Overexpression or suppression of plastidial disproportionating enzyme affected amylose content, amylopectin structure, and morphological and physicochemical properties of starch granules in rice endosperm.
Abstract
Plastidial disproportionating enzyme1 (DPE1), an α-1,4-d-glucanotransferase, has been thought to be involved in storage starch synthesis in cereal crops. However, the precise function of DPE1 remains to be established. We present here the functional identification of DPE1 in storage starch synthesis in rice (Oryza sativa) by endosperm-specific gene overexpression and suppression. DPE1 overexpression decreased amylose content and resulted in small and tightly packed starch granules, whereas DPE1 suppression increased amylose content and formed heterogeneous-sized, spherical, and loosely packed starch granules. Chains with degree of polymerization (DP) of 6 to 10 and 23 to 38 were increased, while chains with DP of 11 to 22 were decreased in amylopectin from DPE1-overexpressing seeds. By contrast, chains with DP of 6 to 8 and 16 to 36 were decreased, while chains with DP of 9 to 15 were increased in amylopectin from DPE1-suppressed seeds. Changes in DPE1 gene expression also resulted in modifications in the thermal and pasting features of endosperm starch granules. In vitro analyses revealed that recombinant DPE1 can break down amylose into maltooligosaccharides in the presence of Glc, while it can transfer maltooligosyl groups from maltooligosaccharide to amylopectin or transfer maltooligosyl groups within and among amylopectin molecules in the absence of Glc. Moreover, a metabolic flow of maltooligosyl groups from amylose to amylopectin was clearly identifiable when comparing DPE1-overexpressing lines with DPE1-suppressed lines. These findings demonstrate that DPE1 participates substantially in starch synthesis in rice endosperm by transferring maltooligosyl groups from amylose and amylopectin to amylopectin.
Starch is important for plant development and is critical for crop quality and nutrition. The starch accumulated in cereal endosperm is the most important dietary source of energy for humans. Starch consists of two types of d-Glc homopolymers, amylose and amylopectin, accounting for 15% to 30% and 70% to 85%, respectively (Hermansson and Svegmark, 1996; Tester et al., 2004). Amylose is a largely linear polymer, in which glucosyl monomers are joined via α-1,4-glycoside bonds. Amylopectin is a highly branched molecule with 5% to 6% nonrandomly distributed α-1,6-glycoside bonds connecting linear chains of various lengths (Nakamura, 2002; James et al., 2003). The amylopectin structural model maintains it to be a cluster with a polymodal chain length distribution (Robin et al., 1974; Manners and Matheson, 1981; Hizukuri, 1986). The conserved architecture is responsible for the semicrystalline nature of starch granules, in which Glc units are densely packed.
The physicochemical properties of starch are determined largely by the ratio of amylose to amylopectin and the structural features of the amylopectin (Buléon et al., 1998; Nakamura 2002; Fujita et al., 2003). Amylose is synthesized by ADP glucose pyrophosphorylase (AGPase) and granule-bound starch synthase I (GBSSI), whereas amylopectin is synthesized by concerted reactions catalyzed by AGPase, soluble starch synthase (SS), starch-branching enzyme (BE), and starch-debranching enzyme (DBE; Smith et al., 1997; Nakamura, 2002; Ball and Morell, 2003; Tetlow, 2006). AGPase catalyzes the first reaction in starch synthesis, producing the activated glucosyl donor ADP-Glc. GBSSI and SSs act specifically to elongate amylose and amylopectin, respectively. BEs generate α-1,6-glycoside bonds by cleaving internal α-1,4-glycoside bonds and transferring the released reducing ends to C6 hydroxyls, thereby forming a new branched chain. DBEs hydrolyze α-1,6-glycoside bonds and play an essential role in the formation of amylopectin. Additionally, α-glucan phosphorylase (Pho) is involved in storage starch synthesis (Dauvillée et al., 2006; Satoh et al., 2008). In rice (Oryza sativa), the loss of plastidial phosphorylase (Pho1) causes smaller starch granules to accumulate and modifies the amylopectin structure, resulting in abnormal endosperm phenotypes, such as white core, shrunken, and pseudonormal endosperms (Satoh et al., 2008). Pho1 may play an important role in the glucan initiation process by synthesizing glucan primers from short-chain maltooligosaccharides (MOSs; Satoh et al., 2008; Hwang et al., 2010).
Disproportionating enzyme (d-enzyme) is an α-1,4-glucanotransferase that catalyzes the cleavage of α-1,4-glucosidic bonds of glucans, transferring the glucosyl groups to the nonreducing end of another glucan or free Glc and releasing Glc or a glucan chain, depending on the cleavage site. d-enzymes have been identified in potato (Solanum tuberosum; Peat et al., 1956; Jones and Whelan, 1969; Takaha et al., 1993, 1998; Lloyd et al., 2004), Arabidopsis (Arabidopsis thaliana; Lin and Preiss, 1988; Critchley et al., 2001; Chia et al., 2004), barley (Hordeum vulgare; Yoshio et al., 1986), sweet potato (Ipomoea batatas; Suganuma et al., 1991), pea (Pisum sativum; Kakefuda and Duke, 1989), and wheat (Triticum aestivum; Bresolin et al., 2006). Two types of d-enzymes are found in plants, plastid-located disproportionating enzyme1 (DPE1) and cytoplasm-distributed DPE2. DPE1 and DPE2 differ in expression pattern and subcellular localization as well as protein structure and reaction properties. Both are members of the glucoside hydrolase family77 (GH77) and possess a unique C-terminal domain responsible for glucoside hydrolysis in GH77. In DPE2, the C-terminal domain is separated by an insert of 151 amino acids, which explains the differing reaction properties of DPE2 and DPE1. Maltotriose and larger MOSs are preferred substrates for DPE1, whereas maltose appears to be the only effective donor for DPE2. The latter can catalyze the transfer of one Glc unit from maltose to a polysaccharide, thereby releasing the other Glc moiety (Chia et al., 2004; Lloyd et al., 2004). Based on these characteristics, DPE2 has been proposed to be an α-1,4-glucosyltransferase rather than an α-1,4-glucanotransferase (Lloyd et al., 2005; Smith et al., 2005).
The disproportionation reaction catalyzed by d-enzymes does not result in a change in total number of α-1,4-glycoside bonds. However, the reaction does lead to a modification in the composition and size distribution of glucans, potentially functioning in the synthesis or degradation of starch. In potato, suppression of 98% of DPE1 activity did not affect the composition or structure of tuberous starch but delayed tuber sprouting and shoot growth, suggesting that starch is less effectively remobilized (Takaha et al., 1998). In Arabidopsis, deficiency of DPE1 increased the starch accumulation in leaves with a high proportion of amylose but did not affect the structure of amylopectin. During starch degradation in prolonged darkness, a higher proportion of amylose was eliminated, accompanied by a larger accumulation of MOSs in the dpe1 mutant, indicating that DPE1 is involved primarily in the metabolism of MOSs during starch degradation in Arabidopsis leaves (Critchley et al., 2001).
The identification of mutants of Chlamydomonas reinhardtii that specifically lack DPE1 activity resulted in a completely different proposal for a critical role of d-enzyme in starch synthesis. Colleoni et al. (1999a, 1999b) discovered that the sta11 mutant had a reduced starch content, altered starch structure, and high levels of MOSs when grown under limiting nitrogen to promote starch accumulation. They argued that DPE1 acted during starch synthesis to transfer maltooligosyl groups from MOSs onto branch chains of amylopectin and played a role in the determination of chain length of amylopectin. Circumstantial evidence also suggests the involvement of DPE1 in storage starch synthesis in cereal crop endosperm. In rice, the transcriptional level of DPE1 is high in developing endosperm (Ohdan et al., 2005; Akdogan et al., 2011), and in wheat, DPE1 activity and protein level are coordinated with production of endosperm starch (Bresolin et al., 2006).
Rice is a staple crop that provides the most important dietary source of carbohydrates for more than half the world’s population. The rice genome contains at least 27 genes encoding starch-biosynthesizing enzymes: six for AGPase, two for GBSSI, eight for SS, three for BE, four for DBE, two for Pho, and two for d-enzyme (Ohdan et al., 2005). Among them, the functions of the former six groups have been studied intensively; however, little is known about the precise role of DPE1 in starch metabolism in developing rice endosperm, although the expression profile of rice DPE1 and enzymatic properties of recombinant rice DPE1 have been examined (Ohdan et al., 2005; Akdogan et al., 2011). Here, we report the functional characterization of DPE1 in rice storage starch synthesis. Endosperm-specific overexpression or suppression of DPE1 affected the amylose content, starch structure, and morphological and physicochemical properties of starch granules. The activities of other major starch synthesizing enzymes were not changed in DPE1-overexpressed or -suppressed seeds. These results demonstrate that DPE1 has a substantial role in the synthesis of starch in rice endosperm. The mechanism by which DPE1 is involved in rice endosperm starch synthesis was investigated.
RESULTS
High Expression of DPE1 Specifically in Endosperm of Developing Rice Seeds
The transcript of DPE1 was plentiful at 1 d after flowering (DAF), the initiation phase of seed development, then increased rapidly to peak by 5 DAF when starch synthesis in endosperm begins and then decreased promptly to a low level until 9 DAF, finally remaining almost constant through the late-milking stage of endosperm development (≥15 DAF; Fig. 1A). These results were consistent with a previous report (Ohdan et al., 2005). The accumulation of DPE1 in seeds was detected by its activity of releasing Glc from maltotriose (Fig. 1B). With the increase of DPE1 transcripts, the activity of DPE1 increased swiftly from 3 to 7 DAF, then increased further to peak at 13 DAF when endosperm starch and grain dry weight accumulated and then declined gradually during the period between 13 and 17 DAF. Subsequently, the activity of DPE1 was maintained at about the same level as 17 DAF throughout endosperm development.
Figure 1.
Expression of DPE1 and activity of DPE1 in developing rice seeds. A, Expression profile of DPE1. Aliquots of first-strand cDNAs corresponding to 5 ng of total RNA were used as templates for quantitative real-time reverse transcription-PCR. Data are means ± sd from three independent measurements. B, DPE1 activity. The activity was detected by its ability to release Glc from maltotriose and is given as nanomoles per minute per kernel. Data are means ± sd from four independent measurements. C, In situ western-blot analysis of DPE1. D, Western-blot analysis of DPE1.
The expression pattern of DPE1 in seeds was determined by in situ western-blot analysis (Qu et al., 2003). When the seed section was directly subjected to alkaline phosphatase-conjugated horse anti-rabbit IgG, some background signals were detected in the embryo. When the section first reacted with rabbit anti-DPE1 antibody and then with alkaline phosphatase-conjugated horse anti-rabbit IgG, extensive signals were observed in the whole endosperm (Fig. 1C). Thus, DPE1 expression was restricted to the whole endosperm. The endosperm-specific expression pattern of DPE1 was further confirmed by western-blot analysis. DPE1 was detected in the endosperm but not in the embryo (Fig. 1D). These results show that DPE1 is expressed specifically in the rice endosperm at a high level.
Subcellular Localization of DPE1 and Its Association with Endosperm Starch Granules
To investigate the subcellular localization of DPE1, we fused GFP and red fluorescent protein (RFP) to the C terminus of DPE1 (DPE1-GFP) and the transit peptide (the first 73 amino acids) of the small subunit of Rubisco (Rubisco, a marker for chloroplasts; Rubisco-S-RFP), respectively. The fusion proteins were expressed transiently in rice protoplasts. Coexpression of DPE1-GFP with Rubisco-S-RFP showed that the green fluorescence of DPE1-GFP was almost completely merged with red fluorescence of Rubisco-S-RFP (Fig. 2A).
Figure 2.
DPE1 is localized in amyloplasts and associated with starch granules in rice endosperm. A, Subcellular localization of DPE1 in rice protoplasts. B and C, Distributions (B) and proportion (C) of DPE1 in soluble protein (SP), starch granule loosely bound protein (LBP), and starch granule tightly bound protein (TBP) from developing endosperm at 12 DAF. Data are means ± sd from three independent preparations. Bars = 5 μm.
To test whether DPE1 was distributed in amyloplasts in rice endosperm, the soluble, starch granule loosely bound, and starch granule tightly bound proteins were fractionated from developing endosperm at 12 DAF as described (Fujita et al., 2006), and the partitioning of DPE1 in the three fractions was determined by western-blot analysis. DPE1 was present in the soluble and loosely bound fractions but not in the tightly bound fraction (Fig. 2B). DPE1 in the soluble and loosely bound fractions accounted for approximately 56% and 44%, respectively, of the total DPE1 in developing rice endosperm (Fig. 2C). Thus, DPE1 is localized in amyloplasts (Bresolin et al., 2006) and loosely bound to starch granules.
Production of Endosperm-Specific DPE1-Overexpressed and -Suppressed lines
To determine the role of DPE1 in rice endosperm starch synthesis, we generated transgenic rice with DPE1 overexpression or suppression specifically in the endosperm, under the control of the glutelin GluC and GluA-2 promoters, respectively. Successful transformants were confirmed by PCR. Twelve DPE1-overexpressed lines and 25 DPE1-suppressed lines were generated. Transgenic rice lines were self-pollinated through four or five generations. Five homozygous DPE1-overexpressed lines (OX-2, -3, -4, -7, and -10) and five homozygous DPE1-suppressed RNA interference (Ri) lines (Ri-1, -2, -4, -8, and -12) were used for further analysis.
Expression and Distribution of DPE1 in DPE1-Overexpressed and -Suppressed Seeds
The expression level of DPE1 in transgenic seeds was evaluated by western-blot analysis. A unique 66-kD band for DPE1 was found in transgenic lines and the wild type. The expression of DPE1 was significantly decreased in Ri lines versus the wild type (Fig. 3A, left section). By contrast, the expression of DPE1 was markedly higher in OX lines than in the wild type (Fig. 3A, right section).
Figure 3.
Expression and activity of DPE1 in transgenic rice seeds. A, Western-blot analysis of DPE1 in dry seeds. The α-tubulin was a loading control. B, In situ western-blot analysis of DPE1 in seeds at 17 DAF. C, Native-PAGE/activity staining analysis of DPE1 in seeds at 12 DAF. The activity band was excised, and identity with DPE1 was confirmed by liquid chromatography-tandem mass spectrometry analysis. D and E, DPE1 activity for developing endosperm at 10 DAF (D) and dry seeds (E). DPE1 activity was detected by its ability to release Glc from maltotriose and is given as nanomoles per minute per milligram weight of tissue. Data are means ± sd from four independent measurements. Different lowercase letters indicate significant differences, by Duncan’s multiple range test, at P < 0.05. DW, Dry weight; FW, fresh weight; WT, wild type.
The expression pattern of DPE1 driven by the GluC or GluA2 promoter was determined by in situ western-blot analysis (Fig. 3B). DPE1 distribution was restricted to the whole endosperm in OX and Ri lines. The expression of DPE1 was evidently stronger in OX lines while markedly weaker in Ri lines than in the wild type. The expression patterns of DPE1 in OX and Ri lines were consistent with the reported tissue specificity of the GluC and GluA2 promoters (Qu et al., 2008).
Activity of DPE1 in DPE1-Overexpressed and -Suppressed Seeds
To determine whether the DPE1 transgene was functional in transformed plants, DPE1 activity in transgenic rice seeds was first measured by native-PAGE/activity staining analysis (Colleoni et al., 1999b). The resolving gel contained 1% (w/v) oyster glycogen, and the reaction mixture included 2 mm maltoheptaose. Upon staining with iodine, a dark-brown band was observed in OX lines, whereas no visible band was found in the wild-type or Ri lines (Fig. 3C, left section). The brown band was excised, digested with trypsin, and subjected to liquid chromatography-tandem mass spectrometry. The tryptic peptides aligned well with the primary sequence of rice DPE1 (Fig. 3C, right section). No other starch-metabolizing enzyme was detected. Thus, the differences in the coloration of the bands reflected differences in the activity of DPE1 in transgenic rice seeds to transfer maltoheptaose to glycogen.
The activity of DPE1 in transgenic rice seeds was further analyzed by its activity of releasing Glc from maltotriose (Akdogan et al., 2011). The OX and Ri lines tested exhibited significantly higher and lower DPE1 activities than did the wild type, respectively. For developing endosperm at 10 DAF, DPE1 activities were 2.62- and 2.76-fold higher in lines OX-3 and OX-7 and 0.43- and 0.28-fold lower in Ri-4 and Ri-12, respectively, than in the wild type (Fig. 3D). For dry seeds, the activities of DPE1 were 2.45- and 2.66-fold higher in lines OX-3 and OX-7 and 0.41- and 0.30-fold lower in Ri-4 and Ri-12, respectively, than those in the wild type (Fig. 3E).
To examine the possible pleiotropic effects of DPE1 overexpression and suppression on other starch-synthesizing enzymes, zymogram analyses were performed. No significant difference in the activity of SS (SSI and SSIIIa; Fig. 4A), DBE (isoamylase and pullulanase; Fig. 4B), Pho (Pho1 and Pho2; Fig. 4C), BE (BEI, BEIIa, and BEIIb; Fig. 4D), or GBSSI (Fig. 4E) was observed between Ri, OX, and wild-type lines.
Figure 4.
Effects of DPE1 overexpression or suppression on activities of enzymes involved in rice endosperm starch synthesis. A to D, Native-PAGE/activity analysis of isoforms of SSs (SSIIIa and SSI; A), DBEs (ISA and Pul; B), Pho (Pho1 and Pho2; C), and BE (BEI, BEIIa, and BEIIb; D) in transgenic rice seeds at 12 DAF; the activity bands are indicated by arrowheads. The activity band for Pho1 was also detected in B. E, GBSSI activity. GBSSI activity was detected by quantifying the amount of ATP that was converted from ADP and is given as picomoles per minute per milligram fresh weight of developing endosperm at 12 DAF. Data are means ± sd from three independent measurements. Different lowercase letters indicate significant differences, by Duncan’s multiple range test, at P < 0.05. ISA1, Isoamylase1; Pul, pullulanase; WT, wild type.
Morphology and Crystallinity of Starch Granules in DPE1-Overexpressed and -Suppressed Seeds
To test whether the altered DPE1 activity affected the granular structure of starch in endosperm, we observed seed cross sections and purified starch granules by scanning electron microscopy (SEM). In the wild type, starch granules were densely packed in amyloplasts (Fig. 5A), and isolated starch granules were similar in size and had irregular polyhedron shapes with sharp edges (Fig. 5B). In Ri lines, starch granules were loosely packed in amyloplasts (Fig. 5A), and isolated starch granules were heterogeneous; some were larger and more spherical, and some were smaller and more irregularly polyhedron-shaped than wild-type granules (Fig. 5B). In OX lines, starch granules were tightly packed in amyloplasts (Fig. 5A), and isolated starch granules were slightly smaller, with less irregular polyhedron shapes than wild-type granules (Fig. 5B). The average size of starch granules was significantly larger in Ri-12 (4.08 µm) and smaller in OX-7 (3.06 µm) than in the wild type (3.62 µm; Supplemental Fig. S1). Thus, both overexpression and suppression of DPE1 resulted in modifications in starch granule morphology and size.
Figure 5.
SEM of starch granules in transgenic rice seeds. A, Cross-sections of developing seeds at 30 DAF. Letters a and b represent the outer and inner regions of the endosperm, respectively. B, Starch granules from mature seeds of transgenic lines. WT, Wild type. Bars = 0.5 mm (A, top section), 10 μm (A, middle section), 10 μm (A, bottom section) and 5 μm (B).
The x-ray diffraction patterns of endosperm starch granules were examined in lines Ri-12 and OX-7. Starch granules from Ri-12 and OX-7 showed the typical A-type x-ray diffraction pattern of wild-type starch granules (Supplemental Fig. S2). Furthermore, Ri-12, OX-7, and wild-type starch granules did not differ in height or sharpness of the major peaks of the x-ray diffraction spectra. Thus, the three starches shared a similar crystalline structure.
Accumulation of Starch in DPE1-Overexpressed and -Suppressed Seeds
Total starch content and apparent amylose content (AAC) were measured in transgenic rice seeds. Total starch contents in Ri-4 (76.1%), Ri-12 (74.6%), OX-3 (75.0%), and OX-7 (74.4%) were largely comparable with that of the wild type (75.2%; Fig. 6A). The AACs in Ri-4, Ri-12, OX-3, and OX-7 were 16.7%, 18.4%, 10.9%, and 11.9%, respectively, while that of the wild type was 14.8% (Fig. 6B). Thus, the AAC was higher, by 12.8% to 24.3% in Ri lines, and lower, by 19.6% to 26.4% in OX lines, respectively, than in the wild type.
Figure 6.
Amylose content and absorbance spectra of amylose in transgenic rice seeds. A and B, Total starch content (A) and amylose content (B). Data are means ± sd from three biological replicates. Different lowercase letters indicate significant differences, by Duncan’s multiple range test, at P < 0.05. C, Absorbance spectra of amylose isolated from transgenic seeds. WT, Wild type.
Fine Structure of Starch in DPE1-Overexpressed and -Suppressed Seeds
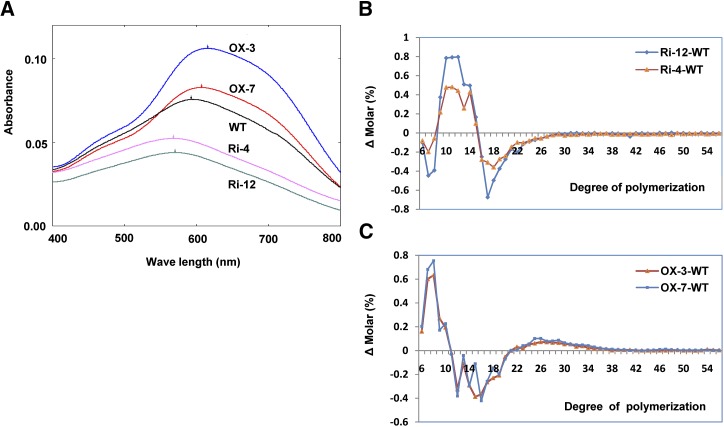
Blue value (BV) and wavelength of maximum absorbance (λmax) are two characteristics of starch representing its ability to combine with iodine. To examine whether changes in DPE1 expression affected the structure of starch, the BV and λmax of amylose and amylopectin were analyzed. The BV and λmax of the amylose-iodine complexes were evidently lower in OX-3 (0.334, 617 nm) and OX-7 (0.465, 616 nm) than in the wild type (0.593, 624 nm; Fig. 6C). Although the λmax of the amylose-iodine complexes in Ri-4 (625 nm) and Ri-12 (625 nm) was similar to that in the wild type (624 nm), the BV was slightly higher in Ri-4 (0.616) and Ri-12 (0.658) than in the wild type (0.593). These results indicate that suppression and overexpression of DPE1 resulted in higher and lower Mrs of amylose, respectively (Hizukuri, 1986).
The BV and λmax of the amylopectin-iodine complexes were evidently lower in Ri-4 (0.036, 568 nm) and Ri-12 (0.029, 570 nm) than in the wild type (0.062, 592 nm), whereas they were distinctly higher in OX-3 (0.096, 615 nm) and OX-7 (0.072, 607 nm; Fig. 7A). These results suggest that DPE1 suppression and overexpression resulted in smaller or larger fractions of long chains of amylopectin, respectively (Hizukuri, 1986).
Figure 7.
Altered structure of amylopectin from DPE1-overexpressed and -suppressed seeds. A, Absorbance spectra of amylopectin isolated from transgenic seeds. B and C, Differences in chain length distributions of amylopectin between wild-type (WT) and Ri lines (B) and between wild-type and OX lines (C).
To further examine changes in the fine structure of amylopectin in transgenic seeds, the chain length distribution of amylopectin was analyzed by measuring 8-amino-1,3,6-pyrenetrisulfonic acid-labeled α-1,4-glucans, using high-resolution capillary electrophoresis. Chains with degree of polymerization (DP) of 6 to 8 and 16 to 36 decreased significantly, whereas chains with DP of 9 to 15 increased markedly in lines Ri-4 and Ri-12 (Fig. 7B, top section). Conversely, chains with DP of 6 to 10 and 23 to 38 increased significantly, whereas those with DP of 11 to 22 decreased clearly in lines OX-3 and OX-7 (Fig. 7B, bottom section).
Soluble Sugars and MOSs in DPE1-Overexpressed and -Suppressed Seeds
Soluble sugars in transgenic seeds were extracted (Pico et al., 2015) and analyzed (Turner and Turner, 1960; Takaha et al., 1993). Soluble sugars in rice seeds consist mainly of Glc, Suc, and soluble MOSs, with Glc and Suc accounting for 85% to 90% and soluble MOSs for 10% to 15% (Supplemental Fig. S3). The contents of Glc and total soluble sugars did not differ between Ri-4, Ri-12, and the wild type, whereas they were significantly higher in OX-3 and OX-7 than in the wild type (Supplemental Figs. S4 and S5).
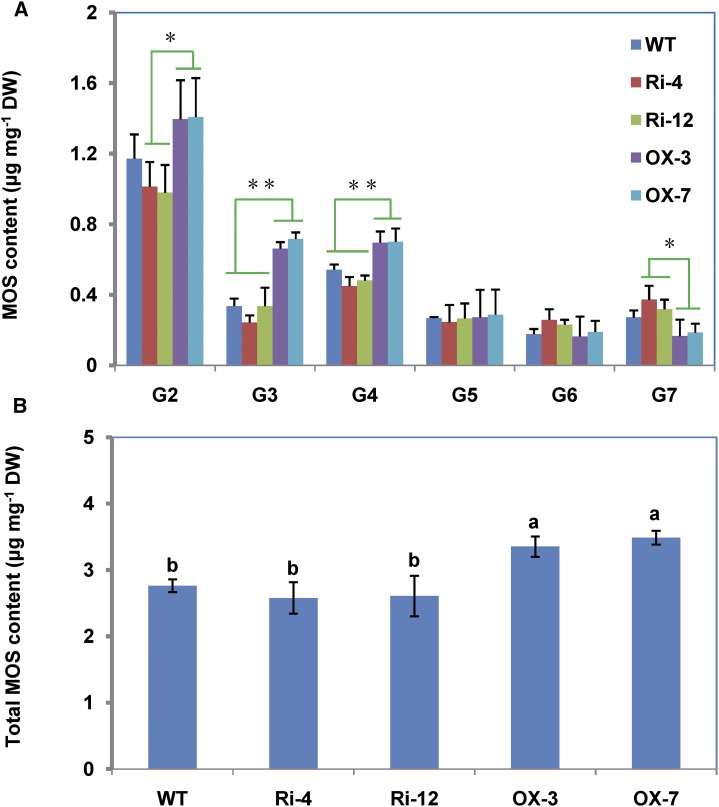
The components of MOSs and their contents were analyzed by high-performance anion-exchange chromatography with pulsed amperometric detection (Critchley et al., 2001). Maltose (G2), maltotriose (G3), and maltotetraose (G4) were the major MOSs in wild-type seeds, accounting for 43%, 12%, and 19%, respectively, while maltopentaose (G5), maltohexaose (G6), and maltoheptaose (G7) were the minor MOSs, accounting for 9%, 6%, and 10%, respectively (Fig. 8). The amounts of G2 to G7 were 1.17, 0.34, 0.54, 0.26, 0.17, and 0.27 µg mg–1 dry weight (DW) in wild-type seeds, respectively. Compared with the wild type, the levels of G2, G3, and G4 decreased, while those of G6 and G7 increased in Ri lines, whereas the contents of G2, G3, and G4 increased, while those of G6 and G7 decreased in OX lines. The contents of G2, G3, and G4 were markedly higher, while the content of G7 was significantly lower in OX than in Ri lines (Fig. 8A). The total amounts of MOSs in Ri lines were generally comparable with that in the wild type; however, they were significantly higher in the OX lines than in the wild type (Fig. 8B). These results show that changes in DPE1 expression affected the accumulation of soluble MOSs in transgenic seeds.
Figure 8.
Analysis of the MOSs present in transgenic rice seeds. A, MOS components and contents in transgenic rice seeds. Data are means ± sd from three biological replicates. The asterisks indicate significant differences, by Duncan’s multiple range test, at *P < 0.05 or **P < 0.01. G2 to G7 indicate maltose, maltotriose, maltotetraose, maltopentaose, maltohexaose, and maltoheptaose, respectively. B, Total MOS content. Different lowercase letters indicate significant differences, by Duncan’s multiple range test, at P < 0.05. WT, Wild type.
Action of DPE1 on Amylose and Amylopectin
To investigate whether amylose or amylopectin is an effective donor or acceptor for maltooligosyl group transfer, recombinant DPE1 devoid of its transit peptide for targeting to the amyloplast was expressed in Escherichia coli and purified sequentially by Ni2+ affinity chromatography and gel filtration chromatography. Recombinant His-tagged DPE1 (rDPE1) migrated at the expected 70 kD on SDS-PAGE (Fig. 9A) and exhibited significant activity, releasing Glc from maltotriose dissolved in double-distilled water and 10% (v/v) dimethyl sulfoxide (DMSO; Fig. 9B).
Figure 9.
Activity of DPE1 on amylose and amylopectin. A, SDS-PAGE of recombinant DPE1 purified from E. coli. Lane M indicates molecular weight markers (170, 130, 100, 70, 55, 40, 35, and 25 kD), and lane 1 indicates recombinant DPE1. B, Activity of recombinant DPE1. The activity was detected by the ability to release Glc from maltotriose in double-distilled water (DDW) and 10% DMSO. Data are means ± sd from three biological replicates. C, TLC of reaction products of DPE1 activity on amylose and amylopectin in the presence of Glc. M indicates standard MOSs, AM indicates amylose, AP indicates amylopectin, a plus sign indicates with DPE1, and a minus sign indicates without DPE1. D, TLC of reaction products of DPE1 activity on amylopectin in the absence of Glc. E, Native-PAGE/activity analysis of recombinant DPE1 on amylopectin in the presence (a) and absence (b) of Glc. The activity bands are indicated by arrowheads. The loading control of recombinant DPE1 in a and b is shown in c. F, Native-PAGE/activity analysis of DPE1 in transgenic rice seeds on amylopectin. The activity bands are indicated by arrowheads.
The activities of rDPE1 on amylose and amylopectin were investigated (Fig. 9, C and D). Amylose and amylopectin were incubated with or without rDPE1 for 12 h in the presence of Glc (the smallest acceptor of a glucosyl moiety; Takaha et al., 1993), respectively. After thin-layer chromatography (TLC), a range of MOSs was observed from the reaction of rDPE1 with amylose or amylopectin; the amounts of amylose or amylopectin were reduced significantly on the TLC plate (Fig. 9C). These results suggest that, in the presence of Glc, DPE1 can transfer maltooligosyl groups from amylose and amylopectin to Glc, forming a range of MOS molecules. In the presence of Glc, the activity of DPE1 was obviously weaker on amylopectin than on amylose because 25-fold more enzyme was needed to catalyze the same amount of amylopectin.
When amylopectin alone or with maltohexaose (G6) was incubated with rDPE1 in the absence of Glc, no visible change in the amount of amylopectin was observed on the TLC plate (Fig. 9D). However, the BV and λmax of the amylopectin-iodine complexes clearly changed. The BV and λmax of amylopectin treated by rDPE1 with or without G6 were 0.778 and 521 nm and 0.769 and 488 nm, respectively, compared with those of untreated amylopectin of 0.899 and 541 nm (Supplemental Fig. S6). These results showed that, in the absence of Glc, rDPE1 can transfer maltooligosyl groups from MOSs to amylopectin or modify directly the fine structure of amylopectin via inter- and/or intra-amylopectin molecular transfers of maltooligosyl groups.
The activity of DPE1 on amylopectin was further measured by native-PAGE/activity staining analysis. Purified rDPE1 appeared as a major band and two minor bands on native-PAGE, indicating that rDPE1 can spontaneously assemble into two other larger homopolymers (Fig. 9E, bottom section). In the presence of Glc, the bands corresponding to the position of rDPE1 were stained white (Fig. 9E, top section), indicating that rDPE1 can almost completely break down the small amount of amylopectin existing in the gel. In the absence of Glc, a distinct phenomenon was observed. The core of the activity band was stained white, whereas the peripheral region was stained dark brown (Fig. 9E, middle section). This suggested that the amylopectin molecules in the core were degraded, and the resulting maltooligosyl groups were transferred to the amylopectin molecules in the peripheral region, modifying the fine structures of these molecules (Colleoni et al., 1999b). Moreover, the ability of native DPE1 in transgenic seeds to modify the fine structure of amylopectin was confirmed by native-PAGE/activity staining analysis. Upon staining with iodine, a dark-brown band for DPE1 was observed in OX lines, whereas no visible band was found in Ri lines (Fig. 9F), suggesting that, in the absence of Glc, the fine structure of amylopectin there was modified directly by native DPE1 in DPE1-overexpressed seeds.
Physiochemical Properties of Starch Granules in DPE1-Overexpressed and -Suppressed Seeds
To evaluate the physicochemical properties of endosperm starch granules in the transgenic lines, the gelatinization characteristics of endosperm starch granules were first examined in terms of solubility in urea solution (Nishi et al., 2001). When the supernatant of starch disintegrated with 5 m urea solution was stained with I2/KI solution, the absorption spectra of the iodine-starch complexes were clearly different between transgenic lines and the wild type (Fig. 10A). The absorbance values of the iodine-starch complexes from 420 to 700 nm were obviously higher in Ri and OX lines than in the wild type. In addition, the λmax of starch was significantly greater in Ri-4 (582 nm) and Ri-12 (589 nm) than in the wild type (564 nm), whereas the λmax of starch was distinctly lower in OX-3 (535 nm) and OX-7 (533 nm). Moreover, the absorbance values at λmax were slightly higher in Ri-4 (1.004) and Ri-12 (1.101) than in OX-3 (0.883) and OX-7 (0.994). These results indicate that the swelling/dissolving ability of starch granules in urea solution was greater in Ri lines than in OX lines.
Figure 10.
Urea gelatinization properties and viscosity profiles of starch granules in transgenic rice seeds. A, Absorbance spectra of starch dissolved in 5 m urea solution. Rice powder (10 mg) was mixed with 1 mL of 5 m urea solution and shaken for 24 h at 25°C. The supernatant was reacted with iodine solution, and the absorbance spectra of the starch-iodine complexes were determined. B, Viscosity profiles of starch from transgenic rice seeds. WT, Wild type.
The thermal gelatinization properties of starch granules were further analyzed by differential scanning calorimetry (Nakamura, 2002). The temperatures of the onset (To), peak (Tp), and conclusion (Tc) of gelatinization of endosperm starch granules were lower in Ri and OX lines than in the wild type (Table I). The To, Tp, and Tc of endosperm starch granules were largely comparable between Ri and OX lines. The gelatinization enthalpy (ΔH) of endosperm starch was approximately 2 and 1 mJ mg–1 lower in Ri and OX lines than in the wild type, respectively. These results suggest that starch granules from Ri and OX lines gelatinized more readily than those from the wild type.
Table I. Effects of DPE1 overexpression or suppression on thermal gelatinization properties of starch granules from transgenic rice seeds.
Data are mean ± sd from triplicate assays from three independent preparations.
| Linea | To | Tp | Tc | ΔH |
|---|---|---|---|---|
| °C | mJ mg–1 | |||
| Wild type | 61.4 ± 1.0 | 68.0 ± 0.6 | 76.2 ± 0.5 | 11.9 ± 1.0 |
| Ri-4 | 60.5 ± 0.6 | 67.4 ± 0.2 | 74.5 ± 0.4 | 9.8 ± 0.6 |
| Ri-12 | 60.3 ± 0.1 | 66.8 ± 0.5 | 74.6 ± 0.2 | 9.6 ± 0.6 |
| OX-3 | 59.8 ± 0.3 | 65.6 ± 0.3 | 75.4 ± 0.7 | 10.9 ± 0.5 |
| OX-7 | 60.0 ± 0.4 | 66.4 ± 0.2 | 74.8 ± 0.3 | 10.9 ± 0.2 |
The wild type (cv Kitaake) was used as an untransformed control; Ri-4, Ri-12, OX-3, and OX-7 are the transgenic lines tested.
The pasting properties of endosperm starch granules were determined by a rapid viscosity analyzer (Fig. 10B). The peak, trough, and final viscosities were 3,122, 2,100, and 3,585 centipoise, respectively, in the wild type. The smallest peak, trough, and final viscosities were observed in OX-3 and OX-7, which were only 35% and 39%, 34% and 45%, and 33% and 44%, respectively, of that of wild-type starch. The viscosity profiles of starch from Ri lines were mostly similar to those of wild-type starch. During heating, the viscosities of pasting starch increased at slower rates in Ri-4 and Ri-12 than in the wild type. During cooling, the viscosities of pasting starch first decreased at slower rates and then increased at higher rates in Ri-4 and Ri-12 than in the wild type. However, the values of these viscosities did not exceed those of wild-type starch at any time point. Thus, the peak, trough, and final viscosities were slightly lower in Ri-4 and Ri-12 than in the wild type and were maintained at levels of approximately 81% and 84%, 90% and 91%, and 95% and 94%, respectively, of that of wild-type starch.
DISCUSSION
Storage starch is synthesized inside amyloplasts and deposited as starch granules in the endosperm. Numerous starch biosynthetic enzymes, such as GBSSI, SSI, Pho1, and ISA1, are localized in amyloplasts and associated with starch granules (Fujita et al., 2006; Satoh et al., 2008; Utsumi et al., 2011). In this study, we demonstrated that DPE1 was localized in amyloplasts and loosely associated with starch granules in rice endosperm. An amyloplast-localized DPE1 was also reported in wheat endosperm (Bresolin et al., 2006). We showed that DPE1 is expressed at a high level, specifically in rice endosperm, and that the activity of DPE1 is substantially high throughout starch accumulation and endosperm development. The temporal and spatial profiles of DPE1 transcripts were consistent with two previous reports (Ohdan et al., 2005; Akdogan et al., 2011). Furthermore, in wheat endosperm, the level of DPE1 was high at 5 to 10 DAF and higher in the later stages of seed development (Bresolin et al., 2006). These results suggest the possibility that DPE1 plays a role in rice endosperm starch biosynthesis.
DPE1 was originally described as an enzyme using and producing soluble oligosaccharides (Peat et al., 1956; Jones and Whelan, 1969). It has been reported that DPE1 has a pivotal role in starch synthesis in C. reinhardtii (Colleoni et al., 1999a, 1999b). However, the identification of a DPE1-deficient Arabidopsis mutant, as well as DPE1-suppressed potato lines, has resulted in a distinct proposal for the critical role of DPE1 in transitory starch degradation (Takaha et al., 1998; Critchley et al., 2001). Our present investigation shows that changes in DPE1 expression affected the amylose content, structure of amylopectin, and morphological and physicochemical properties of starch granules, indicating that DPE1 functions substantially in storage starch synthesis in rice endosperm. Together, these results suggest that DPE1 may have different roles in the metabolism of transitory and storage starch in plants. We conclude that a primary function of DPE1 in cereal crops involves storage starch synthesis.
The amylose content of endosperm starch increased significantly, from 14.8% to 17.6%, in our DPE1-suppressed lines, whereas it decreased from 14.8% to 11.4% in our DPE1-overexpressed lines (Fig. 6B). Enzymatic analyses revealed that overexpression and suppression of DPE1 had no effect on GBSSI activity (Fig. 4E). Thus, these changes in amylose content in transgenic lines would seem to be attributable to overexpression or suppression of DPE1, rather than the activity of GBSSI. These changes were generally predictable from in vitro experiments. On treatment with α-1,4-glucanotransferase (a counterpart of DPE1 in bacteria), the amylose contents of rice and corn (Zea mays) starch decreased from 30.0% to 21.8% and from 21.6% to 15.6%, respectively (Cho et al., 2009; Do et al., 2012). Furthermore, suppression and overexpression of DPE1 also resulted in higher or lower Mr amylose, as revealed by the absorbance spectra of the amylose-iodine complexes (Fig. 6C). Recombinant DPE1 can almost completely break down amylose by transferring a range of maltooligosyl groups to Glc (Fig. 9C). Moreover, the contents of G2, G3, and G4 were evidently higher in OX lines than in Ri lines (Fig. 8A), indicating that DPE1 in rice endosperm can also catalyze amylose into short-chain MOSs. These results show that amylose is an effective substrate in vivo and in vitro for DPE1. We conclude that changes in DPE1 gene expression modified not only the content, but also the Mr of amylose.
The contents of total starch in our transgenic lines and the wild type were comparable, while the content of amylose was elevated in DPE1-suppressed lines and reduced in DPE1-overexpressed lines (Fig. 6). Thus, the content of amylopectin would be expected, accordingly, to be increased in DPE1-overexpressed lines and correspondingly decreased in DPE1-suppressed lines. In the presence of Glc, both amylose and amylopectin can be catalyzed by recombinant DPE1 into a range of MOSs (Fig. 9C). In the absence of Glc, recombinant DPE1 in E. coli or transgenic seeds can transfer maltooligosyl groups from MOSs to amylopectin or directly modify the fine structure of amylopectin by transferring maltooligosyl groups from one molecule to itself or to another (Fig. 9, D and E). In the presence of Glc, the activity of DPE1 on amylose was much stronger than on amylopectin in degrading them into MOSs. A metabolic flow of maltooligosyl groups from amylose to amylopectin was clearly identifiable when comparing OX lines to Ri lines (Fig. 8A). The elevated level of DPE1 in OX lines should enhance, theoretically, its activity in transferring maltooligosyl groups from amylose to Glc, leading to a higher level of each MOS (G2–G7). However, the contents of the major MOSs (G2–G4) were higher, while that of G6 and G7 were lower in OX lines than in Ri lines (Fig. 8A). Furthermore, compared with the significant differences in amylose and amylopectin (≤75 mg g–1 DW for each) between Ri and OX lines, the changes in MOS contents were much smaller (≤0.5 mg g–1 DW for each MOS). These results indicate that, under DPE1 overexpression, a larger proportion of amylose was catalyzed by DPE1 into a range of MOSs, and these MOSs were mostly transferred by DPE1 to amylopectin, as revealed by the elevated content of amylopectin in OX lines. This hypothesis is supported by the report that plastidial d-enzyme is involved in amylopectin synthesis, by transferring maltooligosyl groups from MOSs onto the peripheral chains of amylopectin (Colleoni et al., 1999b). It has been proposed that MOSs may act as a primer for amylose synthesis in pea and potato (Denyer et al., 1996). However, this seems unlikely in rice seeds because the MOS was increased in the OX lines and decreased in the Ri lines, with amylose content reduced and increased, respectively (Figs. 6 and 8). A previous study also suggested that MOS is used as a substrate to synthesize long linear glucans, which serve as linear substrates for BE to form branched glucans in rice endosperm (Satoh et al., 2008).
In this study, DPE1-suppressed lines showed lower proportions of chains with DP of 6 to 8 and 16 to 36 and higher proportions of chains with DP of 9 to 15. Conversely, DPE1-overexpressed lines showed higher proportions of chains with DP of 6 to 10 and 23 to 38 and lower proportions of chains with DP of 11 to 22 (Fig. 7B). The profiles of chain length distribution of amylopectin in DPE1-suppressed lines are largely similar to those in rice SSIIIa mutants (Fujita et al., 2007) and RNA interference-suppressed SSIIIa lines (Zhang et al., 2011). Zymogram analysis revealed that overexpression and suppression of DPE1 had no effect on SSIIIa activity. Thus, these changes in chain length distribution in DPE1-suppressed lines would seem to be attributable to the suppression of DPE1 rather than repression of SSIIIa or deficiency of SSIIIa. The profiles of chain length distribution of amylopectin in DPE1-overexpressed lines are very similar to those of amylopectin from corn starch treated with α-1,4-glucanotransferase from Synechocystis sp. PCC 6803. The latter exhibited higher proportions of chains with DP of 4 to 9 and 22 to 40 and lower proportions of chains with DP of 10 to 21 (Lee et al., 2009). On treatment with α-1,4-glucanotransferase, rice starch also showed a similar profile of chain length distribution for amylopectin (Cho et al., 2009). These results show that amylopectin is an effective substrate in vivo and in vitro for DPE1. Together with the fact that the content and chain length distribution of amylopectin changed in transgenic seeds, we conclude that changes in DPE1 gene expression modified not only the content, but also the fine structure of amylopectin.
The fine structure of amylopectin is essential for the morphology and crystallinity of starch granules. We found that both DPE1 overexpression and suppression altered the appearance of starch granules and their overall arrangement (Fig. 5). In DPE1-overexpressed lines, starch granules were tightly packed in the endosperm, whereas in DPE1-suppressed lines, starch granules were loosely arranged (Fig. 5A). Starch granules from DPE1-suppressed lines were heterogeneous; some were larger and more spherical, and some were smaller and more irregularly polyhedron shaped. By contrast, starch granules from DPE1-overexpressed lines were obviously smaller and less irregularly polyhedron shaped than wild-type starch granules (Fig. 5B). The x-ray diffraction patterns of endosperm starch granules were almost equivalent between DPE1-overexpressed and -suppressed lines and the wild type (Supplemental Fig. S2). The similar crystallinity is consistent with relatively small changes in the fine structure of amylopectin between transgenic lines and the wild type. We conclude that DPE1 in cereal endosperm has an indispensable function in the formation of starch granule morphology.
It has been noted that there is a correlation between the gelatinization properties of starch granules and the molecular structure of amylopectin (Kalichevsky et al., 1990; Tester and Morrison, 1990; Shi and Seib, 1992; Fredriksson et al., 1998; Jane et al., 1999; Singh et al., 2003; Sodhi and Singh, 2003; Schirmer et al., 2013). The chains of amylopectin are grouped into A chains that carry no additional chains, B chains that carry A chains or other B chains, and a single C chain that possesses the sole reducing end group. A and B1 chains form a single cluster, and B2 and B3 chains extend to two and three clusters, respectively (Hizukuri, 1986), with DPs of 6 to 12, 13 to 24, and 25 to 36, respectively (Hanashiro et al., 1996). It has been suggested that A and B1 chains correlate negatively and positively with the gelatinization of the crystals, respectively (Inouchi et al., 2005; Kong et al., 2008; Wang et al., 2010). Starch granules from DPE1-overexpressed lines gelatinized more readily than those from the wild type (Fig. 10A; Table I). The elevation of A chains and the depletion of B1 chains of amylopectin in DPE1-overexpressed lines would be expected to be responsible for the lower gelatinization temperature and higher solubility in urea solution. Unexpectedly, starch granules from DPE1-suppressed lines also showed higher solubility in urea solution and lower gelatinization temperatures than did wild-type starch granules (Fig. 8A; Table I). These results were not predictable from the decrease in A chains and increase in B1 chains of amylopectin in DPE1-suppressed lines, indicating that some other factor(s) may cooperate to determine the gelatinization properties of starch granules. It has been suggested that amylose inhibits the swelling of starch granules and that heterogeneous granule size and irregular granule shapes destroy granule-granule interactions (Tester and Morrison, 1990; Jane et al., 1999; Singh et al., 2003). Thus, these inconsistent results may be due to high amylose contents, a heterogeneous size distribution, and/or the irregular appearance of starch granules in DPE1-suppressed lines.
The viscosity profiles of pasting starch from the DPE1-suppressed lines largely resembled those of wild-type starch. The peak, trough, and final viscosities of pasting starch were lower by 17.5%, 9.5%, and 5.5%, respectively, in DPE1-suppressed lines than in the wild type (Fig. 8B). The viscosities of pasting starch from DPE1-overexpressed lines were the smallest, and the peak, trough, and final viscosities were maintained at only 37%, 39.5%, and 38.5%, respectively, of those of wild-type starch (Fig. 10B). These results show that overexpression and suppression of DPE1 in rice endosperm had substantial effects on the pasting properties of starch granules.
Several studies have shown that the size and appearance of starch granules, granule-granule interactions, amylose content, and the fine structure of amylopectin exert effects on the pasting properties of starch collectively (Okechukwu and Rao, 1995; Singh et al., 2003; Singh and Kaur, 2004; Cai et al., 2006; Schirmer et al., 2013). Under DPE1 suppression, the decreased A chains, increased B1 chains, and elevated amylose content were able to restrain the swelling of starch granules and maintain the integrity of swollen starch granules (Tester and Morrison, 1990; Jane et al., 1999; Singh et al., 2003), which is mostly associated with the higher pasting viscosity (Hermansson and Svegmark, 1996; Schirmer et al., 2013). However, heterogeneous granule size and irregular granule appearance could destroy granule-granule interactions, which are predominately related to the lower pasting viscosity. These may explain the slightly lower peak, trough, and final viscosities in DPE1-suppressed lines than in the wild type.
Under DPE1 overexpression, the increased A chains, decreased B chains, and low amylose content could promote the swelling of starch granules, facilitating the disintegration of starch granules and the rapidly developed viscosities during heating. Moreover, the low amylose content was able to reduce the amount of leached-out amylose during heating and suppress the increase in viscosity induced by leached-out amylose rearrangement during cooling (Flipse et al., 1996; Gunaratne et al., 2007; Schirmer et al., 2013). These findings may explain why the starch from DPE1-overexpressed lines exhibited a viscosity profile with the smallest peak, trough, and final viscosities.
In conclusion, our data demonstrate that DPE1 has an essential role in rice endosperm starch synthesis by transferring maltooligosyl groups from amylose and amylopectin to amylopectin. This transferring of maltooligosyl groups may be a complement for the model of stacking Glc one after another during the chain elongation of amylopectin. We suggest that DPE1 plays an important role in maintaining the ratio of amylose to amylopectin in rice seeds as well. These findings extend our understanding of the molecular mechanism of starch biosynthesis in cereal endosperm.
MATERIALS AND METHODS
Isolation of Rice DPE1
The entire open reading frame of DPE1 was amplified by reverse transcription-PCR. Total RNA was extracted from developing rice (Oryza sativa ‘Kitaake’) seeds at 12 DAF using the TRIzol reagent (Invitrogen) and treated with RNase-Free DNase I (Takara). First-strand complementary DNA (cDNA) was generated using a PrimeScript First-Strand cDNA Synthesis Kit (Takara) and was used as a template for PCR. The resulting PCR products, encoding rice DPE1 with its transit peptide, were cloned into pMD18-T (Takara). The primers are listed in Supplemental Table S1.
Real-Time Quantitative PCR
Total RNA extraction, treatment, and first-strand cDNA synthesis were performed as described above. Real-time quantitative PCR was performed on a Light Cycler 480 (Roche). Rice Actin-1 was used as an endogenous control, and all experiments had at least three biological replicates. The primers are listed in Supplemental Table S1.
Assay of DPE1 Activity
Rice powder (20 mg) or a single endosperm at the milking stage was homogenized with 500 μL of cold solution containing 100 mm sodium phosphate, pH 6.8, and 50 mm 2-mercaptoethanol. The homogenate was centrifuged (20,000g for 10 min at 4°C), and the supernatant was used for the assay of DPE1.
DPE1 activity was assayed by a method adapted from Akdogan et al. (2011). Enzyme extract (50 μL) was mixed with 50 μL of 20 mm maltotriose. The reaction mixture was incubated at 30°C for 30 min. The reaction was terminated by immersing the tube in boiling water for 10 min. The released Glc was measured.
Western-Blot Analysis
A synthetic peptide (SVGVGEDLPEGYEQM) was used to produce an antiserum against DPE1 in rabbits. The polyclonal antibody was purified by affinity chromatography with the synthetic peptide coupled to Sulfolink Coupling Gel (Pierce). Western-blot analysis was performed as described by Qu et al. (2005).
GFP and RFP Fusion Constructs for Transient Expression in Rice Protoplasts
mRFP was fused to the C terminus of the transit peptide of the Rubisco small subunit (Rubisco:mRFP; Liu et al., 2012). GFP was fused to the C terminus of DPE1 (DPE1:GFP). The primers are listed in Supplemental Table S1. The chimeric genes were subcloned into pBI221 by replacing the sequence encoding GUS. The resulting two transient expression vectors were cotransformed into rice protoplasts as described by Tian et al. (2013). Transformed cells were examined under a confocal microscope (TCS SP5, Leica), and digital images were recorded.
Proportion of Soluble and Starch Granule-Bound DPE1
The soluble, starch granule loosely bound, and starch granule tightly bound proteins were fractionated from developing endosperm as described by Fujita et al. (2006). The distribution of DPE1 in the three fractions was determined by western-blot analysis. The relative amount of DPE1 was quantified by measuring the intensity of bands using Quantity One (Bio-Rad).
Binary Vector Construction and Rice Genetic Transformation
To construct an endosperm-specific DPE1-overexpressing vector, the full-length cDNA was subcloned into the binary vector pGPTV-GluC-GUS-35S-HPT (Qu et al., 2008). To construct an endosperm-specific DPE1-suppressed vector, the sequence from nucleotides 254 to 638 was ligated to the RNA interference vector GluA2-pTCK303 (Tian et al., 2013). The primers are listed in Supplemental Table S1.
The DPE1 overexpression and suppression vectors were individually introduced into Agrobacterium tumefaciens strain EHA105. Transformation of rice was performed as described by Qu et al. (2008). Rice ‘Kitaake’ was used as the recipient of the transformation. The primers for genotyping are listed in Supplemental Table S1.
Zymogram Analyses of DPE1, Pho, DBE, BE, and SS
The crude enzyme extract for DPE1, Pho, DBE, BE, and SS was prepared as described by Satoh et al. (2008). Native-PAGE/activity staining analyses for BE, DPE1, and DBE were as described by Yamanouchi and Nakamura (1992), Colleoni et al. (1999a), and Fujita et al. (1999), respectively, and for Pho and SS were as described by Satoh et al. (2008).
GBSSI Activity Assay
The activity of GBSSI was determined as described by Liu et al. (2013).
SEM of Starch Granules
The milled rice was ground to a powder and used to isolate starch granules according to Sodhi and Singh (2003). Seed sections and purified starch granules were used for SEM analyses, as described by Fujita et al. (2003), with a Hitachi S-4800 scanning electron microscope at 10.0 kV.
Measurement of Starch and Amylose Contents
The AAC of the sample was determined as described by Juliano (1971). Powder (50 mg) was washed twice in 80% (v/v) ethanol and then extracted sequentially with 9.2 and 4.6 m perchloric acid. The extract was collected and diluted to 50 mL with water. An aliquot of the solution was analyzed for starch content as described by Turner and Turner (1960).
Determination of BV and λmax of Amylose and Amylopectin
The milled rice was ground to powder and used to isolate starch granules according to Sodhi and Singh (2003). Purified starch granules were fractionated into amylose and amylopectin and used for spectrum analysis of the amylose-iodine or the amylopectin-iodine complex, as described by Cai et al. (2006). λmax was determined, and BV was expressed as A600 for amylose and A680 for amylopectin.
Chain Length Distribution Profile of Amylopectin
The milled rice was ground to a powder and used for the analysis of chain length distribution of amylopectin. Extraction of starch and its following digestion with Pseudomonas amyloderamosa isoamylase was performed as described by Fujita et al. (2001). The reduction of isoamylolyzate was performed with sodium borohydride as described by Nagamine and Komae (1996). The chain length distribution of amylopectin was determined by measuring 8-amino-1,3,6-pyrenetrisulfonic acid-labeled isoamylolyzate using high-resolution capillary electrophoresis (PA800 Plus, Beckman) as described by O’Shea and Morell (1996).
Extraction of Soluble Sugars and Quantification of MOSs
Extraction of soluble sugars in rice seeds was performed as described by Pico et al. (2015). MOS was measured by high-performance anion-exchange chromatography with pulsed amperometric detection (DX-600, Dionex) on a CarboPac PA100 (4- × 250-mm) column, using a method adapted from Critchley et al. (2001). Eluants were 50 mm NaOH (A) and 500 mm sodium acetate in 50 mm NaOH (B). Gradients of the eluants were 0 to 15 min, 95% to 80% A/5% to 20% B; 15 to 25 min, 80% A/20% B; 25 to 28 min, 80% to 95% A/20% to 5% B; and 28 to 38 min, 95% A/5% B. For each run, 10 μL of sample was injected, and the flow rate was 0.8 mL min–1. Peaks were identified by MOS standards. Peak areas were determined using Peaknet software (Dionex).
Expression and Purification of Recombinant DPE1
The DNA sequence encoding mature DPE1 was amplified by PCR and inserted between the BamHI and XhoI sites in pET28a (Novagen), generating plastid pHis6-DPE1. pHis6-DPE1 was then transformed into BL21 (DE3, Novagen). Recombinant DPE1 was expressed upon induction of isopropylthio-β-galactoside and purified by Ni2+ affinity chromatography. The fractions containing DPE1 were further purified by gel filtration chromatography and concentrated using an Amicon Ultra-15 (10-kD cutoff; Millipore). The primers are listed in Supplemental Table S1.
TLC
TLC was performed using a method adapted from Takaha et al. (1993). Samples (5 µL) were spotted onto a silica gel 60 thin-layer plate (20 × 20 cm, 250 μm) and developed three times in 1-butanol/ethanol/water (5:5:3). After chromatography, compounds were detected by spraying 5% (v/v) sulfuric acid in methanol and baking at 130°C for 10 min.
Activity of DPE1 on Amylose and Amylopectin
For DPE1 activity on amylose in the presence of Glc, 50 μL of reaction mixture containing 500 μg of amylose dissolved in 5 μL of DMSO and 200 μg of Glc was incubated with or without 1 μg of DPE1 at 30°C for 12 h. For DPE1 activity on amylopectin in the presence of Glc, 50 μL of reaction mixture containing 250 μg of amylopectin dissolved in 5 μL of DMSO and 200 μg of Glc was incubated with or without 25 μg of DPE1 at 30°C for 12 h. Then, 5 μL of the reaction mixture was used for TLC.
For DPE1 activity on amylopectin in the absence of Glc, 50 μL of reaction mixture containing 150 μg of maltohexaose, 250 μg of amylopectin dissolved in 5 μL of DMSO, or both of them were incubated with or without 25 μg of DPE1 at 30°C for 12 h. Then, 5 μL of the reaction mixture was used for TLC.
Native-PAGE/activity staining analysis of DPE1 was performed using a method adapted from Colleoni et al. (1999b). The resolving gel contained 7.5% (w/v) acrylamide (acrylamide/bisacrylamide [30:0.8]), 375 mm Tris-HCl, pH 8.8, and 0.3% (w/v) potato (Solanum tuberosum) amylopectin. The stacking gel contained 3.3% (w/v) acrylamide and 130 mm Tris-HCl, pH 6.8. After electrophoresis, the resolving gel was washed twice with 40 mL of 100 mm Tris-HCl, pH 7.0, 1 mm MgCl2, 1 mm EDTA, and 1 mm dithiothreitol for 15 min and then incubated for 2 h at 30°C for 3 h in 25 mL of this medium with or without 20 mm Glc. After the reaction, the gel was stained with 0.2% (w/v) I2 and 2% (w/v) KI.
Physicochemical Properties of Starch
The urea gelatinization properties of starch were measured as described by Nishi et al. (2001). The solubility of starch granules was expressed in terms of absorbance spectra of the iodine-starch complex of the supernatant from a 5 m urea solution. Thermal gelatinization properties of starch were determined using a differential scanning calorimeter (DSC-821e) as described by Nakamura (2002). The To, Tp, and Tc, together with the ΔH, were measured.
The pasting properties of starch were measured using Rapid Visco Analyzer (NSW 2102). Viscosity profiles of starch were recorded in starch-water suspensions (12% [w/w] and 25 g total weight). The temperature-time conditions included a heating step from 50°C to 95°C at 12°C min–1 (after an equilibration time of 1 min at 50°C), a holding phase at 95°C for 2.5 min, a cooling step from 95°C to 50°C at 12°C min–1, and a holding phase at 50°C for 1.5 min. Average values for peak, trough, and final viscosities were obtained by quadruplicate rapid viscosity analysis measurements.
Sequence data from this article can be found in GenBank/EMBL/DDBJ databases under accession number AP004306 (DPE1, Os07g43390).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Box plot of starch granule size in transgenic rice seeds.
Supplemental Figure S2. The x-ray diffraction patterns of endosperm starch granules in transgenic rice seeds.
Supplemental Figure S3. TLC of soluble sugars in transgenic rice seeds.
Supplemental Figure S4. Glc content in transgenic rice seeds.
Supplemental Figure S5. Soluble sugar contents in transgenic rice seeds.
Supplemental Figure S6. Absorbance spectra of reaction products of DPE1 activity on amylopectin.
Supplemental Table S1. PCR primers.
Acknowledgments
We thank Dr. Kang Chong (Institute of Botany, Chinese Academy of Sciences) for kindly providing the pTCK303 vector, Jianping Gao and Siming Jiao (Institute of Process Engineering, Chinese Academy of Sciences) for the help with MOS measurement, and Juan Li and Changquan Zhang (Key Laboratory of Plant Functional Genomics, Yangzhou University) for the help with starch measurement.
Glossary
- DP
degree of polymerization
- MOS
maltooligosaccharide
- DAF
days after flowering
- AGPase
ADP glucose pyrophosphorylase
- GBSSI
granule-bound starch synthase I
- SS
soluble starch synthase
- BE
starch-branching enzyme
- DBE
starch-debranching enzyme
- Pho
α-glucan phosphorylase
- SEM
scanning electron microscopy
- AAC
apparent amylose content
- λmax
wavelength of maximum absorbance
- DW
dry weight
- DMSO
dimethyl sulfoxide
- TLC
thin-layer chromatography
- To
temperature of the onset of gelatinization
- Tp
temperature of the peak of gelatinization
- Tc
temperature of the conclusion of gelatinization
- ΔH
gelatinization enthalpy
- cDNA
complementary DNA
- BV
blue value
Footnotes
This work was supported by the Natural Science Foundation of China (grant no. 31070226 to X.D.) and the National Program of Transgenic Variety Development of China (grant no. 2014ZX08001–006 to L.Q.Q.).
References
- Akdogan G, Kubota J, Kubo A, Takaha T, Kitamura S (2011) Expression and characterization of rice disproportionating enzymes. J Appl Glycosci 58: 99–105 [Google Scholar]
- Ball SG, Morell MK (2003) From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu Rev Plant Biol 54: 207–233 [DOI] [PubMed] [Google Scholar]
- Bresolin NS, Li Z, Kosar-Hashemi B, Tetlow IJ, Chatterjee M, Rahman S, Morell MK, Howitt CA (2006) Characterisation of disproportionating enzyme from wheat endosperm. Planta 224: 20–31 [DOI] [PubMed] [Google Scholar]
- Buléon A, Colonna P, Planchot V, Ball S (1998) Starch granules: structure and biosynthesis. Int J Biol Macromol 23: 85–112 [DOI] [PubMed] [Google Scholar]
- Cai Y, Wang W, Zhu Z, Zhang Z, Yang J, Zhu Q (2006) The physiochemical characteristics of amylopectin and their relationships to pasting properties of rice flour in different varieties. Sci Agric Sin 39: 1122–1129 [Google Scholar]
- Chia T, Thorneycroft D, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM (2004) A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J 37: 853–863 [DOI] [PubMed] [Google Scholar]
- Cho KH, Auh JH, Ryu JH, Kim JH, Park KH, Park CS, Yoo CH (2009) Structural modification and characterization of rice starch treated by Thermus aquaticus 4-α-glucanotransferase. Food Hydrocoll 23: 2403–2409 [Google Scholar]
- Colleoni C, Dauvillée D, Mouille G, Buléon A, Gallant D, Bouchet B, Morell M, Samuel M, Delrue B, d’Hulst C, et al. (1999a) Genetic and biochemical evidence for the involvement of α-1,4 glucanotransferases in amylopectin synthesis. Plant Physiol 120: 993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleoni C, Dauvillée D, Mouille G, Morell M, Samuel M, Slomiany MC, Liénard L, Wattebled F, d’Hulst C, Ball S (1999b) Biochemical characterization of the Chlamydomonas reinhardtii α-1,4 glucanotransferase supports a direct function in amylopectin biosynthesis. Plant Physiol 120: 1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley JH, Zeeman SC, Takaha T, Smith AM, Smith SM (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J 26: 89–100 [DOI] [PubMed] [Google Scholar]
- Dauvillée D, Chochois V, Steup M, Haebel S, Eckermann N, Ritte G, Ral JP, Colleoni C, Hicks G, Wattebled F, et al. (2006) Plastidial phosphorylase is required for normal starch synthesis in Chlamydomonas reinhardtii. Plant J 48: 274–285 [DOI] [PubMed] [Google Scholar]
- Denyer K, Clarke B, Hylton C, Tatge H, Smith AM (1996) The elongation of amylose and amylopectin chains in isolated starch granules. Plant J 10: 1135–1143 [Google Scholar]
- Do HV, Lee EJ, Park JH, Park KH, Shim JY, Mun SM, Kim YR (2012) Structural and physicochemical properties of starch gels prepared from partially modified starches using Thermus aquaticus 4-α-glucanotransferase. Carbohydr Polym 87: 2455–2463 [Google Scholar]
- Flipse E, Keetels CJAM, Jacobsen E, Visser RGF (1996) The dosage effect of the wild-type GBSS allele is linear for GBSS activity but not for amylose content: Absence of amylose has a distinct influence on the physico-chemical properties of starch. Theor Appl Genet 92: 121–127 [DOI] [PubMed] [Google Scholar]
- Fredriksson H, Silverio J, Andersson R, Eliasson AC, Aman P (1998) The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr Polym 35: 119–134 [Google Scholar]
- Fujita N, Hasegawa H, Taira T (2001) The isolation and characterization of a waxy mutant of diploid wheat (Triticum monococcum L.). Plant Sci 160: 595–602 [DOI] [PubMed] [Google Scholar]
- Fujita N, Kubo A, Francisco PB Jr, Nakakita M, Harada K, Minaka N, Nakamura Y (1999) Purification, characterization, and cDNA structure of isoamylase from developing endosperm of rice. Planta 208: 283–293 [DOI] [PubMed] [Google Scholar]
- Fujita N, Kubo A, Suh DS, Wong KS, Jane JL, Ozawa K, Takaiwa F, Inaba Y, Nakamura Y (2003) Antisense inhibition of isoamylase alters the structure of amylopectin and the physicochemical properties of starch in rice endosperm. Plant Cell Physiol 44: 607–618 [DOI] [PubMed] [Google Scholar]
- Fujita N, Yoshida M, Asakura N, Ohdan T, Miyao A, Hirochika H, Nakamura Y (2006) Function and characterization of starch synthase I using mutants in rice. Plant Physiol 140: 1070–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Yoshida M, Kondo T, Saito K, Utsumi Y, Tokunaga T, Nishi A, Satoh H, Park JH, Jane JL, et al. (2007) Characterization of SSIIIa-deficient mutants of rice: the function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol 144: 2009–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaratne A, Ranaweera S, Corke H (2007) Thermal, pasting, and gelling properties of wheat and potato starches in the presence of sucrose, glucose, glycerol, and hydroxypropyl β-cyclodextrin. Carbohydr Polym 70: 112–122 [Google Scholar]
- Hanashiro I, Abe J, Hizukuri S (1996) A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr Res 283: 151–159 [Google Scholar]
- Hermansson AM, Svegmark K (1996) Developments in the understanding of starch functionality. Trends Food Sci Technol 7: 343–353 [Google Scholar]
- Hizukuri S. (1986) Polymodal distribution of the chain lengths of amylopectins and its significance. Carbohydr Res 147: 342–347 [Google Scholar]
- Hwang SK, Nishi A, Satoh H, Okita TW (2010) Rice endosperm-specific plastidial α-glucan phosphorylase is important for synthesis of short-chain malto-oligosaccharides. Arch Biochem Biophys 495: 82–92 [DOI] [PubMed] [Google Scholar]
- Inouchi N, Hibiu H, Li T, Horibata T, Fuwa H, Itani T (2005) Structure and properties of endosperm starches from cultivated rice of Asia and other countries. J Appl Glycosci 52: 239–246 [Google Scholar]
- James MG, Denyer K, Myers AM (2003) Starch synthesis in the cereal endosperm. Curr Opin Plant Biol 6: 215–222 [DOI] [PubMed] [Google Scholar]
- Jane J, Chen YY, Lee LF, McPherson AE, Wong KS, Radosavljevic M, Kasemsuwan T (1999) Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem 76: 629–637 [Google Scholar]
- Jones G, Whelan GJ (1969) The action pattern of d-enzyme, a transmaltodextrinylase from potato. Carbohydr Res 9: 483–490 [Google Scholar]
- Juliano BO. (1971) A simplified assay for milled-rice amylose. Cereal Sci Today 16: 334–340 [Google Scholar]
- Kakefuda G, Duke SH (1989) Characterization of pea chloroplast d-enzyme. Plant Physiol 91: 136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichevsky MT, Orford PD, Ring SG (1990) The retrogradation and gelation of amylopectins from various botanical sources. Carbohydr Res 198: 49–55 [Google Scholar]
- Kong X, Bertoft E, Bao J, Corke H (2008) Molecular structure of amylopectin from Amaranth starch and its effect on physicochemical properties. Int J Biol Macromol 43: 377–382 [DOI] [PubMed] [Google Scholar]
- Lee BH, Oh DK, Yoo SH (2009) Characterization of 4-α-glucanotransferase from Synechocystis sp. PCC 6803 and its application to various corn starches. N Biotechnol 26: 29–36 [DOI] [PubMed] [Google Scholar]
- Lin TP, Preiss J (1988) Characterization of d-enzyme (4-α-glucanotransferase) in Arabidopsis leaf. Plant Physiol 86: 260–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DR, Huang WX, Cai XL (2013) Oligomerization of rice granule-bound starch synthase 1 modulates its activity regulation. Plant Sci 210: 141–150 [DOI] [PubMed] [Google Scholar]
- Liu HL, Yin ZJ, Xiao L, Xu YN, Qu LQ (2012) Identification and evaluation of ω-3 fatty acid desaturase genes for hyperfortifying α-linolenic acid in transgenic rice seed. J Exp Bot 63: 3279–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR, Blennow A, Burhenne K, Kossmann J (2004) Repression of a novel isoform of disproportionating enzyme (stDPE2) in potato leads to inhibition of starch degradation in leaves but not tubers stored at low temperature. Plant Physiol 134: 1347–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR, Kossmann J, Ritte G (2005) Leaf starch degradation comes out of the shadows. Trends Plant Sci 10: 130–137 [DOI] [PubMed] [Google Scholar]
- Manners DJ, Matheson NK (1981) The fine structure of amylopectin. Carbohydr Res 90: 9–110 [Google Scholar]
- Nagamine T, Komae K (1996) Improvement of a method for chain-length distribution analysis of wheat amylopectin. J Chromatogr A 732: 255–259 [Google Scholar]
- Nakamura Y. (2002) Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue. Plant Cell Physiol 43: 718–725 [DOI] [PubMed] [Google Scholar]
- Nishi A, Nakamura Y, Tanaka N, Satoh H (2001) Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol 127: 459–472 [PMC free article] [PubMed] [Google Scholar]
- Ohdan T, Francisco PB Jr, Sawada T, Hirose T, Terao T, Satoh H, Nakamura Y (2005) Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J Exp Bot 56: 3229–3244 [DOI] [PubMed] [Google Scholar]
- Okechukwu PE, Rao MA (1995) Influence of granule size on viscosity of corn starch suspension. J Texture Stud 26: 501–516 [Google Scholar]
- O’Shea MG, Morell MK (1996) High-resolution slab gel electrophoresis of 8-amino-1,3, 6-pyrenetrisulfonic acid (APTS) tagged oligosaccharides using a DNA sequencer. Electrophoresis 17: 681–686 [DOI] [PubMed] [Google Scholar]
- Peat S, Whelan WJ, Rees WR (1956) The enzymic synthesis and degradation of starch. Part XX. The disproportionating enzyme (d-enzyme) of the potato. J Chem Soc 44–53 [Google Scholar]
- Pico J, Martínez MM, Martín MT, Gómez M (2015) Quantification of sugars in wheat flours with an HPAEC-PAD method. Food Chem 173: 674–681 [DOI] [PubMed] [Google Scholar]
- Qu LQ, Tada Y, Takaiwa F (2003) In situ western hybridization: a new, highly sensitive technique to detect foreign and endogenous protein distribution in rice seeds. Plant Cell Rep 22: 282–285 [DOI] [PubMed] [Google Scholar]
- Qu LQ, Xing YP, Liu WX, Xu XP, Song YR (2008) Expression pattern and activity of six glutelin gene promoters in transgenic rice. J Exp Bot 59: 2417–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu LQ, Yoshihara T, Ooyama A, Goto F, Takaiwa F (2005) Iron accumulation does not parallel the high expression level of ferritin in transgenic rice seeds. Planta 222: 225–233 [DOI] [PubMed] [Google Scholar]
- Robin JP, Mercier C, Charbonnière R, Guilbot A (1974) Linerized starches. Gel filtration and enzymatic studies of insoluble residues from prolonged acid treatment of potato starch. Cereal Chem 51: 389–406 [Google Scholar]
- Satoh H, Shibahara K, Tokunaga T, Nishi A, Tasaki M, Hwang SK, Okita TW, Kaneko N, Fujita N, Yoshida M, et al. (2008) Mutation of the plastidial α-glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell 20: 1833–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer M, Höchstötter A, Jekle M, Arendt E, Becker T (2013) Physicochemical and morphological characterization of different starches with variable amylose/amylopectin ratio. Food Hydrocoll 32: 52–63 [Google Scholar]
- Shi YC, Seib PA (1992) The structure of four waxy starches related to gelatinization and retrogradation. Carbohydr Res 227: 131–145 [Google Scholar]
- Singh N, Kaur L (2004) Morphological, thermal and rheological properties of potato starch fractions varying in granule size. J Sci Food Agric 84: 1241–1252 [Google Scholar]
- Singh N, Singh J, Kaur L, Sodhi NS, Gill BS (2003) Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem 81: 219–231 [Google Scholar]
- Smith AM, Denyer K, Martin C (1997) The synthesis of the starch granule. Annu Rev Plant Physiol Plant Mol Biol 48: 67–87 [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeeman SC, Smith SM (2005) Starch degradation. Annu Rev Plant Biol 56: 73–98 [DOI] [PubMed] [Google Scholar]
- Sodhi NS, Singh N (2003) Morphological, thermal and rheological properties of starches separated from rice cultivars grown in India. Food Chem 80: 99–108 [Google Scholar]
- Suganuma T, Setoguchi S, Fujimoto S, Nagahama T (1991) Analysis of the characteristic action of d-enzyme from sweet potato in terms of subsite theory. Carbohydr Res 212: 201–212 [Google Scholar]
- Takaha T, Critchley J, Okada S, Smith SM (1998) Normal starch content and composition in tubers of antisense potato plants lacking d-enzyme (4-α-glucanotransferase). Planta 205: 445–451 [Google Scholar]
- Takaha T, Yanase M, Okada S, Smith SM (1993) Disproportionating enzyme (4-α-glucanotransferase; EC 2.4.1.25) of potato. Purification, molecular cloning, and potential role in starch metabolism. J Biol Chem 268: 1391–1396 [PubMed] [Google Scholar]
- Tester RF, Karkalas J, Qi X (2004) Starch–composition, fine structure and architecture. J Cereal Sci 39: 151–165 [Google Scholar]
- Tester RF, Morrison WR (1990) Swelling and gelatinization of cereal starches. II. Waxy rice starches. Cereal Chem 67: 558–563 [Google Scholar]
- Tetlow IJ. (2006) Understanding storage starch biosynthesis in plants: a means to quality improvement. Can J Bot 84: 1167–1187 [Google Scholar]
- Tian L, Dai LL, Yin ZJ, Fukuda M, Kumamaru T, Dong XB, Xu XP, Qu LQ (2013) Small GTPase Sar1 is crucial for proglutelin and α-globulin export from the endoplasmic reticulum in rice endosperm. J Exp Bot 64: 2831–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DH, Turner JF (1960) The hydrolysis of glucose monophosphates by a phosphatase preparation from pea seeds. Biochem J 74: 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi Y, Utsumi C, Sawada T, Fujita N, Nakamura Y (2011) Functional diversity of isoamylase oligomers: The ISA1 homo-oligomer is essential for amylopectin biosynthesis in rice endosperm. Plant Physiol 156: 61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xie B, Shi J, Xue S, Deng Q, Wei Y, Tian B (2010) Physicochemical properties and structure of starches from Chinese rice cultivars. Food Hydrocoll 24: 208–216 [Google Scholar]
- Yamanouchi H, Nakamura Y (1992) Organ specificity of isoforms of starch branching enzyme (Q-enzyme) in rice. Plant Cell Physiol 33: 985–991 [Google Scholar]
- Yoshio N, Maeda I, Taniguchi H, Nakamura M (1986) Purification and properties of d-enzymes from malted barley. J Jpn Soc Starch Sci 33: 244–252 [Google Scholar]
- Zhang G, Cheng Z, Zhang X, Guo X, Su N, Jiang L, Mao L, Wan J (2011) Double repression of soluble starch synthase genes SSIIa and SSIIIa in rice (Oryza sativa L.) uncovers interactive effects on the physicochemical properties of starch. Genome 54: 448–459 [DOI] [PubMed] [Google Scholar]