An Arabidopsis peroxidase promotes the production of reactive oxygen species and negatively regulates growth during physiological development and in response to alterations of cell wall integrity.
Abstract
The structure of the cell wall has a major impact on plant growth and development, and alteration of cell wall structural components is often detrimental to biomass production. However, the molecular mechanisms responsible for these negative effects are largely unknown. Arabidopsis (Arabidopsis thaliana) plants with altered pectin composition because of either the expression of the Aspergillus niger polygalacturonase II (AnPGII; 35S:AnPGII plants) or a mutation in the QUASIMODO2 (QUA2) gene that encodes a putative pectin methyltransferase (qua2-1 plants), display severe growth defects. Here, we show that expression of Arabidopsis PEROXIDASE71 (AtPRX71), encoding a class III peroxidase, strongly increases in 35S:AnPGII and qua2-1 plants as well as in response to treatments with the cellulose synthase inhibitor isoxaben, which also impairs cell wall integrity. Analysis of atprx71 loss-of-function mutants and plants overexpressing AtPRX71 indicates that this gene negatively influences Arabidopsis growth at different stages of development, likely limiting cell expansion. The atprx71-1 mutation partially suppresses the dwarf phenotype of qua2-1, suggesting that AtPRX71 contributes to the growth defects observed in plants undergoing cell wall damage. Furthermore, AtPRX71 seems to promote the production of reactive oxygen species in qua2-1 plants as well as plants treated with isoxaben. We propose that AtPRX71 contributes to strengthen cell walls, therefore restricting cell expansion, during normal growth and in response to cell wall damage.
The cell wall is a complex, multifunctional and dynamic structure that provides mechanical support to plant cells, and it is involved in cell adhesion, defense against pathogens, regulation of metabolic functions, and cell-to-cell communication (Keegstra, 2010). Cell walls are usually composed of polysaccharides (cellulose, hemicelluloses, and pectins), phenolic compounds (e.g. ferulic acid and lignin), and proteins (Carpita and McCann, 2000). In the apoplast, cellulose microfibrils are associated to hemicelluloses, such as xyloglucans (XGs), producing a network embedded in a matrix of pectins. The latter are mainly composed of linear chains of homogalacturonan (HG) and branched chains of rhamnogalacturonans. Pectins are abundant in the middle lamella, where they ensure cell adhesion, as well as in primary and, to a lesser degree, secondary walls (Willats et al., 2001). HG is synthesized in a highly esterified form in the Golgi apparatus (Zhang and Staehelin, 1992) and then secreted in the apoplast, where pectin methylesterases remove part of the methyl groups (Pelloux et al., 2007). Free carboxylic groups allow the formation of so-called egg box structures, in which adjacent HG chains are linked by calcium-mediated ionic bridges, making the pectin matrix more rigid (Micheli, 2001). Pectins can also form other types of interactions, such as covalent cross links with other cell wall polysaccharides, phenolic compounds, and proteins (Caffall and Mohnen, 2009; Tan et al., 2013).
The wall structure influences both the extent and the direction of cell expansion (Mirabet et al., 2011). Growth takes place perpendicularly to the direction of cellulose microfibrils, which are deposited along the perpendicular axis of the cell, providing resistance to turgor pressure and extensibility along the longitudinal axis (Crowell et al., 2010). Two major classes of proteins have been proposed to promote cell wall expansion: XG endotransglycosylases, which cleave XG chains and link together the newly generated reduced end to a new XG chain (Fry et al., 1992), and expansins, which promote primary cell walls relaxation by disrupting cellulose-hemicellulose noncovalent links (Cosgrove, 2000). During expansion, the cell wall is relaxed, whereas turgor forces induce its deformation; subsequently, expansin inhibition and formation of cross links between structural proteins (such as extensins), polysaccharides, and/or monolignols cause wall stiffening and, consequently, slow down expansion (Wolf et al., 2009).
Regulation of apoplastic levels of reactive oxygen species (ROS) is important to determine cell expansion rate and organ size (Gapper and Dolan, 2006). ROS production in the cell wall is controlled, both under physiological conditions and in response to environmental stimuli, by several classes of enzymes, most prominently plasma membrane NADPH oxidases (Torres and Dangl, 2005) and class III peroxidases (CIII Prxs; Bolwell et al., 1999; Cosio and Dunand, 2009). NADPH oxidases, commonly known as respiratory burst oxidase homologs (Rbohs), are transmembrane proteins that oxidize cytoplasmic NADPH, translocate electrons across the plasma membrane, and generate superoxide radicals in the cell wall (Torres et al., 2002; Torres and Dangl, 2005). Superoxide radicals are then rapidly converted into hydrogen peroxide (H2O2) either spontaneously or in a reaction catalyzed by superoxide dismutases (Bolwell et al., 1999).
CIII Prxs are heme-containing enzymes secreted in the extracellular space or the vacuole, where they perform two different enzymatic cycles, namely the peroxidative and hydroxylic cycles (Welinder et al., 2002; Passardi et al., 2004). During the peroxidative cycle, the enzyme uses H2O2 as an oxidant in a two-step reaction to convert different substrates, including cell wall phenolic compounds and structural proteins, into free radicals that can subsequently combine together to form covalent linkages. This activity contributes to cell wall stiffening and therefore limits growth. CIII Prxs can also cause cell wall loosening through the hydroxylic cycle, in which H2O2 and O2− are used in a Fenton-type reaction to generate hydroxyl radicals, including •OH, that lead to nonenzymatic cleavage of polysaccharides (Chen and Schopfer, 1999; Dunand et al., 2003; Passardi et al., 2005). CIII Prxs can, therefore, play opposite roles in cell expansion, being able to cause both wall stiffening and loosening, depending on the growth conditions. CIII Prxs can also generate O2−, which is then dismutated into H2O2, through the oxidation of NAD(P)H. These enzymes can both positively and negatively modulate apoplastic ROS levels (Passardi et al., 2004).
The enzymatic characteristics of CIII Prxs allow them to participate in a wide range of physiological processes, including seed germination, plant growth, and elongation and defense against pathogens, as well as the catabolism of several extracellular molecules, including auxins (Hiraga et al., 2001). CIII Prxs are also involved in lignification through the H2O2-dependent generation of monolignol phenoxy radicals that spontaneously form lignin polymers (Marjamaa et al., 2009). Lignin monomers can also cross link cell wall polysaccharides, including pectins, through ferulate bridges or diferulate bonds formed by CIII Prxs in the presence of H2O2 (Iiyama et al., 1994). Notably, in some plant species, CIII Prxs-mediated cross links between ferulic acid and pectins arrest cell expansion (Iiyama et al., 1994). Lastly, CIII Prxs can cross link Tyr and Lys residues of extensins, contributing to the formation of a dense network within the cell wall (Schnabelrauch et al., 1996).
CIII Prxs likely appeared when plants colonized land and subsequently underwent a high rate of gene duplication, with the consequent increase of functional specialization (Passardi et al., 2005). For instance, the Arabidopsis (Arabidopsis thaliana) genome encodes 73 putative CIII Prxs, with various spatiotemporal expression profiles, suggesting that different isoforms play specific roles in growth, development, and adaptation to the environment (Tognolli et al., 2002; Welinder et al., 2002). In this article, we show that the Arabidopsis CIII Prx gene AtPRX71, which is strongly expressed upon loss of cell wall integrity (CWI), negatively affects growth and cell size and positively regulates ROS levels. In addition, when AtPRX71 is lacking, both ROS accumulation and growth defects caused by cell wall alterations are significantly reduced. We propose that accumulation of ROS-generating CIII Prxs is a mechanism to cope with loss of CWI both during normal development and in response to stress, leading to wall stiffening and therefore limiting cell expansion and growth.
RESULTS
Levels of AtPRX71 Transcripts Are Higher in Plants with Altered Cell Walls
We have previously shown that tobacco (Nicotiana tabacum) and Arabidopsis plants expressing an attenuated version of the Aspergillus niger polygalacturonase II (AnPGII; 35S:AnPGII plants) have a reduced content of deesterified HG and a significant reduction of growth that correlates with the levels of expression of the transgene (Capodicasa et al., 2004). Furthermore, 35S:AnPGII plants show constitutive activation of defense responses and accumulation of high levels of ROS in their tissues (Ferrari et al., 2008). To obtain insights into the mechanisms underlying the growth defect of 35S:AnPGII plants, whole-genome transcript analysis of rosette leaves of two independent transgenic lines, 35S:AnPGII 57 and 26, that show high levels of expression of the transgene (Lionetti et al., 2010) was performed using custom Arabidopsis 28K microarrays (GenBank Gene Expression Omnibus [GEO] Platform ID no. GPL15543). Differentially expressed transcripts were identified using a false discovery rate (FDR) < 0.05 and a module of logged fold change (FC) on base 2 (|log2 FC|) ≥ 1 (GEO accession no. GSE66980). With this filtering criteria, the transcript levels of 16 genes increased, and those of 4 genes decreased in a statistically significant way in rosette leaves of both transgenic lines compared with the wild type (Table I). Gene Ontology functional categories of these genes were enriched in response to stress and defense against other organisms (P < 0.001). Notably, one-third of the genes significantly induced in both 35S:AnPGII lines compared with the wild type encoded proteins putatively involved in cell wall structure and/or ROS homeostasis, including EXTENSIN4 (EXT4; At1g76930), a pectin esterase (At4g02330), copper/zinc superoxide dismutase1 (CSD1; At1g08830), and a CIII Prx (At5g64120).
Table I. Genes differentially expressed in both 35S:AnPGII 57 and 26 plants compared with the wild type.
Differentially expressed transcripts were identified using Limma R package using an FDR < 0.05 and a module of logged FC on base 2 |log2 FC| ≥ 1.0.
| Arabidopsis Genome Initiative Code | Annotation | 57a | 26a |
|---|---|---|---|
| At1g77510 | PROTEIN DISULFIDE ISOMERASE6 | 3.3 | 2.4 |
| At1g76930 | AtEXT4 | 2.8 | 3.0 |
| At1g76960 | Unknown protein | 2.8 | 2.9 |
| At5g64120 | AtPRX71 | 2.7 | 2.7 |
| At4g02330 | PECTIN METHYLESTERASE41 | 2.4 | 1.6 |
| At1g21520 | Unknown protein | 2.4 | 2.3 |
| At1g08450 | CALRETICULIN3 | 2.2 | 2.4 |
| At5g50460 | Protein transport protein SEC61 γ-subunit | 2.1 | 1.9 |
| At1g30720 | RetOx-like flavin adenine dinucleotide-binding domain-containing protein | 2.1 | 1.9 |
| At4g21620 | Gly-rich protein | 1.8 | 1.8 |
| At3g62600 | ER-LOCALIZED DnaJ-LIKE PROTEIN 3b | 1.7 | 1.4 |
| At1g19380 | Unknown protein | 1.5 | 1.4 |
| At1g08830 | COPPER/ZINC SUPEROXIDE DISMUTASE1 (CSD1) | 1.4 | 1.4 |
| At2g38870 | Ser-type endopeptidase inhibitor | 1.4 | 2.2 |
| At1g72070 | DnaJ heat shock N-terminal domain-containing protein | 1.3 | 1.5 |
| At1g71697 | CHOLINE KINASE1 | 1.3 | 1.7 |
| At3g52500 | Aspartyl protease | −2.1 | −1.6 |
| At3g47340 | GLUTAMINE-DEPENDENT ASPARAGINE SYNTHASE1 | −1.9 | −1.6 |
| At3g15570 | NON-PHOTOTROPIC HYPOCOTYL3 family protein | −1.7 | −1.4 |
| At1g25550 | MYB family transcription factor | −1.6 | −1.7 |
Log2 FC of the expression in the transgenic line compared with the wild type.
At5g64120, which was among the genes whose expressions increased the most in 35S:AnPGII plants (Table I), encodes the apoplastic CIII Prx ATP15a/AtPRX71, previously shown to produce ROS in response to hypoosmolarity (Rouet et al., 2006) and recently implicated in the regulation of stem lignin composition (Shigeto et al., 2013, 2015). AtPRX71 is expressed under physiological conditions in rosette leaves and, to a lesser extent, roots and stems (Shigeto et al., 2015). Because we have previously observed high levels of ROS and apoplastic Prx activity in 35S:AnPGII plants (Ferrari et al., 2008), we hypothesized that AtPRX71 might contribute to the growth reduction observed in these plants by promoting ROS production. This gene was, therefore, selected for further characterization.
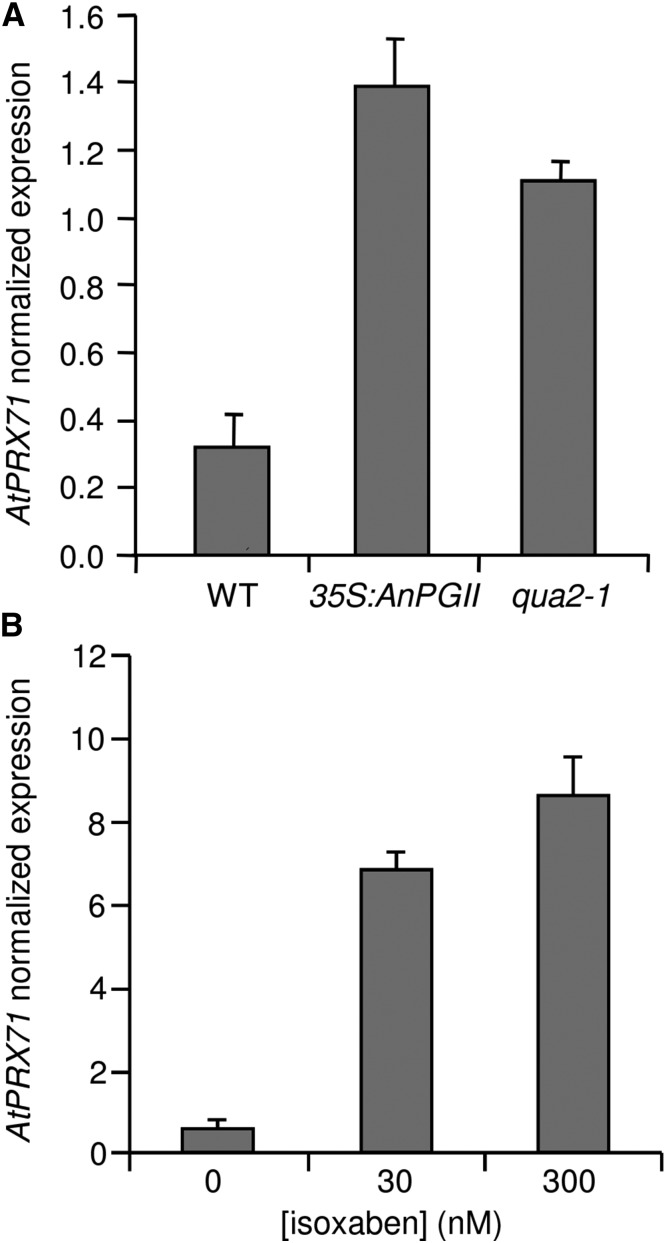
Higher levels of AtPRX71 transcripts in rosette leaves of 35S:AnPGII 57 plants compared with the wild type were confirmed by quantitative PCR (qPCR; Fig. 1A). Moreover, AtPRX71 mRNA levels also increased in the quasimodo2-1 (qua2-1) mutant (Fig. 1A), which is impaired in a putative pectin methyltransferase and, like 35S:AnPGII plants, shows reduced levels of deesterified HG and impaired growth (Mouille et al., 2007). Moreover, AtPRX71 transcript levels increased in wild-type Arabidopsis seedlings treated with the herbicide isoxaben (IXB; Fig. 1B), which inhibits cellulose synthases and affects cellulose deposition (Scheible et al., 2001). Taken together, these data indicate that alterations in different cell wall structural components promote the expression of AtPRX71.
Figure 1.
Elevated AtPRX71 expression in plants with altered cell walls. Expression of AtPRX71 in rosette leaves of 4-week-old wild-type (WT), 35S:AnPGII, and qua2-1 plants (A) or 10-d-old wild-type seedlings treated for 24 h with 0, 30, or 300 nm IXB (B) was determined by qPCR and normalized using the UBQ5 gene. Bars represent average arbitrary units ± sd of three technical replicates. This experiment was repeated three times with similar results.
AtPRX71 Negatively Regulates Arabidopsis Growth
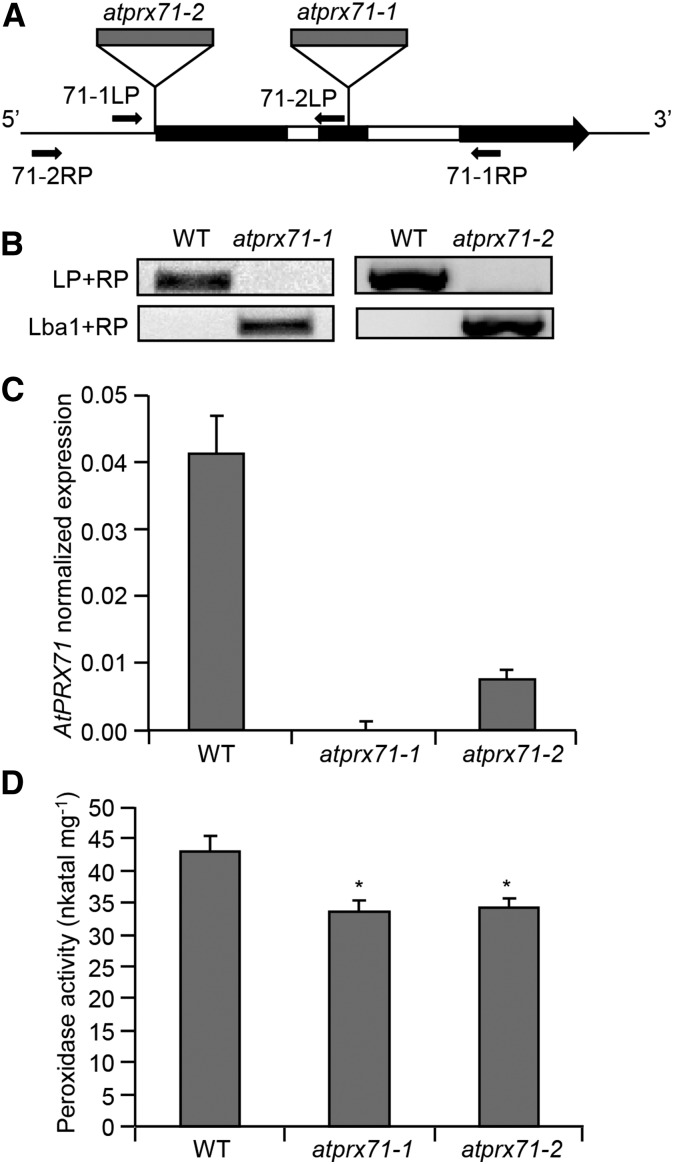
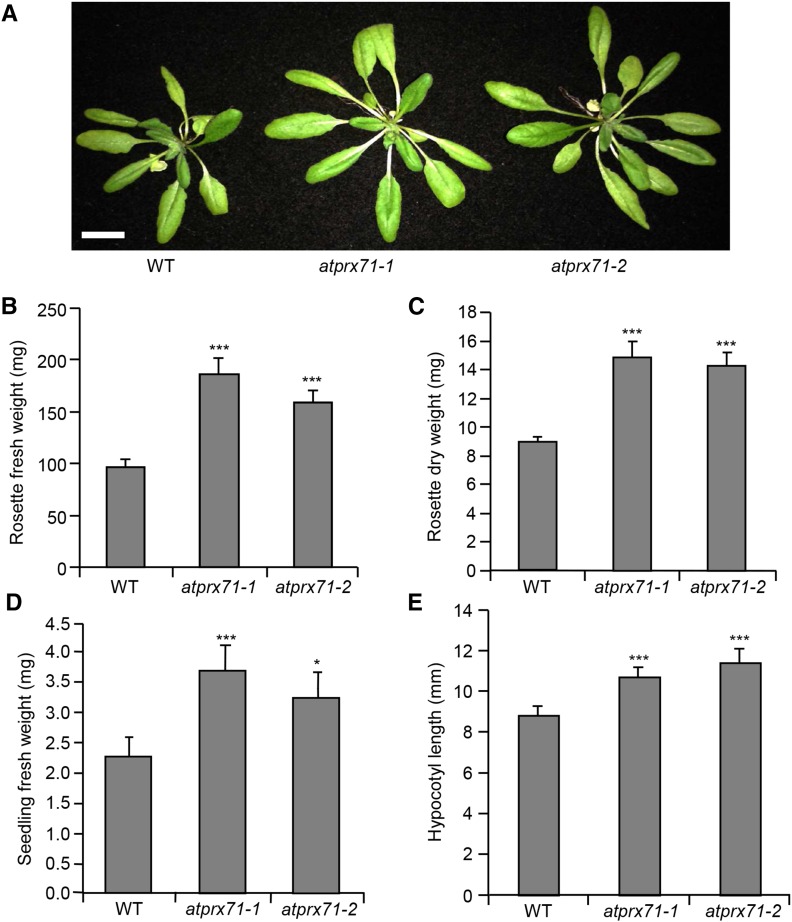
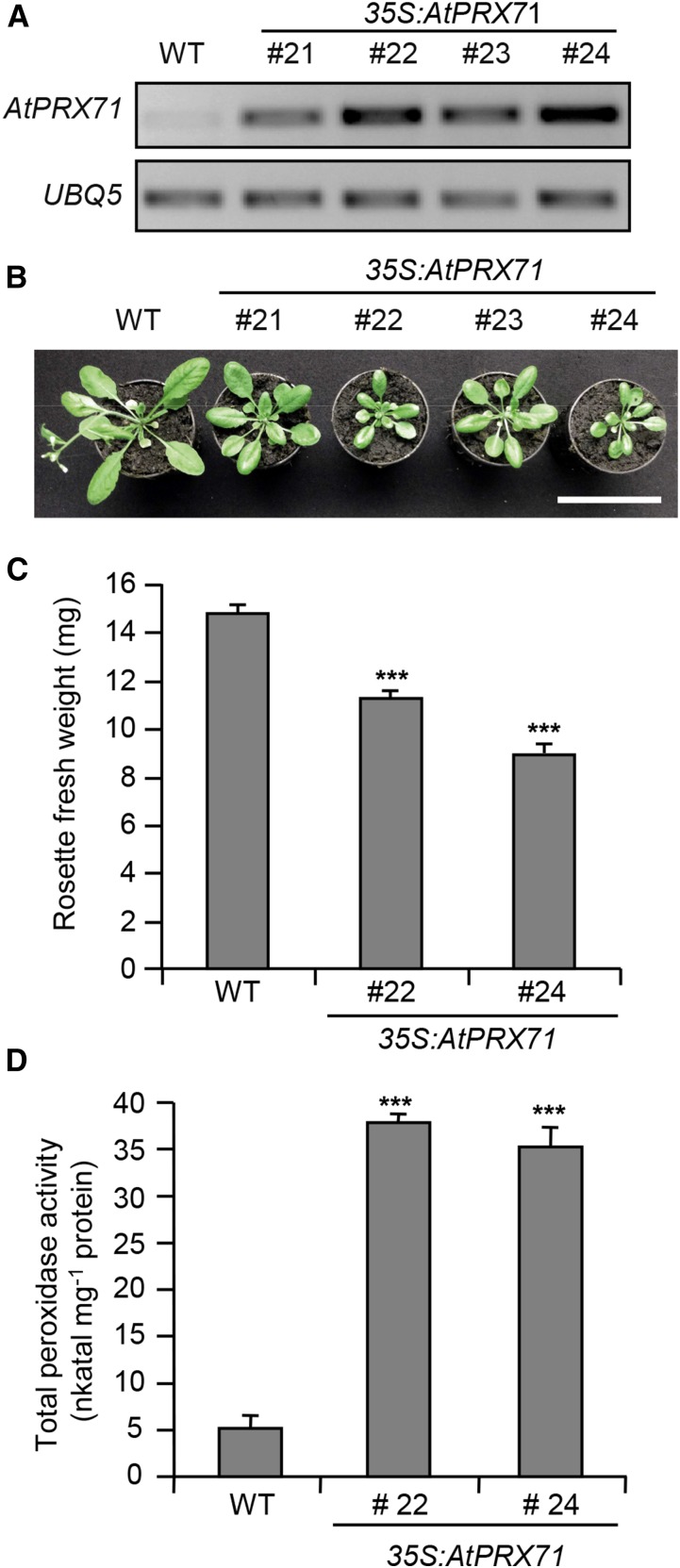
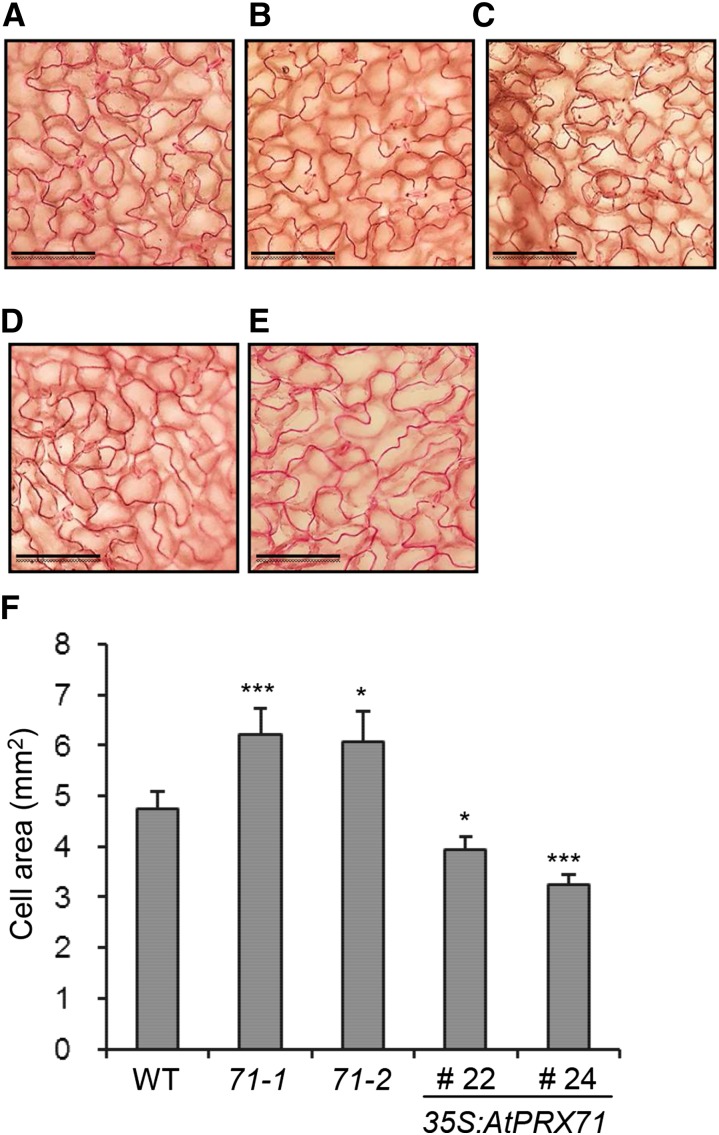
To investigate the function of AtPRX71, two independent insertion lines for this gene were isolated; these lines, named atprx71-1 and atprx71-2, carry a transfer DNA (T-DNA) insertion in the second exon and the promoter of the gene, respectively (Fig. 2, A and B). AtPRX71 transcript levels were undetectable in atprx71-1 and strongly reduced in atprx71-2 seedlings (Fig. 2C). Basal guaiacol Prx activity, which characterizes CIII Prxs, was reduced in seedlings of both mutants by about 20%, compared with the wild type (Fig. 2D). Adult atprx71-1 and atprx71-2 plants displayed enhanced rosette size (Fig. 3A) and a significant increase in both fresh (Fig. 3B) and dry (Fig. 3C) biomass. An increase in biomass was also observed in 10-d-old mutant seedlings compared with the wild type (Fig. 3D). Hypocotyls of etiolated seedlings were significantly longer in both mutants than in the wild type (Fig. 3E), whereas average length and fresh weight of floral stems of wild-type and atprx71-1 plants were not significantly different (Supplemental Fig. S1). These results indicated that AtPRX71 negatively affects Arabidopsis growth. To corroborate this conclusion, we generated transgenic plants expressing AtPRX71 under the control of the constitutive Cauliflower mosaic virus 35S promoter. Four independent 35S:AtPRX71 lines (21–24) accumulating high levels of AtPRX71 transcripts (Fig. 4A) were selected for further analysis. When grown in soil, transgenic plants had smaller, more compact rosettes compared with the wild type (Fig. 4B). Lines 22 and 24, which showed higher expression of the transgene (Fig. 4A), exhibited a marked reduction of rosette size (Fig. 4B) and a significant reduction of rosette fresh weight (Fig. 4C). These lines also accumulated very high levels of guaiacol Prx activity in their leaves (Fig. 4D) compared with untransformed plants. Taken together, these data indicate that high levels of AtPRX71 expression have a negative impact on plant growth. It has been proposed that CIII Prxs can restrict cell expansion by the formation of cell wall cross links (Passardi et al., 2004, 2005). We therefore hypothesized that the effects of AtPRX71 on Arabidopsis growth may depend on its ability to influence cell size. To verify this hypothesis, the area of the epidermal cells of rosette leaves was measured in wild-type, atprx71-1, atprx71-2, and 35S:AtPRX71 plants. Average cell area in both atprx71 mutants increased by approximately 30% with respect to the wild type, whereas 35S:AtPRX71 plants showed a reduction of cell size of 20% to 30% (Fig. 5, A–F). These results, therefore, suggest that AtPRX71 negatively regulates Arabidopsis growth, because its activity limits cell expansion.
Figure 2.
Isolation of insertional mutants for AtPRX71. A, Schematic representation of the AtPRX71 locus. Exons and introns are represented in black and white, respectively. Localization of T-DNA insertions (gray) and the primers used for genotyping of atprx71-1 and atprx71-2 are shown. B, Genotyping of atprx71-1 and atprx71-2. Genomic DNA from the wild type (WT) and mutants was subjected to PCR using primer pairs for the wild-type allele (LP + RP) or the T-DNA insertion (Lba1 + RP). LP, 71-1LP or 71-2LP; RP, 71-1RP or 71-2RP. C, Expression of AtPRX71 was analyzed in wild-type, atprx71-1, and atprx71-2 rosette leaves by qPCR using UBQ5 as the reference gene. Bars represent average arbitrary units ± sd of three technical replicates. This experiment was repeated twice with similar results. D, Total peroxidase activity in protein extracts from wild-type, atprx71-1, and atprx71-2 10-d-old seedlings was determined by a guaiacol oxidation-based assay. Bars represent average activity ± se of at least six independent samples. This experiment was repeated twice with similar results. *, Statistically significant differences between the wild type and mutants according to Student’s t test (P < 0.05).
Figure 3.
Loss of AtPRX71 increases growth. A to C, Representative picture (A), fresh weight (B), and dry weight (C) of 4-week-old soil-grown wild-type (WT), atprx71-1, and atprx71-2 rosettes. Bar =1.5 cm. D, Fresh weight of 10-d-old wild-type, atprx71-1, and atprx71-2 seedlings grown in vitro on solid medium in the light. E, Hypocotyl length of 5-d-old wild-type, atprx71-1, and atprx71-2 etiolated seedlings grown in vitro on solid medium. B to E, Bars represent average weight ± se (n > 10 in each experiment); asterisks indicate statistically significant differences between the wild type and mutants according to Student’s t test. *, P < 0.05; ***, P < 0.01.
Figure 4.
Overexpression of AtPRX71 causes reduced growth. A, RNA was extracted from leaves of four-week-old wild-type (WT) plants and four independent lines expressing AtPRX71 under the control of the 35S promoter (35S:AtPRX71). Expression of AtPRX71 was determined by reverse transcription PCR using UBQ5 as reference gene. B, Representative picture of rosettes of the same lines described in A. Bar = 5 cm. C, Average rosette fresh weight ± se (n > 10) of the wild type and 35S:AtPRX71 lines 22 and 24 4-week-old soil-grown plants. D, Peroxidase activity in leaves of wild-type and 35S:AtPRX71 plants grown as in C. Bars represent average enzymatic activity (nanokatal milligram−1 protein) ± sd (n = 3). ***, Significant differences between wild-type and transgenic plants according to Student’s t test (P < 0.01).
Figure 5.
AtPRX71 negatively affects leaf epidermal cell size. Rosette leaves from 3-week-old wild-type (WT; A), atprx71-1 (B), and atprx71-2 (C) plants and two lines overexpressing AtPRX71 (35S:AtPRX71 lines 22 [D] and 24 [E]) were cleared and stained with ruthenium red, and images of the epidermis were taken with a microscope. Bars = 100 µm. F, Bars represent the average area of epidermal cells ± se (n > 20). Asterisks indicate significant differences between the wild type and mutants or transgenic plants according to Student’s t test. 71-1, atprx71-2; 71-2, atprx71-2. *, P < 0.05; ***, P < 0.01.
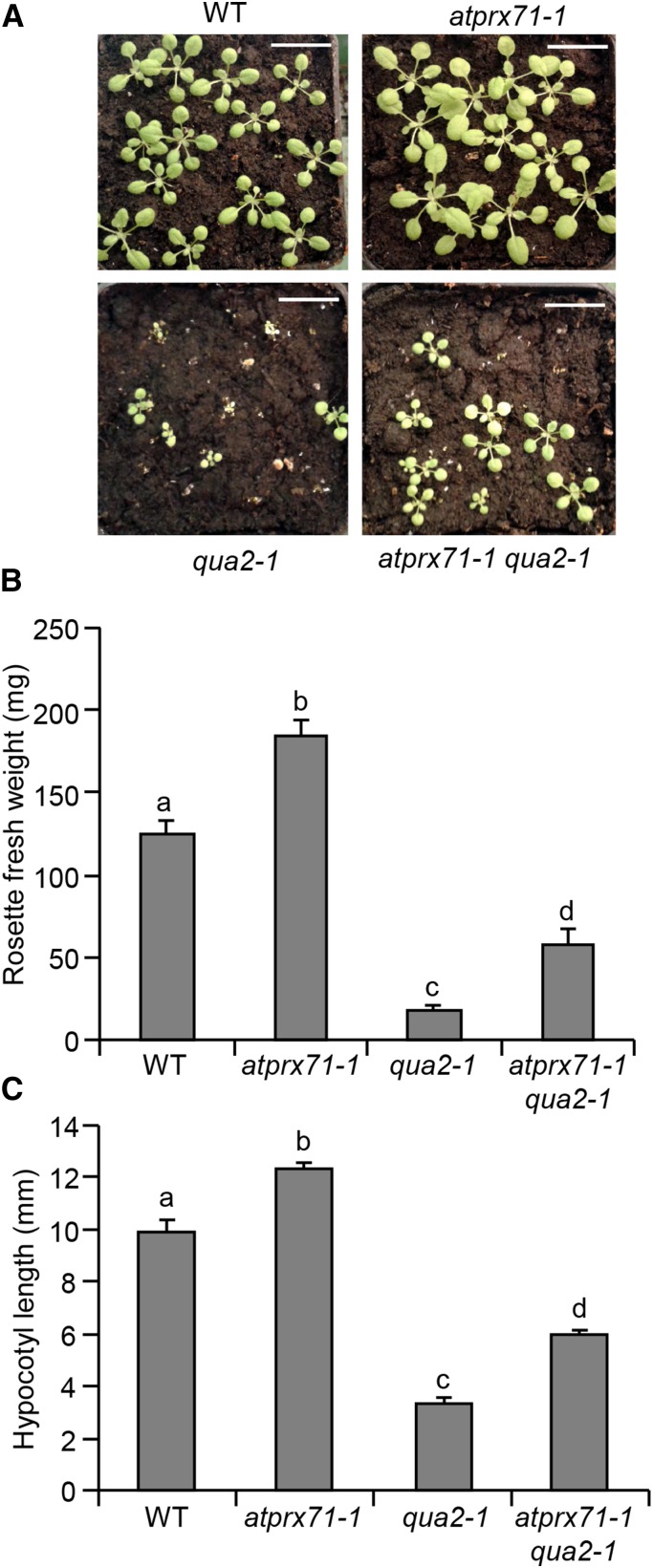
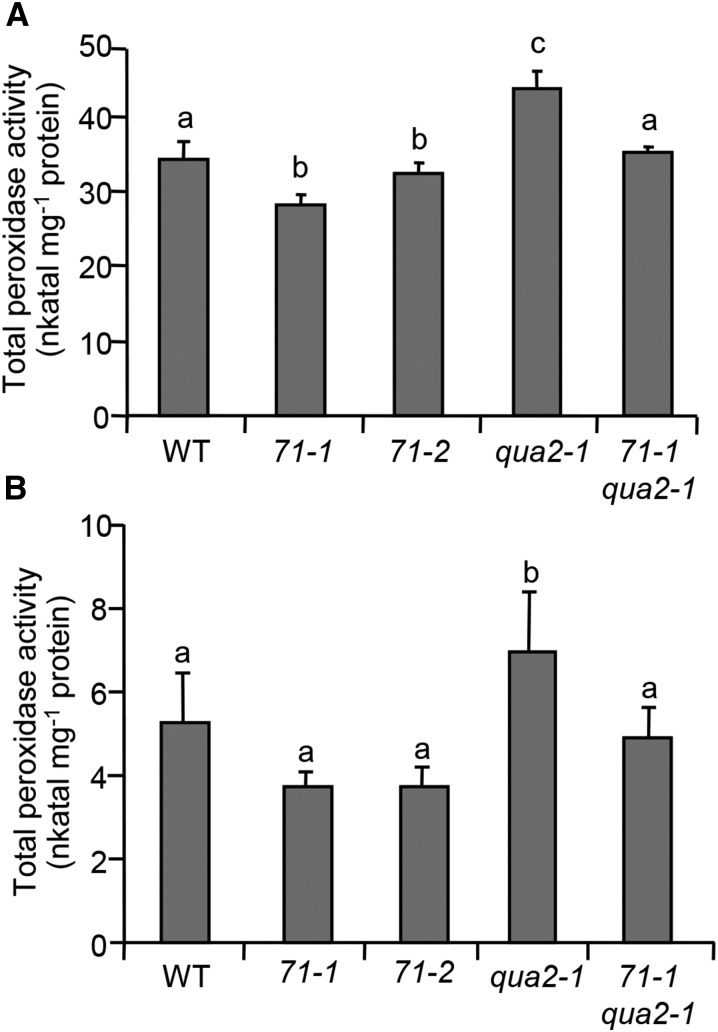
To determine if the elevated levels of expression of AtPRX71 contribute to the dwarf phenotype observed in plants with impaired CWI, we crossed atprx71-1 and 35S:AnPGII plants; however, expression of the transgene progressively declined in the subsequent generations, likely as a consequence of gene silencing, and homozygous F3 double-mutant plants did not show detectable PG activity (data not shown). For this reason, we crossed atprx71-1 with qua2-1 and analyzed the growth of homozygous double-mutant plants. Rosettes of soil-grown atprx71-1 qua2-1 plants were bigger (Fig. 6A), and rosette fresh weight was almost 4 times greater (Fig. 6B) than in single qua2-1 mutants. Hypocotyls of etiolated seedlings were also significantly longer in the double mutant compared with qua2-1 seedlings (Fig. 6C). Total guaiacol peroxidase activity was higher in qua2-1 plants than in the wild type at both seedling and adult stages, whereas activity in the qua2-1 atprx71-1 double mutant was similar to that of the wild type, although not as low as in the atprx71 mutants (Fig. 7). These data indicate that high levels of AtPRX71 expression contribute to the dwarf phenotype of qua2-1 plants, likely as a consequence of increased CIII Prx activity.
Figure 6.
Loss of AtPRX71 partially restores qua2-1 growth defects. A, Representative picture of wild-type (WT), atprx71-1, qua2-1, and atprx71-1 qua2-1 3-week-old soil-grown rosettes. Bar = 1 cm. B, Rosette fresh weight of wild-type, atprx71-1, qua2-1, and atprx71-1 qua2-1 4-week-old soil-grown plants. Bars indicate average weight ± se (n > 10). C, Hypocotyl length of 5-d-old wild-type, atprx71-1, and atprx71-2 etiolated seedlings grown in vitro on solid medium (n > 10). Different letters in B and C indicate significant differences according to one-way ANOVA followed by Tukey’s significance test (P < 0.05).
Figure 7.
Peroxidase activity in atprx71 and qua2-1 mutants. Total proteins were extracted from 10-d-old in vitro-grown seedlings (A) or rosette leaves from 4-week-old soil-grown plants (B) and assayed for peroxidase activity. Bars represent average enzyme activity (nanokatal milligram−1 protein) ± sd (n = 3). Letters indicate significant differences according to one-way ANOVA followed by Tukey’s significance test (P < 0.05). 71-1, atprx71-1; 71-2, atprx71-2; WT, wild type.
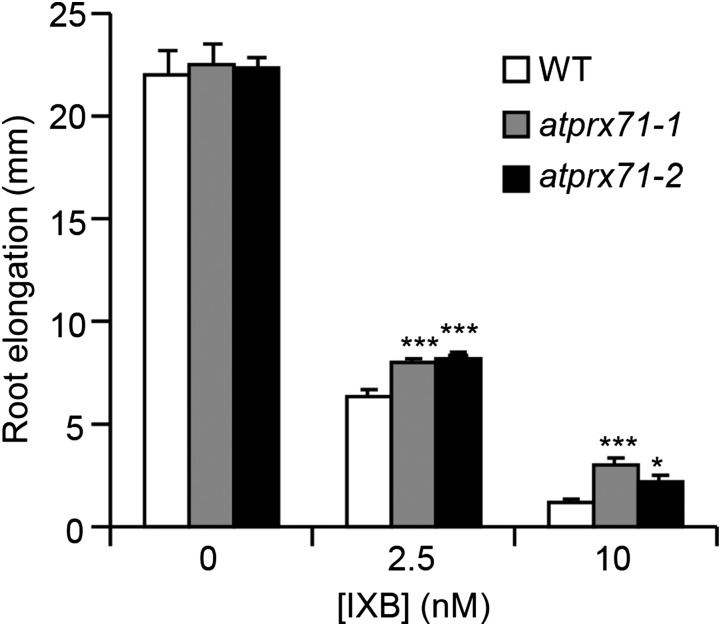
We also investigated whether AtPRX71 contributes to the growth defects caused by IXB treatment. Because this inhibitor strongly impairs Arabidopsis primary root elongation (Caño-Delgado et al., 2003; Tsang et al., 2011), we measured root length of wild-type, atprx71-1, and atprx71-2 seedlings grown for 3 d in the presence of 2.5 and 10 nm IXB. In the absence of IXB, root length was comparable in all three genotypes (Fig. 8), indicating that AtPRX71 does not play a major role in regulating the growth of this organ. However, root elongation of atprx71-1 and atprx71-2 seedlings was less inhibited by IXB compared with the wild type (Fig. 8), suggesting that increased expression of AtPRX71 triggered by the disruption of cellulose deposition contributes to restrict root growth. Transcript levels of two defense-related genes, PHYTOALEXIN DEFICIENT3 (PAD3/At3g26830), encoding an enzyme catalyzing the biosynthesis of camalexin (Zhou et al., 1999), and At1g26380 (RetOx), encoding a protein with homology to reticuline oxidases (Denoux et al., 2008), were also analyzed in wild-type, atprx71-1, and atprx71-2 seedlings to determine whether AtPRX71 is required also for IXB-induced gene expression. However, transcripts of both genes increased in response to IXB to a similar extent in wild-type and mutant plants (Supplemental Fig. S2). Therefore, partial resistance of atprx71-1 and atprx71-2 seedlings to IXB is likely not caused by an altered uptake or perception of the inhibitor. Taken together, these results lead to the conclusion that up-regulation of AtPRX71 expression contributes to the reduced growth observed in plants undergoing loss of CWI.
Figure 8.
Inhibition of root elongation by IXB is partially reduced in atprx71 mutants. Wild-type (WT), atprx71-1, and atprx71-2 seedlings grown for 5 d on solid medium were transferred to solid medium containing the indicated doses of IXB. Primary root elongation was measured after 3 d. Bars indicate average elongation ± se (n > 12). Asterisks indicate significant differences between the wild type and mutants according to Student’s t test. This experiment was repeated twice with similar results. *, P < 0.05; ***, P < 0.01.
AtPRX71 Promotes the Accumulation of ROS in Response to Cell Wall Alterations
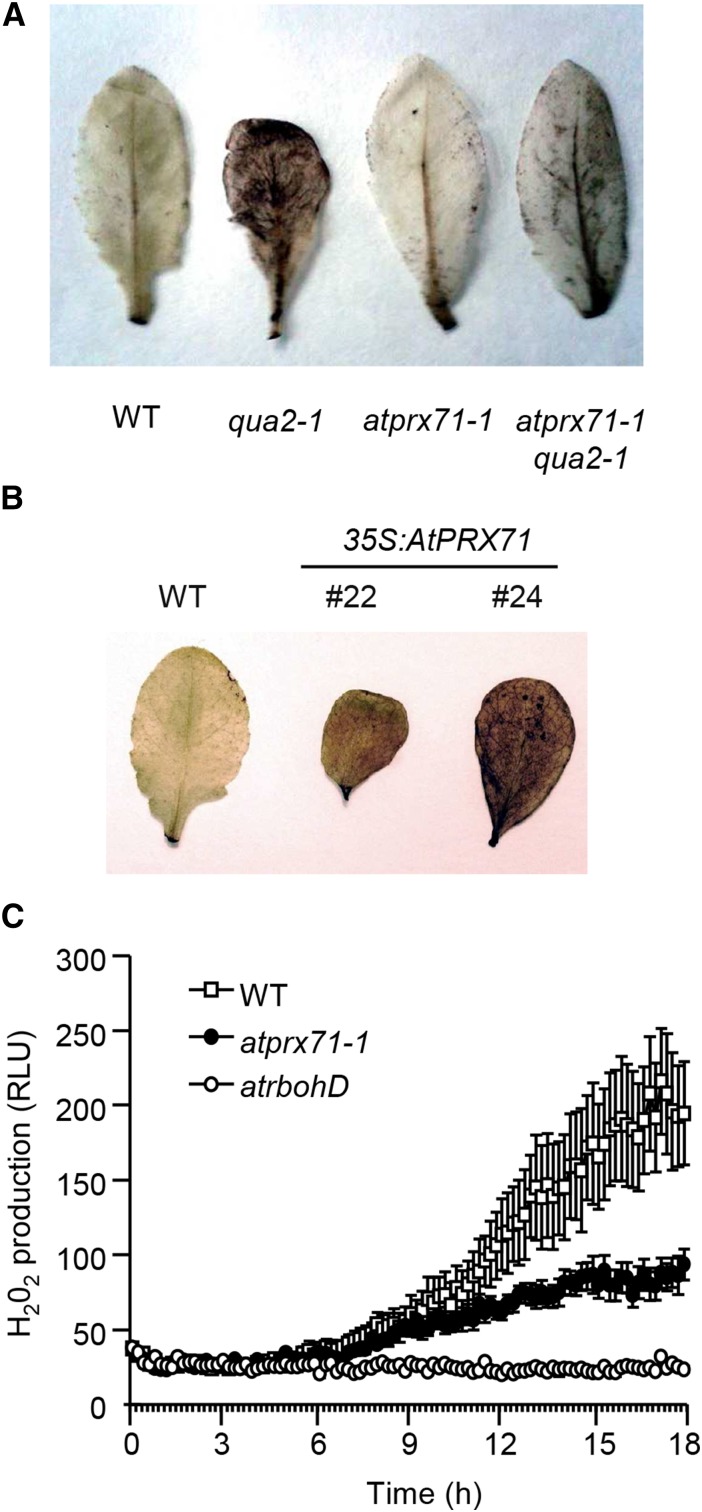
Because CIII Prxs can inhibit cell expansion by promoting the accumulation of H2O2 in the apoplast (Lu et al., 2014), we investigated whether high levels of AtPRX71 expression are associated with increased ROS accumulation. Staining of leaves of 35S:AnPGII plants with 3′,3′-diaminobenzidine (DAB) results in a strong brown coloration of the whole lamina, indicative of high levels of H2O2 and CIII Prxs (Ferrari et al., 2008). Here, we show that an intense DAB staining can also be observed in leaves of qua2-1 plants (Fig. 9A), indicating that accumulation of high level of H2O2 is a common feature of plants with altered pectin. Notably, staining of rosette leaves was strongly reduced in leaves of the atprx71-1 qua2-1 double mutant compared with qua2-1 plants (Fig. 9A). This observation suggests that elevated levels of expression of AtPRX71 are at least partially responsible for the accumulation of ROS caused by altered pectin composition. Consistently, leaves of 35S:AtPRX71 plants showed a strong DAB staining (Fig. 9B), supporting the conclusion that high levels of expression of AtPRX71 promote the accumulation of ROS.
Figure 9.
AtPRX71 promotes ROS accumulation in response to cell wall modifications. ROS accumulation in rosette leaves of 4-week-old qua2-1, atprx71-1, and atprx71-1 qua2-1 plants (A) and 35S:AtPRX71 plants belonging to lines 22 and 24 (B) was revealed by DAB staining. A representative picture for each genotype is shown. This experiment was repeated three (A) and two (B) times with similar results. C, Wild-type (WT), atprx71-1, and atrbohD seedlings grown for 3 d in liquid medium were treated with 600 nm IXB, and H2O2 production was measured every 10 min for 18 h with a luminol-based assay. Each point represents the average luminescence of 12 seedlings ± sd. This experiment was repeated three times with similar results. RLU, Relative luminescence unit.
It has been reported that IXB induces the accumulation of H2O2 in Arabidopsis seedlings and that this accumulation is dependent on the NADPH oxidase AtrbohD (Denness et al., 2011). To determine whether AtPRX71 also contributes to this IXB-induced oxidative burst, wild-type and atprx7-1 seedlings were treated with IXB using the atrbohD mutant as a negative control. H2O2 started to accumulate in the wild type 3 to 4 h after treatment and steadily increased at least for the subsequent 14 to 15 h, whereas as expected, its accumulation was completely abolished in atrbohD seedlings (Fig. 9C). H2O2 production in atprx71-1 and wild-type seedlings was comparable during the first 10 to 12 h of treatment, but then it increased much more slowly in the mutant, and after 18 h of treatment it was about one-half of that observed in the wild type (Fig. 9C). This observation leads to the conclusion that AtPRX71 is not necessary for the initial onset of the IXB-induced oxidative burst, but it is required to ensure a sustained production of high levels of H2O2 in the apoplast. Taken together, these data indicate that AtPRX71 contributes to the accumulation of ROS in plants with altered cell walls.
Production of ROS mediated by AtPRX71 may affect not only growth but also resistance to biotic stresses. Indeed, it was previously reported that Arabidopsis plants overexpressing AtPRX71 show increased resistance to the fungus Botrytis cinerea (Chassot et al., 2007), suggesting that this CIII Prx can protect against pathogen infections. However, susceptibility of both atprx71-1 and atprx71-2 mutants to B. cinerea was comparable with that of the wild type (Supplemental Fig. S3), indicating that lack of AtPRX71 alone is not sufficient to impair resistance to this fungus.
DISCUSSION
The plant cell wall is constantly subjected to modification and reorganization, both under physiological conditions and in response to abiotic and biotic stresses (Cheung and Wu, 2011). These changes allow cells to adapt their shape and size during development and in response to environmental stimuli. However, changes in cell wall composition can have detrimental effects, and plants, therefore, must constantly adjust cell walls to maintain their mechanical properties. Increasing evidence indicates that plants possess one or more mechanisms to perceive changes in the wall that may undermine its integrity and activate proper responses, including new structural modifications aimed at maintaining CWI (Hamann, 2012). CIII Prxs are likely among the effectors of this CWI maintenance system, contributing to strengthen the cell wall and, as a consequence, limiting cell expansion and plant growth. Indeed, CIII Prxs play important roles in the regulation of cell wall mechanical properties and cell expansion (Passardi et al., 2004, 2005). Here, we have shown that expression of an Arabidopsis gene encoding a CIII Prxs, namely AtPRX71, is elevated in plants with altered pectin composition (35S:AnPGII and qua2-1 plants) or impaired in cellulose deposition (plants treated with IXB) and that lack of AtPRX71 partially restores growth defects observed in these plants. Moreover, AtPRX71 limits growth not only upon alterations of CWI but also under physiological conditions. Finally, AtPRX71 likely acts by promoting the accumulation of apoplastic ROS, which in turn may catalyze the formation of cross links, therefore stiffening the cell wall and restricting cell expansion.
The conclusion that AtPRX71 encodes a CIII Prx that negatively affects growth is based on the observations that reduced expression of this gene leads to both reduced levels of peroxidase activity and increased rosette size and biomass, whereas 35S:AnPGII plants show high levels of peroxidase activity and a significant reduction of biomass. Our findings are in agreement with previous reports describing other Arabidopsis CIII Prxs having negative effects on growth. For instance, overexpression of AtPRX37 causes dwarfism (Pedreira et al., 2011), and plants mutated for or overexpressing AtPRX53 exhibit longer or shorter hypocotyls, respectively (Jin et al., 2011). We also observed enhanced hypocotyl elongation in atprx71-1 and atprx71-2 etiolated seedlings; this latter result indicates that AtPRX71 regulates plant growth by restricting cell size rather than cell number, because it has been shown that elongation of etiolated hypocotyls occurs mainly by cell expansion (Gendreau et al., 1997). This conclusion is supported by the observation that, compared with the wild type, plants with reduced or increased levels of expression of AtPRX71 display increased or decreased leaf epidermal cell size, respectively. Indeed, it has been recently reported that increased peroxidase activity is responsible for the defective cell expansion caused by the kuoda1 mutant, which is impaired in a myeloblastosis oncoprotein (MYB)-like transcriptional repressor of different CIII Prx genes (Lu et al., 2014).
In addition to its role as a negative regulator of growth during normal development, AtPRX71 seems to limit growth in plants with altered pectin composition, which was initially suggested by the elevated expression of this gene and the increased CIII Prx activity detected in 35S:AnPGII plants (Ferrari et al., 2008) and qua2-1 (this work) and subsequently, confirmed by the observation that the constitutively high peroxidase activity and the dwarf phenotype of qua2-1 plants are partially reverted by the atprx71-1 mutation. Furthermore, AtPRX71 seems to contribute to the growth defects caused by an altered cellulose deposition, because roots of atprx71 seedlings are partially resistant to the inhibitory effect of IXB. Indeed, it has been previously shown that bean (Phaseolus vulgaris) cells habituated to dichlobenil, another inhibitor of cellulose synthesis, show increased guaiacol Prx activity (García-Angulo et al., 2009). It is, therefore, likely that accumulation of CIII Prxs is a conserved response of higher plants to cell wall damage.
The effects of AtPRX71 on Arabidopsis cell expansion and growth are likely mediated by its ability to promote the accumulation of apoplastic H2O2. Four lines of evidence support this hypothesis: (1) leaves of plants overexpressing AtPRX71 accumulate high levels of ROS, (2) leaves of plants with altered pectin composition (35S:AnPGII plants and qua2-1) also accumulate high levels of ROS, (3) the qua2-1 atprx71-1 double mutant shows reduced ROS accumulation compared with qua2-1, and (4) production of H2O2 in Arabidopsis seedlings treated with IXB is partially dependent on AtPRX71. ROS-mediated cross linking of wall structural components may be directly or indirectly catalyzed by AtPRX71 and, possibly, other CIII Prx isoforms to counteract cell wall damage. This mechanism may also act during normal development; indeed, ROS homeostasis controlled by CIII Prxs has been proposed to regulate leaf size in Arabidopsis (Lu et al., 2014). Notably, AtPRX71 affects only the late phase of H2O2 production in response to IXB, indicating that this and possibly, other CIII Prxs may not be required for the initial onset of the oxidative burst triggered by cell wall damage, which is indeed dependent on NAPDH oxidases (Denness et al., 2011). However, CIII Prx may be necessary to maintain a robust and prolonged production of ROS for longer times after the initial burst is triggered.
The exact biochemical mechanism responsible for the effects of AtPRX71 on Arabidopsis growth still needs to be elucidated. AtPRX71 has been implicated in stem lignification, although no alterations in stem lignin content but just a slight increase in the syringyl/guaiacyl lignin ratio was observed in knockout plants for this gene (Shigeto et al., 2013). Consistently, we did not detect significant differences in stem length and weight between wild-type and atprx71 plants, confirming that AtPRX71 has a negligible role in the lignification, at least in this organ. Characterization of AtPRX71 activity in vitro, however, indicates that this protein is able to oxidize 2,6-dimethoxyphenol and syringaldazine, which are model monolignol compounds (Shigeto et al., 2014), suggesting that this PRX may promote polymerization of lignin under specific conditions or in specific tissues. Moreover, AtPRX71 is also able to form protein radicals in vitro (Shigeto et al., 2014); the significantly increased expression of EXT4 observed in 35S:AnPGII plants, therefore, suggests that AtPRX71-mediated extensin cross links may be formed in response to altered CWI to strengthen the cell wall.
In addition to their role in plant growth, ROS-producing CIII Prxs have also been implicated in defense against pathogens. In Arabidopsis, AtPRX33 and AtPRX34 contribute to a significant proportion of the H2O2 generated in response to microbe-associated molecular patterns and are required for resistance to different pathogens and for the activation of some defense responses (Bindschedler et al., 2006; Daudi et al., 2012; O’Brien et al., 2012) and in particular, salicylic acid-mediated gene expression (Mammarella et al., 2015). ROS generated by AtPRX33 and AtPRX34 during infection can therefore participate in Arabidopsis defense not only by promoting cell wall reinforcement but also indirectly through the modulation of the immune response. Additional CIII Prx isoforms, including AtPRX71, that are able to favor the accumulation of ROS in the apoplast, may also contribute to Arabidopsis resistance against microbes. Indeed, it was previously shown that overexpression of AtPRX71 as well as two other CIII Prx genes confers strong resistance to B. cinerea infection (Chassot et al., 2007). However, AtPRX71 is dispensable for basal resistance to this pathogen, because atprx71-1 and atprx71-2 plants show wild-type susceptibility (this work). This apparent incongruence can be explained if expression of multiple CIII Prxs is induced during infection, acting redundantly to promote ROS production and resistance to microbes. If this is the case, lack of a single isoform would not significantly decrease the plant ability to limit pathogen growth; however, overexpression of any of these Prxs would be sufficient to boost accumulation of ROS, which we have observed in the case of 35S:AtPRX71 plants, therefore conferring a strong resistant phenotype.
The results presented in this work indicate that modifications of different cell wall structural components increase the expression of AtPRX71, which in turns, leads to ROS production and cell wall reinforcement. This implies that CWI perturbations may activate common responses, regardless of the specific component that is affected. For example, alterations of different structural polysaccharides would result in cell wall weakening, which may be perceived through the stretching of the plasma membrane under the effect of turgor pressure (Hamann, 2012). Mechanosensitive channels may thus be activated and trigger downstream responses, including accumulation of CIII Prxs. This model is supported by the observation that some phenotypes induced by IXB, such as ectopic deposition of lignin, can be reverted by osmotic support (Hamann et al., 2009). Moreover, hypoosmotic stress alone can lead to AtPRX71-mediated production of ROS (Rouet et al., 2006), suggesting that changes in turgor pressure can directly induce the expression of this peroxidase. Alternatively, loss of CWI may result in the release of elicitor-active cell wall fragments, which may be responsible for the induction of AtPRX71 expression. For instance, oligogalacturonides produced by partial degradation of HG trigger the activation of several defense responses in Arabidopsis (Ferrari et al., 2013; Benedetti et al., 2015), including the expression of AtPRX71 (Ferrari et al., 2007; Denoux et al., 2008). Further studies are required to discriminate between these two hypotheses, which are not, however, mutually exclusive.
Other than providing unique insights into the biological mechanisms regulating plant growth, our findings can be relevant to improve crops dedicated to the production of biofuels and other industrial products. Increasing evidence supports the idea that targeted modifications of cell wall structural components can increase the efficiency of conversion of lignocellulosic biomasses (Chen and Dixon, 2007; Kaida et al., 2009; Selig et al., 2009; Lionetti et al., 2010). Knowledge about how to manipulate cell wall composition without detrimental effects on growth and resistance to stresses is, therefore, needed. We have previously shown that 35S:AnPGII and qua2-1 plants are more efficiently degraded by cellulase (Lionetti et al., 2010; Francocci et al., 2013), indicating that pectin composition strongly affects biomass utilization. However, a major reduction in HG content severely impairs growth, strongly limiting the application of this approach. According to the results presented here, the detrimental effects of pectin modification on growth might be avoided by reducing the expression of specific CIII Prx isoforms. Notably, we found that lack of AtPRX71 increases biomass production without negative effects on resistance to pathogens, at least in the case of B. cinerea. Because CIII Prxs also negatively regulate saccharification efficiency (Kavousi et al., 2010), crops with both targeted modifications of cell wall composition and reduced levels of selected CIII Prxs may be more efficiently processed without detrimental effects or even a positive impact on biomass production.
In conclusion, our results indicate that wall stiffening and the consequent inhibition of growth mediated by CIII Prxs like AtPRX71 may be an important component of CWI maintenance (Hamann, 2012), both during physiological developmental processes (e.g. expansion of new organs) and in response to stresses (e.g. wounding or pathogen attack). These findings provide unique insights into the mechanisms of regulation of plant growth and may be used in the development of crop varieties with improved biomass production and utilization.
MATERIALS AND METHODS
Biological Material and Growth Conditions
All Arabidopsis (Arabidopsis thaliana) plants used in this work are in the Columbia-0 (Col-0) background. Wild-type Col-0 seeds were obtained from Lehle Seeds. The insertion lines atprx71-1 (SALK_123643) and atprx71-2 (SALK_121202C) were obtained from the Salk Institute Genomic Analysis Laboratory (Alonso et al., 2003). The generation of Col-0 plants expressing the Aspergillus niger PGII gene (35S:AnPGII plants; lines 26 and 57) and the isolation and characterization of the qua2-1 mutant have been previously described (Mouille et al., 2007; Lionetti et al., 2010).
Plants were grown in soil (Einheitserde) in a growth chamber at 22°C and 70% relative humidity with a 16-h-light/8-h-dark photoperiod using fluorescent lamps (Osram). Light intensity was about 120 μmol m−2 s−1. Before sowing, seeds were always stratified in sterile water for 3 d in the dark at +4°C.
For in vitro growth, seeds were surface sterilized as previously described (Ferrari et al., 2007) and stratified as described above. For growth on solid medium, seeds were germinated on plates containing one-half-strength Murashige and Skoog medium (MS; Murashige and Skoog, 1962) basal salts supplemented with 1% (w/v) Suc unless otherwise stated and 0.7% (w/v) plant agar, pH 5.0. For analysis of gene expression after IXB treatments, sterilized seeds were distributed in 12-well plates (about 10 seeds per well) containing 1 mL of 2.2 g L−1 basal MS supplemented with 0.5% (w/v) Suc, pH 5.5. After 9 d, the medium was replaced with fresh medium, and after 1 d, seedlings were treated for 24 h with filter-sterilized 0.01% (v/v) dimethyl sulfoxide or IXB (Sigma) at the indicated concentrations.
Gene Expression Analysis
Leaves or seedlings were frozen in liquid nitrogen and homogenized with an MM301 Ball Mill (Retsch) for about 2 min at 30 Hz. Total RNA was extracted with Isol-RNA Lysis Reagent (5′-Prime) according to the manufacturer’s instructions.
Full-genome transcriptome analysis was performed on custom Arabidopsis 28K v2.0 Microarrays (GenBank GEO Platform ID no. GPL15543) synthesized using a CombiMatrix B3 Synthesizer (Combimatrix) according to manufacturer’s instructions. Total RNA (1 µg) was amplified, and 6 µg of antisense RNA was labeled using the RNA AmpULSe Amplification and Labeling Kit with Cy5 for CombiMatrix Arrays (Kreatech Biotechnology) according to the manufacturer’s instructions. Labeled samples were hybridized to the microarrays according to CombiMatrix protocols. Scanning was performed on a GenePix 4000B Scanner (Molecular Devices). Data extraction was done using Microarray Imager software (Combimatrix), and quantile normalization of data was performed using Blist v0.6 software (Combimatrix). Differentially expressed transcripts were identified using Limma R package using an FDR < 0.05 and a module of logged FC on base 2 |log2 FC| ≥ 1 (Smyth, 2004). Expression data are available from the National Center for Biotechnology Information (GEO accession no. GSE66980). Gene Ontology categories enrichment of differentially expressed genes was determined with the aid of the AgriGO Toolkit (Du et al., 2010).
For reverse transcription PCR analysis, RNA was treated with RQ1 DNase (Promega), and first-strand complementary DNA was obtained using Improm II Reverse Transcriptase (Promega). PCR was performed using Taq DNA Polymerase (RBC-Bioscience) with primers specific for the genes of interest (Supplemental Table S1) using the following conditions: 94°C for 2 min; 28 cycles as follows: 30 s at 94°C, 30 s at 58°C, and 30 s at 72°C; and 72°C for 5 min. Real-time PCR was performed using a CFX96 Real-Time System (Bio-Rad). Complementary DNA (corresponding to 50 ng of total RNA) was amplified using GoTaq qPCR Master Mix (Promega) and 0.4 mm each primer. Expression levels of each gene, relative to UBIQUITIN5 (UBQ5) were determined using a modification of the method by Pfaffl (2001)) as previously described (Ferrari et al., 2006).
Plant Growth Measurement
Rosette fresh weight was measured in 4-week-old soil-grown plants. Dry weight measurements were performed on the same material after incubation for 8 h at 60°C. Fresh weight of seedlings was determined after 10 d of growth in solid MS.
For measurements of hypocotyls in etiolated seedlings, sterilized seeds were sown on solid MS containing 1% (w/v) plant agar. Plates were incubated for 4 h under constant light at 25°C, wrapped with aluminum foil, and transferred to a growth chamber. Hypocotyl length was measured after incubation in the dark at 22°C for 5 d.
For cell area determination, the fifth leaf of 3-week-old soil-grown plants (at least four plants for genotype) was harvested, and chlorophyll was extracted in 100% boiling ethanol. Cleared leaves were stained with 0.05% (w/v) ruthenium red. Excess dye was removed by washing leaves with water. Leaves were mounted in 20% (v/v) glycerol and examined using a Nikon Eclipse E200 Microscope. Images were taken with a Nikon Digital Sight DS-Fi1c Camera, and cell area was measured using ImageJ (http://rsbweb.nih.gov/ij/index.html).
Fungal Infections
Growth of Botrytis cinerea and inoculation of Arabidopsis leaves were conducted as previously described (Ferrari et al., 2003).
Isolation and Genotyping of Mutants
Genomic DNA was extracted from 4-week-old plants using the method by Edwards et al. (1991). Genomic DNA was subjected to PCR to detect the wild-type allele of AtPRX71 using 71-1RP and 71-1LP primers (Supplemental Table S1) for atprx71-1 and 71-2RP and 71-2LP for atprx71-2. To detect the insertion of the T-DNA, the Lba1 primer (Supplemental Table S1) was used in place of the LP primer. Samples were subjected to amplification using Taq DNA Polymerase (RCB-Bioscience) under the following conditions: 2 min at 94°C; 35 cycles as follows: 30 s at 94°C, 30 s at 60°C, and 45 s at 72°C; and 7 min at 72°C. PCR products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining.
Generation of double atprx71-1 qua2-1 mutants was achieved by crossing of single-mutant plants. F2 individuals were screened for the atprx71-1 mutation as described above, whereas the qua2-1 mutation was detected by high-resolution DNA melting analysis (Vossen et al., 2009). Briefly, genomic DNA was amplified by real-time PCR as described above with the QUA2FW and QUA2REV primers (Supplemental Table S1) using an annealing temperature of 59°C. Melting curves of the PCR products from 70°C to 80°C were obtained using 0.2°C increments in temperature at each fluorescence measurement step.
Generation of Plants Overexpressing AtPRX71
The AtPRX71 genomic region, starting from the predicted translation start codon and ending in correspondence of the predicted translation stop codon, was amplified using Col-0 genomic DNA as a template. PCR was performed with High-Fidelity PCR Master Mix (Roche) using the OEXPOX1FW and OEXPOX1REV primers (Supplemental Table S1). The purified PCR fragment was cloned into the pGEM T-Easy Vector (Promega), and the obtained plasmid was introduced in Escherichia coli DH5α by electroporation. The insert was excised from the plasmid using XbaI and SacI (Fermentas), gel purified, and subcloned into the binary vector pBI121 (Stratagene) in place of the beta-glucuronidase uidA gene. After ligation and electroporation into E. coli DH5α cells, the obtained plasmid (pBI121-AtPRX71), which contains the AtPRX71 gene under the control of the Cauliflower mosaic virus 35S promoter, was introduced in Agrobacterium tumefaciens GV3101 cells. Arabidopsis Col-0 plants were transformed with the obtained bacterial strain using the floral dip method (Clough and Bent, 1998). Transformants were selected on solid MS plates supplemented with 50 μg mL−1 kanamycin.
Oxidative Burst Assay
For determination of the oxidative burst elicited by IXB, a luminol-based protocol (Denness et al., 2011) was used. Arabidopsis seedlings were grown in sterile 96-well plates (Costar) on Gamborg’s medium supplemented with 1% (w/v) Suc, pH 5.5, for 6 d at 23°C. Before the experiment, the medium was removed, and 100 μL of assay solution (17 mm luminol [Sigma], 1 μm horseradish PRX [Sigma], and 600 nM IXB) was added to each well. Plates were placed in a GloMax 96 Microplate Luminometer (Promega), and luminescence was measured for 18 h at 10-min intervals using an integration time of 1 s.
Histochemical Analyses
For H2O2 visualization, rosette leaves from 4-week-old plants were incubated for 12 h in a solution containing 1 mg mL−1 DAB, pH 5.0. Chlorophyll was extracted for 10 min with boiling ethanol and 2 h with ethanol at room temperature before photography (Orozco-Cardenas and Ryan, 1999).
Measurement of CIII Prx Activity
Soluble proteins were extracted from 10-d-old seedlings and 4-week-old rosette leaves by grinding in the following extraction buffer (1 mL for 100 mg of seedlings and 50 µL for 100 mg of leaves): 20 mm HEPES, pH 7.0, 1 mm EGTA, 10 mm vitamin C, and PVP PolyclarAT (100 mg g−1 fresh weight). The extract was centrifuged two times (10 min at 10,000g and 4°C) to remove insoluble material. Subsequent experiments were carried out on the supernatant. The protein content was determined using the Bradford Reagent (Serva) with bovine serum albumin (Sigma-Aldrich) as a standard (Bradford, 1976). PRX activity was measured at 25°C by following the oxidation of 8 mm guaiacol (Fluka) at 470 nm in the presence of 2 mm H2O2 (Carlo Erba) in a phosphate buffer (200 mm, pH 6.0).
Sequence data from this article are as follows: At5g64120 (AtPRX71), At3g62250 (UBQ5), At3g26830 (PAD3), and At1g26380 (RetOx).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Lack of AtPRX71 does not affect stem growth.
Supplemental Figure S2. Gene expression induced by IXB is not altered in atprx71 plants.
Supplemental Figure S3. AtPRX71 is not required for basal resistance to B. cinerea.
Supplemental Table S1. Primers used in this work.
Acknowledgments
We thank Dr. Grégory Mouille and Stéphane Verger (Institut National de la Recherche Agronomique, Centre de Recherche de Versailles-Grignon, France) for providing qua2-1 seeds and assistance in the genotyping of this mutant.
Glossary
- Col-0
Columbia-0
- CWI
cell wall integrity
- DAB
3′,3′-diaminobenzidine
- FC
fold change
- FDR
false discovery rate
- GEO
Gene Expression Omnibus
- H2O2
hydrogen peroxide
- HG
homogalacturona
- IXB
isoxaben
- MS
Murashige and Skoog medium
- qPCR
quantitative PCR
- ROS
reactive oxygen species
- T-DNA
transfer DNA
- XG
xyloglucan
Footnotes
This work was supported by the Fondazione Cariverona Completamento e attività del Centro di Genomica Funzionale Vegetale (to A.F. and M.D.), the Université Paul Sabatier Toulouse 3 (to C.D.), the Centre National de la Recherche Scientifique (to P.R.), the Ministero delle Politiche Agricole, Alimentari e Forestali Grant BIOMASSVAL (to F.C.), and the Institute Pasteur-Fondazione Cenci Bolognetti (to F.C.).
Articles can be viewed without a subscription.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Benedetti M, Pontiggia D, Raggi S, Cheng Z, Scaloni F, Ferrari S, Ausubel FM, Cervone F, De Lorenzo G (2015) Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc Natl Acad Sci USA 112: 5533–5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindschedler LV, Dewdney J, Blee KA, Stone JM, Asai T, Plotnikov J, Denoux C, Hayes T, Gerrish C, Davies DR, et al. (2006) Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J 47: 851–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F, Rowntree EG, Wojtaszek P (1999) Recent advances in understanding the origin of the apoplastic oxidative burst in plant cells. Free Radic Res (Suppl) 31: S137–S145 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344: 1879–1900 [DOI] [PubMed] [Google Scholar]
- Caño-Delgado A, Penfield S, Smith C, Catley M, Bevan M (2003) Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J 34: 351–362 [DOI] [PubMed] [Google Scholar]
- Capodicasa C, Vairo D, Zabotina O, McCartney L, Caprari C, Mattei B, Manfredini C, Aracri B, Benen J, Knox JP, et al. (2004) Targeted modification of homogalacturonan by transgenic expression of a fungal polygalacturonase alters plant growth. Plant Physiol 135: 1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N, McCann M (2000) The cell wall. In Buchanan BB, Gruissem W, Jones RL, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 52–108 [Google Scholar]
- Chassot C, Nawrath C, Métraux JP (2007) Cuticular defects lead to full immunity to a major plant pathogen. Plant J 49: 972–980 [DOI] [PubMed] [Google Scholar]
- Chen F, Dixon RA (2007) Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol 25: 759–761 [DOI] [PubMed] [Google Scholar]
- Chen SX, Schopfer P (1999) Hydroxyl-radical production in physiological reactions. A novel function of peroxidase. Eur J Biochem 260: 726–735 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2011) THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr Opin Plant Biol 14: 632–641 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2000) Expansive growth of plant cell walls. Plant Physiol Biochem 38: 109–124 [DOI] [PubMed] [Google Scholar]
- Cosio C, Dunand C (2009) Specific functions of individual class III peroxidase genes. J Exp Bot 60: 391–408 [DOI] [PubMed] [Google Scholar]
- Crowell EF, Gonneau M, Vernhettes S, Höfte H (2010) Regulation of anisotropic cell expansion in higher plants. C R Biol 333: 320–324 [DOI] [PubMed] [Google Scholar]
- Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24: 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denness L, McKenna JF, Segonzac C, Wormit A, Madhou P, Bennett M, Mansfield J, Zipfel C, Hamann T (2011) Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol 156: 1364–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J (2008) Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol Plant 1: 423–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38: W64–W70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunand C, de Meyer M, Crevecoeur M, Penel C (2003) Expression of a peroxidase gene in zucchini in relation with hypocotyl growth. Plant Physiol Biochem 41: 805–811 [Google Scholar]
- Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J (2007) Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol 144: 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Pontiggia D, Manfredini C, Lionetti V, Bellincampi D, Cervone F, De Lorenzo G (2008) Transgenic expression of a fungal endo-polygalacturonase increases plant resistance to pathogens and reduces auxin sensitivity. Plant Physiol 146: 669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Vairo D, Cervone F, De Lorenzo G (2006) Antisense expression of the Arabidopsis thaliana AtPGIP1 gene reduces polygalacturonase-inhibiting protein accumulation and enhances susceptibility to Botrytis cinerea. Mol Plant Microbe Interact 19: 931–936 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM (2003) Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 35: 193–205 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Savatin DV, Sicilia F, Gramegna G, Cervone F, Lorenzo GD (2013) Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front Plant Sci 4: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francocci F, Bastianelli E, Lionetti V, Ferrari S, De Lorenzo G, Bellincampi D, Cervone F (2013) Analysis of pectin mutants and natural accessions of Arabidopsis highlights the impact of de-methyl-esterified homogalacturonan on tissue saccharification. Biotechnol Biofuels 6: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ (1992) Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem J 282: 821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141: 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Angulo P, Alonso-Simón A, Mélida H, Encina A, Acebes JL, Alvarez JM (2009) High peroxidase activity and stable changes in the cell wall are related to dichlobenil tolerance. J Plant Physiol 166: 1229–1240 [DOI] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T. (2012) Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front Plant Sci 3: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Bennett M, Mansfield J, Somerville C (2009) Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J 57: 1015–1026 [DOI] [PubMed] [Google Scholar]
- Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42: 462–468 [DOI] [PubMed] [Google Scholar]
- Iiyama K, Lam T, Stone BA (1994) Covalent cross-links in the cell wall. Plant Physiol 104: 315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Hewezi T, Baum TJ (2011) Arabidopsis peroxidase AtPRX53 influences cell elongation and susceptibility to Heterodera schachtii. Plant Signal Behav 6: 1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida R, Kaku T, Baba K, Oyadomari M, Watanabe T, Nishida K, Kanaya T, Shani Z, Shoseyov O, Hayashi T (2009) Loosening xyloglucan accelerates the enzymatic degradation of cellulose in wood. Mol Plant 2: 904–909 [DOI] [PubMed] [Google Scholar]
- Kavousi B, Daudi A, Cook CM, Joseleau JP, Ruel K, Devoto A, Bolwell GP, Blee KA (2010) Consequences of antisense down-regulation of a lignification-specific peroxidase on leaf and vascular tissue in tobacco lines demonstrating enhanced enzymic saccharification. Phytochemistry 71: 531–542 [DOI] [PubMed] [Google Scholar]
- Keegstra K. (2010) Plant cell walls. Plant Physiol 154: 483–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V, Francocci F, Ferrari S, Volpi C, Bellincampi D, Galletti R, D’Ovidio R, De Lorenzo G, Cervone F (2010) Engineering the cell wall by reducing de-methyl-esterified homogalacturonan improves saccharification of plant tissues for bioconversion. Proc Natl Acad Sci USA 107: 616–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Wang T, Persson S, Mueller-Roeber B, Schippers JH (2014) Transcriptional control of ROS homeostasis by KUODA1 regulates cell expansion during leaf development. Nat Commun 5: 3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammarella ND, Cheng Z, Fu ZQ, Daudi A, Bolwell GP, Dong X, Ausubel FM (2015) Apoplastic peroxidases are required for salicylic acid-mediated defense against Pseudomonas syringae. Phytochemistry 112: 110–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjamaa K, Kukkola EM, Fagerstedt KV (2009) The role of xylem class III peroxidases in lignification. J Exp Bot 60: 367–376 [DOI] [PubMed] [Google Scholar]
- Micheli F. (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6: 414–419 [DOI] [PubMed] [Google Scholar]
- Mirabet V, Das P, Boudaoud A, Hamant O (2011) The role of mechanical forces in plant morphogenesis. Annu Rev Plant Biol 62: 365–385 [DOI] [PubMed] [Google Scholar]
- Mouille G, Ralet MC, Cavelier C, Eland C, Effroy D, Hématy K, McCartney L, Truong HN, Gaudon V, Thibault JF, et al. (2007) Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. Plant J 50: 605–614 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) Revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15: 437–479 [Google Scholar]
- O’Brien JA, Daudi A, Finch P, Butt VS, Whitelegge JP, Souda P, Ausubel FM, Bolwell GP (2012) A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol 158: 2013–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96: 6553–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24: 255–265 [DOI] [PubMed] [Google Scholar]
- Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9: 534–540 [DOI] [PubMed] [Google Scholar]
- Pedreira J, Herrera MT, Zarra I, Revilla G (2011) The overexpression of AtPrx37, an apoplastic peroxidase, reduces growth in Arabidopsis. Physiol Plant 141: 177–187 [DOI] [PubMed] [Google Scholar]
- Pelloux J, Rustérucci C, Mellerowicz EJ (2007) New insights into pectin methylesterase structure and function. Trends Plant Sci 12: 267–277 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet MA, Mathieu Y, Barbier-Brygoo H, Laurière C (2006) Characterization of active oxygen-producing proteins in response to hypo-osmolarity in tobacco and Arabidopsis cell suspensions: identification of a cell wall peroxidase. J Exp Bot 57: 1323–1332 [DOI] [PubMed] [Google Scholar]
- Scheible WR, Eshed R, Richmond T, Delmer D, Somerville C (2001) Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis Ixr1 mutants. Proc Natl Acad Sci USA 98: 10079–10084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabelrauch LS, Kieliszewski M, Upham BL, Alizedeh H, Lamport DT (1996) Isolation of pl 4.6 extensin peroxidase from tomato cell suspension cultures and identification of Val-Tyr-Lys as putative intermolecular cross-link site. Plant J 9: 477–489 [DOI] [PubMed] [Google Scholar]
- Selig MJ, Adney WS, Himmel ME, Decker SR (2009) The impact of cell wall acetylation on corn stover hydrolysis by cellulolytic and xylanolytic enzymes. Cellulose (Lond) 16: 711–722 [Google Scholar]
- Shigeto J, Itoh Y, Hirao S, Ohira K, Fujita K, Tsutsumi Y (2015) Simultaneously disrupting AtPrx2, AtPrx25 and AtPrx71 alters lignin content and structure in Arabidopsis stem. J Integr Plant Biol 57: 349–356 [DOI] [PubMed] [Google Scholar]
- Shigeto J, Kiyonaga Y, Fujita K, Kondo R, Tsutsumi Y (2013) Putative cationic cell-wall-bound peroxidase homologues in Arabidopsis, AtPrx2, AtPrx25, and AtPrx71, are involved in lignification. J Agric Food Chem 61: 3781–3788 [DOI] [PubMed] [Google Scholar]
- Shigeto J, Nagano M, Fujita K, Tsutsumi Y (2014) Catalytic profile of Arabidopsis peroxidases, AtPrx-2, 25 and 71, contributing to stem lignification. PLoS One 9: e105332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK. (2004) Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Molec Biol 3: 1–25 [DOI] [PubMed] [Google Scholar]
- Tan L, Eberhard S, Pattathil S, Warder C, Glushka J, Yuan C, Hao Z, Zhu X, Avci U, Miller JS, et al. (2013) An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 25: 270–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognolli M, Penel C, Greppin H, Simon P (2002) Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene 288: 129–138 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8: 397–403 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang DL, Edmond C, Harrington JL, Nühse TS (2011) Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiol 156: 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen RH, Aten E, Roos A, den Dunnen JT (2009) High-resolution melting analysis (HRMA): more than just sequence variant screening. Hum Mutat 30: 860–866 [DOI] [PubMed] [Google Scholar]
- Welinder KG, Justesen AF, Kjaersgård IVH, Jensen RB, Rasmussen SK, Jespersen HM, Duroux L (2002) Structural diversity and transcription of class III peroxidases from Arabidopsis thaliana. Eur J Biochem 269: 6063–6081 [DOI] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Mackie W, Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47: 9–27 [PubMed] [Google Scholar]
- Wolf S, Rausch T, Greiner S (2009) The N-terminal pro region mediates retention of unprocessed type-I PME in the Golgi apparatus. Plant J 58: 361–375 [DOI] [PubMed] [Google Scholar]
- Zhang GF, Staehelin LA (1992) Functional compartmentation of the Golgi apparatus of plant cells : immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol 99: 1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Tootle TL, Glazebrook J (1999) Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell 11: 2419–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]