Foliar metabolite levels, including myoinositol, show correlation with grain yield in tropical maize field trials during drought, heat, and simultaneous drought/heat stresses.
Abstract
The development of abiotic stress-resistant cultivars is of premium importance for the agriculture of developing countries. Further progress in maize (Zea mays) performance under stresses is expected by combining marker-assisted breeding with metabolite markers. In order to dissect metabolic responses and to identify promising metabolite marker candidates, metabolite profiles of maize leaves were analyzed and compared with grain yield in field trials. Plants were grown under well-watered conditions (control) or exposed to drought, heat, and both stresses simultaneously. Trials were conducted in 2010 and 2011 using 10 tropical hybrids selected to exhibit diverse abiotic stress tolerance. Drought stress evoked the accumulation of many amino acids, including isoleucine, valine, threonine, and 4-aminobutanoate, which has been commonly reported in both field and greenhouse experiments in many plant species. Two photorespiratory amino acids, glycine and serine, and myoinositol also accumulated under drought. The combination of drought and heat evoked relatively few specific responses, and most of the metabolic changes were predictable from the sum of the responses to individual stresses. Statistical analysis revealed significant correlation between levels of glycine and myoinositol and grain yield under drought. Levels of myoinositol in control conditions were also related to grain yield under drought. Furthermore, multiple linear regression models very well explained the variation of grain yield via the combination of several metabolites. These results indicate the importance of photorespiration and raffinose family oligosaccharide metabolism in grain yield under drought and suggest single or multiple metabolites as potential metabolic markers for the breeding of abiotic stress-tolerant maize.
The increasing world population coupled to environmental deterioration is creating ever greater pressure on our capacity for sustainable food productivity. Alongside biotic stresses, abiotic stresses such as drought, heat, salinity, and nutrient deficiency greatly reduce yields in crop fields either when present alone or in combination. Breeding for more resilient crops, therefore, is one of the major approaches to cope with the increasing challenges in world agriculture. Considerable research effort has thus been invested in order to dissect plant responses to individual stresses at various levels (for review, see Urano et al., 2010; Lopes et al., 2011; Obata and Fernie, 2012), but the interaction between different stresses has been far less investigated (Cairns et al., 2012b, 2013; Suzuki et al., 2014). In general, the combination of stresses additively affects plant physiology (i.e. the symptoms of the individual stresses appear simultaneously) and synergistically diminishes the yield and productivity of plants (Keleş and Öncel, 2002; Giraud et al., 2008; Vile et al., 2012; Suzuki et al., 2014). The molecular responses, however, are not simply additive and are rarely predicted from the responses to individual stresses (Rizhsky et al., 2002, 2004; Prasch and Sonnewald, 2013; Rasmussen et al., 2013). Information from carefully controlled greenhouse experiments has begun to dissect the molecular mechanisms by which plants, in particular Arabidopsis (Arabidopsis thaliana), respond to drought and temperature stresses (Skirycz et al., 2010, 2011; Skirycz and Inzé, 2010; Bowne et al., 2012; Tardieu, 2012; Verkest et al., 2015). Our knowledge of the molecular basis of the responses of crop species in a field environment, however, is considerably less well advanced (Araus et al., 2008; Cabrera-Bosquet et al., 2012). That said, a large number of genotypes have been generated on the basis of their resistance to both biotic and abiotic stresses (for review, see Bänziger et al., 2006; Takeda and Matsuoka, 2008; Cooper et al., 2014), and the genome sequencing and molecular characterization of a range of stress-tolerant plant species have recently been reported (Wu et al., 2012; Ma et al., 2013; Bolger et al., 2014; Tohge et al., 2014). These studies are not only important as basic research for further studies in crops but also are a prerequisite in the development of molecular marker-based approaches to improve crop tolerance to stress.
As a first step toward this goal, a deeper understanding of the plant responses to the stressful environment, especially those to multiple stress conditions under field conditions, is crucial for the improvement of stress-tolerant crops. This is important on two levels: (1) in the field, singular abiotic stresses are rare; and (2) yield and stress adaptation are complex traits that render breeding gains slower than would be expected under optimal conditions (Bruce et al., 2002). Recent studies have revealed that the response of plants to combinations of two or more stress conditions is unique and cannot be directly extrapolated from their responses to the different stresses when applied individually. This would be a result of complex combinations of different, and sometimes opposing, responses in signaling pathways, including those that may interact and inhibit one another (Prasch and Sonnewald, 2013; Rasmussen et al., 2013; Suzuki et al., 2014).
Maize (Zea mays) is grown in over 170 million ha worldwide, of which 130 million ha are in less-developed countries (FAO, 2014). In sub-Saharan Africa, maize is a staple crop; however, yields in this region have stagnated at less than 2 tons ha−1, while maize yields worldwide have continued to increase (Cairns et al., 2012a). Low yields in sub-Saharan Africa are largely associated with drought stress (DS) and low soil fertility (Bänziger and Araus, 2007). Additionally, simulation studies indicate that maize yield in Africa is likely to be significantly impaired by heat stress (HS; Lobell and Burke, 2010; Lobell et al., 2011), such as can be anticipated as a result of the changes in climate predicted for the coming decades (Müller et al., 2011). Moreover, the sensitivity of maize yield to heat is exacerbated under drought conditions (Lobell et al., 2011; Cairns et al., 2012a, 2012b, 2013). Therefore, the development of maize germplasm tolerant to drought and heat conditions is of utmost importance to both increase yields and offset predicted yield losses under projected climate change scenarios (Easterling et al., 2007), especially in sub-Saharan Africa. While direct selection for grain yield under DS has resulted in admirable gains in grain yield under stress (Bänziger et al., 2006; Cairns et al., 2013), further improvement requires the incorporation of additional selection traits (Cairns et al., 2012a, 2012b). In recent years, genetic and phenotypic markers have been searched extensively for drought tolerance of maize by high-throughput genomic and phenotyping approaches, respectively (Tuberosa and Salvi, 2006; Wen et al., 2011; Araus et al., 2012; Cairns et al., 2013; Prasanna et al., 2013; Araus and Cairns, 2014; Tsonev et al., 2014). Moreover, metabolic markers started to draw attention due to their close relationship with yield phenotypes (Fernie and Schauer, 2009; Redestig et al., 2011; Riedelsheimer et al., 2012a, 2012b; Witt et al., 2012; Degenkolbe et al., 2013). The accumulation of some metabolites has been reported to be directly related to the performance of potato (Solanum tuberosum) cultivars in beetle resistance in the field (Tai et al., 2014). Additionally, identical genomic regions were mapped as both agronomic and metabolic quantitative trait loci in field-grown maize and wheat (Triticum aestivum), indicating the utility of metabolic traits for breeding selection (Riedelsheimer et al., 2012b; Hill et al., 2015). A recent study showed that genetic gains in maize grain yield under DS were higher using a molecular marker-based approach than conventional breeding (Beyene et al., 2015).
Here, we focused on the relationship between leaf metabolites and grain yield under drought, heat, and simultaneous drought and heat conditions in the field. The negative effect of DS on maize yield is especially acute during the reproductive stage between tassel emergence and early grain filling (Grant et al., 1989), when it is believed to induce premature seed desiccation and to limit grain filling. Grain is more susceptible to DS than vegetative tissues; therefore, the prediction of grain yield from the physiological parameter of leaves is a challenge (Sangoi and Salvador, 1998; Khodarahmpour and Hamidi, 2011). Nevertheless, maize yield is dependent on both the assimilate supply to the kernel (source) and the potential of the kernel to accommodate this assimilate (sink potential; Jones and Simmons, 1983). Breeding for modern temperate hybrids has focused more on the sink potential, particularly under stress conditions (Tollenaar and Lee, 2006); therefore, there should be considerable potential remaining to improve source ability. DS and HS would be anticipated largely to affect leaf metabolism and especially photosynthesis, compromising the source capacity of leaves (Chaves et al., 2009; Lawlor and Tezara, 2009; Osakabe et al., 2014). In keeping with this, drought was found to have the most dramatic effect on the metabolite composition in leaves compared with other organs in our previous greenhouse experiments (Witt et al., 2012). Since the source ability is closely related to leaf metabolism, the leaf metabolite profile should have a close relationship to grain yield particularly under conditions of stress. Given that several recent studies have indicated the importance of metabolic preadaptation to various stress tolerances in plants (Sanchez et al., 2011; Benina et al., 2013), we also postulate that basal metabolite levels under optimal growth conditions could be correlated to stress tolerance. In order to test this, metabolite profiles of the leaf blades of 10 hybrids were analyzed in field experiments conducted at the International Maize and Wheat Improvement Center (CIMMYT) subtropical experimental station in 2010 and 2011 in which the plants were exposed to singular or combined drought and heat stresses (DS+HS; Cairns et al., 2012a, 2013). The results are discussed both in the context of current models of stress tolerance and with respect to their practical implications for future breeding strategies.
RESULTS
Grain Yield Was Affected by Stress Treatments
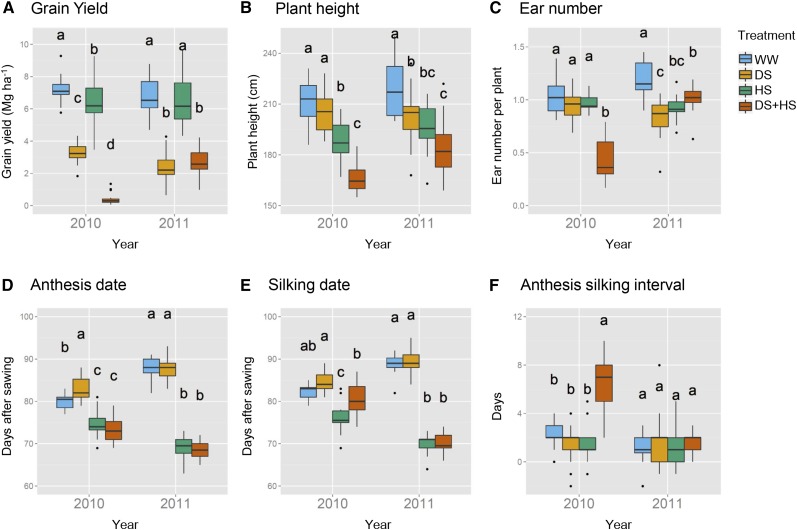
Maize plants were grown as part of the CIMMYT field trials, in which a collection of 300 hybrid lines representing the genetic diversity within the CIMMYT and International Institute of Tropical Agriculture tropical and subtropical maize improvement programs (Wen et al., 2011) were tested for tolerance to DS, HS, and DS+HS (Cairns et al., 2013). Ten maize experimental hybrids (Table I) were chosen to cover a wide range of drought and heat tolerance on grain yield observed in previous field trials (Cairns et al., 2013). DS was imposed by stopping irrigation before flowering to achieve water deficiency at anthesis stage. This treatment induces mild DS, which increases canopy temperature 1°C to 2°C in the absence of HS (Romano et al., 2011; Zia et al., 2013). However, climate conditions varied slightly between the two years of evaluation. In 2010, one rainfall event (with 56 mm of precipitation) occurred at the start of February, 1 week before drought was applied under optimal temperature (and 3 weeks before trial mean anthesis). For the DS+HS trial, one rainfall event of 35 mm occurred 2 weeks before the start of anthesis and the day before the last irrigation to apply DS. In 2011, one rainfall event of 13 mm occurred 10 d before the start of anthesis in the DS+HS trial and 4 d after DS was applied (Table II). Drought treatment significantly decreased average grain yields to 45.8% and 35.2% of well-watered (WW) plants in the 2010 and 2011 seasons, respectively (Fig. 1A). Grain yield across treatments varied in genotypes from 1.83 to 4.32 Mg ha−1 in 2010 and from 0.65 to 4.28 Mg ha−1 in 2011 (Supplemental Fig. S1). The grain yield of individual genotypes tended to be in agreement with tolerance levels determined from the previous field experiments (Table I; Supplemental Fig. S1; Supplemental Table S1). Plant height did not differ between tolerant and susceptible genotypes, in accordance with the occurrence of the stress around flowering, when plants have already achieved their maximum height (Supplemental Fig. S1; Supplemental Table S1). Other parameters did not differ between tolerant and susceptible genotypes, whereas tolerant genotypes reached earlier anthesis and silking under HS. Drought treatment did not evoke consistent effects on silking date, anthesis date, anthesis/silking interval, ear height, and ear number across the two harvests (Fig. 1, B–E, and G; Supplemental Fig. S1). HS was applied by growing plants in the dry season. A group of plants were additionally treated by DS+HS (for details, see “Materials and Methods”). HS decreased plant height and shortened the time until silking and anthesis in both years (Fig. 1, D and E; Supplemental Fig. S1). However, grain yield was decreased significantly in 2010 but not in 2011 (Fig. 1A). The grain yield under DS+HS was also the same as that in the drought condition in 2011 (Fig. 1A), indicating that the heat treatment in 2011 was not severe enough to affect grain yield. Therefore, the heat treatment in 2011 was recognized as mild HS, which does not cause yield reduction. DS+HS exclusively affected ear number and anthesis silking interval (Fig. 1, D and E) and led to a severe yield reduction in 2010 (Fig. 1A). Two-way ANOVA indicated that grain yield was influenced significantly by genotype, treatment, and also their interaction in both years (Supplemental Table S2). Ear number and anthesis date were also influenced by the interaction of genotype and treatment in 2010 but not in 2011 (Supplemental Table S2). These results indicate that grain yield is the most suitable parameter to assess the genotypic variation of stress tolerance in this study and were mainly used for correlation analysis with metabolite profile.
Table I. Summary of maize genotypes.
| Genotype | Entrya | Tolerance tob |
||
|---|---|---|---|---|
| DS | DS+HS | HS | ||
| La Posta Seq C7-F64-2-6-2-2 | 147 | Tolerant | Tolerant | Not tested |
| DTPWC9-F31-1-3-1-1 | 102 | Tolerant | Tolerant | Tolerant |
| DTPYC9-F143-5-4-1-2 | 8 | Moderate | Tolerant | Tolerant |
| La Posta Seq C7-F18-3-2-1-1 | 37 | Tolerant | Moderate | Tolerant |
| CML-486 | 72 | Moderate | Tolerant | Moderate |
| La Posta Seq C7-F96-1-2-1-3 | 89 | Susceptible | Tolerant | Tolerant |
| CML311/MBR C3 Bc F95-2-2-1 | 87 | Susceptible | Moderate | Moderate |
| CML311/MBR C2 Bc F41-2 | 34 | Tolerant | Tolerant | Susceptible |
| DTPYC9-F69-3-5-1-1 | 91 | Tolerant | Susceptible | Susceptible |
| [GQL5/[GQL5/[MSRXPOOL9]C1F2-205-1(OSU23i)-5-3-X-X-1-BB]F2-4sx]-11-3-1-1 | 117 | Moderate | Susceptible | Susceptible |
The nos. used to identify the genotypes in this article. bBased on Cairns et al. (2013).
Table II. Weather records for December to May in 2010 and 2011 at the experimental site at Tlaltizapán, Mexico.
Monthly average figures are given for maximum, minimum, and daily average air temperatures and total monthly rainfall. All measurements were taken at 2 m.
| Month |
Temperature |
Rainfall |
||||||
|---|---|---|---|---|---|---|---|---|
| Average | Minimum | Maximum | 2010 |
2011 |
||||
| 2010 |
2011 |
2010 |
2011 |
2010 |
2011 |
|||
| °C | mm | |||||||
| December | 25.2 | 22.2 | 20.8 | 16.1 | 29.7 (0)a | 28.3 (0) | 0 | 0 |
| January | 24.2 | 25.0 | 20.1 | 19.4 | 28.3 (0) | 30.5 (0) | 20.4 | 0 |
| February | 25.0 | 27.5 | 20.8 | 22.4 | 29.2 (0) | 32.6 (0) | 60.0 | 0 |
| March | 28.7 | 29.8 | 23.3 | 24.5 | 34.1 (6) | 35.1 (10) | 0 | 0 |
| April | 30.7 | 32.2 | 25.5 | 27.4 | 35.8 (29) | 37.0 (12) | 34.8 | 12.8 |
| May | 33.1 | 32.4 | 28.7 | 28.2 | 37.8 (30) | 36.6 (10) | 1.4 | 12.1 |
No. of days with an air temperature greater than 35°C.
Figure 1.
Yield-related parameters in the two years of field stress trials. Box plots show grain yield (A), plant height (B), number of ears per plant (C), anthesis date (D), silking date (E), and interval between anthesis and silking dates (F) in the 2010 and 2011 seasons. Ten maize hybrids were grown in WW (blue), DS (orange), WW and HS (green), and DS+HS (red) conditions in two independent plots (n = 20). Letters indicate the results of Tukey’s test comparing the conditions in each year (P < 0.05).
Metabolite Profiling Revealed Differential Metabolic Responses of Genotypes to Stress Conditions
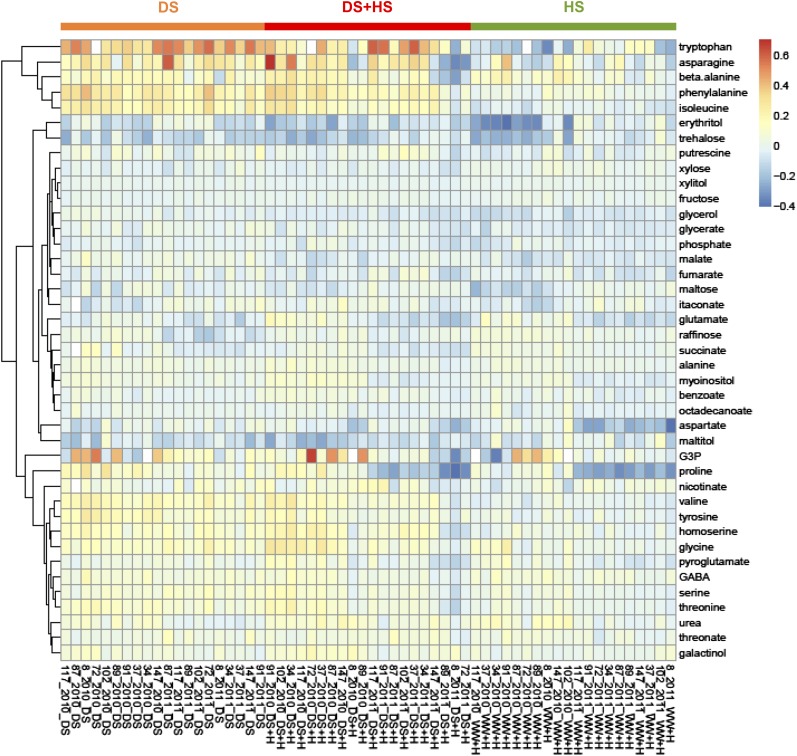
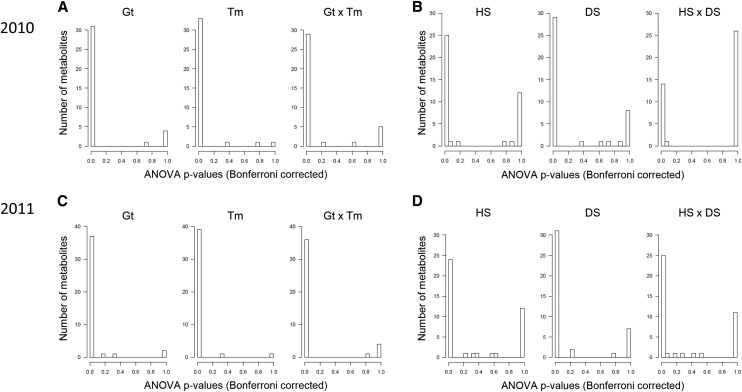
Leaf material was harvested for metabolite profiling 2 weeks after the cessation of irrigation. Gas chromatography-mass spectroscopy (GC-MS)-based metabolite profiling was conducted to analyze the metabolites of central carbon metabolism. Metabolite levels were analyzed on a dry weight basis to avoid the effect of differential water contents. Forty-one metabolites were detected in more than half of the samples. Some highly abundant metabolites, including Suc and Glc, could not be quantified due to being above the dynamic range limit of the specified settings of the GC-MS apparatus. The metabolites were clustered into three groups when analyzed by hierarchical clustering (Fig. 2). The first cluster includes amino acids (e.g. Trp, Asn, β-Ala, Phe, and Ile) that accumulated highly under DS. A second large cluster includes various metabolites that tended to decrease (e.g. erythritol, maltose, malate, and fumarate) or showed relatively minor responses (e.g. Xyl, raffinose, and putrescine) in all stress conditions. By contrast, metabolites in the third cluster tended to accumulate in all stress conditions (e.g. Ser, Gly, and 4-aminobutanoate [GABA]; Fig. 2). These metabolic responses were similar across the genotypes but varied in magnitude (Fig. 2). The clear separation among samples from the four growth conditions in principal component analysis (PCA) indicates the differential metabolic effect of DS, HS, and DS+HS on maize leaf in 2010 (Supplemental Fig. S2A). DS contributed to the separation on principal component 2, while HS contributed to principal component 1 (Supplemental Fig. S2A). Separation between single and multiple stress conditions was not clear in 2011, most likely due to the mild HS (Supplemental Fig. S2B). Two-way ANOVA indicated that most metabolites were influenced significantly by genotype, treatment, and their interaction, suggesting differential responses of individual genotypes to each treatment in both years (Fig. 3, A and C). The effects on levels of individual metabolites are summarized in Supplemental Table S3. When the effect of treatments was tested, ANOVA revealed that most metabolites were separately influenced by each stress (Fig. 3, B and D). However, just 13 metabolites were significantly affected by the interaction of HS and DS in 2010 (Fig. 3B), suggesting that limited metabolites responded to combined stresses in a specific manner or that the effects of individual stress components compensated each other in some metabolites. Some metabolites showed a clear tendency of differential accumulation in stress-tolerant and -sensitive hybrids under stress conditions. Among them, galactinol levels were lower in tolerant genotypes than in susceptible ones under DS in both years (Supplemental Table S4). Accumulation of this metabolite varied between genotypic groups with different tolerance levels under all stress conditions tested (Supplemental Table S4), suggesting a relationship with stress tolerance.
Figure 2.
Heat map of metabolic responses to stress conditions in each genotype. Metabolite levels in each stress condition were normalized to that in the WW condition and log2 transformed. Values are means of up to 12 biological replicates. Red and blue colors represent increase and decrease of metabolites using a false-color scale, respectively. Samples from DS, DS+HS, and WW and HS conditions are arranged from the left. Genotypes are ordered by the grain yield in the corresponding stress condition (smallest at the left) in each year.
Figure 3.
Effects of treatments and genotypes on the levels of individual metabolites. Histograms show the number of metabolites whose levels were altered, with indicated P values by Bonferroni-corrected two-way ANOVA analyzing the effects of genotypes (Gt) and treatments (Tm; A and C) or HS and DS (B and D). Each bar indicates a range of 0.05. Data from 2010 (A and B) and 2011 (C and D) were independently analyzed. Metabolite levels from six biological replicates in two independent plots (n = 12) were used for analysis.
DS and HS Conditions Evoke Increases in the Levels of Many Amino Acids
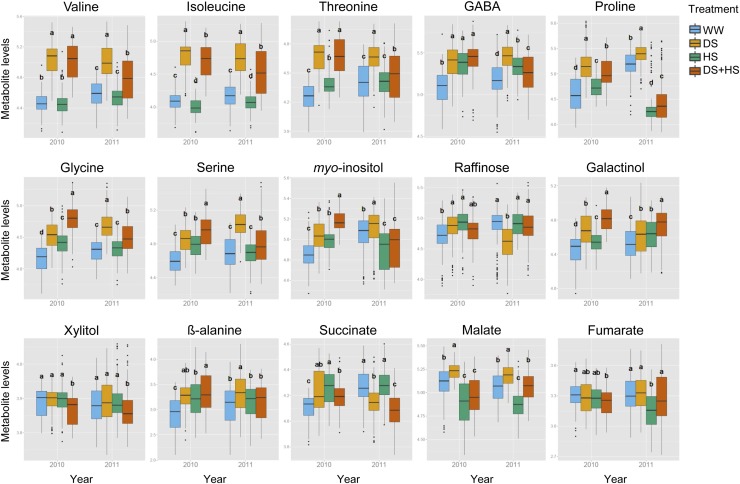
Among the 41 metabolites, 20 accumulated and three were reduced under DS in both years (Fig. 4; Supplemental Fig. S3). Those showing increased levels included many amino acids (Phe, Trp, Asn, Ser, Thr, Ile, Ala, Pro, Val, Gly, Tyr, GABA, pyro-Glu, β-Ala, and homo-Ser), sugar and sugar alcohols (maltose, myoinositol, and galactinol), and organic acids (glycerate and threonate). By contrast, two sugar alcohols (erythritol and maltitol) and trehalose were reduced by the drought treatment (Fig. 4; Supplemental Fig. S3). Under HS, Phe, Ala, GABA, threonate, Xyl, and galactinol accumulated and Ile, glycerol, malate, glycerate, and phosphate were reduced in both years (Fig. 4; Supplemental Fig. S3). These metabolites can thus be considered as responding to even mild HS. Some other metabolites, including Trp, Ser, Thr, β-Ala, Pro, Glu, pyro-Glu, raffinose, myoinositol, succinate, and urea, accumulated only in the HS condition of 2010, suggesting that they responded only to severe stress (Fig. 4; Supplemental Fig. S3). Maltose, erythritol, maltitol, and trehalose were decreased under HS only in 2010 (Fig. 4; Supplemental Fig. S3). These metabolites are possibly related to grain yield under HS, since they responded only to severe HS affecting grain yield but not to the mild stress without an effect on yield.
Figure 4.
Foliar metabolite levels in the two years of field stress trials. Box plots show the relative levels of selected metabolites in the 2010 and 2011 seasons. Only the metabolites discussed in “Discussion” are shown. Plots for all metabolites are shown in Supplemental Figure S3. Ten maize hybrids were grown in WW (blue), DS (orange), WW and HS (green), and DS+HS (red) conditions in two independent plots (n = 20). Letters indicate the results of Tukey’s test comparing the conditions in each year (P < 0.05).
Stress Combination Additively Affected the Metabolite Profile
Metabolic responses under DS+HS shared similar changes with the individual stress treatments. Only three (benzoate, fumarate, and xylitol) and two (urea and xylitol) metabolites changed specifically under DS+HS in 2010 and 2011, respectively, while no significant effects on the levels of these metabolites were observed in DS or HS compared with WW plants individually (Supplemental Fig. S4). Of the 34 metabolites affected under the DS+HS condition, 21 were also affected in both DS and HS conditions in 2010 (Supplemental Fig. S4). In 2011, the number of metabolites in this category was reduced to 12 and the metabolites shared between DS and DS+HS increased to 14, most likely due to mild HS (Supplemental Fig. S4). K-means clustering was performed in order to classify the metabolites according to the responses to stress conditions in 2010 (Supplemental Fig. S5). Most of the metabolites fitted well into five clusters, with some exceptions in which the responses were not clear. Those clustered into the first three and the latter two clusters tended to accumulate and decrease under DS+HS, respectively. Metabolites in clusters 1 and 2 were increased in all stress conditions, although those in cluster 1 accumulated further by stress combination. Cluster 3 includes many amino acids highly accumulated in both DS and DS+HS conditions. The metabolites in cluster 4 are characterized by the reduction in HS, while cluster 5 includes those specifically decreased under DS+HS (Supplemental Fig. S5). According to the criteria of response modes defined for describing transcript responses under stress combination by Rasmussen et al. (2013), most of the metabolites in clusters 1 and 2 and some in cluster 4 are assigned to the similar response mode. Clusters 3 to 5 mostly contain metabolites that responded in independent mode, while just three metabolites, namely benzoate in cluster 1 and fumarate and xylitol in cluster 5, could be classified as belonging to the combinatorial mode. Interestingly, metabolic responses in the 2010 trial could be well classified into just five of 20 scenarios that were predefined to the responses against stress combinations (Rasmussen et al., 2013). It should also be noted that most of the metabolic changes in DS+HS should be predictable from the metabolic responses to each single stress treatment, since similar and independent response modes are considered to be predictable (Rasmussen et al., 2013).
Pro is a metabolite whose function in the DS+HS condition has been reported (Rizhsky et al., 2004). The accumulation of Pro differed between 2010 and 2011 in our maize field trial. While Pro levels in DS+HS were reduced in comparison with DS in both years, it was much lower in 2011 along with the Pro level under single HS. It should also be noted that the level of Pro in DS+HS was still significantly higher than that in the WW condition in 2010 (Fig. 4).
Metabolic Responses under Stress Combination Could Be Predicted from the Sum of Those in Single Stresses
Many of the metabolic responses, especially those in clusters 1, 2, and 3, seem to be predictable not only qualitatively but also quantitatively by the simple sum of responses in DS and HS. In order to test this hypothesis, the response factor was calculated by dividing the metabolite level under stress conditions by that in the WW condition. Following log2 transformation, correlations between the sum of the response factors in DS and HS (predicted response factor in DS+HS) and the actual response factor in DS+HS were tested (Table III; Supplemental Table S5). Surprisingly, the predicted response factors correlated significantly with actual response factors in 17 of 41 metabolites in 2010 (Table III). The means of the predicted and actual values were fairly similar in most of the metabolites, and Student’s t test showed significant differences between these two values in only 11 of the 41 metabolites (Table III). This analysis suggested that a large part of the metabolic response under stress combination could be explained by the additive effects of individual treatments. Predicted and actual response factors were correlated in more metabolites in 2011, but this is most likely due to the weak effect of HS (Supplemental Table S5). On the other hand, the predicted response factor was significantly different from the actual one in eight metabolites, including Tyr, succinate, urea, GABA, raffinose, and Xyl (Table III). The levels of these metabolites are most likely determined by regulatory mechanisms specifically operating under combined stress conditions.
Table III. Actual and predicted metabolic responses of maize leaves to DS, HS, and DS+HS in 2010.
| Metabolite | DSa | HSa | DS+HSa | Predictedb | rc |
|---|---|---|---|---|---|
| Ala | 0.703 ± 0.098 | 0.625 ± 0.113 | 0.928 ± 0.141 | 1.379 ± 0.193 | 0.78 |
| Asn | 1.147 ± 0.223 | 0.357 ± 0.310 | 1.887 ± 0.475 | 1.442 ± 0.457 | 0.64 |
| Asp | −0.344 ± 0.228 | 0.129 ± 0.157 | −0.135 ± 0.183 | −0.428 ± 0.294 | 0.40 |
| Benzoate | 0.103 ± 0.077 | 0.142 ± 0.078 | 0.500 ± 0.066 | 0.234 ± 0.141 | 0.50 |
| β-Ala | 1.059 ± 0.095 | 0.980 ± 0.198 | 1.406 ± 0.221 | 2.035 ± 0.253 | 0.76 |
| Erythritol | −0.477 ± 0.145 | −1.847 ± 0.229 | −1.109 ± 0.161 | −2.224 ± 0.365 | 0.45 |
| Fru | −0.035 ± 0.067 | 0.101 ± 0.061 | 0.049 ± 0.053 | 0.067 ± 0.127 | 0.69 |
| Fumarate | −0.062 ± 0.123 | −0.097 ± 0.087 | −0.171 ± 0.080 | −0.220 ± 0.199 | 0.18 |
| Glycerol-3-P | 1.612 ± 0.454 | 0.855 ± 0.553 | 1.436 ± 0.362 | 4.014 ± 0.853 | 0.98 |
| GABA | 1.023 ± 0.162 | 0.957 ± 0.145 | 1.011 ± 0.164 | 1.973 ± 0.266 | 0.41 |
| Galactinol | 0.706 ± 0.124 | 0.227 ± 0.082 | 1.164 ± 0.109 | 1.027 ± 0.170 | 0.75 |
| Glu | 0.004 ± 0.143 | 0.627 ± 0.222 | 0.814 ± 0.209 | 0.555 ± 0.315 | 0.73 |
| Glycerate | 0.098 ± 0.115 | −0.389 ± 0.109 | −0.147 ± 0.137 | −0.268 ± 0.199 | 0.69 |
| Glycerol | 0.271 ± 0.099 | −0.882 ± 0.128 | −0.516 ± 0.070 | −0.560 ± 0.212 | 0.18 |
| Gly | 1.129 ± 0.103 | 0.760 ± 0.216 | 2.032 ± 0.277 | 1.909 ± 0.247 | 0.43 |
| Homo-Ser | 0.957 ± 0.104 | 0.220 ± 0.110 | 1.272 ± 0.180 | 1.171 ± 0.174 | −0.15 |
| Ile | 2.328 ± 0.146 | −0.319 ± 0.135 | 1.988 ± 0.253 | 2.008 ± 0.219 | 0.01 |
| Itaconate | −0.138 ± 0.146 | 0.125 ± 0.198 | 1.128 ± 0.110 | 0.036 ± 0.302 | 0.42 |
| Malate | 0.417 ± 0.116 | −0.662 ± 0.168 | −0.573 ± 0.162 | −0.296 ± 0.206 | 0.63 |
| Maltitol | −0.494 ± 0.178 | −0.277 ± 0.146 | −1.093 ± 0.184 | −0.956 ± 0.255 | 0.66 |
| Maltose | 0.022 ± 0.244 | −1.181 ± 0.156 | −0.497 ± 0.182 | −1.274 ± 0.340 | 0.71 |
| Myoinositol | 0.620 ± 0.083 | 0.445 ± 0.084 | 1.067 ± 0.098 | 1.056 ± 0.156 | 0.37 |
| Nicotinate | 0.264 ± 0.092 | −0.020 ± 0.122 | 0.517 ± 0.117 | 0.178 ± 0.215 | −0.02 |
| Octadecanoate | 0.108 ± 0.087 | 0.162 ± 0.138 | 0.204 ± 0.110 | 0.263 ± 0.187 | 0.40 |
| Phe | 2.467 ± 0.178 | 0.266 ± 0.111 | 2.355 ± 0.236 | 2.785 ± 0.241 | −0.43 |
| Phosphate | −0.213 ± 0.097 | −0.299 ± 0.141 | −0.134 ± 0.135 | −0.535 ± 0.194 | 0.36 |
| Pro | 1.897 ± 0.269 | 0.311 ± 0.182 | 1.365 ± 0.265 | 1.968 ± 0.405 | 0.38 |
| Putrescine | 0.198 ± 0.124 | −0.195 ± 0.134 | 0.001 ± 0.116 | 0.094 ± 0.236 | 0.60 |
| Pyro-Glu | 0.389 ± 0.134 | 0.568 ± 0.149 | 0.899 ± 0.236 | 1.003 ± 0.254 | 0.59 |
| Raffinose | 0.482 ± 0.097 | 0.704 ± 0.114 | 0.402 ± 0.113 | 1.149 ± 0.182 | −0.04 |
| Ser | 0.834 ± 0.110 | 0.570 ± 0.139 | 1.202 ± 0.204 | 1.435 ± 0.208 | 0.60 |
| Succinate | 0.513 ± 0.185 | 0.524 ± 0.071 | 0.364 ± 0.122 | 1.134 ± 0.191 | 0.21 |
| Threonate | 0.356 ± 0.090 | 0.595 ± 0.086 | 0.480 ± 0.089 | 0.954 ± 0.161 | 0.76 |
| Thr | 1.321 ± 0.105 | 0.414 ± 0.140 | 1.359 ± 0.213 | 1.728 ± 0.192 | 0.44 |
| Trehalose | −1.083 ± 0.240 | −1.790 ± 0.243 | −1.641 ± 0.230 | −2.746 ± 0.451 | 0.70 |
| Trp | 2.874 ± 0.269 | −0.940 ± 0.337 | 1.961 ± 0.403 | 1.985 ± 0.567 | 0.46 |
| Tyr | 1.988 ± 0.148 | 0.186 ± 0.154 | 1.307 ± 0.243 | 2.135 ± 0.257 | 0.50 |
| Urea | 0.624 ± 0.108 | 0.874 ± 0.144 | 1.026 ± 0.099 | 1.548 ± 0.215 | 0.36 |
| Val | 1.913 ± 0.127 | −0.014 ± 0.139 | 1.798 ± 0.238 | 1.912 ± 0.200 | 0.06 |
| Xylitol | 0.009 ± 0.015 | 0.025 ± 0.019 | −0.087 ± 0.014 | 0.038 ± 0.025 | 0.69 |
| Xyl | −0.140 ± 0.056 | 0.176 ± 0.078 | −0.197 ± 0.061 | 0.072 ± 0.106 | 0.26 |
Response factors showing log2 fold change in the metabolite level against the WW condition. Values are means ± se (n = 20). bPredicted response factors to DS+HS are sums of those to individual DS and HS. Values shown in boldface are significantly different from actual response factors in Student’s t test (P < 0.05). cCorrelation coefficients between actual and predicted response factors. Values shown in boldface are correlations at P < 0.05.
Correlation Analysis Revealed a Close Relationship between Leaf Metabolite Levels and Grain Yield under Stress Conditions
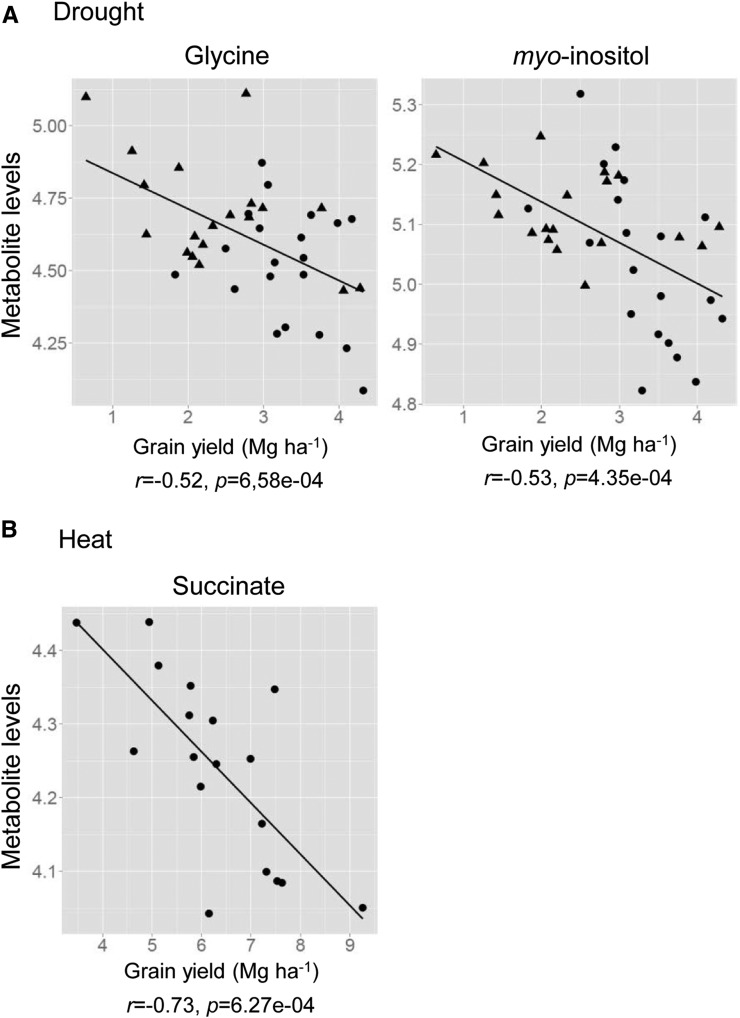
In order to identify metabolites closely related to grain yield under stress conditions, the correlation between levels of each metabolite and grain yield in each growth condition was tested by Pearson correlation analysis (Table IV). When the relationship between metabolite levels and grain yield in optimal growth conditions was tested, only β-Ala and maltitol displayed significant correlation (Table IV). Under the DS condition, levels of glycerol and glycerate showed positive correlation and those of Asn, Ser, pyro-Glu, phosphate, itaconate, and galactinol were negatively correlated to grain yield. Gly, myoinositol, threonate, glycerol-3-P, and nicotinate showed strong negative correlation to grain yield, with P < 0.01 (Table IV; Fig. 5). Correlation analysis in HS and DS+HS conditions was conducted only for 2010 data, since heat treatment had no effect on grain yield in 2011 (Fig. 1A). The results from each year are shown in Supplemental Table S6. Thr, Val, erythritol, xylitol, trehalose, glycerol, phosphate, and nicotinate showed a positive correlation and fumarate, succinate, and raffinose showed a negative correlation with grain yield under HS in 2010 (Table IV). Maltitol is the only metabolite showing a positive correlation with grain yield in the DS+HS condition (Table IV). Levels of Phe, Thr, Ile, Val, Asp, and benzoate (P < 0.01) as well as Trp, homo-Ser, Ala, Gly, Tyr, Glu, pyro-Glu, myoinositol, malate, and GABA (P < 0.05) were negatively correlated to this trait.
Table IV. Correlation between grain yield and metabolite levels.
Correlation coefficients are shown between grain yield and metabolite levels under each growth condition. Ten genotypes grown in two plots were analyzed (n = 20 for 1 year). Data from both years were analyzed for the WW and DS conditions, whereas only 2010 data were analyzed for the HS and DS+HS conditions.
| Metabolite | WW | DS | HS | DS+HS |
|---|---|---|---|---|
| Ala | −0.05 | −0.08 | 0.17 | −0.52* |
| Asn | 0.27 | −0.36* | 0.36 | −0.55* |
| Asp | 0.29 | −0.30 | 0.33 | −0.63** |
| Benzoate | 0.04 | 0.11 | −0.44 | −0.66** |
| β-Ala | 0.43** | −0.05 | 0.42 | −0.21 |
| Erythritol | 0.25 | 0.06 | 0.58* | 0.07 |
| Fru | −0.06 | −0.03 | −0.16 | 0.17 |
| Fumarate | 0.18 | 0.15 | −0.58* | −0.43 |
| Glycerol-3-phosphate | −0.22 | −0.51** | 0.00 | −0.25 |
| GABA | −0.13 | 0.10 | −0.19 | −0.55* |
| Galactinol | −0.10 | −0.32* | −0.25 | 0.15 |
| Glu | 0.09 | −0.16 | 0.21 | −0.60* |
| Glycerate | −0.06 | 0.36 | −0.05 | 0.05 |
| Glycerol | 0.14 | 0.38 | 0.67* | 0.17 |
| Gly | 0.14 | −0.52*** | −0.35 | −0.59* |
| Homo-Ser | 0.26 | −0.25 | 0.31 | −0.56* |
| Ile | −0.05 | −0.21 | 0.43 | −0.71** |
| Itaconate | −0.14 | −0.40* | −0.31 | 0.22 |
| Malate | −0.10 | 0.20 | −0.45 | −0.59* |
| Maltitol | 0.43** | 0.18 | 0.35 | 0.64** |
| Maltose | 0.10 | 0.29 | 0.25 | 0.44 |
| Myoinositol | −0.22 | −0.54*** | −0.21 | −0.51* |
| Nicotinate | −0.27 | −0.47** | 0.51* | −0.02 |
| Octadecanoate | −0.26 | −0.20 | −0.42 | 0.00 |
| Phe | −0.08 | −0.26 | 0.38 | −0.67** |
| Phosphate | −0.02 | −0.37* | 0.56* | −0.24 |
| Pro | 0.03 | −0.10 | 0.42 | −0.44 |
| Putrescine | −0.03 | 0.15 | 0.36 | −0.05 |
| Pyro-Glu | 0.24 | −0.40* | −0.28 | −0.60* |
| Raffinose | 0.05 | 0.29 | −0.59* | 0.12 |
| Ser | 0.10 | −0.37* | 0.26 | −0.49 |
| Succinate | −0.16 | 0.11 | −0.73*** | −0.13 |
| Threonate | 0.14 | −0.47** | −0.05 | 0.31 |
| Thr | 0.18 | 0.12 | 0.52* | −0.62** |
| Trehalose | 0.11 | 0.01 | 0.60** | −0.34 |
| Trp | 0.05 | 0.08 | 0.01 | −0.50* |
| Tyr | 0.06 | 0.14 | 0.38 | −0.52* |
| Urea | 0.02 | 0.17 | 0.00 | −0.30 |
| Val | −0.06 | −0.17 | 0.55* | −0.69** |
| Xylitol | 0.16 | 0.10 | 0.56* | 0.23 |
| Xyl | 0.02 | 0.28 | 0.32 | 0.06 |
Correlation at P < 0.05.
Correlation at P < 0.01.
Correlation at P < 0.001.
Figure 5.
Correlation between metabolite levels and grain yield under DS (A) and HS (B) conditions. Levels of selected metabolites showing significant correlation (P < 0.001; Table IV) were plotted against grain yield under stress conditions. Ten maize hybrids were grown in two independent plots (n = 20 per year), and means of metabolite levels from six biological replicates were plotted. No metabolite showed significant correlation to grain yield under DS+HS. Circles and triangles indicate data from 2010 and 2011, respectively. Due to a minor effect of heat treatment on grain yield in 2011, only 2010 data were used for the analysis of HS. r and p are the correlation coefficient and P value from Pearson correlation analysis, respectively.
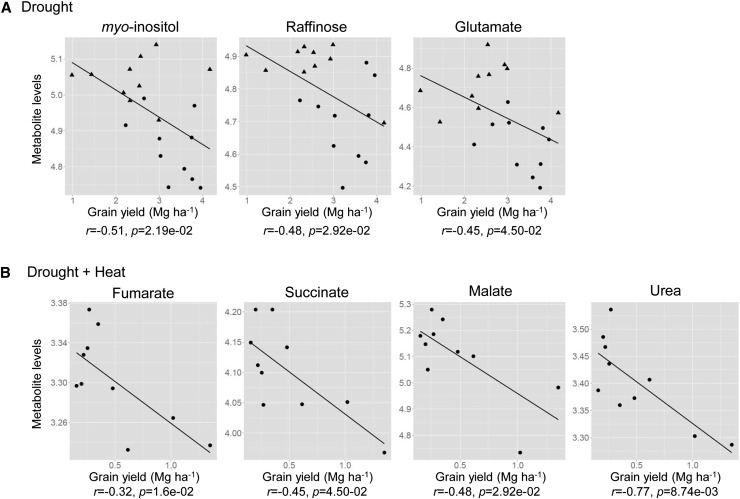
The correlation of metabolite levels under control conditions with grain yield under stress conditions was next tested in order to identify the metabolites that could be important components of metabolic preadaptation (Table V). Levels of Glu, raffinose, myoinositol, nicotinate, and octadecanoate in the control condition were significantly correlated to grain yield in DS, although no metabolite other than phosphate was correlated to grain yield in HS in 2010 (Table V; Fig. 6). Tricarboxylic acid cycle intermediates, namely succinate, fumarate, malate, and urea, displayed a negative correlation to grain yield in DS+HS (Table V; Fig. 6). Interestingly, grain yield under HS also showed significant correlation to that in the WW condition (Table V). This suggests that genotypes with better yield under the optimal condition also produce more grain even under HS, while stress tolerance affects the grain yield more under DS.
Table V. Correlation between grain yield under stressed conditions and grain yield and metabolite levels under the WW condition.
Correlation coefficients are shown between grain yield under stress conditions and grain yield and metabolite levels in the WW condition. Each genotype was analyzed as a data point (n = 10 for 1 year). Data from both years were analyzed for DS, whereas only 2010 data were analyzed for HS and DS+HS.
| Yield/Metabolite | DS | HS | DS+HS |
|---|---|---|---|
| Grain yield | 0.32 | 0.85** | 0.32 |
| Metabolite | |||
| Ala | −0.27 | −0.11 | 0.17 |
| Asn | 0.17 | 0.04 | 0.60 |
| Asp | −0.29 | 0.33 | 0.30 |
| Benzoate | −0.31 | −0.27 | −0.45 |
| β-Ala | −0.13 | 0.43 | 0.19 |
| Erythritol | 0.35 | −0.18 | 0.03 |
| Fru | −0.07 | −0.66 | −0.53 |
| Fumarate | 0.09 | −0.66 | −0.70* |
| Glycerol-3-P | −0.39 | −0.65 | −0.64* |
| GABA | −0.16 | −0.45 | −0.18 |
| Galactinol | −0.34 | 0.32 | −0.09 |
| Glu | −0.45* | 0.20 | 0.08 |
| Glycerate | 0.31 | 0.12 | −0.05 |
| Glycerol | 0.11 | 0.37 | −0.04 |
| Gly | −0.44 | 0.25 | 0.45 |
| Homo-Ser | −0.27 | 0.09 | 0.12 |
| Ile | −0.25 | −0.29 | 0.11 |
| Itaconate | −0.38 | 0.62 | 0.33 |
| Malate | 0.12 | −0.58 | −0.72* |
| Maltitol | −0.03 | 0.15 | 0.08 |
| Maltose | 0.08 | −0.06 | 0.59 |
| Myoinositol | −0.51* | −0.49 | −0.18 |
| Nicotinate | −0.55* | −0.25 | −0.59 |
| Octadecanoate | −0.51* | −0.39 | −0.55 |
| Phe | −0.33 | −0.29 | −0.19 |
| Phosphate | −0.38 | −0.78* | −0.41 |
| Pro | −0.27 | 0.53 | 0.00 |
| Putrescine | −0.32 | −0.61 | −0.37 |
| Pyro-Glu | −0.41 | 0.06 | 0.35 |
| Raffinose | −0.49* | −0.12 | −0.14 |
| Ser | −0.15 | 0.06 | 0.30 |
| Succinate | −0.33 | −0.53 | −0.74* |
| Threonate | −0.29 | −0.28 | 0.11 |
| Thr | −0.13 | 0.22 | 0.38 |
| Trehalose | 0.19 | −0.23 | −0.19 |
| Trp | 0.44 | −0.33 | −0.35 |
| Tyr | −0.30 | 0.24 | 0.27 |
| Urea | −0.36 | −0.24 | −0.77** |
| Val | −0.26 | −0.17 | 0.14 |
| Xylitol | 0.02 | −0.31 | 0.01 |
| Xyl | −0.04 | −0.29 | −0.40 |
Correlations at P < 0.05.
Correlations at P < 0.01.
Figure 6.
Correlation between metabolite levels in the control condition and grain yield under DS (A) and DS+HS (B) conditions. Levels of selected metabolites under the WW condition showing significant correlation (P < 0.05; Table V) were plotted against grain yield under stress conditions. No organic metabolite showed significant correlation to grain yield under HS. Mean values from each of 10 maize hybrids (n = 10 per year) were plotted. Circles and triangles indicate data from 2010 and 2011, respectively. Due to a minor effect of heat treatment on grain yield in 2011, only 2010 data were used for the analysis of DS+HS. r and p are the correlation coefficient and P value from Pearson correlation analysis, respectively.
In order to gain insight into the sequential effects of multiple stresses on the relationship between metabolite levels and grain yield, the correlation between grain yield and levels of individual metabolites was tested using the results from two growth conditions in 2010 (Table VI). The results from the 2011 trial are shown in Supplemental Table S7. Four pairs of conditions, namely WW/DS, DS/DS+HS, WW/HS, and HS/DS+HS, were tested to compare the effects of a stress in the presence and absence of the other stress. It should be noted that the results rather reflect treatment effects than genotypic ones, due to the larger contribution of treatments on the changes in both grain yield and metabolite levels. Twenty metabolites showed significant correlation with grain yield commonly in the WW/DS and HS/DS+HS condition pairs, indicating that these metabolites were similarly affected by DS regardless of the presence of HS (Table VI). Asp, maltose, xylitol, and Xyl were four metabolites that showed correlation to grain yield only in the presence of HS. Contrary to DS, only six metabolites were correlated with grain yield commonly when WW/HS and HS/DS+HS pairs were tested (Table VI). Thirteen and eight metabolites were significantly correlated with grain yield under HS specifically in the presence (DS/DS+HS) and absence (WW/HS) of DS, respectively. This suggests that the effects of metabolites on grain yield under HS are largely dependent on the presence of DS.
Table VI. Correlation between grain yield and metabolite levels under two growth conditions in 2010.
Correlation coefficients between grain yield and metabolite levels under each growth condition were calculated by using data from two growth conditions. Conditions were chosen to dissect the effects of stress combination. Ten genotypes grown in two growth conditions were analyzed (n = 20). Correlation coefficients shown are correlations at P < 0.05. ns, Not significant (P > 0.05).
| Metabolite | WW/DS | DS/DS+HS | WW/HS | HS/DS+HS |
|---|---|---|---|---|
| Ala | −0.68** | −0.39 | ns | −0.47* |
| Asn | −0.61 | −0.38 | ns | −0.55* |
| Asp | ns | ns | ns | 0.37 |
| Benzoate | −0.32 | −0.71* | −0.36 | −0.63* |
| β-Ala | −0.45* | ns | ns | ns |
| Erythritol | 0.36 | 0.54* | 0.49* | −0.34 |
| Fru | ns | ns | −0.38 | ns |
| Fumarate | ns | ns | −0.33 | ns |
| Glycerol-3-P | −0.43 | −0.63* | ns | −0.57* |
| GABA | −0.69* | ns | −0.40 | ns |
| Galactinol | −0.60* | −0.38 | ns | −0.72 |
| Glu | ns | −0.61* | ns | ns |
| Glycerate | ns | ns | ns | ns |
| Glycerol | −0.34 | 0.71* | 0.65* | ns |
| Gly | −0.69* | −0.60* | −0.34 | −0.68* |
| Homo-Ser | −0.66 | ns | ns | −0.57* |
| Ile | −0.87** | ns | ns | −0.82** |
| Itaconate | ns | −0.36 | ns | −0.54* |
| Malate | −0.52* | 0.63* | ns | ns |
| Maltitol | 0.52* | 0.71* | 0.40 | 0.73* |
| Maltose | ns | 0.37 | 0.43* | −0.42 |
| Myoinositol | −0.66* | −0.69* | ns | −0.71* |
| Nicotinate | −0.41 | ns | ns | −0.42 |
| Octadecanoate | ns | ns | −0.36 | ns |
| Phe | −0.90** | ns | ns | −0.80* |
| Phosphate | ns | ns | 0.36 | ns |
| Pro | −0.56 | ns | ns | −0.48* |
| Putrescine | −0.48 | ns | ns | ns |
| Pyro-Glu | −0.36 | −0.54* | ns | −0.41 |
| Raffinose | −0.35 | ns | −0.40 | ns |
| Ser | −0.54* | −0.41 | ns | −0.45 |
| Succinate | −0.51* | ns | −0.63 | ns |
| Threonate | −0.50* | ns | ns | ns |
| Thr | −0.77* | ns | ns | −0.59* |
| Trehalose | 0.48* | ns | 0.43 | ns |
| Trp | −0.83** | ns | ns | −0.74* |
| Tyr | −0.78* | 0.42 | ns | −0.61* |
| Urea | −0.67* | −0.36 | ns | ns |
| Val | −0.86** | ns | ns | −0.75* |
| Xylitol | ns | 0.64* | ns | 0.72* |
| Xyl | ns | ns | ns | 0.56* |
Correlation at P < 0.01.
Correlation at P < 0.001.
Combination of Metabolite Levels Could Explain the Variation of Grain Yield by Multiple Regression Modeling
Additionally, a multiple linear regression model was constructed in order to identify groups of parameters that coordinately affect grain yield in each growth condition for the 2010 data. Grain yield was used as a dependent variable, and a minimum number of independent variables were selected from all parameters measured in this study, only metabolite levels, and at last metabolite levels under the WW condition, which contributed to fully explain the variation of grain yield among genotypes (Table VII). The models require 10 to 15 parameters to explain the variation of grain yield in most cases, but only seven were used for the DS condition (Table VII). The models were quite similar when all parameters (agronomical variables and metabolites) or only the metabolites were used as independent variables. Especially the models selected for DS were identical in both cases (Table VII). Similar sets of metabolites under the WW condition explained the variation of grain yield in WW and stress conditions (Table VII). In the case of DS, exactly the same sets of metabolites as in the WW condition were selected (Table VII). Interestingly, galactinol was selected for all models (Table VII). When the same analysis was conducted for 2011 data, galactinol was again selected as a parameter in all models, whereas the models required more independent variables than for the 2010 data to explain yield variation (Supplemental Table S8).
Table VII. The set of variables selected by multiple regression analysis to explain the 100% variance in grain yield under each growth condition in 2010.
Multiregression analysis showed the set of both agronomical parameters and metabolite levels, only metabolite levels, and metabolite levels under the WW condition explaining the 100% variance in grain yield. The results of the same analysis for 2011 are shown in Supplemental Table S8. Agronomic traits are shown in boldface. SEE, se of the estimate.
| Treatment | All Parameters | Only Metabolites | Metabolites in the WW Condition |
|---|---|---|---|
| WW | Galactinol, Pro, octadecanoate, raffinose, xylitol, Thr, succinate, Phe, myoinositol, erythritol, glycerol, anthesis silking interval, itaconate, urea, silking date | Galactinol, Pro, octadecanoate, raffinose, xylitol, Thr, succinate, Phe, myoinositol, erythritol, glycerol, itaconate, glycerate, trehalose, Asp | Galactinol, Pro, octadecanoate, raffinose, xylitol, Thr, succinate, Phe, myoinositol, erythritol, glycerol, itaconate, glycerate, trehalose, Asp |
| SEE | 0.84 | 0.84 | 0.84 |
| DS | Galactinol, urea, octadecanoate, maltitol, glycerol-3-P, benzoate, putrescine | Galactinol, urea, octadecanoate, maltitol, glycerol-3-P, benzoate, putrescine | Galactinol, Pro, octadecanoate, raffinose, xylitol, Thr, succinate, Phe, myoinositol, erythritol, glycerol, itaconate, glycerate, trehalose, Asp |
| SEE | 0.59 | 0.59 | 0.65 |
| HS | Galactinol, xylitol, itaconate, Ala, anthesis date, Ser, urea, GABA, Tyr, Ile | Galactinol, xylitol, itaconate, Ala, raffinose, Ser, urea, GABA, Trp, Ile | Galactinol, glycerate, phosphate, Pro, erythritol, nicotinate, itaconate, glycerol, trehalose, Trp, succinate, xylitol, octadecanoate |
| SEE | 0.83 | 0.83 | 1.45 |
| DS+HS | Galactinol, trehalose, threonate, maltitol, glycerate, plant height, Fru, malate, Trp, β-Ala, urea | Galactinol, trehalose, threonate, maltitol, glycerate, nicotinate, Fru, malate, benzoate, phosphate, urea | Galactinol, Ala, raffinose, octadecanoate, succinate, myoinositol, Phe, Pro, xylitol, pyro-Glu, phosphate, urea |
| SEE | 0.37 | 0.37 | 0.40 |
DISCUSSION
Comparison of DS Responses in Field and Greenhouse Experiments
Large-scale metabolite analyses under stress conditions in the field remain rare. To our best knowledge, this is the first study reporting metabolic effects of simultaneous abiotic stresses in field-grown plants. HS was applied by altering the planting date to ensure that the reproductive phase coincided with high temperatures (Craufurd et al., 2013). Despite limitations in fine climate control, large-scale field trials are still valuable, since it is often reported that important agronomical traits are masked in greenhouse-grown crops (Alexandersson et al., 2014). In our previous study in controlled greenhouse conditions, genotypes chosen to cover a wide range of DS tolerance based on field results did not display differential effects of DS on physiological traits (Witt et al., 2012). Further field studies showed that these contrasting genotypes demonstrate differential physiological responses to DS (Cairns et al., 2012a, 2013). Additionally, in this study, genotypes showed differential physiological responses to DS, albeit all six genotypes tested in the greenhouse experiment were also included and the other four were selected by the same criteria. These results reaffirmed the importance of conducting field experiments to understand the effects of abiotic stresses on crops. On the other hand, some metabolic responses were shared in both greenhouse and field trials. The accumulation of amino acids, including Ile, Val, Thr, and GABA, is a metabolic response common in many abiotic stress environments in Arabidopsis (Obata and Fernie, 2012). These metabolites were also accumulated in maize in both greenhouse and field trials under all stress conditions tested, although Thr and GABA were not annotated in greenhouse samples (Sicher and Barnaby, 2012; Witt et al., 2012; Barnaby et al., 2013). Other amino acids, such as Pro, Phe, and Trp, also accumulated under DS in both conditions (Sicher and Barnaby, 2012; Witt et al., 2012; Barnaby et al., 2013) as well as in Arabidopsis (Urano et al., 2009). The accumulation of these metabolites was much lower in this field study than in the other greenhouse studies (Sicher and Barnaby, 2012; Witt et al., 2012; Barnaby et al., 2013), most likely depending on the severity of DS due to the soil structure and coincident rainfalls. As the accumulation of amino acids under DS has been reported in various plant species (Evers et al., 2010; Degenkolbe et al., 2013; Barchet et al., 2014; Hatzig et al., 2014; Suguiyama et al., 2014), it can be considered as a well-conserved and robust metabolic response to DS in plants. This response might be due to less dilution effect caused by the diminished growth under stress conditions (Génard et al., 2014). However, our field study was performed in fully grown plants and DS was imposed near flowering, which is the most sensitive stage of maize grain production to DS, but leaf expansion had finished at that period. It should also be noted the plant height was not affected significantly by DS in 2010, indicating that the dilution effect played a minor role in amino acid accumulation. Pro is one of many well-known compatible solutes in plants (Hare and Cress, 1997). Branched-chain amino acids (Val, Leu, and Ile) and other amino acids sharing synthetic pathways with branched-chain amino acids (Lys, Thr, and Met) accumulate in various abiotic stress conditions (Obata and Fernie, 2012) and have also been proposed as compatible solutes (Joshi et al., 2010) or alternative electron donors for the respiratory electron transport chain (Araújo et al., 2011), although comparative assessment of these functions under DS conditions remains elusive.
Metabolic Effects of Individual DS and HS
In addition to the metabolites described in the previous paragraph, many metabolites accumulated under DS in both years. Although some of them, including GABA and galactinol, have been suggested to function in abiotic stress tolerance (Fait et al., 2008; Nishizawa et al., 2008), we focus mainly on Gly and Ser here and on myoinositol in a later paragraph. Apart from other amino acids, Gly and Ser are closely related to photorespiration (Bauwe et al., 2010). Even in C4 plants like maize, in which the Rubisco oxygenation reaction should take place at a lower rate than in C3 plants, recent studies have indicated the essentiality of photorespiration for growth under normal air (Zelitch et al., 2009; Maurino and Peterhansel, 2010). Both Gly and Ser were accumulated under DS in this study, suggesting altered photorespiratory flux. This might be related to yield performance under DS, since these metabolites, especially Gly, correlated to grain yield. Photorespiration has actually been proposed to function in protection from photoinhibition under drought, salt, and high-light stresses as a sink of excess reducing equivalent (Wingler et al., 2000) and/or by preventing the excess accumulation of reactive oxygen species (Voss et al., 2013). It has also been shown to contribute to the tolerance to moderate water deficiency in tomato (Solanum lycopersicum) plants by ameliorating nitrogen use efficiency reduced by lower nitrogen assimilation (Sánchez-Rodríguez et al., 2011). It should also be noted that Gly and Ser are principal sources of one carbon unit largely consumed to synthesize an osmoprotectant, Gly betaine, in some plant tissues (Hanson and Roje, 2001). The levels of this osmolyte differ among maize varieties (Brunk et al., 1989) and positively correlate to the degree of salt tolerance (Saneoka et al., 1995). Negative correlations between grain yield in DS and levels of Gly and Ser are possibly related to the levels of consumption of these metabolites to synthesize Gly betaine, leading to the variation of yield performance under drought in maize genotypes. Trehalose is another well-known osmoprotectant in some insects, plants, and yeast, but its accumulation and function are species specific (Iturriaga et al., 2009). The decrease of trehalose under DS in this study suggests a function other than as an osmoprotectant in maize.
Among the metabolites that responded to HS, succinate accumulated and its level negatively correlated to grain yield under severe HS in 2010. This is a novel observation to our knowledge, although the functional background is hardly explained. Since succinate is a metabolite connecting the tricarboxylic acid cycle and the GABA shunt (Fait et al., 2008), the balance between these two pathways might affect succinate level. Interestingly, the GABA shunt-related metabolites, namely GABA and Glu, and the tricarboxylic acid cycle organic acids, malate and fumarate, were increased and decreased under HS, respectively.
Effects of DS+HS
The effects of simultaneous application of drought and heat have been relatively well studied in comparison with other stress combinations, due to its economic impact and increasing risk by global climate change in the near future (Suzuki et al., 2014). There are two studies so far in which metabolite profiles under DS+HS conditions in Arabidopsis were examined with application of relatively mild (Prasch and Sonnewald, 2013) and severe (Rizhsky et al., 2004) HS. In both studies, Pro was accumulated under DS but not in DS+HS (Rizhsky et al., 2004; Prasch and Sonnewald, 2013). This is explained as a consequence of the avoidance of the toxic effect of Pro under HS (Rizhsky et al., 2004). Such clear regulation was not observed in this study, and Pro levels were differentially affected by stresses in the two years. While this might be related to varied environmental conditions between the two years, including stress levels, it is more likely due to different adaptation strategies to DS between maize and Arabidopsis. It is becoming clear that plant species have specific preferences in the selection of compatible solutes to accumulate under stress conditions (Gong et al., 2005; Benina et al., 2013). While Pro is one of the well-known compatible solutes in Arabidopsis (Hare and Cress, 1997), this species accumulates only small amounts of Gly betaine (Missihoun et al., 2011), which has been proven to be involved in stress tolerance in maize (Brunk et al., 1989). Therefore, it is conceivable that the degree of dependence on Pro for DS tolerance and/or cellular Pro concentration is different between Arabidopsis and maize (Spoljarević et al., 2011; Sperdouli and Moustakas, 2012), resulting in the different regulation of Pro level under the DS+HS condition.
Similar to both Arabidopsis studies, only a few metabolites specifically responded to DS+HS in our field maize experiment. Interestingly, most of the metabolic changes in DS+HS were quantitatively predictable from the sum of responses to each single stress, in contrast to transcript responses in Arabidopsis (Rasmussen et al., 2013). In fact, the Arabidopsis metabolite profiling results from milder stress treatments show a similar tendency (Prasch and Sonnewald, 2013), but not in the severe stress experiment (Rizhsky et al., 2004). It is possible that metabolic pathways are regulated to meet the metabolic demands under each stress condition, resulting in an additive metabolite profile under stress combination unless the metabolic network is collapsed by severe stress treatments. Given that the naturally feasible stresses are imposed more mildly than typical stress treatments in greenhouse experiments (Romano et al., 2011; Zia et al., 2013), the general metabolic response in stress combination should be considered as the sum of individual stresses in the field. This is also supported by PCA, in which drought and heat contribute the majority of the variance observed in the metabolic data, with principal component 1 separating DS from the WW condition, principal component 2 separating HS from no HS, and DS+HS being separated from the WW condition in an additive fashion. Another result supporting this argument comes from the correlation analysis using two conditions in which the effect of DS was well conserved regardless of the presence of HS. However, HS treatments were differently affected in the presence or absence of DS, indicating specific effects of HS under stress combination on both metabolite levels and grain yield. This might be due to stomatal closure, which would be anticipated to occur under DS and which would be expected to induce effects of HS on plant metabolism. Indeed, the negative effect of heat on photosynthesis has been reported to be apparent only in the presence of DS in European oak (Quercus robur), tobacco (Nicotiana tabacum), and wheat (Suzuki et al., 2014).
Myoinositol as a Potential Metabolic Marker for Breeding of Drought-Tolerant Maize
One of our main goals was to find metabolic markers useful for the selection of maize genotypes giving better grain yield under abiotic stress conditions. The preferential choice of metabolite levels in the variable selection by multiple linear regression analysis suggests metabolic traits to be promising markers that might behave stronger than classical agronomical yield components for explaining variability in grain yield. Although further validation of the results and efficient methods for screening are required for the actual use of candidate metabolite markers in breeding, new strategies of molecular breeding such as marker-assisted recurrent selection, which require only one cycle of phenotyping and subsequently focus on selection based on genotypic data, potentially open up new avenues for high-cost, low-throughput phenotyping options (Jannink et al., 2010; Bohra, 2013). Selection markers that can be determined in optimal growth conditions are desired because it is very difficult to control stress conditions in the field. Metabolic markers would be a promising target, because the species-specific metabolite profile under nonstress conditions has been recognized to be closely related to stress tolerance and the adaptation strategy of plant species (Benina et al., 2013). Myoinositol is the most promising candidate of a single marker metabolite for yield performance under drought found in this study. It was accumulated, and its level was negatively correlated to grain yield under DS. Additionally, its level in the WW condition was also negatively correlated to grain yield in DS. These results suggest a possibility for marker-assisted breeding to choose maize genotype, raising better grain yield under DS by the selection of a genotype containing less myoinositol in WW leaves. Myoinositol itself is implicated to function as an osmolyte (Kaur et al., 2013), like other sugar alcohols. However, the importance of myoinositol in plant stress tolerance is rather related to its function as a precursor of many metabolites involved in abiotic stress tolerance. Raffinose family oligosaccharides (RFOs), especially raffinose, are ubiquitous in the plant kingdom and contribute to stress tolerance likely by membrane stabilization and antioxidative functions (Van den Ende, 2013). Raffinose is synthesized by adding a Gal residue from galactinol to Suc, and myoinositol is used to synthesize galactinol. Therefore, cellular myoinositol metabolism is closely related to the accumulation of RFOs and, further, to stress tolerance (Elsayed et al., 2014). Actually, galactinol and raffinose were accumulated under DS as myoinositol, although raffinose was reduced in the 2011 season. Galactinol levels exhibited a relationship with the tolerance levels of the genotypes, and the levels of galactinol and raffinose negatively correlated to the grain yield in DS and HS, respectively. Galactinol level was chosen for all models explaining the genotypic variation of grain yield in all growth conditions by multiple linear regression analysis. Additionally, the raffinose level under the WW condition showed correlation to grain yield in DS as well as myoinositol. These observations indicate a close relationship between the metabolism of myoinositol and RFOs and the yield performance of maize under DS. The negative correlation between the levels of these metabolites and grain yield under DS indicates that the yield performance is not due to the osmoprotective functions of these compounds but rather to the metabolism of these compounds. One possible explanation is that the genotypes showing lower accumulation of myoinositol and raffinose synthesize higher degrees of RFOs such as stachyose. The pathways of RFO metabolism reconstructed from genomic information revealed that most of the key enzymes are encoded by multiple gene members with different expression patterns (Zhou et al., 2012), indicating the operation and importance of RFO metabolism in maize. RFOs can also serve as mobile and storage carbon sources with advantages in osmolytic and mobile flexibility over Suc and starch, respectively (Van den Ende, 2013). Therefore, it is also possible that the lower myoinositol and raffinose levels in tolerant genotypes are due to their use of RFOs as carbon sources. Altogether, metabolite profiles from field DS experiments indicate the importance of RFO metabolism in yield performance under drought in field-grown maize, although the mechanism underlying this remains to be investigated.
Possible Relationship between Basal Respiration and Yield Performance under DS+HS
Levels of three tricarboxylic acid cycle-related metabolites in the WW condition showed negative correlation to grain yield in DS+HS. Although this result should be considered with special caution due to the uneven distribution of grain yield among genotypes, this is an interesting observation because these metabolites showed completely different responses against each stress treatment. It might be that the basal operation of the tricarboxylic acid cycle is related to the yield performance under DS+HS and, therefore, that these metabolites can also be used as metabolic markers. The down-regulation of respiratory pathways including the tricarboxylic acid cycle was reported under DS+HS in a previous Arabidopsis study (Prasch and Sonnewald, 2013), supporting this possibility.
It should be noted that the correlation coefficient in this study is relatively low; however, this is most likely due to the fact that the data are highly variable, since they were obtained from field-grown samples where the control of growth conditions is difficult. There are some previously reported correlation analyses in field studies on metabolite levels that corroborate this statement (Robinson et al., 2007; Degenkolbe et al., 2013). That said, the coefficient values of most of the correlations discussed here range from −0.77 to −0.45 with P values lower than 0.05 or 0.001, which can be considered as highly reliable.
Multiple Metabolic Features as Biochemical Markers
The use of multiple metabolites as biochemical markers is another possible way to improve grain yield under stress conditions. Indeed, a strategy of employing multiple markers has been proposed for molecular marker-assisted breeding (Jannink et al., 2010). The results of this study indicate the potentiality of this approach, since the combination of metabolites explained the variation of grain yield very well, especially under stress conditions, in the multiple linear regression models. The metabolic traits showed performance in yield prediction superior to conventional agronomical parameters that have been shown to be correlated to grain yield, suggesting the potential of metabolite profiling in breeding programs. GC-MS-based metabolite profiling is especially promising due to its high-throughput, robust nature and its ability to analyze a wide range of primary metabolites (Obata and Fernie, 2012). We employed multiple linear regression for model establishment in this study, but other regression methods, including multilevel response analyses, the random forest model, and correlation network analyses, should also be useful. This study also indicated the potential of the metabolite levels in the WW condition to predict grain yield under stress conditions. Interestingly, galactinol was selected in all models as the variable contributing to yield prediction. As described above, galactinol functions as a galactosyl donor especially for the synthesis of RFOs, including raffinose and stachyose (Loewus and Murthy, 2000). The contribution of galactinol in yield explanation emphasizes the importance of RFO metabolism in grain yield performance.
CONCLUSION
The metabolite profiles of maize leaves from field DS, HS, and DS+HS trials were analyzed in this study. The metabolite profiling study using field samples is still rare, and to our knowledge this is the first study reporting metabolite responses to a stress combination in field-grown crops, making our results a good reference for future studies. One of the interesting findings is that the metabolic responses to DS+HS were rather the sum of the effects of two individual stresses than novel or divergent effects. This is likely due to the progressive nature of field stress treatments and needs to be considered as a general trend under field conditions. In contrast to the phenotypic and transcriptomic profiles monitored in previous studies (Witt et al., 2012; Alexandersson et al., 2014), some typical stress responses of primary metabolism in field-grown plants are fairly similar to those of greenhouse-grown plants and seem well conserved between growth conditions and even among species. This robustness of metabolic change renders it a good candidate for marker-assisted breeding. The metabolite profiling of field stress samples successfully identified metabolite signatures closely related to grain yield under abiotic stress conditions. It highlights the importance of photorespiration and RFO metabolism for yield performance under DS. Especially myoinositol and RFO levels are quite promising metabolic markers for maize breeding, since those in the WW condition were correlated to grain yield in DS, allowing selection under normal growth conditions. There are some conventional HPLC-based methods available to analyze myoinositol and RFOs, and the recent optimization of high-performance anion-exchange chromatography coupled with pulsed amperometric detection would allow higher throughput analysis for biochemical marker-assisted breeding (Gangola et al., 2014). Additionally, multiple linear regression analysis suggested the possible interplay between metabolic pathways in stress tolerance and the potential use of multiple metabolic markers for yield prediction. Further trials should be conducted to confirm the relationship between these metabolic traits and yield performance under stress and to test the effectiveness of metabolites for biochemical maker-assisted breeding.
MATERIALS AND METHODS
Plant Materials and Experimental Conditions
Ten maize (Zea mays) lines were chosen based on their contrasting responses to DS and DS+HS (Cairns et al., 2013; Table I). Single cross hybrids were generated by crossing lines with the tropical tester CML-539.
Experiments were conducted at the CIMMYT experimental station in Tlaltizapán, Mexico (18°41′N, 99°07′W, 940 m above sea level). A total of four experiments were planted each year composed of two different water and temperature regimes. Optimal temperature experiments were planted at the end of the wet season (late November), and higher temperature experiments were planted at the start of the dry season (mid-February). Due to the low latitude of the experimental station, this experimental design facilitated the application of different temperature conditions without large effects on daylength and irradiation. Two different water treatments were used at each temperature regime: a WW control and anthesis stage DS. DS was imposed by stopping irrigation before flowering to ensure stress at anthesis. In 2010, trials under the WW condition, DS, HS, and DS+HS received 1,037, 520, 790, and 576 mm of irrigation, respectively. In 2011, trials under the WW condition, DS, HS, and DS+HS received 1,151, 550, 639, and 600 mm of irrigation, respectively. Rainfall and temperature data during the experiments are presented in Table II. Experiments were planted in two-row plots, with a final plant density of 6.67 plants m−2. An α-lattice design was used, replicated two times. All plots received 80 kg nitrogen ha−1 (as urea) and 80 kg phosphorus ha−1 [as triple calcium superphosphate; Ca(H2PO4)·2H2O] at sowing. A second application of nitrogen (80 kg ha−1) was applied 5 weeks after sowing (V6 stage; Ritchie et al., 1998). Recommended plant, weed, and insect control measures were used.
Field Measurements
Days to anthesis and silking were recorded when 50% of the plants had shed pollen and 50% of the plants had silks, respectively. The anthesis silking interval was calculated as days to silking minus days to anthesis. At physiological maturity, plant height was measured on two representative plants per plot, then all plants were hand harvested and grain yield was measured. Grain weights were adjusted to 12.5% moisture content.
Metabolite Profiling
Metabolite profiling was performed as described by Witt et al. (2012). Leaf blades were harvested 2 weeks after the water stress was applied at the flowering stage. Samples were collected from six individual plants from each plot and treated as biological replicates. Materials were snap frozen in liquid nitrogen and lyophilized for 1 week. One hundred milligrams of lyophilized powder was used for GC-MS-based metabolite profiling following the protocol of Lisec et al. (2006). Peaks were manually annotated, and ion intensity was determined by the aid of TagFinder (Luedemann et al., 2012) using a reference library derived from the Golm Metabolome Database (Kopka et al., 2005). The parameters used for peak annotation are shown in Supplemental Table S9 following the recommended reporting format (Fernie et al., 2011).
Data Analysis
Metabolite levels were represented by the observed ion intensity of a selected unique ion. Ion intensity was log10 transformed and normalized using an ANOVA-based model for the removal of measurement bias (Lisec et al., 2011). The normalized data set used for the analysis is shown in Supplemental Table S10.
Statistical analyses and graphical representations (ANOVA, Tukey’s honestly significant difference test, heat map, hierarchical clustering, Bonferroni correction, PCA, box plot, Venn diagram, correlation analysis, and Student’s t test) were performed using the R software environment 3.1.1 (http://cran.r-project.org/). The box plots were drawn by the ggplot function in the ggplot2 package. PCA was conducted after paretonormalization using the rnipals-algorithm of the pcaMethods package (Stacklies et al., 2007). ANOVA was conducted using the anova function on linear models incorporating either genotype and treatment or HS and DS status as factors and allowing interactions. The resulting P values were corrected for multiple testing using the stringent Bonferroni method. Tukey’s honestly significant difference test was conducted by the glht function in the multcomp package. Correlation was tested by the cor.test function, and scatterplots were drawn by the ggplot function. Venn diagram was drawn using the venn.diagram function in the VennDiagram package. The effects of stress combination on metabolite levels were predicted from responses to single stresses as follows. Response factors were calculated by dividing metabolite levels transformed to linear values and divided by those from WW plants grown in the same field replicate followed by log2 transformation. Predicted response factors in the DS+HS condition are the sum of those in the DS and HS conditions. The values calculated from each genotype and field were considered as replicates (n = 20) and used for the calculation of the mean and se. Correlations between actual and predicted response factors were tested by the cor.test function in R. Furthermore, the metabolic and agronomic data set was subjected to multiple linear regression analysis to ascertain which combination of metabolites alone or metabolites plus agronomic traits fully explained differences in grain yield across the whole set of genotypes and replications within each growing condition. Models were selected using the linear regression function of SPSS (SPSS) with the Entre method to gain an r2 value approaching 1. Multiple linear regression analyses were also performed using the levels of metabolites under the WW condition as independent variables and grain yield under the three different stress conditions as dependent variables.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Yield related parameters of individual genotypes in the 2 years of field stress trials.
Supplemental Figure S2. PCA of metabolite profiles under field stress conditions.
Supplemental Figure S3. Foliar levels of all detected metabolites in the 2 years of field stress trials.
Supplemental Figure S4. Number of metabolites that altered its level by either heat, drought, or simultaneous heat and drought treatments.
Supplemental Figure S5. Clustering of metabolites according to the responses to drought, heat, and simultaneous drought and heat treatments.
Supplemental Table S1. Effect of treatments on yield parameters of the subsets of maize genotypes grouped by stress tolerance.
Supplemental Table S2. Effects of treatments and genotypes on the yield parameters.
Supplemental Table S3. Genotype and treatment effects on metabolite levels in each year.
Supplemental Table S4. Effect of treatments on metabolite levels of the subsets of maize genotypes grouped by stress tolerance.
Supplemental Table S5. Actual and predicted metabolic responses of maize leaves to drought, heat, and simultaneous drought and heat stresses in 2011.
Supplemental Table S6. Correlation between grain yield and metabolite levels under each growth condition in each year.
Supplemental Table S7. Correlation between grain yield and metabolite levels under two growth conditions in 2011.
Supplemental Table S8. The set of variables selected by multiregression analysis to explain the 100% variance in grain yield under each growth condition in 2011.
Supplemental Table S9. Parameters used for peak annotation in GC-MS analysis.
Supplemental Table S10. Normalized data set used for statistical analysis.
Acknowledgments
We thank Ciro Sanchez for coordinating the field experiments in Mexico.
Glossary
- CIMMYT
International Maize and Wheat Improvement Center
- DS
drought stress
- HS
heat stress
- DS+HS
combined drought and heat stresses
- GC-MS
gas chromatography-mass spectrometry
- GABA
4-aminobutanoate
- PCA
principal component analysis
- WW
well-watered
- RFO
raffinose family oligosaccharide
Footnotes
This work was supported by the European Union FP7 Coordination and Support Action OPTICHINA (grant no. FP7–KBBE 26604 to T.O., S.W., N.P.-R., J.L.A., J.E.C., and A.R.F.), the European Union integrative project 3TO4 (grant no. FP7–KBBE 289582 to T.O. and A.R.F.), the Max Planck Society (to T.O. and A.R.F.), and the Alexander von Humboldt Foundation (to I.F.-S.).
Articles can be viewed without a subscription.
References
- Alexandersson E, Jacobson D, Vivier MA, Weckwerth W, Andreasson E (2014) Field-omics: understanding large-scale molecular data from field crops. Front Plant Sci 5: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo WL, Tohge T, Ishizaki K, Leaver CJ, Fernie AR (2011) Protein degradation: an alternative respiratory substrate for stressed plants. Trends Plant Sci 16: 489–498 [DOI] [PubMed] [Google Scholar]
- Araus JL, Cairns JE (2014) Field high-throughput phenotyping: the new crop breeding frontier. Trends Plant Sci 19: 52–61 [DOI] [PubMed] [Google Scholar]
- Araus JL, Serret MD, Edmeades GO (2012) Phenotyping maize for adaptation to drought. Front Physiol 3: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus JL, Slafer GA, Royo C, Serret MD (2008) Breeding for yield potential and stress adaptation in cereals. CRC Crit Rev Plant Sci 27: 377–412 [Google Scholar]
- Bänziger M, Araus J (2007) Recent advances in breeding maize for drought and salinity stress tolerance. In Jenks MA, Hasegawa PM, Jain SM, eds, Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops. Springer, Dordrecht, The Netherlands, pp 587–601 [Google Scholar]
- Bänziger M, Setimela PS, Hodson D, Vivek B (2006) Breeding for improved abiotic stress tolerance in maize adapted to southern Africa. Agric Water Manage 80: 212–224 [Google Scholar]
- Barchet GLH, Dauwe R, Guy RD, Schroeder WR, Soolanayakanahally RY, Campbell MM, Mansfield SD (2014) Investigating the drought-stress response of hybrid poplar genotypes by metabolite profiling. Tree Physiol 34: 1203–1219 [DOI] [PubMed] [Google Scholar]
- Barnaby JY, Kim M, Bauchan G, Bunce J, Reddy V, Sicher RC (2013) Drought responses of foliar metabolites in three maize hybrids differing in water stress tolerance. PLoS One 8: e77145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwe H, Hagemann M, Fernie AR (2010) Photorespiration: players, partners and origin. Trends Plant Sci 15: 330–336 [DOI] [PubMed] [Google Scholar]
- Benina M, Obata T, Mehterov N, Ivanov I, Petrov V, Toneva V, Fernie AR, Gechev TS (2013) Comparative metabolic profiling of Haberlea rhodopensis, Thellungiella halophyla, and Arabidopsis thaliana exposed to low temperature. Front Plant Sci 4: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyene Y, Semagn K, Mugo S, Tarekegne A, Babu R, Meisel B, Sehabiague P, Makumbi D, Magorokosho C, Oikeh S, et al. (2015) Genetic gains in grain yield through genomic selection in eight bi-parental maize populations under drought stress. Crop Sci 55: 154–163 [Google Scholar]
- Bohra A. (2013) Emerging paradigms in genomics-based crop improvement. ScientificWorldJournal 2013: 585467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A, Scossa F, Bolger ME, Lanz C, Maumus F, Tohge T, Quesneville H, Alseekh S, Sørensen I, Lichtenstein G, et al. (2014) The genome of the stress-tolerant wild tomato species Solanum pennellii. Nat Genet 46: 1034–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowne JB, Erwin TA, Juttner J, Schnurbusch T, Langridge P, Bacic A, Roessner U (2012) Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol Plant 5: 418–429 [DOI] [PubMed] [Google Scholar]
- Bruce WB, Edmeades GO, Barker TC (2002) Molecular and physiological approaches to maize improvement for drought tolerance. J Exp Bot 53: 13–25 [PubMed] [Google Scholar]
- Brunk DG, Rich PJ, Rhodes D (1989) Genotypic variation for glycinebetaine among public inbreds of maize. Plant Physiol 91: 1122–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Bosquet L, Crossa J, von Zitzewitz J, Serret MD, Araus JL (2012) High-throughput phenotyping and genomic selection: the frontiers of crop breeding converge. J Integr Plant Biol 54: 312–320 [DOI] [PubMed] [Google Scholar]
- Cairns JE, Crossa J, Zaidi PH, Grudloyma P, Sanchez C, Araus JL, Thaitad S, Makumbi D, Magorokosho C, Bänziger M, et al. (2013) Identification of drought, heat, and combined drought and heat tolerant donors in maize. Crop Sci 53: 1335–1346 [Google Scholar]
- Cairns JE, Sanchez C, Vargas M, Ordoñez R, Araus JL (2012a) Dissecting maize productivity: ideotypes associated with grain yield under drought stress and well-watered conditions. J Integr Plant Biol 54: 1007–1020 [DOI] [PubMed] [Google Scholar]
- Cairns JE, Sonder K, Zaidi PH, Verhulst N, Mahuku G, Babu R, Nair SK, Das B, Govaerts B, Vinayan MT, et al. (2012b) Maize production in a changing climate: impacts, adaptation, and mitigation strategies. Adv Agron 114: 1–58 [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot (Lond) 103: 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M, Messina CD, Podlich D, Totir LR, Baumgarten A, Hausmann NJ, Wright D, Graham G (2014) Predicting the future of plant breeding: complementing empirical evaluation with genetic prediction. Crop Pasture Sci 65: 311–336 [Google Scholar]
- Craufurd PQ, Vadez V, Jagadish SVK, Prasad PVV, Zaman-Allah M (2013) Crop science experiments designed to inform crop modeling. Agric Meteorol 170: 8–18 [Google Scholar]
- Degenkolbe T, Do PT, Kopka J, Zuther E, Hincha DK, Köhl KI (2013) Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS One 8: e63637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling W, Aggarwal P, Batima P, Brander K, Erda L, Howden M, Kirilenko A, Morton J, Soussana J, Schmidhuber J, et al. (2007) Food, fibre and forest products. In Parry M, Canziani O, Palutikof J, van der Lindin P, Hanson C, eds, Climate Change 2007: Impacts, Adaptation, and Vulnerability. Cambridge University Press, Cambridge, UK, pp 273–313 [Google Scholar]
- Elsayed AI, Rafudeen MS, Golldack D (2014) Physiological aspects of raffinose family oligosaccharides in plants: protection against abiotic stress. Plant Biol (Stuttg) 16: 1–8 [DOI] [PubMed] [Google Scholar]
- Evers D, Lefèvre I, Legay S, Lamoureux D, Hausman JF, Rosales ROG, Marca LRT, Hoffmann L, Bonierbale M, Schafleitner R (2010) Identification of drought-responsive compounds in potato through a combined transcriptomic and targeted metabolite approach. J Exp Bot 61: 2327–2343 [DOI] [PubMed] [Google Scholar]
- Fait A, Fromm H, Walter D, Galili G, Fernie AR (2008) Highway or byway: the metabolic role of the GABA shunt in plants. Trends Plant Sci 13: 14–19 [DOI] [PubMed] [Google Scholar]
- FAO (2014) FAO Statistical Database. http://faostat3.fao.org/ (July 24, 2015)
- Fernie AR, Aharoni A, Willmitzer L, Stitt M, Tohge T, Kopka J, Carroll AJ, Saito K, Fraser PD, DeLuca V (2011) Recommendations for reporting metabolite data. Plant Cell 23: 2477–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Schauer N (2009) Metabolomics-assisted breeding: a viable option for crop improvement? Trends Genet 25: 39–48 [DOI] [PubMed] [Google Scholar]
- Gangola MP, Jaiswal S, Khedikar YP, Chibbar RN (2014) A reliable and rapid method for soluble sugars and RFO analysis in chickpea using HPAEC-PAD and its comparison with HPLC-RI. Food Chem 154: 127–133 [DOI] [PubMed] [Google Scholar]
- Génard M, Baldazzi V, Gibon Y (2014) Metabolic studies in plant organs: don’t forget dilution by growth. Front Plant Sci 5: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud E, Ho LHM, Clifton R, Carroll A, Estavillo G, Tan YF, Howell KA, Ivanova A, Pogson BJ, Millar AH, et al. (2008) The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol 147: 595–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Li P, Ma S, Rupassara SI, Bohnert HJ (2005) Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J 44: 826–839 [DOI] [PubMed] [Google Scholar]
- Grant RF, Jackson BS, Kiniry JR, Arkin GF (1989) Water deficit timing effects on yield components in maize. Agron J 81: 61–65 [Google Scholar]
- Hanson AD, Roje S (2001) One-carbon metabolism in higher plants. Annu Rev Plant Physiol Plant Mol Biol 52: 119–137 [DOI] [PubMed] [Google Scholar]
- Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21: 79–102 [Google Scholar]
- Hatzig S, Zaharia LI, Abrams S, Hohmann M, Legoahec L, Bouchereau A, Nesi N, Snowdon RJ (2014) Early osmotic adjustment responses in drought-resistant and drought-sensitive oilseed rape. J Integr Plant Biol 56: 797–809 [DOI] [PubMed] [Google Scholar]
- Hill CB, Taylor JD, Edwards J, Mather D, Langridge P, Bacic A, Roessner U (2015) Detection of QTL for metabolic and agronomic traits in wheat with adjustments for variation at genetic loci that affect plant phenology. Plant Sci 233: 143–154 [DOI] [PubMed] [Google Scholar]
- Iturriaga G, Suárez R, Nova-Franco B (2009) Trehalose metabolism: from osmoprotection to signaling. Int J Mol Sci 10: 3793–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannink JL, Lorenz AJ, Iwata H (2010) Genomic selection in plant breeding: from theory to practice. Brief Funct Genomics 9: 166–177 [DOI] [PubMed] [Google Scholar]
- Jones RJ, Simmons SR (1983) Effect of altered source-sink ratio on growth of maize kernels. Crop Sci 23: 129–134 [Google Scholar]
- Joshi V, Joung JG, Fei Z, Jander G (2010) Interdependence of threonine, methionine and isoleucine metabolism in plants: accumulation and transcriptional regulation under abiotic stress. Amino Acids 39: 933–947 [DOI] [PubMed] [Google Scholar]
- Kaur H, Verma P, Petla BP, Rao V, Saxena SC, Majee M (2013) Ectopic expression of the ABA-inducible dehydration-responsive chickpea L-myo-inositol 1-phosphate synthase 2 (CaMIPS2) in Arabidopsis enhances tolerance to salinity and dehydration stress. Planta 237: 321–335 [DOI] [PubMed] [Google Scholar]
- Keleş Y, Öncel I (2002) Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci 163: 783–790 [Google Scholar]
- Khodarahmpour Z, Hamidi J (2011) Evaluation of drought tolerance in different growth stages of maize (Zea mays L.) inbred lines using tolerance indices. Afr J Biotechnol 10: 13482–13490 [Google Scholar]
- Kopka J, Schauer N, Krueger S, Birkemeyer C, Usadel B, Bergmüller E, Dörmann P, Weckwerth W, Gibon Y, Stitt M, et al. (2005) GMD@CSB.DB: the Golm Metabolome Database. Bioinformatics 21: 1635–1638 [DOI] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W (2009) Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann Bot (Lond) 103: 561–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisec J, Römisch-Margl L, Nikoloski Z, Piepho HP, Giavalisco P, Selbig J, Gierl A, Willmitzer L (2011) Corn hybrids display lower metabolite variability and complex metabolite inheritance patterns. Plant J 68: 326–336 [DOI] [PubMed] [Google Scholar]
- Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1: 387–396 [DOI] [PubMed] [Google Scholar]
- Lobell DB, Bänziger M, Magorokosho C, Vivek B (2011) Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat Clim Chang 1: 42–45 [Google Scholar]
- Lobell DB, Burke MB (2010) On the use of statistical models to predict crop yield responses to climate change. Agric Meteorol 150: 1443–1452 [Google Scholar]
- Loewus F, Murthy PPN (2000) myo-Inositol metabolism in plants. Plant Sci 150: 1–19 [Google Scholar]
- Lopes MS, Araus JL, van Heerden PDR, Foyer CH (2011) Enhancing drought tolerance in C(4) crops. J Exp Bot 62: 3135–3153 [DOI] [PubMed] [Google Scholar]
- Luedemann A, von Malotky L, Erban A, Kopka J (2012) TagFinder: preprocessing software for the fingerprinting and the profiling of gas chromatography-mass spectrometry based metabolome analyses. Methods Mol Biol 860: 255–286 [DOI] [PubMed] [Google Scholar]
- Ma T, Wang J, Zhou G, Yue Z, Hu Q, Chen Y, Liu B, Qiu Q, Wang Z, Zhang J, et al. (2013) Genomic insights into salt adaptation in a desert poplar. Nat Commun 4: 2797. [DOI] [PubMed] [Google Scholar]
- Maurino VG, Peterhansel C (2010) Photorespiration: current status and approaches for metabolic engineering. Curr Opin Plant Biol 13: 249–256 [DOI] [PubMed] [Google Scholar]
- Missihoun TD, Schmitz J, Klug R, Kirch HH, Bartels D (2011) Betaine aldehyde dehydrogenase genes from Arabidopsis with different sub-cellular localization affect stress responses. Planta 233: 369–382 [DOI] [PubMed] [Google Scholar]
- Müller C, Cramer W, Hare WL, Lotze-Campen H (2011) Climate change risks for African agriculture. Proc Natl Acad Sci USA 108: 4313–4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa A, Yabuta Y, Shigeoka S (2008) Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol 147: 1251–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Fernie AR (2012) The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol Life Sci 69: 3225–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2014) ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol 202: 35–49 [DOI] [PubMed] [Google Scholar]
- Prasanna BM, Araus JL, Crossa J, Cairns JE (2013) High-throughput and precision phenotyping for cereal breeding programs. In Gupta PK, Varshney RK, eds, Cereal Genomics II. Springer, Dordrecht, The Netherlands, pp 341–374 [Google Scholar]
- Prasch CM, Sonnewald U (2013) Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol 162: 1849–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S, Barah P, Suarez-Rodriguez MC, Bressendorff S, Friis P, Costantino P, Bones AM, Nielsen HB, Mundy J (2013) Transcriptome responses to combinations of stresses in Arabidopsis. Plant Physiol 161: 1783–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redestig H, Kusano M, Ebana K, Kobayashi M, Oikawa A, Okazaki Y, Matsuda F, Arita M, Fujita N, Saito K (2011) Exploring molecular backgrounds of quality traits in rice by predictive models based on high-coverage metabolomics. BMC Syst Biol 5: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedelsheimer C, Czedik-Eysenberg A, Grieder C, Lisec J, Technow F, Sulpice R, Altmann T, Stitt M, Willmitzer L, Melchinger AE (2012a) Genomic and metabolic prediction of complex heterotic traits in hybrid maize. Nat Genet 44: 217–220 [DOI] [PubMed] [Google Scholar]
- Riedelsheimer C, Lisec J, Czedik-Eysenberg A, Sulpice R, Flis A, Grieder C, Altmann T, Stitt M, Willmitzer L, Melchinger AE (2012b) Genome-wide association mapping of leaf metabolic profiles for dissecting complex traits in maize. Proc Natl Acad Sci USA 109: 8872–8877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J, Singh U, Godwin D, Bowen W (1998) Cereal growth, development and yield. In Tsuji GY, Hoogenboom G, Thornton PK, eds, Understanding Options for Agricultural Production. Springer, Dordrecht, The Netherlands, pp 79–98 [Google Scholar]
- Rizhsky L, Liang H, Mittler R (2002) The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130: 1143–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide: the response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134: 1683–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AR, Ukrainetz NK, Kang KY, Mansfield SD (2007) Metabolite profiling of Douglas-fir (Pseudotsuga menziesii) field trials reveals strong environmental and weak genetic variation. New Phytol 174: 762–773 [DOI] [PubMed] [Google Scholar]
- Romano G, Zia S, Spreer W, Sanchez C, Cairns J, Araus JL, Müller J (2011) Use of thermography for high throughput phenotyping of tropical maize adaptation in water stress. Comput Electron Agric 79: 67–74 [Google Scholar]
- Sanchez DH, Pieckenstain FL, Escaray F, Erban A, Kraemer U, Udvardi MK, Kopka J (2011) Comparative ionomics and metabolomics in extremophile and glycophytic Lotus species under salt stress challenge the metabolic pre-adaptation hypothesis. Plant Cell Environ 34: 605–617 [DOI] [PubMed] [Google Scholar]
- Sánchez-Rodríguez E, Rubio-Wilhelmi MdelM, Ríos JJ, Blasco B, Rosales MÁ, Melgarejo R, Romero L, Ruiz JM (2011) Ammonia production and assimilation: its importance as a tolerance mechanism during moderate water deficit in tomato plants. J Plant Physiol 168: 816–823 [DOI] [PubMed] [Google Scholar]
- Saneoka H, Nagasaka C, Hahn DT, Yang WJ, Premachandra GS, Joly RJ, Rhodes D (1995) Salt tolerance of glycinebetaine-deficient and -containing maize lines. Plant Physiol 107: 631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangoi L, Salvador RJ (1998) Maize susceptibility to drought at flowering: a new approach to overcome the problem. Cienc Rural 28: 699–706 [Google Scholar]
- Sicher RC, Barnaby JY (2012) Impact of carbon dioxide enrichment on the responses of maize leaf transcripts and metabolites to water stress. Physiol Plant 144: 238–253 [DOI] [PubMed] [Google Scholar]
- Skirycz A, De Bodt S, Obata T, De Clercq I, Claeys H, De Rycke R, Andriankaja M, Van Aken O, Van Breusegem F, Fernie AR, et al. (2010) Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress. Plant Physiol 152: 226–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirycz A, Inzé D (2010) More from less: plant growth under limited water. Curr Opin Biotechnol 21: 197–203 [DOI] [PubMed] [Google Scholar]
- Skirycz A, Vandenbroucke K, Clauw P, Maleux K, De Meyer B, Dhondt S, Pucci A, Gonzalez N, Hoeberichts F, Tognetti VB, et al. (2011) Survival and growth of Arabidopsis plants given limited water are not equal. Nat Biotechnol 29: 212–214 [DOI] [PubMed] [Google Scholar]
- Sperdouli I, Moustakas M (2012) Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J Plant Physiol 169: 577–585 [DOI] [PubMed] [Google Scholar]
- Spoljarević M, Agić D, Lisjak M, Gumze A, Wilson ID, Hancock JT, Teklić T (2011) The relationship of proline content and metabolism on the productivity of maize plants. Plant Signal Behav 6: 251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacklies W, Redestig H, Scholz M, Walther D, Selbig J (2007) pcaMethods: a Bioconductor package providing PCA methods for incomplete data. Bioinformatics 23: 1164–1167 [DOI] [PubMed] [Google Scholar]
- Suguiyama VF, Silva EA, Meirelles ST, Centeno DC, Braga MR (2014) Leaf metabolite profile of the Brazilian resurrection plant Barbacenia purpurea Hook. (Velloziaceae) shows two time-dependent responses during desiccation and recovering. Front Plant Sci 5: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 203: 32–43 [DOI] [PubMed] [Google Scholar]
- Tai HH, Worrall K, Pelletier Y, De Koeyer D, Calhoun LA (2014) Comparative metabolite profiling of Solanum tuberosum against six wild Solanum species with Colorado potato beetle resistance. J Agric Food Chem 62: 9043–9055 [DOI] [PubMed] [Google Scholar]
- Takeda S, Matsuoka M (2008) Genetic approaches to crop improvement: responding to environmental and population changes. Nat Rev Genet 9: 444–457 [DOI] [PubMed] [Google Scholar]
- Tardieu F. (2012) Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario. J Exp Bot 63: 25–31 [DOI] [PubMed] [Google Scholar]
- Tohge T, de Souza LP, Fernie AR (2014) Genome-enabled plant metabolomics. J Chromatogr B Analyt Technol Biomed Life Sci 966: 7–20 [DOI] [PubMed] [Google Scholar]
- Tollenaar M, Lee EA (2006) Dissection of physiological processes underlying grain yield in maize by examining genetic improvement and heterosis. Maydica 51: 399–408 [Google Scholar]
- Tsonev S, Todorovska E, Avramova V, Kolev S, Abu-Mhadi N, Christov NK (2014) Genomics assisted improvement of drought tolerance in maize: QTL approaches. Biotechnology and Biotechnological Equipment 23: 1410–1413 [Google Scholar]
- Tuberosa R, Salvi S (2006) Genomics-based approaches to improve drought tolerance of crops. Trends Plant Sci 11: 405–412 [DOI] [PubMed] [Google Scholar]
- Urano K, Kurihara Y, Seki M, Shinozaki K (2010) ‘Omics’ analyses of regulatory networks in plant abiotic stress responses. Curr Opin Plant Biol 13: 132–138 [DOI] [PubMed] [Google Scholar]
- Urano K, Maruyama K, Ogata Y, Morishita Y, Takeda M, Sakurai N, Suzuki H, Saito K, Shibata D, Kobayashi M, et al. (2009) Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J 57: 1065–1078 [DOI] [PubMed] [Google Scholar]
- Van den Ende W. (2013) Multifunctional fructans and raffinose family oligosaccharides. Front Plant Sci 4: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkest A, Byzova M, Martens C, Willems P, Verwulgen T, Slabbinck B, Rombaut D, Van de Velde J, Vandepoele K, Standaert E, et al. (2015) Selection for improved energy use efficiency and drought tolerance in canola results in distinct transcriptome and epigenome changes. Plant Physiol 168: 1338–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile D, Pervent M, Belluau M, Vasseur F, Bresson J, Muller B, Granier C, Simonneau T (2012) Arabidopsis growth under prolonged high temperature and water deficit: independent or interactive effects? Plant Cell Environ 35: 702–718 [DOI] [PubMed] [Google Scholar]
- Voss I, Sunil B, Scheibe R, Raghavendra AS (2013) Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol (Stuttg) 15: 713–722 [DOI] [PubMed] [Google Scholar]
- Wen W, Araus JL, Shah T, Cairns J, Mahuku G, Bänziger M, Torres JL, Sánchez C, Yan J (2011) Molecular characterization of a diverse maize inbred line collection and its potential utilization for stress tolerance improvement. Crop Sci 51: 2569–2581 [Google Scholar]
- Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond B Biol Sci 355: 1517–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt S, Galicia L, Lisec J, Cairns J, Tiessen A, Araus JL, Palacios-Rojas N, Fernie AR (2012) Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress. Mol Plant 5: 401–417 [DOI] [PubMed] [Google Scholar]
- Wu HJ, Zhang Z, Wang JY, Oh DH, Dassanayake M, Liu B, Huang Q, Sun HX, Xia R, Wu Y, et al. (2012) Insights into salt tolerance from the genome of Thellungiella salsuginea. Proc Natl Acad Sci USA 109: 12219–12224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I, Schultes NP, Peterson RB, Brown P, Brutnell TP (2009) High glycolate oxidase activity is required for survival of maize in normal air. Plant Physiol 149: 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ML, Zhang Q, Zhou M, Sun ZM, Zhu XM, Shao JR, Tang YX, Wu YM (2012) Genome-wide identification of genes involved in raffinose metabolism in maize. Glycobiology 22: 1775–1785 [DOI] [PubMed] [Google Scholar]
- Zia S, Romano G, Spreer W, Sanchez C, Cairns J, Araus JL, Müller J (2013) Infrared thermal imaging as a rapid tool for identifying water-stress tolerant maize genotypes of different phenology. J Agron Crop Sci 199: 75–84 [Google Scholar]