Abstract
STUDY QUESTION
Are lifestyle factors (smoking, BMI, alcohol use and oral contraceptive pill use) associated with the human ovarian reserve as determined by the total ovarian non-growing follicle number?
SUMMARY ANSWER
Light to moderate alcohol use was significantly associated with greater ovarian non-growing follicle (NGF) count, whereas other lifestyle factors were not significantly related.
WHAT IS KNOWN ALREADY
A single previous investigation has suggested that smoking and alcohol use are associated with lower ovarian follicle density. However, this investigation utilized follicle density as the outcome of interest rather than the estimated total ovarian NGF count.
STUDY DESIGN, SIZE, DURATION
This cross-sectional investigation included a convenience sample of premenopausal women from two different academic sites, the University of Washington (n = 37, from 1999–2004) and the University of Oklahoma (n = 73, from 2004–2013), undergoing incidental oophorectomy at the time of hysterectomy (total n = 110, age range 21–52 years).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Prior to undergoing oophorectomy, participants completed detailed questionnaires regarding lifestyle exposures. Following surgery, total ovarian NGF counts were determined with systematic random sampling rules and a validated fractionator/optical dissector technique. Associations between lifestyle factors and log-transformed ovarian follicle counts were determined using multivariable linear regression.
MAIN RESULTS AND THE ROLE OF CHANCE
After controlling for age, BMI, oral contraceptive pill (OCP) use, tobacco use and site of collection, cumulative alcohol use (measured in alcoholic drinks per day multiplied by years of drinking) was associated with ovarian NGF count. Women reporting light (>0 to <1 drink-years) and moderate (1–3 drink-years) alcohol use had greater NGF counts (β = 0.75, P = 0.04, and β = 1.00, P = 0.03; light and moderate use, respectively) as compared with non-users. Neither heavier alcohol use (>3 drink-years), BMI, OCP use, nor tobacco use were significantly associated with the ovarian NGF count. Similar patterns of association with moderate cumulative alcohol use were observed when evaluating associations with pre-antral follicles and total follicle counts.
LIMITATIONS, REASONS FOR CAUTION
All participants in this convenience sample had a benign indication for hysterectomy, and therefore may not be broadly representative of the population without such an indication. Additionally, lifestyle factors were self-reported, and the sample size of the present investigation limits our ability to detect associations of smaller magnitude.
WIDER IMPLICATIONS OF THE FINDINGS
While our findings are in disagreement with a single investigation that utilized human follicle density as the outcome of interest, they are consistent with many studies investigating the relationship between lifestyle factors and the age of spontaneous menopause. Furthermore, they suggest a mechanism that does not involve accelerated follicular atresia to explain the association between smoking and an earlier age of menopause.
STUDY FUNDING/COMPETING INTEREST(S)
This investigation was funded by NIA R29-HD37360-04 (N.A.K.) and OCAST HR04-115 (K.R.H.) and by the National Institute of General Medical Sciences, Grant 1 U54GM104938 (J.D.P.). There is no conflict of interest.
Keywords: ovarian follicle count, menopause, alcohol use, tobacco use, oral contraceptive pill use, ovarian reserve, morphometric, primordial follicles, non-growing follicles
Introduction
The human ovarian non-growing follicle pool is estimated to reach a maximum of ∼7 million around 20 weeks of fetal life, followed by a progressive depletion throughout fetal life, childhood and the reproductive years (Wise et al., 1996; Fauser, 2000). At the time of birth the number of non-growing follicles (NGFs: primordial, intermediate, and primary follicles), has decreased to ∼500 000–1 million, and by menarche only 350–500 000 remain (Hansen et al., 2008). Ultimately the progressive loss of follicles results in menopause at an estimated NGF count of 750–1000 (Block, 1951; McKinlay et al., 1992; Hansen et al., 2008; Depmann et al., 2015).
Given that the above follicle dynamics affect the age of menopause, and the wide variations in the age at which menopause occurs (40–60 years (McKinlay et al., 1992)), considerable differences between individuals exist in the pattern of follicle pool depletion and/or the number of follicles at birth. It is likely that a combination of genetic and environmental factors are responsible for this inter-individual variation (Fauser, 2000).
Multiple studies have investigated the impact of lifestyle choices (tobacco use, oral contraceptives [OCP] use, BMI and alcohol use) on the reproductive lifespan by correlating the impact of these exposures with the age of spontaneous menopause. Although occasionally in agreement, many of these studies report contradictory findings.
The strongest epidemiologic evidence for an association of a single environmental factor and the age of spontaneous menopause exists for smoking. Numerous epidemiologic studies have demonstrated an association between current smoking and the age of menopause (Kaufman et al., 1980; Adena and Gallagher, 1982; Willett et al., 1983; McKinlay et al., 1985; Everson et al., 1986; Hiatt and Fireman, 1986; Brambilla and McKinlay, 1989; Parazzini et al., 1992; Kato et al., 1998; Cooper et al., 1999; Brett and Cooper, 2003; van Asselt et al., 2004; Kinney et al., 2006). However, most investigations have suggested that former smokers experience menopause at a similar time point as do never smokers (Kaufman et al., 1980; Adena and Gallagher, 1982; Willett et al., 1983; Midgette and Baron, 1990; Kato et al., 1998; Cooper et al., 1999; Hardy et al., 2000; van Asselt et al., 2004).
The association between alcohol exposure and age of spontaneous menopause has been studied to a lesser extent, with mixed results. Evidence suggests that alcoholism is associated with an earlier age of menopause (Gavaler, 1985). When comparing women with any regular alcohol intake with abstinent women, most studies suggest no difference in the age of menopause (Adena and Gallagher, 1982; Everson et al., 1986; Cramer et al., 1995; Nilsson et al., 1997; Nagata et al., 1998). The studies that did show a difference in this context suggested an association with any alcohol intake and later age of menopause (Kato et al., 1988; Torgerson et al., 1997; Cooper et al., 2001; Brett and Cooper, 2003).
If lifestyle factors influence the age of spontaneous menopause (either positively or negatively), one possible mechanism would be a direct effect of these exposures on the rate of ovarian follicle depletion. While addressing this potential mechanism seems straightforward and fundamental, direct morphological studies on the human ovary examining the effect of these factors on the ovarian reserve are rare because of technical challenges and the scarcity of human specimens (Block, 1951; Fauser, 2000; Hansen et al., 2008; Knowlton et al., 2014).
In 2000, Westhoff et al. provided the first morphologic evidence suggesting that smoking was associated with decreased human ovarian follicle number (Westhoff et al., 2000). In their study examining the (morphologically normal) ovaries of 85 women undergoing incidental oophorectomy at the time of hysterectomy, follicle density decreased with age, current or past cigarette smoking, and ever alcohol use. Other factors such as body mass index and oral contraceptive use were not significantly associated with lower follicle number (Westhoff et al., 2000). The purpose of this investigation was to determine the relationship between lifestyle factors and the true ovarian reserve, the ovarian NGF number, in premenopausal women undergoing oophorectomy for benign indications.
Materials and Methods
Participants
Normal ovaries were collected from 133 premenopausal women (age 21–52 years) undergoing oophorectomy for benign indications at two clinical sites. As part of a series of studies investigating the reproductive aging process in women, ovaries were collected between 1999 and 2004 at the University of Washington (UW) and between 2004 and 2013 at the University of Oklahoma Health Sciences Center (OU). The investigators were not involved in the decision to perform surgery, nor the surgery itself. Additionally, the investigators did not perform an endocrine evaluation of the women prior to surgery. Since potential participants were identified by gynecological surgeons and not systematically evaluated for participation by the investigators, the included women represent a convenience sample. Exclusion criteria included gynecological malignancy, prior radiation or chemotherapy, autoimmune disease, and prior ovarian surgery. Additionally, ovarian pathology such as endometriomas, dermoid cysts, and other cystic masses of the ovary >2 cm also excluded subjects from participation as did a solid ovarian mass of any size. For the 110 subjects with complete covariate data, indications for surgery were as follows: pelvic pain (26), menorrhagia (20), endometriosis (7), prolapse (1), leiomyoma (16), and combined diagnoses (40).
Ethical approval
This study was approved by the OU and UW Institutional Review Boards.
Tissue preparation and follicle counting with modern stereology techniques
Tissue preparation, follicle identification, and counting were performed as previously described using a validated technique including the fractionator and optical dissector morphometric tools (Charleston et al., 2007; Hansen et al., 2008). These tools, combined with systematic random sampling, provide for the most thorough and statistically valid method for the determination of bias-free estimates of ovarian follicle counts. Briefly, the fractionator/optical dissector method is based on directly counting the particles of interest (in this case, the oocyte nucleoli) in a known fraction of the original structure. The total number of nucleoli encountered in this fraction is then multiplied by the inverse of a hierarchy of systematic random sampling fractions in order to generate an estimate of the total number in the original specimen.
The first sample fraction (F1) consisted of the ovary minus the small portion previously removed for pathological examination. Each ovary was cut into 1 mm slabs perpendicular to the long axis of the ovary. Approximately 8 slabs were selected out of the total generated (yielding a second fraction, F2) using systematic random sampling rules. The selected slabs were dehydrated and embedded as a group in one or more (2″ × 3″) blocks of glycol methacrylate (GMA, Technovit 8100, Energy Beam Sciences, Inc., Agawam, MA, USA). The blocks were sectioned at a thickness of 25 µm using a rotary microtome. Every 10th section from a random start (the third fraction, F3) was collected in the order generated on glass slides for staining. Sections were stained with Richardson's stain and then mounted with cover slips using Cytoseal 280 (Stephens Scientific, Kalamazoo, MI, USA).
Sections representing the largest 2-dimensional profile of each slab were then selected for counting with the optical dissector. The fraction that this section represented from the entire collected stack of sections from each slab (F4) was determined by placing a point grid over the section and summing the points that fell over the sections. This value was then divided by the total number of points landing over all collected sections.
Optical dissector counting frames were placed over the selected stained sections using systematic random sampling rules (Gundersen et al., 1988). Placement of optical dissectors and delineation of the areas of interest was accomplished by use of StereoInvestigator software (MicroBright Field, Colchester, MA, USA) operating on a PC computer coupled to a Nikon (OU) or Zeiss Photomicroscope II (UW). Sequential placement of optical dissector frames was performed by a motor driven microscope stage directed by the StereoInvestigator software.
The entire ovarian surface of each section in the counting sample was outlined under low magnification for placement of the dissector frames. The area of the dissector frame divided by the area of the steps between placements (representing a grid) represented a fifth sampling fraction (F5). The next sampling fraction (F6) consisted of the height of the optical dissector divided by the height of the tissue section. This fraction accounts for the portion of the tissue section represented by the guard area in which no counting was performed. Raw counts (Q-) for each class of follicles were then converted to an estimate of the total number (N) of follicles in the entire ovary by the following equation (where “Q-” = number of each class of follicles identified in the fraction of tissue counted):
The largest antral follicles (2–10 mm in diameter) spanned more than one ovarian 1 mm slab and therefore counts were determined with a different methodology. All slabs were examined under low magnification in sequence. Large antral follicles were identified and counted by their appearance and subsequent disappearance from sequenced slabs.
Follicle identification
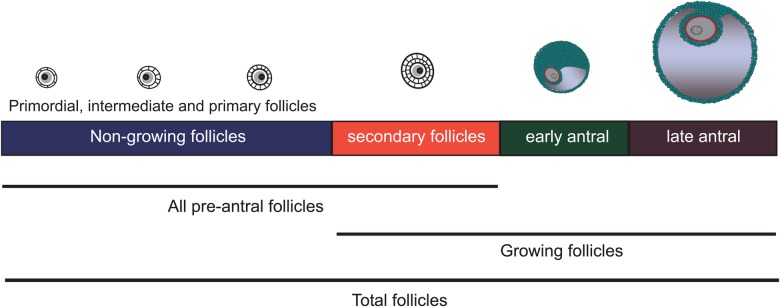
Follicles were classified according to the morphologic criteria as described by Gougeon (1996). The population of NGFs consisted of primordial (PF), intermediate, and primary follicles. PFs were defined as containing a single layer of flattened granulosa cells; intermediate follicles were defined by a single layer of granulosa cells with at least one cuboidal and one flattened granulosa cell; and primary follicles were defined as containing a single layer of cuboidal granulosa cells without any flattened granulosa cells. The population of pre-antral follicles included NGFs and secondary follicles. Secondary follicles consisted of a primary oocyte with multiple layers of granulosa cells but without an antrum. Antral follicles, classified as ‘early’ (<2 mm diameter) or ‘late’ (2–10 mm in diameter), were assessed at only one study site (OU); thus, a sub-analysis of total follicle count, which summed NGF, secondary, early and late antral follicles, was conducted among the subsample of 73 subjects who had antral follicle counts available.
Lifestyle factors
BMI, tobacco, alcohol and OCP use were determined with a detailed questionnaire administered within 2 weeks prior to surgery. BMI was categorized as normal (<25 kg/m2), overweight (25–29.9 kg/m2) and obese (>30 kg/m2). Pack-years of tobacco use (amount smoked per day × duration in years) was defined as none, up to 5 and >5 pack-years. The pack-years unit of measurement is a measure of cumulative exposure which incorporates components of both dose and duration. One pack-year is equivalent to smoking one pack (20 cigarettes) per day for 1 year, or half a pack per day for 2 years or numerous other equivalent combinations of the product of smoking dose and duration. Alcohol intake was reported as amount (drinks per week) and duration (years) of current use. A drink was defined as one 12-oz. (340 g) beer, a 1.5-oz. (42.5 g) shot of liquor or a 5-oz. (142 g) glass of wine, which is equivalent to ∼1.8 units of alcohol. Past use was recorded separately when a change in consumption patterns was reported.
Similar to tobacco exposure, cumulative alcohol intake was measured in drink-years, calculated as average drinks per day multiplied by years of alcohol use. When past alcohol intake patterns differed from current use, past drink-years were calculated separately and current and past drink-years were summed [(current drinks per week/7 × current years of use) + (past drinks per week/7 × past years of use)]. Cumulative alcohol intake was categorized as none, light (>0 to <1 drink-years), moderate (1–3 drink-years) and heavy (>3 drink-years). Thus, one drink-year would be equal to one drink per day for 1 year, or one drink per week for 7 years and so on. In order to consider behaviors proximate to the time of specimen collection, smoking and alcohol status were also evaluated as current, past or never use. OCP use was classified as none, ≤5 years or >5 years of use, with the upper category reflecting the top quartile of the distribution of duration of use. OCP use was also evaluated according to current use (yes/no). The analyses included the 110 participants (n = 73, OU and n = 37, UW) with complete covariate data.
Statistical analyses
The relationships between log-transformed ovarian follicle counts and lifestyle factors were determined by multivariable linear regression. The continuous measures of ovarian follicle counts were log transformed to meet the linear regression model assumptions of normally distributed errors and homoscedasticity. Separate models were estimated for three ovarian follicle populations: NGFs, all pre-antral follicles, and total follicles (Fig. 1). All models controlled for age (<40 years versus ≥40 years), study site and all other lifestyle factors. Five year age categories were initially evaluated to assess the proper specification of age in all models, collapsing the <30-year age group with those age 30–34 years due to the small number of subjects in their twenties (n = 5). In the absence of a monotonic relationship with age, age was defined as a binary indicator to parsimoniously reflect the pattern of point estimates with similar magnitude among categories <40 years and those ≥40 years.
Figure 1.
Schematic representation of ovarian follicle populations. Follicles are more advanced in development from left to right and are not drawn to scale.
Interaction between alcohol and smoking was explored by entering interaction terms into the linear regression model. A P-value of <0.05 was considered statistically significant. Statistical analysis was performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient characteristics are described in Table I. The study population included women who were mostly age 40 years or older (mean age 41.7, SD 6.6) and who were overweight or obese (mean BMI 30.2, SD 7.6). Approximately half reported a history of tobacco use (55.4%) and most reported previous use of alcohol (86.4%). One-quarter of the study population reported oral contraceptive use for >5 years. Fifteen percent (n = 17) were current OCP users at the time of ovary collection. The distribution of non-growing, pre-antral and total follicle counts are displayed in Table II.
Table I.
Characteristics of 110 women undergoing oophorectomy for benign indications at two clinical sites.
| n (%)a | |
|---|---|
| Age (years) | |
| 20–29 | 5 (4.6) |
| 30–39 | 28 (25.5) |
| 40–49 | 62 (56.4) |
| ≥50 | 10 ( 9.1) |
| Body mass index (kg/m2) | |
| <24.9 | 30 (27.3) |
| 25–29.9 | 31 (28.2) |
| ≥30 | 49 (44.6) |
| Alcohol use | |
| 0 drink-years | 15 (13.6) |
| >0–<1 drink-years | 51 (46.4) |
| 1–3 drink-years | 15 (13.6) |
| >3 drink-years | 29 (26.4) |
| Tobacco use | |
| 0 pack-years | 49 (44.6) |
| >0–5 pack-years | 26 (23.6) |
| > 5 pack-years | 35 (31.8) |
| Oral contraceptive use | |
| 0 months | 8 (7.3) |
| 1–60 months | 75 (68.2) |
| ≥61 months | 27 (24.6) |
| Study site | |
| OK | 73 (66.4) |
| WA | 37 (33.6) |
aPercentage may exceed 100% due to rounding.
Table II.
Distribution of follicle counts for women undergoing oophorectomy for benign indications at two clinical sites.
| n | Percentile |
Minimum | Maximum | |||||
|---|---|---|---|---|---|---|---|---|
| 10th | 25th | 50th (median) | 75th | 90th | ||||
| Non-growing follicles | 110 | 1447 | 3128 | 10 578 | 33 008 | 126 135 | 223 | 455 102 |
| Pre-antral follicles | 110 | 1464 | 3558 | 11 717 | 33 008 | 133 581 | 223 | 474 572 |
| Total folliclesa | 73 | 1746 | 4986 | 12 730 | 30 452 | 139 814 | 894 | 474 594 |
aTotal follicle count includes antral follicles which were measured at only one clinical site, n = 73.
All follicle counts were highly statistically significantly negatively associated with age (β = −2.41, P < 0.0001; β = −2.35, P < 0.0001; β = −2.19, P < 0.0001 for NGF, pre-antral and total follicles, respectively). Cumulative alcohol use was positively associated with ovarian NGF count controlling for age, BMI, OCP use, tobacco use and site of collection (Table III). Women reporting light (>0 to <1 drink-years) and moderate (1–3 drink-years) alcohol use had greater NGF counts (β = 0.75, P = 0.04, and β = 1.00, P = 0.03; light and moderate use, respectively) compared with non-users. The association with heavier alcohol use (>3 drink-years), although positive, was weaker and not significantly associated with ovarian NGF count (β = 0.44, P = 0.32). When alcohol use was evaluated as binary indicators of current use within the last year, past use or no previous use, current (β = 0.75, P = 0.05) and past users (β = 0.78, P = 0.05) had higher NGF counts compared with non-users, although the associations reached only marginal significance (Table IV). Similar associations with higher NGF counts were observed among ever users of alcohol compared with never users (β = 0.76, P = 0.03). However, when comparing NGF counts between those with and without intake within the last year, differences were not detected (current users versus current non-users: β = 0.22, P = 0.43). Furthermore, BMI, OCP use (measured as current use or months of use) and tobacco use were not associated with ovarian NGF count (Table III).
Table III.
Linear regression coefficientsa and 95% confidence intervals (CI) for associations between lifestyle characteristics and natural log transformed follicle counts among 110 subjects from two clinical sites.
| Non-growing follicles (n = 110) Beta (95% CI) |
P | Pre-antral follicles (n = 110) Beta (95% CI) |
P | Total folliclesc (n = 73) Beta (95% CI) |
P | |
|---|---|---|---|---|---|---|
| Alcohol use | ||||||
| 0 drink-years | Refb | Ref | Ref | |||
| >0 to < 1 drink-years | 0.75 (0.02,1.47) | 0.04 | 0.62 (−0.11,1.35) | 0.09 | 0.33 (−0.44,1.10) | 0.39 |
| 1 to 3 drink-years | 1.00 (0.11,1.90) | 0.03 | 0.87 (−0.03,1.77) | 0.06 | 1.41 (0.41,2.41) | 0.007 |
| >3 drink-years | 0.44 (−0.43,1.32) | 0.32 | 0.31 (−0.56,1.19) | 0.48 | 0.17 (−0.89,1.23) | 0.75 |
| Tobacco use | ||||||
| 0 pack-years | Ref | Ref | Ref | |||
| >0 to 5 pack-years | 0.18 (−0.43,0.78) | 0.57 | 0.15 (−0.46,0.77) | 0.62 | 0.37 (−0.34,1.08) | 0.30 |
| >5 pack-years | 0.27 (−0.33,0.87) | 0.37 | 0.30 (−0.30,0.90) | 0.33 | 0.55 (−0.17,1.28) | 0.13 |
| Body mass index | ||||||
| <24.9 | Ref | Ref | Ref | |||
| 25 to 29.9 | −0.28 (−0.90,0.33) | 0.36 | −0.30 (−0.92,0.31) | 0.33 | −0.61 (−1.37,0.15) | 0.12 |
| ≥30 | −0.08 (−0.64,0.48) | 0.78 | −0.12 (−0.69,0.44) | 0.66 | −0.21 (−0.88,0.45) | 0.53 |
| Oral contraceptive use | ||||||
| 0 months | Ref | Ref | Ref | |||
| 1 to 60 months | 0.70 (−0.21,1.62) | 0.13 | 0.66 (−0.26,1.57) | 0.16 | 0.46 (−0.58,1.51) | 0.38 |
| ≥61 months | 0.43 (−0.56,1.43) | 0.39 | 0.39 (−0.61,1.39) | 0.44 | 0.72 (−0.47,1.91) | 0.23 |
aAll models control for age, study site and all other lifestyle factors in the table.
bReferent group.
cTotal follicle count includes antral follicles which were measured at only one clinical site, n = 73.
Table IV.
Linear regression coefficientsa and 95% confidence intervals (CI) for associations between alcohol use and follicle counts among 110 subjects from two clinical sites.
| Alcohol use | Non-growing follicles (n = 110) Betaa (95% CI) |
P | Pre-antral follicles (n = 110) Beta (95% CI) |
P | Total folliclesc (n = 73) Beta (95% CI) |
P |
|---|---|---|---|---|---|---|
| None | Refb | Ref | Ref | |||
| Currentd | 0.75 (−0.01,1.51) | 0.05 | 0.63 (−0.13,1.39) | 0.11 | 0.60 (−0.25,1.46) | 0.16 |
| Past | 0.78 (−0.00,1.55) | 0.05 | 0.64 (−0.14,1.42) | 0.11 | 0.50 (−0.34,1.33) | 0.24 |
| Never use | Ref | Ref | Ref | |||
| Ever use | 0.76 (0.06,1.47) | 0.03 | 0.63 (−0.07,1.34) | 0.08 | 0.54 (−0.23,1.31) | 0.16 |
| No use in last year | Ref | Ref | Ref | |||
| Use in last year | 0.22 (−0.33,0.76) | 0.43 | 0.19 (−0.36,0.73) | 0.50 | 0.25 (−0.37,0.87) | 0.42 |
aControlling for age, BMI, tobacco use, oral contraceptive use, and study site.
bReferent group.
cTotal follicle count includes antral follicles which were measured at only one clinical site, n = 73.
dCurrent use is defined as alcohol use within last year.
Similar patterns of increased follicle counts with moderate cumulative alcohol use were observed for total follicle counts (Table III) and positive associations with pre-antral follicle counts approached statistical significance. Associations with current and past alcohol use or with ever use remained in the positive direction, but did not achieve statistical significance. Consistent with results for NGF, significant associations with BMI, OCPs and tobacco use were not observed for the other follicle populations.
In the absence of an inverse association between cumulative smoking and ovarian follicle counts, smoking status was also evaluated according to current or past use as compared with never use. However, no patterns of association with smoking status were observed for any of the follicle populations examined.
Although small sample size limits the exploration of potential modifying effects of smoking, interaction terms for alcohol and smoking were not statistically significant indicating no suggestive evidence of interaction. Furthermore, when models for each follicle group were estimated separately for smokers and non-smokers, the stratum-specific point estimates for moderate alcohol consumption remained positive and were generally of similar magnitude across strata; although, stratum-specific results were somewhat attenuated compared with the overall results and did not achieve statistical significance (e.g. for NGF, β = 0.79, P = 0.16 for moderate alcohol use among non-smokers and β = 0.84, P = 0.32 among smokers).
Discussion
In this investigation, light to moderate alcohol use was associated with a greater ovarian NGF count. This finding is consistent with investigations that have suggested that light to moderate alcohol use is associated with a delayed age of spontaneous menopause (Kato et al., 1988; Torgerson et al., 1997; Cooper et al., 2001; Brett and Cooper, 2003; Kinney et al., 2006). Log transformations of continuous outcomes are frequently applied when estimating linear regression models to ensure the assumptions that underlie the model are met. Although differences in mean follicle counts expressed on the log scale are not intuitive, back-transformations of the regression coefficients using the anti-log are interpretable as ratios of geometric means (Bland and Altman, 1996). Therefore, our results indicate that light and moderate alcohol users have geometric mean NGF counts that are more than two times greater than non-users (e0.75 = 2.1 and e1.00 = 2.7 for light and moderate alcohol users, respectively). While this difference in NGF count appears large in absolute terms, according to the power model of ovarian NGF depletion {log(NGF) = (−0.00019)*(age in years)2.452 + 5.717}, this difference would correspond to a delayed age of menopause of ∼3 years in the average age participant in the present investigation (Hansen et al., 2008). These findings are in line with Kinney et al.'s observations of a delayed age of menopause of 2.2 years in moderate alcohol users (Kinney et al., 2006).
Our study is preceded by only one other morphological study examining the effects of lifestyle factors on the ovarian reserve (Westhoff et al., 2000). Our results differ from Westhoff et al. (2000), who reported lower follicle counts among those who had ever used alcohol compared with never users. However, in that study, average follicle density per slide, not total ovarian follicle number, was the outcome of interest. Because of the unequal distribution of follicles in the human ovary, random biopsies or serial sections are not indicative of the total NGF count (Block, 1951; Lass, 2004). Additionally, the Westhoff investigation utilized systematic sampling of some, but not all ovaries, pooled follicle populations together, and whether or not randomized systematic sampling was performed was not specified in the report.
Given the limitations of ovarian follicle density measurements, we used modern morphometric techniques with validated tools to determine the total ovarian follicle counts. Our study population also differed from patients studied by Westhoff et al. in that our participants were younger and more obese (45 versus 20%), with a greater proportion of current smokers (29 versus 15%) and alcohol users (86 versus 29%). Additionally, alcohol use was reported as a dichotomous variable (‘never’ or ‘ever’) in the Westhoff study (Westhoff et al., 2000), whereas it was calculated as ‘drink-years’ in our study in order to examine the relationship between ovarian follicle counts and cumulative alcohol exposure. However, when we examined comparisons of ever versus never users, NGF counts remained higher among women with any history of alcohol use. Our failure to detect NGF count differences between those who did and did not use alcohol within the last year suggests that the association between alcohol and follicle count may be attributed to long-term rather than recent use.
In addition to consistency with reports of light to moderate alcohol use being associated with delayed onset of menopause (Kato et al., 1988; Torgerson et al., 1997; Cooper et al., 2001; Brett and Cooper, 2003; Kinney et al., 2006), our findings are also consistent with previous reports that former smoking is not associated with a different age of menopause as compared with never smoking (Kaufman et al., 1980; Adena and Gallagher, 1982; Willett et al., 1983; Midgette and Baron, 1990; Kato et al., 1998; Cooper et al., 1999; Hardy et al., 2000; van Asselt et al., 2004; Kinney et al., 2006). The results are also in agreement with most studies reporting no association of BMI and OCP use (Cooper et al., 2001; Brett and Cooper, 2003) with the age of spontaneous menopause.
To our knowledge, this is the only investigation to assess the relationship between human ovarian follicle count and lifestyle factors using modern morphometric techniques. Our investigation utilized the total NGF count as the outcome of interest rather than the last menstrual period, which could be considered a surrogate marker for the former. Studies using the described technique are rare, and will be even more so in the future due to changes in practice patterns resulting in fewer elective oophorectomies.
Limitations of this study include the small sample size and the fact that patients undergoing surgery may not be representative of the overall population. It is possible that unmeasured characteristics of the convenience sample may have influenced associations between lifestyle factors and follicle counts. In addition, the design of the study was cross-sectional which limits causal inference. Although lifestyle factors were collected prior to surgery and subjects were unaware of their actual follicle counts, it is possible that knowledge of menstrual cycle characteristics or other factors that may be related to follicle counts may have influenced the accuracy of reporting of certain health behaviors. Thus, the potential for exposure misclassification bias cannot be ruled out. In order for such measurement error to explain the observed results for alcohol, women with reduced NGF counts would have had to systematically under-report their alcohol use or alcohol intake would have to be over-reported by those with higher NGF counts. In a recent study assessing the reliability and validity of lifetime alcohol measures, Greenfield et al. undertook extended test-retest analyses of retrospective lifetime drinking measures in various age groups (Greenfield et al., 2014). Reliability was lower for respondents under 30 and higher for Whites versus Blacks and Hispanics (rho = 0.68 versus rho = 0.56 versus rho = 0.56, both P = 0.01), but did not differ by gender. The authors concluded that lifetime alcohol assessments are reasonably reliable, albeit with some individual imprecision related to demographic factors (Greenfield et al., 2014). Although alcohol use may be inaccurately reported, it is important to note that we identified significant differences in NGF count between ‘ever’ and ‘never’ users. These use categories would be much less likely to be inaccurately reported.
In terms of the mechanistic implications of our findings, the association between current smoking and an advanced (i.e. earlier) age of spontaneous menopause may be explained by a mechanism that does not involve direct toxicity to ovarian follicles. Furthermore, our findings suggest that a potential mechanism involved in the delayed age of menopause in light to moderate alcohol users may be the delayed entry of non-growing follicles into the growing follicle pool.
Due to the cross-sectional design of this study, we cannot conclude with certainty that moderate alcohol use is protective of early onset of menopause. However, our results suggest that light to moderate use is not harmful from an ovarian reserve perspective. Furthermore, our findings suggest that smokers who quit are likely to not have suffered permanent damage to their ovarian reserve. This finding is consistent with literature suggesting no acceleration in the age of spontaneous menopause in former smokers.
Authors' roles
J.D.P. contributed to the data analysis, interpretation of results and writing the manuscript. A.M.Q. contributed to the interpretation of the data and writing the manuscript. L.B.C. contributed to the enrollment and evaluation of women participating in the study, data collection, and manuscript revision. M.R.S. contributed to subject recruitment at the University of Washington, the development of the morphometric technique, and manuscript revision. N.A.K. contributed to subject recruitment at the University of Washington, the development of the morphometric technique, and manuscript revision. K.R.H. developed the research question and study protocol, collected data, interpreted results and contributed to writing the manuscript.
Funding
This investigation was funded by NIA R29-HD37360-04 (N.A.K.) and OCAST HR04-115 (K.R.H.) and by the National Institute of General Medical Sciences, Grant 1 U54GM104938 (J.D.P.).
Conflict of interest
None declared.
References
- Adena MA, Gallagher HG. Cigarette smoking and the age at menopause. Ann Hum Biol 1982;9:121–130. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. The use of transformation when comparing two means. BMJ 1996;312:1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block E. Quantitative morphological investigations of the follicular system in women; methods of quantitative determinations. Acta Anat (Basel) 1951;12:267–285. [DOI] [PubMed] [Google Scholar]
- Brambilla DJ, McKinlay SM. A prospective study of factors affecting age at menopause. J Clin Epidemiol 1989;42:1031–1039. [DOI] [PubMed] [Google Scholar]
- Brett KM, Cooper GS. Associations with menopause and menopausal transition in a nationally representative US sample. Maturitas 2003;45:89–97. [DOI] [PubMed] [Google Scholar]
- Charleston JS, Hansen KR, Thyer AC, Charleston LB, Gougeon A, Siebert JR, Soules MR, Klein NA. Estimating human ovarian non-growing follicle number: the application of modern stereology techniques to an old problem. Hum Reprod 2007;22:2103–2110. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Sandler DP, Bohlig M. Active and passive smoking and the occurrence of natural menopause. Epidemiology 1999;10:771–773. [PubMed] [Google Scholar]
- Cooper GS, Baird DD, Darden FR. Measures of menopausal status in relation to demographic, reproductive, and behavioral characteristics in a population-based study of women aged 35–49 years. Am J Epidemiol 2001;153:1159–1165. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Harlow BL, Xu H, Fraer C, Barbieri R. Cross-sectional and case-controlled analyses of the association between smoking and early menopause. Maturitas 1995;22:79–87. [DOI] [PubMed] [Google Scholar]
- Depmann M, Faddy MJ, van der Schouw YT, Peeters PHM, Broer SL, Kelsey TW, Nelson SM, Broekmans FJM. The relationship between variation in size of the primordial follicle pool and age at natural menopause. J Clin Endocrinol Metab 2015;100:E845–E851. [DOI] [PubMed] [Google Scholar]
- Everson RB, Sandler DP, Wilcox AJ, Schreinemachers D, Shore DL, Weinberg C. Effect of passive exposure to smoking on age at natural menopause. Br Med J (Clin Res Ed) 1986;293:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser BC. Follicle pool depletion: factors involved and implications. Fertil Steril 2000;74:629–630. [DOI] [PubMed] [Google Scholar]
- Gavaler JS. Effects of alcohol on endocrine function in postmenopausal women: a review. J Stud Alcohol 1985;46:495–516. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 1996;17:121–155. [DOI] [PubMed] [Google Scholar]
- Greenfield TK, Nayak MB, Bond J, Kerr WC, Ye Y. Test-retest reliability and validity of life-course alcohol consumption measures: the 2005 National Alcohol Survey follow-up. Alcohol Clin Exp Res 2014;38:2479–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B et al. . The new stereological tools: dissector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 1988;96:857–881. [DOI] [PubMed] [Google Scholar]
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod 2008;23:699–708. [DOI] [PubMed] [Google Scholar]
- Hardy R, Kuh D, Wadsworth M. Smoking, body mass index, socioeconomic status and the menopausal transition in a British national cohort. Int J Epidemiol 2000;29:845–851. [DOI] [PubMed] [Google Scholar]
- Hiatt RA, Fireman BH. Smoking, menopause, and breast cancer. J Natl Cancer Inst 1986;76:833–838. [PubMed] [Google Scholar]
- Kato I, Tominaga S, Suzuki T. Factors related to late menopause and early menarche as risk factors for breast cancer. Jpn J Cancer Res 1988;79:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol 1998;51:1271–1276. [DOI] [PubMed] [Google Scholar]
- Kaufman DW, Slone D, Rosenberg L, Miettinen OS, Shapiro S. Cigarette smoking and age at natural menopause. Am J Public Health 1980;70:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney A, Kline J, Levin B. Alcohol, caffeine and smoking in relation to age at menopause. Maturitas 2006;54:27–38. [DOI] [PubMed] [Google Scholar]
- Knowlton NS, Craig LB, Zavy MT, Hansen KR. Validation of the power model of ovarian nongrowing follicle depletion associated with aging in women. Fertil Steril 2014;101:851–856. [DOI] [PubMed] [Google Scholar]
- Lass A. Assessment of ovarian reserve: is there still a role for ovarian biopsy in the light of new data? Hum Reprod 2004;19:467–469. [DOI] [PubMed] [Google Scholar]
- McKinlay SM, Bifano NL, McKinlay JB. Smoking and age at menopause in women. Ann Intern Med 1985;103:350–356. [DOI] [PubMed] [Google Scholar]
- McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas 1992;14:103–115. [DOI] [PubMed] [Google Scholar]
- Midgette AS, Baron JA. Cigarette smoking and the risk of natural menopause. Epidemiology 1990;1:474–480. [DOI] [PubMed] [Google Scholar]
- Nagata C, Takatsuka N, Inaba S, Kawakami N, Shimizu H. Association of diet and other lifestyle with onset of menopause in Japanese women. Maturitas 1998;29:105–113. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Moller L, Koster A, Hollnagel H. Social and biological predictors of early menopause: a model for premature aging. J Intern Med 1997;242:299–305. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Negri E, La Vecchia C. Reproductive and general lifestyle determinants of age at menopause. Maturitas 1992;15:141–149. [DOI] [PubMed] [Google Scholar]
- Torgerson DJ, Thomas RE, Campbell MK, Reid DM. Alcohol consumption and age of maternal menopause are associated with menopause onset. Maturitas 1997;26:21–25. [DOI] [PubMed] [Google Scholar]
- Van Asselt KM, Kok HS, van Der Schouw YT, Grobbee DE, te Velde ER, Pearson PL, Peeters PH. Current smoking at menopause rather than duration determines the onset of natural menopause. Epidemiology 2004;15:634–639. [DOI] [PubMed] [Google Scholar]
- Westhoff C, Murphy P, Heller D. Predictors of ovarian follicle number. Fertil Steril 2000;74:624–628. [DOI] [PubMed] [Google Scholar]
- Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, Cramer D, Hennekens CH. Cigarette smoking, relative weight, and menopause. Am J Epidemiol 1983;117:651–658. [DOI] [PubMed] [Google Scholar]
- Wise PM, Krajnak KM, Kashon ML. Menopause: the aging of multiple pacemakers. Science 1996;273:67–70. [DOI] [PubMed] [Google Scholar]