation of gene expression but that RAD1 function is partially redundant.
Figure 7.
Colonization levels in M. truncatula rad1. A, Quantitation of total infection units, with hyphopodia only, infection units with intercellular hyphae only, and infection units with arbuscules in rad1 and a wild-type segregant (WT-seg) from the rad1 backcrossed population at 2 and 6 dpi with G. versiforme in a double-cone assay system. For each line, the data are the means of three biological replicate cones each containing two plants. B, The total percentage of colonized root length in rad1 and R108 at 3 and 5 wpp with G. versiforme. Plants were fertilized with a modified one-half-strength Hoagland’s solution with 20 µm phosphate. C, The total percentage of colonized root length in rad1 and R108 at 3, 5, and 7 wpp with G. versiforme. Plants were fertilized with a modified one-half-strength Hoagland’s solution with 200 µm phosphate. Error bars show sem (n ≥ 3). Asterisks indicate significant differences (Student’s t test). *, P < 0.05; **, P < 0.01.
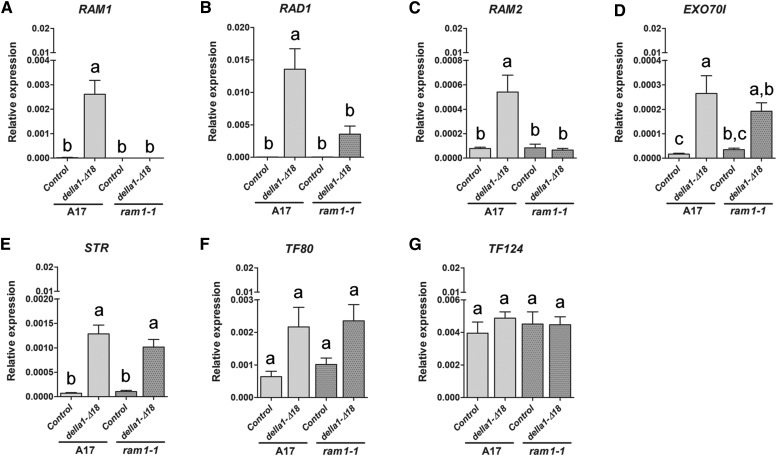
Overexpression of della1-Δ18 Induces Expression of RAM1
RAM1 is necessary to enable arbuscule branching, and its expression is induced in colonized cortical cells. In M. truncatula and pea, DELLA proteins are also required to enable arbuscule development, and in della double mutants, AM fungi colonize the root cortex but fail to make arbuscules (Floss et al., 2013; Foo et al., 2013; Yu et al., 2014; Takeda et al., 2015). Because it is known that DELLAs act by regulating the expression of TFs or modulating their activity (Davière and Achard, 2013), we considered the possibility that DELLA1 regulates expression of RAM1. To evaluate this, we generated roots overexpressing a constitutively active DELLA protein (della1-Δ18; Floss et al., 2013) and analyzed expression of RAM1 and a selection of other genes associated with arbuscule development. Constitutive expression of della1-Δ18, in mock-inoculated roots, was sufficient to result in small but significant increases in transcript levels of RAM1 and RAD1 but not TF80 or TF124. Additionally, RAM2, EXO70I, and STR transcripts were also increased by della1-Δ18 overexpression but to much lower levels than RAM1 or RAD1 (Fig. 8). Thus, DELLA1 promotes increases in RAM1 expression, and we speculated that increases in RAM1 subsequently led to increased expression of RAM2, EXO70I, and STR. To test this hypothesis, we overexpressed della1-Δ18 in ram1-1 and evaluated RAM2, EXO70I, and STR transcript levels. This revealed that della1-Δ18-induced increases in RAM2, RAD1, and EXO70I transcripts but not STR were dependent on RAM1 (Fig. 8, B–D). Because STR shows expression before symbiosis, it is likely that the basal expression is regulated differently than the symbiosis-induced expression. The observation that a della1-Δ18-induced increase in STR transcripts in colonized roots was RAM1 dependent supports this argument (data not shown). Taken together with the results of RAM1 overexpression (Fig. 6), we suggest that the symbiosis-induced expression of RAM2, RAD1, EXO70I, and STR genes is regulated by RAM1 and that RAM1 expression is regulated by DELLA (Fig. 9).
Figure 8.
Overexpression of della1-Δ18 increases RAM1 transcripts. A to G, Relative expression of a selection of genes in A17 and ram1-1 roots overexpressing a dominant della gene, 35Spro:della1-Δ18 (della-Δ18) or a 35Spro:GFP vector control (Control). Error bars indicate sem (n ≥ 4). Different letters indicate significant transcript levels among della1-Δ18 overexpression and vector control A17 or ram1-1 roots differences (all pairs; Tukey’s HSD mean-separation test; P < 0.05 in B; P < 0.01 in A and C–G). Gene expression is relative to M. truncatula elongation factor1α.
Figure 9.

RAM1 regulates genes required to support arbuscule branching. DELLA1 increases expression of RAM1, and RAM1 regulates transcription of EXO70I and STR either directly or indirectly and may do so as a complex with RAD1. RAM1 interacts with two GRAS TFs, RAD1 and TF80 (thick lines). The expression of STR and RAM2 is decreased in rad1 mutant roots (thin arrow lines).
DISCUSSION
Arbuscules are generally described as hyphae that have a tree-like structure, which includes a trunk from which thick branches and gradually finer branches arise. The periarbuscular membrane that surrounds the arbuscule is one continuous membrane, but its protein composition defines two large domains: a trunk domain, which surrounds the hyphal trunk and the first thick branches, and a branch domain, which encompasses the fine hyphal branches. Here, we provide evidence that RAM1 is essential to enable arbuscule branching and regulates expression of some genes associated with the branch domain of the periarbuscular membrane. In contrast to previous analyses, we do not find evidence that RAM1 is necessary to support hyphopodium formation, and it is possible that this interpretation arose from the late time points used for the original phenotypic analyses (Gobbato et al., 2012). In wild-type roots, a productive symbiosis results in secondary infection events, and at late time points, colonization levels are high. In contrast, at late time points, colonization levels in ram1 are low because of the failing symbiosis, and it is possible that this was interpreted as a defect in entry into the root rather than a failure in cortical colonization and establishment of the symbiosis. Here, a detailed analysis of ram1-3, beginning at 48 h and extending to 5 weeks postinoculation, revealed that the fungal symbiont is capable of entering ram1-3 roots and also, the cortical cells but that the hyphae fail to undergo the repeated dichotomous branching typical of an arbuscule in wild-type roots. Because RAM1 is a GRAS protein, it is reasonable to infer that it controls essential transcriptional events required specifically for the branching phase of arbuscule development. Furthermore, the ram1-3 phenotype suggests that the cellular commitment to a program that supports arbuscule development occurs not at the point of hyphal penetration of the cortical cell but after the hypha has entered the cell. A transcriptional program coordinated with arbuscule branching also correlates with previous findings of membrane protein distribution in the periarbuscular membrane trunk and branch domains (Pumplin et al., 2012), and therefore, genes encoding membrane proteins of the branch domain are potential candidates for regulation by RAM1.
RAM1 is predicted to encode a transcriptional regulator of the GRAS family, and in previous studies of GRAS factors, a transcriptional approach that combined analysis of loss-of-function mutants and ectopic expression proved a viable way to predict downstream target genes (Levesque et al., 2006). Taking a similar approach, transcript analyses of ram1-3 mycorrhizal roots and also, roots overexpressing RAM1 predict that AM-induced expression of STR, EXO70I, RAM2, and RAD1 is regulated, at least in part, by RAM1. We were unable to show direct binding of RAM1 to EXO70I or STR upstream regulatory sequences, but because GRAS factors typically work in complexes, it is possible that the RAM1 interaction occurs through a complex with other DNA binding proteins. RAD1 is one potential candidate because STR transcript levels show a small but significant reduction in rad1, and EXO70I transcripts show a similar trend. In L. japonicus, RAD1 and RAM1 interact when coexpressed in N. benthamiana cells (Xue et al., 2015), and here, we observed interaction of RAD1 and RAM1 in yeast two-hybrid assays. However, although an RAM1/RAD1 complex shows transcriptional activation activity in yeast, we did not find evidence for activation of EXO70I or STR promoters. Previously, RAM1 was shown to associate with the RAM2 promoter through interaction with a second GRAS factor NSP2 (Gobbato et al., 2012), and our yeast two-hybrid assays point to an interaction of RAM1 with an additional GRAS factor, TF80. Consequently, RAM1 in association with one or more of these other factors might be needed to enable interaction with the EXO70I and STR promoters. Chromatin immunoprecipitation assays would help to address these questions, and we prepared epitope-tagged RAM1 to enable these analyses. However, the tagged RAM1 proteins did not fully complement the ram1 mutants, and therefore, it will be necessary to pursue these studies in another way.
EXO70I and STR are both required to support arbuscule branching (Zhang et al., 2010; Gutjahr et al., 2012; Zhang et al., 2015): EXO70I for development of the branch domain of the periarbuscular membrane and STR for export of an unknown substrate across the periarbuscular membrane. In exo70i and str mutants, arbuscule development is arrested early in the branching process. These phenotypes are also consistent with the proposal that RAM1 regulates EXO70I and STR expression. However, neither the exo70i nor the str phenotype is as severe as that of ram1-3, which suggests that expression of other genes involved in early arbuscule branching is also regulated by RAM1. Large numbers of mycorrhiza-induced TFs were identified in transcriptome analyses (Hogekamp and Küster, 2013; Xue et al., 2015), and therefore, TFs other than RAM1 must also regulate gene expression during symbiosis. Expression of some of these TFs may be regulated by RAM1, and we provide initial evidence that RAM1 can induce expression of RAD1. Whether this results in a second wave of gene regulation associated with later phases of arbuscule development or possibly, arbuscule degeneration remains to be determined.
RAM1 itself shows strong transcriptional induction in mycorrhizal roots, specifically in the colonized regions of the root cortex. Therefore, we considered the question of what controls RAM1 expression. It has been shown that DELLA proteins are essential for arbuscule development, but the TFs through which DELLAs act to promote arbuscule development have not been reported. Here, we show that ectopic expression of a dominant della gene increases RAM1 and RAD1 transcript levels but not TF80 or TF124, the other two genes in the RAM1 clade. There are prior examples of DELLAs regulating transcription of other GRAS factors and also, GRAS factors that activate transcription of other GRAS factors, which we potentially see here with RAM1 and RAD1 (Levesque et al., 2006; Heo et al., 2011; Zhang et al., 2011). Additionally, some GRAS proteins bind to their own promoters and autoregulate their transcription (Cui et al., 2007; Zhang et al., 2011). Furthermore, a single GRAS factor can activate or repress gene expression depending on its interaction partner and in some cases, potentially through modification of heterochromatin (Cui and Benfey, 2009). Thus, there is much potential for complex regulation mediated by this class of TFs.
In conclusion, these data indicate that RAM1 regulates at least two genes required to enable arbuscule branching and also, increases expression of RAD1. Transcript levels of RAM1 are modulated by DELLA activity; thus, the data also provide information about the TFs through which DELLAs act to enable development of arbuscules (Fig. 9). Yeast two-hybrid data indicate potential interactions between these GRAS proteins, and future studies should focus on evaluating these complexes and their roles in regulating gene expression during AM symbiosis.
MATERIALS AND METHODS
Plant Growth and Inoculation with Glomus versiforme
Medicago truncatula wild-type or mutant seedlings or transformed plants were grown with Glomus versiforme in either cones or a double-cone system. The cones contained a mixture of gravel and sand with 100 or 150 G. versiforme spores positioned approximately 6 cm below the top of the gravel-sand mix in a 2- to 3-mm sand layer. Seedlings were transplanted into the cones and harvested at 3 to 5 wpp. The double-cone system was established as described previously (Lopez-Meyer and Harrison, 2006), and seedlings were transplanted into the upper cone and grown for 2 weeks before being placed over the lower cone containing 300 G. versiforme spores and grown together for an additional 9 d before pushing two cones together to establish contact between the germinated spores and root mat. Roots were harvested at 2, 4, 6, and 8 dpi. In this system, the inoculation day is known, hence the terminology dpi.
All plants were grown in a growth chamber in a 16-h/8-h and 25°C/22°C day-night cycle and fertilized with a modified one-half-strength Hoagland’s nutrient solution containing 20 µm potassium phosphate twice a week (Javot et al., 2011) unless indicated otherwise.
For the experiment involving different nutrient treatments, ram1-3 seedlings were transplanted into gravel-sand mix containing G. versiforme spores and fertilized with the above nutrient fertilizer for 1 week and then one-half-strength Hoagland’s solution with 20 µm phosphate and 1.5 mm N, one-half-strength Hoagland’s solution with 20 µm phosphate and 7.5 mm N, or one-half-strength Hoagland’s solution with 20 mm phosphate and 7.5 mm N for another 3 weeks (Javot et al., 2011). The plants were harvested, and the roots were stained with Alexa Fluor 488 WGA (Molecular Probes) to allow visualization of the fungal structures (Hong et al., 2012).
Tnt1 Insertion Lines and Cosegregation Analysis
The Tnt1 insertion mutant lines (ram1-3; NF5445 and rad1; NF9554) were identified from the M. truncatula mutant database (http://bioinfo4.noble.org/mutant/). Homozygous plants were identified by genotyping and crossed with the R108 wild type. The F1 generation was selfed, and homozygotes were identified from the F2. These homozygotes and also, wild-type segregants from the backcrossed population were used for experiments, except for the double-cone assays, in which homozygous plants before backcross were used.
For the genotype analysis, genomic DNA was extracted from an M. truncatula leaf as described (Dellaporta et al., 1983) with minor modifications. The leaf was frozen in liquid N and homogenized with two metal beads in a 2-mL tube. PCR-based genotyping was carried out with primers B2439/B2550 to detect the wild-type RAM1 allele and B2439/B2377 for the Tnt1 insertion in RAM1, B2548/B2549 for the wild-type RAD1 allele, and B2548/B2501 for the Tnt1 insertion in RAD1. The primer sequences are shown in Supplemental Table S1.
For the cosegregation study, 120 segregating F2 plants were transplanted into cones (one plant per cone) containing G. versiforme spores, and both the genotype and phenotype were assessed.
Plasmid Construction
For the complementation tests, the RAM1pro:RAM1 construct was created using MultiSite gateway cloning; 800 bp of DNA directly upstream of the first ATG of the RAM1 gene was amplified by PCR with primers B3386 and B3387 from M. truncatula Jemalong A17 genomic DNA and cloned into pDONR P4-P1R (Invitrogen). The coding region of RAM1 was amplified by PCR with primers B3249 and B3250 from G. versiforme-colonized root complementary DNA (cDNA) of M. truncatula A17 and cloned into pDONR221. pDONR4.1 RAM1 promoter, pDONR221 RAM1 coding sequence (CDS), pDONR2.3 Ubiquitin (UBQ) terminator (Ivanov and Harrison, 2014), and pKm43GW (Karimi et al., 2005) were mixed in a MultiSite gateway cloning reaction (Invitrogen), resulting in the pRAM1:RAM1 binary vector.
To overexpress RAM1, 35Spro:RAM1 was generated from pDNOR221 RAM1 CDS, pDONR4.1 35S promoter, pDONR2.3 35S terminator, and pK7m34GW-RR in a MultiSite gateway reaction. pK7m34GW-RR was created from K7m34GW (Karimi et al., 2005) by amplifying the UBQ10pro:DsRED-NOSterm from pRedRoot (Limpens et al., 2005) using primers B3701 and B3702 and ligating into the ApaI site in pK7m34GW (Karimi et al., 2005; S.Ivanov and M.J.Harrison, unpublished data).
For promoter analysis of RAM1, two constructs were generated. Two regions, 2 kb and 800 bp upstream of the first ATG of the RAM1 gene, were amplified by PCR with primers B3842, B3843, and B3844, digested with KpnI and PstI, and then ligated into pCAMBIA2301-Δ35S promoter (Pumplin et al., 2010) to create RAM1pro2kb:UidA and RAM1pro800:UidA.
For yeast (Saccharomyces cerevisiae) assays, pEG202-SC and pJG4-5-SC plasmids containing SMART III and CDS III (SC) sequences were created. The SC sequences were amplified from pGADT7-Rec2 (Clontech) using the primers B1881/B1882 with EcoRI/SmaI sites and B1883/B1884 with SmaI/XhoI sites for pJG4-5 and B1905/B1906 with EcoRI/PmlI sites and B1907/B1908 with PmlI/XhoI sites for pEG202 and cloned into pEG202 to create a binding domain fusion plasmid and pJG4-5 for an activation domain (LexA Repressor) fusion plasmid. pEG202-SC digested with PmlI or pJG4-5-SC digested with SmaI was mixed with RAD1, RAM1, and NSP2 coding sequences amplified from M. truncatula cDNA (primers described in Supplemental Table S1) to generate binding domain-fusion and activation domain-fusion plasmids, which were then transformed into yeast strains EGY48 or Y864. To construct pEG202-GW and pJG4-5-GW, a gateway sequence (GW) was amplified from pDEST22 (Invitrogen) using the primers B3757 with EcoRI and B3758 with XhoI and cloned into the multiple cloning site of each plasmid. pEG202-GW or pJG4-5-GW was mixed with coding sequences (TF80 and TF124) amplified from M. truncatula cDNA (using primers described in Supplemental Table S1) to create binding domain-fusion and activation domain-fusion plasmids.
Agrobacterium rhizogenes-Mediated Transformation of M. truncatula Roots
M. truncatula root transformation was performed as described in Boisson-Dernier et al. (2001) with slight modifications as follows: the cut ends of M. truncatula (A17, R108, or mutants) seedling roots were cocultivated with Agrobacterium rhizogenes containing a binary vector. After cocultivation, the plants with transformed roots were transplanted in Turface (Profile Products) and grown for 10 d to allow more root growth. The plants were then transplanted to cones with 100 to 150 spores of G. versiforme and grown for 3 to 7 weeks as described above.
Staining Roots with Alexa Fluor 488 WGA and Propidium Iodide and Quantification of Colonization
Roots were fixed with 50% (v/v) ethanol for 4 h, cleared in 20% (v/v) potassium hydroxide solution for 4 d at room temperature, rinsed thoroughly with distilled water, and then incubated with 0.2 µg mL−1 Alexa Fluor 488 WGA in phosphate-buffered saline for 1 d in the dark. In some instances, roots were incubated in propidium iodide (20 µg mL−1 in distilled water) to stain the cell wall. Stained roots were examined microscopically using either a fluorescence stereomicroscope or a confocal microscope. For infection unit analysis of ram1-3 grown in the double cones and harvested at 2, 4, and 6 dpi, all colonization events (hyphopodia, intercellular hyphae, arbuscules, trunks, and undeveloped arbuscules) on the root systems of plants grown in the double cones were counted. At the 8-dpi, 3-wpp, and 5-wpp time points, the percentage of infected root pieces containing hyphopodia, intercellular hyphae, arbuscules, or arbuscule trunks/undeveloped arbuscules was scored using the modified gridline intersect method (McGonigle et al., 1990).
RNA Isolation, Semi-qRT-PCR, and qRT-PCR
Total RNA was extracted from roots with TRIzol reagent according to the manufacturer’s instruction manual (www.lifetechnologies.com). cDNA was synthesized using SuperScript III Reverse Transcriptase (Invitrogen) from 500 ng of total RNA. The cDNA equivalent of 10 ng of RNA was used for semi-qRT-PCR and real-time qRT-PCR.
Histochemical GUS Staining
For GUS staining of M. truncatula roots transformed with RAM1pro2kb:UidA and RAM1pro800:UidA, the plant roots were harvested at 3 and 5 wpp and incubated in GUS staining buffer (0.5 mg mL−1 5-bromo-4-chloro-3-indolyl-β-d-glucuronide cyclohexylammonium salts, 100 mm NaH2PO4, pH 7.0, and 5 mm Na2EDTA) for 2 to 4 h at 37°C and then fixed with fixation buffer of 50 mm PIPES, pH 6.9, 5 mm MgSO4, 50 mm EGTA, 11% (v/v) formaldehyde, and 5% (v/v) dimethyl sulfoxide for 1 d (Liu et al., 2008) before clearing with potassium hydroxide and staining with Alexa Fluor 488 WGA as outlined above.
P Content Analyses
The phosphate content of the shoots of M. truncatula plants grown under three different nutrient regimes was measured to confirm the nutrient treatment in Figure 4. Leaf tissue was ground in liquid N, and the soluble phosphate content was measured as described (Ames, 1966).
The data are as follows: 20 µm inorganic phosphate (Pi): 1.5 mm N-R108 (22.04 ± 3.71 [mean ± sd; nanomoles Pi per milligram dry weight]); 20 µm Pi: 1.5 mm N-wild-type-seg (25.19 ± 5.76 [mean ± sd; nanomoles Pi per milligram dry weight]); 20 µm Pi: 1.5 mm N-ram1-3 (25.29 ± 1.07 [mean ± sd; nanomoles Pi per milligram dry weight]); 20 µm Pi: 7.5 mm N-R108 (21.73 ± 5.59 [mean ± sd; nanomoles Pi per milligram dry weight]); 20 µm Pi: 7.5 mm N-wild-type-seg (19.49 ± 0.89 [mean ± sd; nanomoles Pi per milligram dry weight]); 20 µm Pi: 7.5 mm N-ram1-3 (21.74 ± 1.89 [mean ± sd; nanomoles Pi per milligram dry weight]); 2 mm Pi: 7.5 mm N-B108 (258.95 ± 9.69 [mean ± sd; nanomoles Pi per milligram dry weight]); 2 mm Pi: 7.5 mm N-wild-type-seg (251.90 ± 37.03 [mean ± sd; nanomoles Pi per milligram dry weight]); and 2 mm Pi: 7.5 mm N-ram1-3 (171.41 ± 28.64 [mean ± sd; nanomoles Pi per milligram dry weight]).
Shoot phosphate level of the M. truncatula plants fertilized with high-Pi fertilizer (2 mm Pi and 7.5 mm N) showed a significant increase compared with those fertilized with two low-Pi fertilizers.
Yeast Two-Hybrid Assay
For yeast two-hybrid assays, plasmids containing the DNA-binding domain fusion TF genes were transformed into yeast strain EGY48 containing the LacZ (β-galactosidase) reporter (pSH18-34). The plasmids of LexA fusion TF genes were transformed into strain Y864. The two yeast strains were mated, resulting in yeast containing the three plasmids. The mated yeasts were grown on the dropout (SD-Ura-Trp-His) plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside for color development (Zeng et al., 2012).
Statistical Analysis
The measured data were analyzed by Student’s t test or pairwise comparison using all pairs Tukey’s honestly significant difference (HSD) mean-separation test on JMP Pro-10 software. The statistical information, including numbers of biological replicates, means, and error bars, is described in each figure legend. To analyze variances between genotypes and nutrient treatments groups in Figure 4B, the experimental data were analyzed using ANOVA test on JMP Pro-10. To determine if there was a significant effect of RAM1 overexpression in Figure 6, data from three independent experiments were analyzed together. The expression data for each candidate target gene were right skewed so a natural log transformation was applied before analysis. The transformed expression data were analyzed using a linear mixed model with a fixed treatment effect and a random experiment effect. F tests were used to determine whether there was a significant treatment effect. All statistical analyses were performed using the JMP statistical software package (JMP, version 10; SAS Institute Inc., Cary, NC).
Phylogenetic Analysis
The predicted full-length amino acid sequences of all GRAS TFs were obtained by iteratively blasting the TF RAM1 (Medtr7g027190) in a database containing the predicted protein models from the genomes of Arabidopsis (Arabidopsis thaliana; TAIR), rice (Oryza sativa; phytozome10), Lotus japonicus (Kazusa DNA Research Institute), and M. truncatula (JCVI). The protein models were aligned using the program MAFFT, version 7.205, with default values, and the columns containing more than 50% of gaps were eliminated from the alignment. The approximately maximum likelihood phylogenetic tree was generated using FastTree, version 2.1.5, with the wag model of amino acid evolution. Two TFs that are closely related to GRAS factors were used to root the tree. Each branch division shows local support values with the Shimodaira-Hasegawa test (Price et al., 2010).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. RAM1 is expressed in the root cortex in regions that are colonized and can also be detected in younger regions of some lateral roots in both mock-inoculated and colonized root systems.
Supplemental Figure S2. Diagram showing the sites of Tnt1 insertions in the RAM1 gene.
Supplemental Figure S3. Confocal microscope images showing the mycorrhizal phenotype of ram1-3 at 4 and 8 dpi with G. versiforme in the double-cone system.
Supplemental Figure S4. Mycorrhizal colonization phenotype of ram1-3 plants colonized with G. versiforme at 4 and 8 dpi.
Supplemental Figure S5. A comparison of colonization in ram1-1 and ram1-3.
Supplemental Figure S6. ram1-3 complementation and cosegregation analysis.
Supplemental Figure S7. Phylogenetic tree of GRAS TFs.
Supplemental Figure S8. Gene expression in ram1-1.
Supplemental Figure S9. Yeast two-hybrid assays to assess the interaction of various TFs and regulators.
Supplemental Figure S10. Gene expression in rad1.
Supplemental Table S1. List of primers and genes.
Supplemental Data Set S1. GRAS family alignment file.
Supplementary Material
Acknowledgments
We thank Lynn Marie Johnson (Cornell Statistical Consulting Unit, Cornell University) for helping with the statistical analysis of gene expression in roots overexpressing RAM1.
Glossary
- AM
arbuscular mycorrhiza
- cDNA
complementary DNA
- dpi
days postinoculation
- HSD
honestly significant difference
- Pi
inorganic phosphate
- qRT
quantitative reverse transcription
- wpp
weeks postplanting
Footnotes
This work was supported by the Office of Science (Biological and Environmental Research), U.S. Department of Energy (grant no. DE FG02–08ER64628), the National Science Foundation (grant nos. IOS–1127155, IOS–1237993 and DBI–0618969 [instrumentation grant for microscopes in the Boyce Thompson Institute for Plant Research, Plant Cell Imaging Center]). Medicago truncatula insertion mutant lines were obtained from the Samuel Roberts Noble Foundation.
Articles can be viewed without a subscription.
References
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 [DOI] [PubMed] [Google Scholar]
- Ames BN. (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8: 115–118 [Google Scholar]
- Amijee F, Tinker PB, Stribley DP (1989) The development of endomycorrhizal root systems. VII. A detailed study of effects of soil phosphorus on colonization. New Phytol 111: 435–446 [DOI] [PubMed] [Google Scholar]
- Baath E, Spokes J (1989) The effect of added nitrogen and phosphorus on mycorrhizal growth response and infection in Allium schoenoprasum. Can J Bot 67: 3227–3232 [Google Scholar]
- Besserer A, Puech-Pagès V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais JC, Roux C, Bécard G, Séjalon-Delmas N (2006) Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol 4: e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Chabaud M, Garcia F, Becard G, Rosenberg C, Barker DG (2001) Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol Plant Microbe Interact 14: 695–700 [DOI] [PubMed] [Google Scholar]
- Bolle C. (2004) The role of GRAS proteins in plant signal transduction and development. Planta 218: 683–692 [DOI] [PubMed] [Google Scholar]
- Breuillin-Sessoms F, Floss DS, Gomez SK, Pumplin N, Ding Y, Levesque-Tremblay V, Noar RD, Daniels DA, Bravo A, Eaglesham JA, et al. (2015) Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter4 mutants is dependent on the Ammonium Transporter 2 family protein AMT2;3. Plant Cell 27: 1352–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Benfey PN (2009) Interplay between SCARECROW, GA and LIKE HETEROCHROMATIN PROTEIN 1 in ground tissue patterning in the Arabidopsis root. Plant J 58: 1016–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Levesque MP, Vernoux T, Jung JW, Paquette AJ, Gallagher KL, Wang JY, Blilou I, Scheres B, Benfey PN (2007) An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 316: 421–425 [DOI] [PubMed] [Google Scholar]
- Davière JM, Achard P (2013) Gibberellin signaling in plants. Development 140: 1147–1151 [DOI] [PubMed] [Google Scholar]
- Delaux PM, Bécard G, Combier JP (2013) NSP1 is a component of the Myc signaling pathway. New Phytol 199: 59–65 [DOI] [PubMed] [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation. Plant Mol Biol Report 1: 19–22 [Google Scholar]
- Devers EA, Branscheid A, May P, Krajinski F (2011) Stars and symbiosis: microRNA- and microRNA*-mediated transcript cleavage involved in arbuscular mycorrhizal symbiosis. Plant Physiol 156: 1990–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devers EA, Teply J, Reinert A, Gaude N, Krajinski F (2013) An endogenous artificial microRNA system for unraveling the function of root endosymbioses related genes in Medicago truncatula. BMC Plant Biol 13: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feddermann N, Muni RRD, Zeier T, Stuurman J, Ercolin F, Schorderet M, Reinhardt D (2010) The PAM1 gene of petunia, required for intracellular accommodation and morphogenesis of arbuscular mycorrhizal fungi, encodes a homologue of VAPYRIN. Plant J 64: 470–481 [DOI] [PubMed] [Google Scholar]
- Floss DS, Levy JG, Levesque-Tremblay V, Pumplin N, Harrison MJ (2013) DELLA proteins regulate arbuscule formation in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A 110: E5025–E5034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fode B, Siemsen T, Thurow C, Weigel R, Gatz C (2008) The Arabidopsis GRAS protein SCL14 interacts with class II TGA transcription factors and is essential for the activation of stress-inducible promoters. Plant Cell 20: 3122–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Ross JJ, Jones WT, Reid JB (2013) Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Ann Bot (Lond) 111: 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel A, Manthey K, Perlick AM, Meyer F, Pühler A, Küster H, Krajinski F (2005) Combined transcriptome profiling reveals a novel family of arbuscular mycorrhizal-specific Medicago truncatula lectin genes. Mol Plant Microbe Interact 18: 771–782 [DOI] [PubMed] [Google Scholar]
- Gaude N, Bortfeld S, Duensing N, Lohse M, Krajinski F (2012) Arbuscule-containing and non-colonized cortical cells of mycorrhizal roots undergo extensive and specific reprogramming during arbuscular mycorrhizal development. Plant J 69: 510–528 [DOI] [PubMed] [Google Scholar]
- Genre A, Chabaud M, Balzergue C, Puech-Pagès V, Novero M, Rey T, Fournier J, Rochange S, Bécard G, Bonfante P, et al. (2013) Short-chain chitin oligomers from arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. New Phytol 198: 190–202 [DOI] [PubMed] [Google Scholar]
- Gobbato E, Marsh JF, Vernié T, Wang E, Maillet F, Kim J, Miller JB, Sun J, Bano SA, Ratet P, et al. (2012) A GRAS-type transcription factor with a specific function in mycorrhizal signaling. Curr Biol 22: 2236–2241 [DOI] [PubMed] [Google Scholar]
- Gobbato E, Wang E, Higgins G, Bano SA, Henry C, Schultze M, Oldroyd GED (2013) RAM1 and RAM2 function and expression during arbuscular mycorrhizal symbiosis and Aphanomyces euteiches colonization. Plant Signal Behav 8: e26049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C, Radovanovic D, Geoffroy J, Zhang Q, Siegler H, Chiapello M, Casieri L, An K, An G, Guiderdoni E, et al. (2012) The half-size ABC transporters STR1 and STR2 are indispensable for mycorrhizal arbuscule formation in rice. Plant J 69: 906–920 [DOI] [PubMed] [Google Scholar]
- Harrison MJ, Dewbre GR, Liu J (2002) A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14: 2413–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JO, Chang KS, Kim IA, Lee MH, Lee SA, Song SK, Lee MM, Lim J (2011) Funneling of gibberellin signaling by the GRAS transcription regulator scarecrow-like 3 in the Arabidopsis root. Proc Natl Acad Sci USA 108: 2166–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch S, Kim J, Muñoz A, Heckmann AB, Downie JA, Oldroyd GED (2009) GRAS proteins form a DNA binding complex to induce gene expression during nodulation signaling in Medicago truncatula. Plant Cell 21: 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofferek V, Mendrinna A, Gaude N, Krajinski F, Devers EA (2014) MiR171h restricts root symbioses and shows like its target NSP2 a complex transcriptional regulation in Medicago truncatula. BMC Plant Biol 14: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogekamp C, Küster H (2013) A roadmap of cell-type specific gene expression during sequential stages of the arbuscular mycorrhiza symbiosis. BMC Genomics 14: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnjec N, Vieweg MF, Pühler A, Becker A, Küster H (2005) Overlaps in the transcriptional profiles of Medicago truncatula roots inoculated with two different Glomus fungi provide insights into the genetic program activated during arbuscular mycorrhiza. Plant Physiol 137: 1283–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JJ, Park YS, Bravo A, Bhattarai KK, Daniels DA, Harrison MJ (2012) Diversity of morphology and function in arbuscular mycorrhizal symbioses in Brachypodium distachyon. Planta 236: 851–865 [DOI] [PubMed] [Google Scholar]
- Ivanov S, Fedorova EE, Limpens E, De Mita S, Genre A, Bonfante P, Bisseling T (2012) Rhizobium-legume symbiosis shares an exocytotic pathway required for arbuscule formation. Proc Natl Acad Sci USA 109: 8316–8321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov S, Harrison MJ (2014) A set of fluorescent protein-based markers expressed from constitutive and arbuscular mycorrhiza-inducible promoters to label organelles, membranes and cytoskeletal elements in Medicago truncatula. Plant J 80: 1151–1163 [DOI] [PubMed] [Google Scholar]
- Javot H, Penmetsa RV, Breuillin F, Bhattarai KK, Noar RD, Gomez SK, Zhang Q, Cook DR, Harrison MJ (2011) Medicago truncatula mtpt4 mutants reveal a role for nitrogen in the regulation of arbuscule degeneration in arbuscular mycorrhizal symbiosis. Plant J 68: 954–965 [DOI] [PubMed] [Google Scholar]
- Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJ (2007) A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 104: 1720–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, De Meyer B, Hilson P (2005) Modular cloning in plant cells. Trends Plant Sci 10: 103–105 [DOI] [PubMed] [Google Scholar]
- Koide R, Li M (1990) On host regulation of the vesicular-arbuscular mycorrhizal symbiosis. New Phytol 114: 59–74 [DOI] [PubMed] [Google Scholar]
- Krajinski F, Courty PE, Sieh D, Franken P, Zhang H, Bucher M, Gerlach N, Kryvoruchko I, Zoeller D, Udvardi M, et al. (2014) The H+-ATPase HA1 of Medicago truncatula is essential for phosphate transport and plant growth during arbuscular mycorrhizal symbiosis. Plant Cell 26: 1808–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar T, Kohlen W, Sasse J, Borghi L, Schlegel M, Bachelier JB, Reinhardt D, Bours R, Bouwmeester HJ, Martinoia E (2012) A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483: 341–344 [DOI] [PubMed] [Google Scholar]
- Levesque MP, Vernoux T, Busch W, Cui H, Wang JY, Blilou I, Hassan H, Nakajima K, Matsumoto N, Lohmann JU, et al. (2006) Whole-genome analysis of the SHORT-ROOT developmental pathway in Arabidopsis. PLoS Biol 4: e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpens E, Mirabella R, Fedorova E, Franken C, Franssen H, Bisseling T, Geurts R (2005) Formation of organelle-like N2-fixing symbiosomes in legume root nodules is controlled by DMI2. Proc Natl Acad Sci USA 102: 10375–10380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Blaylock LA, Endre G, Cho J, Town CD, VandenBosch KA, Harrison MJ (2003) Transcript profiling coupled with spatial expression analyses reveals genes involved in distinct developmental stages of an arbuscular mycorrhizal symbiosis. Plant Cell 15: 2106–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Versaw WK, Pumplin N, Gomez SK, Blaylock LA, Harrison MJ (2008) Closely related members of the Medicago truncatula PHT1 phosphate transporter gene family encode phosphate transporters with distinct biochemical activities. J Biol Chem 283: 24673–24681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Kohlen W, Lillo A, Op den Camp R, Ivanov S, Hartog M, Limpens E, Jamil M, Smaczniak C, Kaufmann K, et al. (2011) Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 23: 3853–3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Meyer M, Harrison MJ (2006) An experimental system to synchronize the early events of development of the arbuscular mycorrhizal symbiosis. In Sánchez F, Quinto C, López-Lara IM, Geiger O, eds, Biology of Molecular Plant-Microbe Interactions, Vol 5 International Society for Molecular Plant Microbe Interactions, St. Paul, pp 546–551 [Google Scholar]
- Ma Z, Hu X, Cai W, Huang W, Zhou X, Luo Q, Yang H, Wang J, Huang J (2014) Arabidopsis miR171-targeted scarecrow-like proteins bind to GT cis-elements and mediate gibberellin-regulated chlorophyll biosynthesis under light conditions. PLoS Genet 10: e1004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet F, Poinsot V, André O, Puech-Pagès V, Haouy A, Gueunier M, Cromer L, Giraudet D, Formey D, Niebel A, et al. (2011) Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 469: 58–63 [DOI] [PubMed] [Google Scholar]
- McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method that gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115: 495–501 [DOI] [PubMed] [Google Scholar]
- Murray JD, Muni RRD, Torres-Jerez I, Tang Y, Allen S, Andriankaja M, Li G, Laxmi A, Cheng X, Wen J, et al. (2011) Vapyrin, a gene essential for intracellular progression of arbuscular mycorrhizal symbiosis, is also essential for infection by rhizobia in the nodule symbiosis of Medicago truncatula. Plant J 65: 244–252 [DOI] [PubMed] [Google Scholar]
- Oldroyd GED. (2013) Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol 11: 252–263 [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP (2010) FastTree 2: approximately maximum-likelihood trees for large alignments. PLoS One 5: e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N, Harrison MJ (2009) Live-cell imaging reveals periarbuscular membrane domains and organelle location in Medicago truncatula roots during arbuscular mycorrhizal symbiosis. Plant Physiol 151: 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N, Mondo SJ, Topp S, Starker CG, Gantt JS, Harrison MJ (2010) Medicago truncatula Vapyrin is a novel protein required for arbuscular mycorrhizal symbiosis. Plant J 61: 482–494 [DOI] [PubMed] [Google Scholar]
- Pumplin N, Zhang X, Noar RD, Harrison MJ (2012) Polar localization of a symbiosis-specific phosphate transporter is mediated by a transient reorientation of secretion. Proc Natl Acad Sci USA 109: E665–E672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech SS, Heidt S, Requena N (2013) A tandem Kunitz protease inhibitor (KPI106)-serine carboxypeptidase (SCP1) controls mycorrhiza establishment and arbuscule development in Medicago truncatula. Plant J 75: 711–725 [DOI] [PubMed] [Google Scholar]
- Rich MK, Schorderet M, Bapaume L, Falquet L, Morel P, Vandenbussche M, Reinhardt D (2015) A petunia GRAS transcription factor controls symbiotic gene expression and fungal morphogenesis in arbuscular mycorrhiza. Plant Physiol 168: 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SM, Menge JA, Leonard RT (1983) Quantitative and qualitative effects of phosphorus on extracts and exudates of sudangrass roots in relation to vesicular-arbuscular mycorrhiza formation. Plant Physiol 73: 761–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Katzer K, Lambert J, Cerri M, Parniske M (2014) CYCLOPS, a DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe 15: 139–152 [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ (2008) Mycorrhizal Symbiosis. Academic Press, Inc., San Diego [Google Scholar]
- Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. In Merchant SS, Briggs WR, Ort D, eds, Annual Review of Plant Biology, Vol 62 Annual Reviews, Palo Alto, CA, pp 227–250 [DOI] [PubMed] [Google Scholar]
- Takeda N, Handa Y, Tsuzuki S, Kojima M, Sakakibara H, Kawaguchi M (2015) Gibberellins interfere with symbiosis signaling and gene expression and alter colonization by arbuscular mycorrhizal fungi in Lotus japonicus. Plant Physiol 167: 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Sato S, Asamizu E, Tabata S, Parniske M (2009) Apoplastic plant subtilases support arbuscular mycorrhiza development in Lotus japonicus. Plant J 58: 766–777 [DOI] [PubMed] [Google Scholar]
- Wang E, Schornack S, Marsh JF, Gobbato E, Schwessinger B, Eastmond P, Schultze M, Kamoun S, Oldroyd GED (2012) A common signaling process that promotes mycorrhizal and oomycete colonization of plants. Curr Biol 22: 2242–2246 [DOI] [PubMed] [Google Scholar]
- Wang E, Yu N, Bano SA, Liu C, Miller AJ, Cousins D, Zhang X, Ratet P, Tadege M, Mysore KS, et al. (2014) A H+-ATPase that energizes nutrient uptake during mycorrhizal symbioses in rice and Medicago truncatula. Plant Cell 26: 1818–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Huang W, Liu F, Tang N, Liu Y, Lin H, Zhao B (2013) Functional analysis of the novel mycorrhiza-specific phosphate transporter AsPT1 and PHT1 family from Astragalus sinicus during the arbuscular mycorrhizal symbiosis. New Phytol 198: 836–852 [DOI] [PubMed] [Google Scholar]
- Xue L, Cui H, Buer B, Vijayakumar V, Delaux PM, Junkermann S, Bucher M (2015) Network of GRAS transcription factors involved in the control of arbuscule development in Lotus japonicus. Plant Physiol 167: 854–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Grønlund M, Jakobsen I, Grotemeyer MS, Rentsch D, Miyao A, Hirochika H, Kumar CS, Sundaresan V, Salamin N, et al. (2012) Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the PHOSPHATE TRANSPORTER1 gene family. Plant Cell 24: 4236–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Yoshida S, Müller J, Singh S, Banba M, Vickers K, Markmann K, White C, Schuller B, Sato S, et al. (2008) CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc Natl Acad Sci USA 105: 20540–20545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N, Luo D, Zhang X, Liu J, Wang W, Jin Y, Dong W, Liu J, Liu H, Yang W, et al. (2014) A DELLA protein complex controls the arbuscular mycorrhizal symbiosis in plants. Cell Res 24: 130–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Velásquez AC, Munkvold KR, Zhang J, Martin GB (2012) A tomato LysM receptor-like kinase promotes immunity and its kinase activity is inhibited by AvrPtoB. Plant J 69: 92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Blaylock LA, Harrison MJ (2010) Two Medicago truncatula half-ABC transporters are essential for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Cell 22: 1483–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Pumplin N, Ivanov S, Harrison MJ (2015) EXO70I is required for development of a sub-domain of the periarbuscular membrane during arbuscular mycorrhizal symbiosis. Curr Biol 25: 2189–2195 [DOI] [PubMed] [Google Scholar]
- Zhang ZL, Ogawa M, Fleet CM, Zentella R, Hu J, Heo JO, Lim J, Kamiya Y, Yamaguchi S, Sun TP (2011) Scarecrow-like 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc Natl Acad Sci USA 108: 2160–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.