Abstract
STUDY QUESTION
Does repeat-associated non-AUG (RAN) translation play a role in fragile X-associated primary ovarian insufficiency (FXPOI), leading to the presence of polyglycine containing protein (FMRpolyG)-positive inclusions in ovarian tissue?
SUMMARY ANSWER
Ovaries of a woman with FXPOI and of an Fmr1 premutation (PM) mouse model (exCGG-KI) contain intranuclear inclusions that stain positive for both FMRpolyG and ubiquitin.
WHAT IS KNOWN ALREADY
Women who carry the FMR1 PM are at 20-fold increased risk to develop primary ovarian insufficiency (FXPOI). A toxic RNA gain-of-function has been suggested as the underlying mechanism since the PM results in increased levels of mRNA containing an expanded repeat, but reduced protein levels of fragile X mental retardation protein (FMRP). Recently, RAN translation has been shown to occur from FMR1 mRNA that contains PM repeat expansions, leading to FMRpolyG inclusions in brain and non-CNS tissues of fragile X-associated tremor/ataxia syndrome (FXTAS) patients.
STUDY DESIGN, SIZE, DURATION
Ovaries of a woman with FXPOI and women without PM (controls), and ovaries from wild-type and exCGG-KI mice were analyzed by immunohistochemistry for the presence of inclusions that stained for ubiquitin and FMRpolyG . The ovaries from wild-type and exCGG-KI mice were further characterized for the number of follicles, Fmr1 mRNA levels and FMRP protein expression. The presence of inclusions was also analyzed in pituitaries of a man with FXTAS and the exCGG-KI mice.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Human ovaries from a woman with FXPOI and two control subjects and pituitaries from a man with FXTAS and a control subjects were fixed in 4% formalin. Ovaries and pituitaries of wild-type and exCGG mice were fixed in Bouin's fluid or 4% paraformaldehyde. Immunohistochemistry was performed on the human and mouse samples using FMRpolyG, ubiquitin and Fmrp antibodies. Fmr1 mRNA and protein expression were determined in mouse ovaries by quantitative RT–PCR and Western blot analysis. Follicle numbers in mouse ovaries were determined in serial sections by microscopy.
MAIN RESULTS AND THE ROLE OF CHANCE
FMRpolyG-positive inclusions were present in ovarian stromal cells of a woman with FXPOI but not in the ovaries of control subjects. The FMRpolyG-positive inclusions colocalized with ubiquitin-positive inclusions. Similar inclusions were also observed in the pituitary of a man with FXTAS but not in control subjects. Similarly, ovaries of 40-week-old exCGG-KI mice, but not wild-type mice, contained numerous inclusions in the stromal cells that stained for both FMRpolyG- and ubiquitin, while the ovaries of 20-week-old exCGG-KI contained fewer inclusions. At 40 weeks ovarian Fmr1 mRNA expression was increased by 5-fold in exCGG-KI mice compared with wild-type mice, while Fmrp expression was reduced by 2-fold. With respect to ovarian function in exCGG-KI mice: (i) although the number of healthy growing follicles did not differ between wild-type and exCGG-KI mice, the number of atretic large antral follicles was increased by nearly 9-fold in 40-week old exCGG-KI mice (P < 0.001); (ii) at 40 weeks of age only 50% of exCGG-KI mice had recent ovulations compared with 89% in wild-type mice (P = 0.07) and (iii) those exCGG-KI mice with recent ovulations tended to have a reduced number of fresh corpora lutea (4.8 ± 1.74 versus 8.50 ± 0.98, exCGG-KI versus wild-type mice, respectively, P = 0.07).
LIMITATIONS, REASONS FOR CAUTION
Although FMRpolyG-positive inclusions were detected in ovaries of both a woman with FXPOI and a mouse model of the FMR1 PM, we only analyzed one ovary from a FXPOI subject. Caution is needed to extrapolate these results to all women with the FMR1 PM. Furthermore, the functional consequence of FMRpolyG-positive inclusions in the ovaries for reproduction remains to be determined.
WIDER IMPLICATIONS OF THE FINDINGS
Our results suggest that a dysfunctional hypothalamic–pituitary–gonadal-axis may contribute to FXPOI in FMR1 PM carriers.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by grants from NFXF, ZonMW, the Netherlands Brain Foundation and NIH. The authors have no conflict of interest to declare.
Keywords: FXPOI, FMR1 premutation, FMRpolyG, RAN translation, inclusions, FXTAS, trinucleotide repeat expansion, CGG-repeat, ovarian failure, HPG-axis
Introduction
Primary ovarian insufficiency (POI) is a disorder of infertility due to cessation of ovarian function before the age of 40 (Coulam et al., 1986; Visser et al., 2012). POI affects ∼1% of women in the general population and with the exception of X chromosome mutations, most identified genetic defects causing POI are rare. The prevalence is substantially higher in women who carry the premutation (PM) of the fragile X mental retardation gene (FMR1), ranging between 12 and 28% (Allingham-Hawkins et al., 1999; Sherman, 2000). POI in PM carriers is therefore also referred to as fragile X-associated POI (FXPOI). In addition, PM carriers, both men and women, are at risk for a late onset progressive neurodegenerative syndrome, known as fragile X-associated tremor/ataxia syndrome (FXTAS) (Hagerman et al., 2001).
The FMR1 PM contains an expanded unstable CGG repeat of 55–200 trinucleotides in the 5′-untranslated region (5′-UTR) of the FMR1 gene (Hagerman et al., 2001). The prevalence of the PM in the general population is high and is estimated to be 1:291 for females and 1:855 for males (Hunter et al., 2014). The major hallmark of FXTAS is the presence of ubiquitin-positive intranuclear inclusions throughout the brain (Greco et al., 2006). In PM carriers, these ubiquitin-positive inclusions are also found in other tissues, which are associated with co-morbidities including thyroid disease, cardiac arrhythmias, hypertension, migraine, impotence and neuropathy (Hunsaker et al., 2011; Willemsen et al., 2011). The general hypothesis for the cause of PM-associated disorders is an RNA gain-of-function mechanism, since the PM results in normal to slightly reduced protein levels, but elevated levels of FMR1 mRNA, which forms hairpin structures in the expanded CGG repeat (Tassone et al., 2000).
Recently, repeat-associated non-AUG (RAN) translation of the CGG repeat in PM carriers was shown to occur (Todd et al., 2013), adding another potential toxic mechanism to the pathogenicity of the expanded CGG repeat. RAN translation is an unconventional kind of translation first described for the CAG repeat found in spinocerebellar ataxia type 8 (SCA8) and myotonic dystrophy type 1 (DM1) (Zu et al., 2011). Since its discovery, RAN translation has been shown to occur for several nucleotide repeat expansions, including the CGG repeat involved in FXTAS (Todd et al., 2013) and the GGGGCC repeat involved in C9orf72-associated amyotrophic lateral sclerosis and frontotemporal dementia (Ash et al., 2013; Mori et al., 2013). Although the exact mechanism of RAN translation remains unknown, evidence suggests that the scanning ribosome stalls along the CGG repeat expansion, which finally results in the use of an alternative non-AUG start of translation and the production of polypeptides potentially in three different reading frames. RAN translation of the FMR1 PM has been proposed to be initiated in the 5′UTR of the gene resulting in the production of polyglycine (FMRpolyG) and polyalanine (FMRpolyA) proteins (Todd et al., 2013), as deletion of 48 nucleotides 5′ of the CGG repeat reduces RAN translation. The presence of FMRpolyG has been demonstrated in cell culture, Drosophila and mouse models, and in brain and other tissues from FXTAS patients, whereas FMRpolyA could only be detected in transfected cells (Todd et al., 2013; Buijsen et al., 2014; Hukema et al., 2014; Oh et al., 2015).
Despite the predisposition of the FMR1 PM to POI, little is known about the molecular pathways underlying FXPOI, including the role of FMR1 in ovarian function. The current hypothesis about FXPOI is that it arises from some gain-of-function mechanism comparable with that of FXTAS. Recently, ubiquitin-positive intranuclear inclusions, the hallmark histopathology of FXTAS, were observed in ovarian stromal cells of women with the PM (Chang et al., 2011). However, whether or not ovaries of PM carriers also stain positive for the RAN translation product FMRpolyG is unknown. Investigation of this question would bring important insight into the underlying pathology of FXPOI.
To address this question, we performed immunohistochemical analyses of the ovaries of a woman with FXPOI to detect these inclusions. To further study the effects of the CGG repeat expansion on ovarian function, we also studied ovaries of the well-established Dutch knock-in mouse (reviewed in Berman et al., 2014) with an expanded CGG repeat in the PM range (exCGG-KI) for the presence of inclusions and to characterize ovarian function.
Materials and Methods
Human samples
Formalin-fixed and paraffin-embedded ovaries of a woman with the FMR1 PM and two control subjects were analyzed in this study. The woman carrying the FMR1 PM was 42 years old and had a 28-day cycle at time of surgery. Surgery was performed for adenomyosis. The control subjects included a 47-year-old woman with unknown menstrual history at time of surgery and a 70-year-old post-menopausal woman. These subjects were previously described (Case 2, and Controls 1 and 3) (Chang et al., 2011).
Formalin-fixed and paraffin-embedded pituitary tissue was obtained from a male FXTAS patient with a 109 CGG repeat with several co-morbidities who died post-operatively after a carotid endarterectomy. The subject was described in an earlier study about non-CNS inclusions (Case 6 in Hunsaker et al., 2011).
Animals
The exCGG-KI mice, also referred to as ‘Dutch knock-in’ mice, used in this study have been described previously (Bontekoe et al., 2001; Willemsen et al., 2003; Brouwer et al., 2007). All mice had a mixed C57BL/6 and FVB/n genetic background. Genotyping of the mice was performed as described previously (Brouwer et al., 2007; Hukema and Oostra, 2013). The animals were sacrificed by cervical dislocation at 20 and 40 weeks of age (n = 6–14 mice per group). Ovaries were removed and fixed overnight in Bouin's fluid or in 4% paraformaldehyde. In addition, ovaries of 40-week-old mice were snap frozen in liquid nitrogen and stored at −80°C until further processing.
RNA isolation and real-time PCR
RNA was isolated from ovaries (wild-type n = 5 mice; exCGG-KI n = 10 mice) using RNA-Bee (Bioconnect, Huissen, the Netherlands) according to the manufacturer's instructions. RNA concentration and purity were determined using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Thermo Scientific, Wilmington, DE, USA). RT–PCR was performed on 1 µg RNA using iScript cDNA Synthesis Kit (BioRad, Veenendaal, the Netherlands) according to manufacturer's instructions. Quantitative real-time PCR using SYBR green (Kapa Biosystems, Boston, MA, USA) was performed using the following primers: Fmr1 (transition exon 5/6, forward: 5′-CCGAACAGATAATCGTCCACG-3′, reverse: 5′-ACGCTGTCTGGCTTTTCCTTC-3′), Fshr (Follicle Stimulating Hormone Receptor) (forward: 5′-CCTTGCTCCTGGTCTCCTTG-3′, reverse: 5′-CTCGGTCACCTTGCTATCTTG-3′), Lhr (Luteinizing Hormone Receptor) (forward: 5′-CGCCCGACTATCTCTCACCTA-3′, reverse: 5′-GACAGATTGAGGAGGTTGTCAAA-3′), and GAPDH (forward 5′-TCAAGAAGGTGGTGAAGCAGG-3′, reverse 5′-GCCCAAGATGCCCTTCAGT-3′) as internal reference. Efficiencies of the different primer sets were checked and found to be comparable. The comparative cycle threshold (Ct) method (ΔΔCt) was used for relative quantification (Livak and Schmittgen, 2001).
Western blot analysis
Ovaries from wild-type and exCGG-KI mice (n = 5 each) were pooled and homogenized in a HEPES buffer (10 mM HEPES, 300 mM KCl, 3 mM MgCl2, 100 µM CaCl2, 0.45% Triton X-100, 0.05% Tween-20, pH 7.6). After homogenization protein concentrations were determined using a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA), and a Varioskan multimode plate reader (Thermo Scientific). Thirty micrograms of total protein were loaded to a Criterion XT precast gel (4–12% bis–tris) (Biorad). Proteins were subsequently transferred to a nitrocellulose membrane and incubated with 2F5 (anti-FMRP) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies. The membrane was scanned with an Odyssey infrared imager and the intensity of the bands of interest was quantified using Odyssey infrared imager software (LI-COR Biosciences, Linkoln, USA). GAPDH expression served as internal loading control.
Immunohistochemistry
For immunohistochemical analysis, paraformaldehyde-fixed ovaries of 20 and 40 weeks old mice were embedded in paraffin. After routine histological procedures, mounted 6 µm sections were deparaffinized. Antigen retrieval was performed, using microwave or pressure cooker treatment in 0.01 M sodium citrate buffer (pH 6.0). In order to better visualize inclusions an extra antigen retrieval step was added, using proteinase K. Following blocking for endogenous peroxidase activity, immunohistochemistry was performed as described previously (Bakker et al., 2000; Kevenaar et al., 2006), using the Fmrp monoclonal antibody 2F5 (1:50), rabbit anti-ubiquitin (Z0458; 1:250, Dako, Heverlee, Belgium), and mouse anti-FMRpolyG (8FM 1:10; Buijsen et al., 2014) antibodies. Next, sections were incubated with Brightvision poly-HRP-Anti Ms/Rb/Rt IgG kit (Immunologic, Duiven, the Netherlands) and the peroxidase activity was developed with 0.07% (v/v) 3,3-diaminobenzidine tetrahydrochloride (DAB) (Sigma–Aldrich Chemie BV, Zwijndrecht, The Netherlands). Finally, all sections were counterstained with hematoxylin. For double immunofluorescence, slides were blocked for autofluorescence with Sudan Black in 70% ethanol. Secondary antibodies used were anti-rabbit Fab Alexa 488 and anti-mouse Cy3. Nuclei were visualized with Hoechst stain and slides were mounted with ProLong® Gold Antifade mountant (Thermo Scientific). Analysis was performed with a Leica confocal microscope and LASAF software.
Follicle count
For histological examination of the follicle population, fixed ovaries were embedded in paraffin, and after routine histological procedures, sections were stained with hematoxylin and eosin by Slide Stainer HMS 70 (Microm International, Walldorf, Germany) and mounted in Entellan rapid embedding agent (EMS, Hatfield, PA, USA). Follicle count was performed as described previously using serial sections (Durlinger et al., 1999). Follicle diameter was calculated as the mean of measurements along two perpendicular diameters in the section in which the nucleolus of the oocyte was present. Growing follicles were divided into four groups based on their mean diameter: small pre-antral follicles (20–170 μm), large pre-antral follicles (171–220 μm), small antral follicles (221–310 μm) and large antral follicles (>311 μm) (Durlinger et al., 1999; Visser et al., 2007). Atretic follicles were identified by the presence of pyknotic nuclei in granulosa cells (Byskov, 1974; Osman, 1985). Follicles in Stages 1a and 1b of atresia are defined as early atretic follicles. In addition, the number of fresh corpora lutea (CL) formed during the last ovarian cycle was determined. Newly formed CL can be distinguished from older ones by their irregular size and the morphology of the granulosaluteal cells (Visser et al., 2007). All follicles were counted in every fifth section and fresh CL in every tenth section.
Ethical approval
Protocols to obtain ovaries from FMR1 PM carriers and control subjects were approved by the Partners Human Research Committee or the Institutional Review Board at Emory University (Chang et al., 2011).The mice were kept at the Animal Facility of the Erasmus MC in Rotterdam (The Netherlands) under standard animal housing conditions in accordance with the NIH and EU guidelines for the Care and Use of Experimental Animals. The experiments were performed with permission of the local ethics committee.
Statistical analysis
Results are presented as the mean ± SEM. Differences in ovarian parameters between genotype groups and RNA expression levels were tested using Student's t-test. P < 0.05 was considered to be significant.
Results
FMRpolyG-positive inclusions in a FXPOI ovary
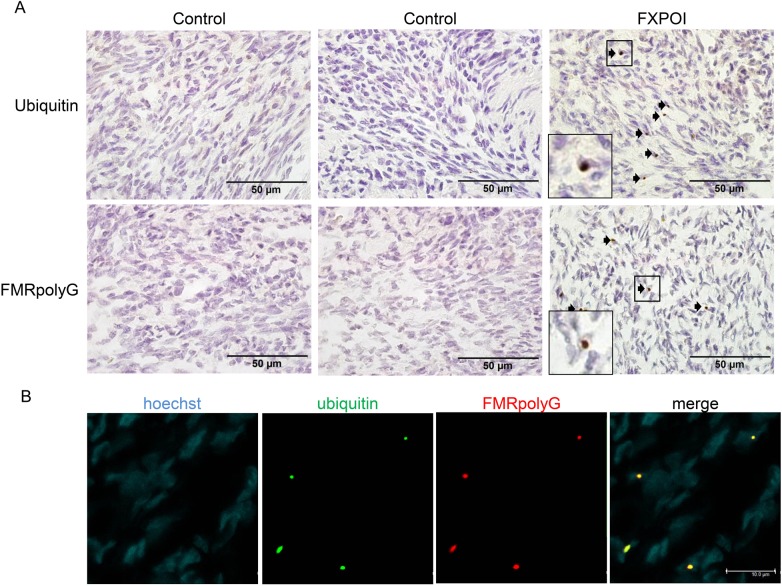
Using paraffin embedded ovarian tissue from a 42-year-old woman with FXPOI, we detected numerous ubiquitin positive inclusions in stromal cells, visible as intranuclear bodies, as previously described (Case 2 in Chang et al., 2011) (Fig. 1A). These inclusions could not be detected in stromal cells of ovaries from control subjects. Since our recent findings indicated that RAN translation occurs for the CGG-repeat in non-CNS tissues (Buijsen et al., 2014), we next determined whether ovaries also contained inclusions positive for the RAN translation product FMRpolyG. Indeed, FMRpolyG-positive staining was detected in ovarian stromal cells of the case with FXPOI, but not in those of control subjects (Fig. 1A). Double labeling revealed that the vast majority (>90%) of the inclusions stained positive for both FMRpolyG and ubiquitin (Fig. 1B).
Figure 1.
Intranuclear inclusions in ovarian stromal cells of a fragile X-associated primary ovarian insufficiency (FXPOI) patient. (A) Ubiquitin- and FMRpolyG-positive inclusions (indicated with arrows) are detected in stromal cells of a FXPOI patient, while two control samples were negative. (B) Co-localization of ubiquitin (green) and FMRpolyG (red) in stromal cells of an ovary from a FXPOI patient, as shown by double immunofluorescence. Nuclei are shown in blue (Hoechst stain); the square indicates of blow up of an inclusion.
FMRpolyG-positive inclusions in exCGG-KI mouse ovaries
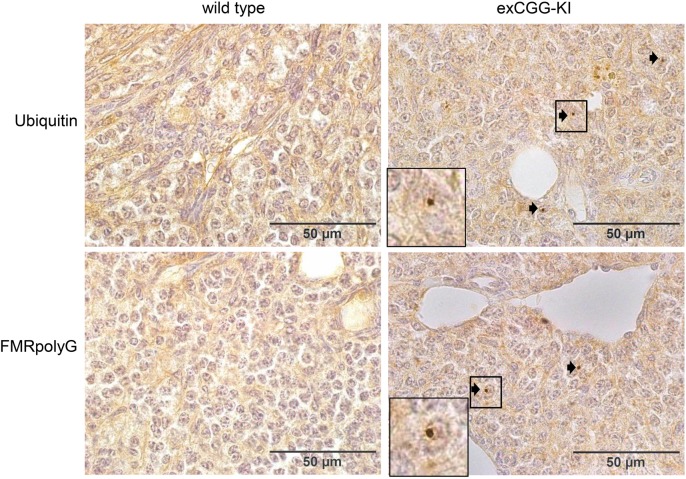
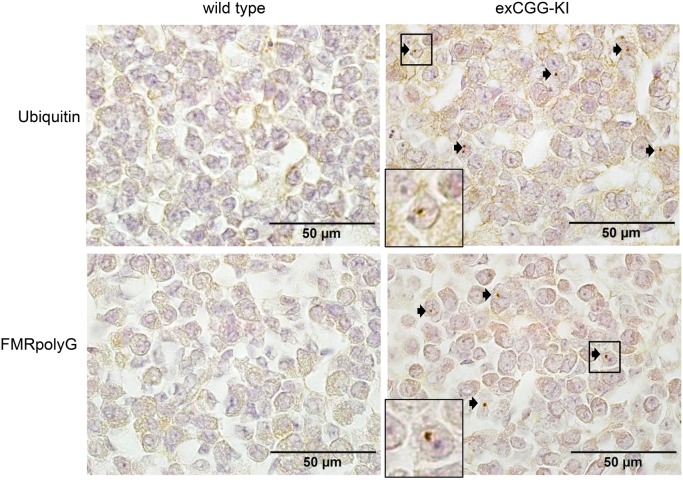
Because the ovarian tissue of the woman with FXPOI did not contain any follicles, we extended our analysis to our exCGG-KI model. Ovaries of 20-week-old exCGG-KI mice contained a few intranuclear ubiquitin- and FMRpolyG-positive intranuclear inclusions in stromal cells, but not in follicles (data not shown). However, in 40-week-old exCGG mice, similar to the human FXPOI case, we detected numerous intranuclear inclusions in the stromal cells of the ovary, both with ubiquitin and FMRpolyG antibodies (Fig. 2). Oocytes, granulosa and theca cells of follicles of all size classes were negative for these inclusions.
Figure 2.
Intranuclear inclusions in ovarian stromal cells of 40-week-old Fmr1 premutation mouse model (exCGG-KI) mice. Immunohistochemistry revealed ubiquitin- and FMRpolyG-positive inclusions (indicated with arrows) in ovarian stromal cells of exCGG-mice (right panels) but not in wild-type mice (left panels). The square indicates of blow up of an inclusion.
Fmr1 expression in exCGG mice
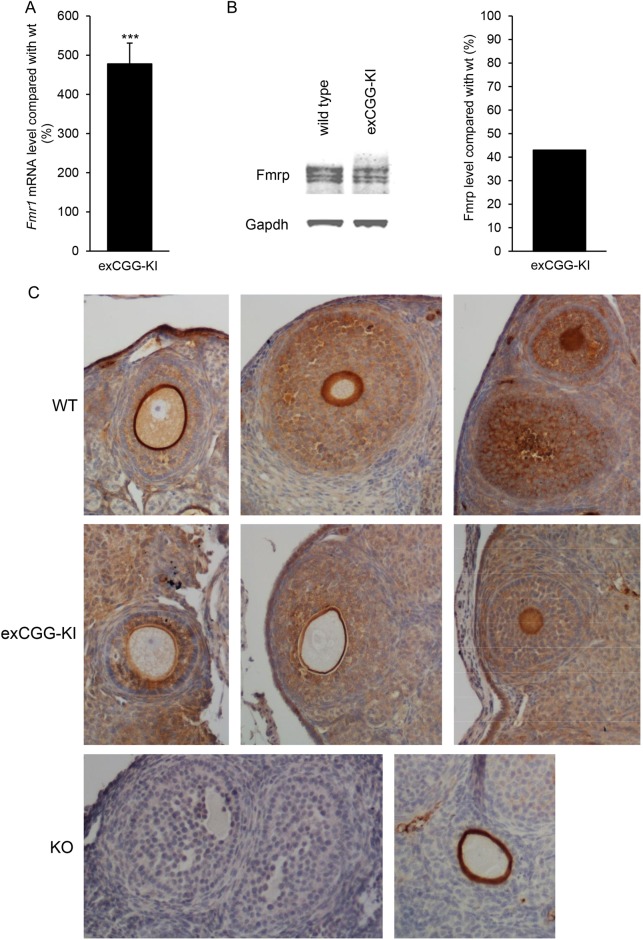
All exCGG-KI mice had a CGG-repeat length in the PM range (100–199 CGGs). To determine the effect of the PM on ovarian Fmr1 mRNA expression, Fmr1 mRNA levels were analyzed in ovaries of 40-week-old exCGG-KI mice. Compared with age-matched wild-type mice, exCGG-KI mice displayed a nearly 5-fold increase in ovarian Fmr1 mRNA expression (Fig. 3A). In contrast, Western blot analysis revealed that Fmrp levels were reduced by 2-fold in exCGG-KI mice compared with wild-type mice (Fig. 3B).
Figure 3.
Ovarian Fmr1 mRNA and fragile X mental retardation protein (Fmrp) expression. (A) Fmr1 mRNA expression in whole ovaries of 40-week-old wild-type (n = 5) and Fmr1 premutation mouse model (exCGG-KI) mice (n = 10). Results are expressed as percentage expression relative to wild-type mice. (B) Western blot analysis of Fmrp expression in 40-week-old whole ovaries of wild-type and exCGG-KI mice. Expression was quantified and expressed relative to wild-type mice (n = 5 per group). (C) Immunohistochemical analysis of Fmrp expression in ovaries of 20-week-old wild-type (WT) and exCGG-KI. Fmrp is expressed in granulosa and theca cells of all growing follicles at different stages. In addition, expression was observed in stromal cells. Ovaries of Fmr1-KO (KO) mice were included as a negative control and did not show specific staining for Fmrp (lower left panel), although some nonspecific staining of the zona pellucida was occasionally seen (lower right panel).
To determine the cellular localization of Fmrp in the ovary, immunohistochemistry was performed using ovaries of 20-week-old mice. Results showed that follicles of all stages expressed Fmrp. Primordial follicles have a strong positive staining for Fmrp. Positive staining was also observed in granulosa and theca cells of growing follicles. In addition, oocytes express Fmrp although the staining intensity weakened in larger growing follicles (Fig. 3C). Fmrp expression was also observed in stromal cells. In ovaries of exCGG-KI mice the staining intensity of Fmrp appeared weaker compared with wild-type mice, in line with the Western blot results. Although some nonspecific staining of the zona pellucida was occasionally seen, the ovaries of knockout mice were negative when stained for Fmrp, illustrating the specificity of the 2F5 antibody.
Follicle numbers in exCGG-KI mice
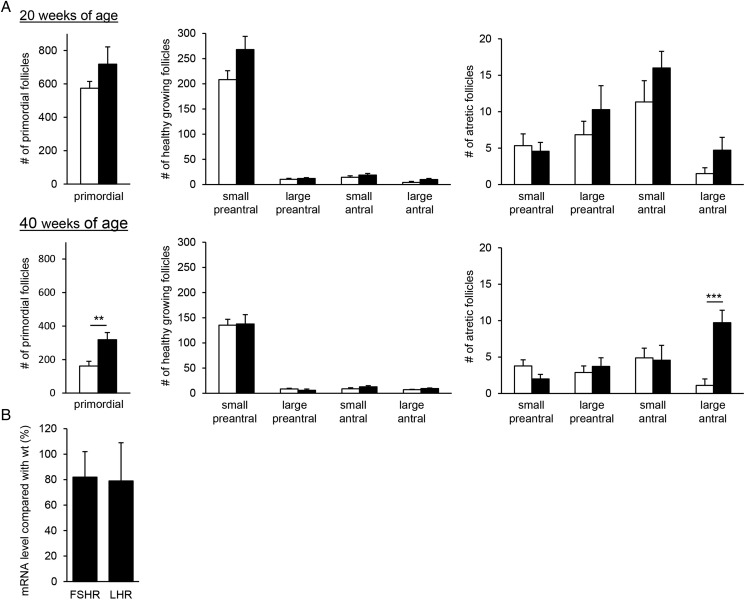
To determine whether the presence of intranuclear inclusions in the ovarian stromal cells of exCGG-KI mice are associated with ovarian function, the entire follicle population was examined at 20 and 40 weeks of age. The number of primordial follicles did not differ between 20-week-old exCGG-KI and wild-type mice, while ovaries of 40-week-old exCGG-KI mice contained 1.5-fold more primordial follicles compared with wild-type mice (P < 0.01) (Fig. 4A). The number of healthy growing follicles did not differ between wild-type and exCGG-KI mice at either age (Fig. 4A). Similarly, no differences were observed in the number of atretic growing follicles between the two genotypes, except for the number of atretic large antral follicles which was increased by nearly 9-fold in 40-week-old exCGG-KI mice (P < 0.001) (Fig. 4A).
Figure 4.
Follicle numbers in Fmr1 premutation mouse model (exCGG-KI) mice. The number of follicles in different size classes was determined in both ovaries for wild-type (open bars) and exCGG-KI (black bars) mice. (A) Number of primordial, healthy and atretic follicles at 20 weeks of age (upper panel) and 40 weeks of age (lower panel). Data represent mean ± SEM (n = 6–9 mice). **P < 0.01; ***P < 0.001. (B) The mRNA expression of FSH and LH receptors (FSHR, LHR) was determined in whole ovaries of 40-week-old wild-type (n = 5) and exCGG-KI (n = 10) mice. Results are expressed as percentage expression relative to wild-type mice. Data represent mean ± SEM.
This increase in number of atretic large antral follicles at 40 weeks of age is suggestive of a failure in ovulation. Therefore, we determined the number of fresh CL, as an index of recent ovulations. At 20 weeks of age, 79% of exCGG-KI mice had recently ovulated compared with 90% of wild-type mice, but this difference was not statistically significant (P = 0.459). Also the number of CL did not significantly differ between exCGG-KI and wild-type mice (Table I). However, at 40 weeks of age, the number of CL in all exCGG-KI mice analyzed was significantly reduced compared with wild types (P < 0.01). This difference is due to the fact that only 50% of exCGG-KI mice had recent ovulations compared with 89% of WT mice, and this difference approached statistical significance (P = 0.07). Furthermore, in those exCGG-KI mice that did have recent ovulations the number of fresh CL tended to be reduced compared with wild-type mice at this age (P = 0.07) (Table I).
Table I.
Number of fresh corpora lutea (CL).
| Age | Genotype | n | % ovulating | No. CL | No. CL in ovulating mice |
|---|---|---|---|---|---|
| 20 weeks | WT | 10 | 90 (9/10) | 8.50 ± 0.98 | 9.44 ± 0.29 |
| exCGG-KI | 14 | 79 (11/14) | 6.43 ± 1.08 | 8.18 ± 0.75 | |
| 40 weeks | WT | 9 | 89 (8/9) | 7.56 ± 1.28 | 8.50 ± 0.98 |
| exCGG-KI | 10 | 50 (5/10)b | 2.40 ± 1.15a | 4.80 ± 1.74b |
The percentage of ovulating mice was determined based on the presence of fresh CL. The number of CL (mean ± SEM) was determined per two ovaries for wild-type (WT) and Fmr1 premutation mouse model (exCGG-KI) mice at 20 and 40 weeks of age.
aP < 0.01.
bP = 0.07.
Because of the reduced number of CL in the exCGG-KI mice, we determined the mRNA expression levels of two receptors known to affect ovulation. However, no differences in mRNA levels for FSH receptor and LH receptor in exCGG-KI mice compared with age matched wild-type mice were found (Fig. 4B).
FMRpolyG and ubiquitin positive inclusions in pituitaries of FMR1 PM carriers
The failure in ovulation may suggest dysfunction of the hypothalamus–pituitary–gonadal (HPG) axis. We previously observed that the hypothalamus–pituitary–adrenal gland (HPA) axis is affected in the exCGG-KI mice and that the pituitary of these mice contained ubiquitin positive inclusions as early as 25 weeks of age (Brouwer et al., 2008). Therefore, we extended our study to determine whether the pituitary also contains FMRpolyG inclusions. Indeed, we found inclusions staining positive for FMRpolyG in pituitaries of exCGG-KI mice but not in wild-type mice (Fig. 5).
Figure 5.
Intranuclear inclusions in Fmr1 premutation mouse model (exCGG-KI) mouse pituitary. Ubiquitin- and FMRpolyG-positive intranuclear inclusions (indicated with arrows) in exCGG-KI mouse pituitary (right panel). Wild-type mouse pituitary was negative for these two markers (left panel). The square indicates of blow up of an inclusion.
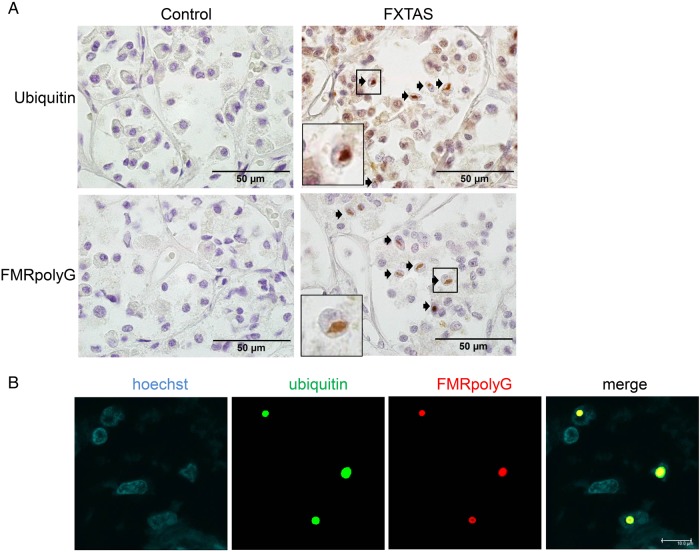
Next, we examined whether there are also FMRpolyG-positive inclusions in the pituitary of PM carriers. For that we used the pituitary of a man with FXTAS from a previous study (Case 6 from Hunsaker et al., 2011) and pituitaries from two control subjects. Indeed, we could detect both ubiquitin- and FMRpolyG-positive inclusions in the PM pituitary, and not in the pituitaries from the control subjects. Ubiquitin- and FMRpolyG-staining co-localized in the vast majority of the inclusions (Fig. 6).
Figure 6.
Intranuclear inclusions in human fragile X-associated tremor/ataxia syndrome (FXTAS) pituitary. (A) Ubiquitin- and FMRpolyG-positive intranuclear inclusions (indicated with arrows) in human FXTAS pituitary (right panel). The control pituitary stains negative for the two markers (left panel). (B) Co-localization of ubiquitin (green) and FMRpolyG (red) in stromal cells of an ovary from a FXPOI patient, as shown by double immunofluorescence. Nuclei are shown in blue (Hoechst stain); the square indicates of blow up of an inclusion.
Discussion
FXPOI is one of the most frequent genetic causes of POI, with nearly 20% of women who carry the PM developing hypergonadotropic hypogonadism compared with 1% of the general population (reviewed in Sherman, 2000; Sullivan et al., 2011). However, little is known of the underlying mechanisms of FXPOI. Intranuclear inclusions are a hallmark for FXTAS, the other PM-associated disorder. Here we confirm that intranuclear inclusions are present in stromal cells of ovaries taken from a woman with FXPOI (Chang et al., 2011). For the first time, we also show that these inclusions stain positive for FMRpolyG, a product of the novel RAN translational mechanism. In addition, we show that the ovaries in a PM-mouse, originally developed as a model of FXTAS, contain ubiquitin- and FMRpolyG-positive inclusions. These FMRpolyG-positive inclusions were also detected in a pituitary from a man with FXTAS as well as in pituitaries of exCGG-KI mice.
The observed expression pattern of Fmrp in all cell types of the ovary is in agreement with previous studies in rodent ovaries (Hoffman et al., 2012; Lu et al., 2012; Ferder et al., 2013), with the exception that we also observed expression in stromal cells of mouse ovaries. This stromal expression pattern is in line with the detection of inclusions in this cell type. Surprisingly, although all follicular stages express Fmrp, they were devoid of inclusions. An explanation for this could be that the development of inclusions requires a prolonged period of time before they become visible, as suggested by the fact that inclusions were observed in the older mice (40-week-old) but were very rarely present in the younger mice (20-week-old). This is consistent with the observations that the formation of intranuclear inclusions in the brain of exCGG-KI mice first appears around 30 weeks of age, with the number and size substantially increasing with age (Willemsen et al., 2003). The formation of inclusions can occur earlier with higher Fmr1 mRNA expression levels, since an inducible mouse model with overexpression of an expanded CGG RNA shows inclusion formation after only 8 weeks of expression (Hukema et al., 2015). Since folliculogenesis is a dynamic process with continuous growth and loss of follicles, the time period to form inclusions may be too short or, alternatively, follicles with inclusions are rapidly removed preventing their visualization.
The elevated levels of Fmr1 mRNA and reduced expression of Fmrp in the ovaries are similar to that found in the brains of these exCGG-KI mice compared with wild-type controls (Willemsen et al., 2003; Brouwer et al., 2007), although the magnitude of the difference in expression in the ovaries is more pronounced. In mice complete loss of Fmrp results in enlarged ovaries with an increased number of follicles compared with wild-type animals (Ascano et al., 2012). Furthermore, ovaries from Fmr1 knock-out mice show increased mTOR protein, whereas the YAC mice with PM CGG repeat show reduced p-mTOR levels (Ascano et al., 2012; Lu et al., 2012). Thus, although the mTOR pathway may be a candidate to target a possible treatment, the ovarian phenotype in the Fmr1 knock-out mice is different from that seen in the YAC and KI mice. This suggests that the ovarian phenotype associated with the PM is not due to reduced FMRP levels. Our finding of ovarian inclusions and the presence of FMRpolyG in these inclusions clearly points the possibility of a pathological gain-of-function mechanism in FXPOI.
In our PM-mouse model we observed, normal or slightly increased numbers of primordial follicles but normal numbers of growing follicles, suggesting that primordial follicle recruitment does not lead to an early exhaustion of the follicle pool. However, aging exCGG-KI mice had no or a reduced number of CL, which, combined with the increased number of atretic large antral follicles, suggests a failure in ovulation. This finding is in agreement with the phenotype observed in two other mouse models that carry the Fmr1 PM (Hoffman et al., 2012; Lu et al., 2012). The presence of the PM may therefore most likely lead to an ovarian defect. However, dysfunction of the HPG axis leading to altered secretion of gonadotrophins cannot be ruled out, particularly since intranuclear inclusions have also been detected in the pituitary gland of the exCGG-KI mice and in a male PM carrier (Greco et al., 2007; Brouwer et al., 2008). The inclusions were detected in the cells of the anterior pituitary, including those that release gonadotrophins (Hunsaker et al., 2011). This suggests that in women with FXPOI ovulation might be defective due to dysfunctioning of the HPG axis. However, in women with the PM, there is no evidence of a disturbance of the HPG axis given their hormonal profile. Unfortunately, serum samples were not available for this study, but in the YAC-TG296 Fmr1 PM mouse model serum LH levels were indeed reduced (Lu et al., 2012).
All three, mouse models used to study FXPOI—the Dutch and NIH KI mice as well as the YAC mouse—can be compared with gain insight into the mechanisms leading to FXPOI. All show increased Fmr1 mRNA expression with normal or reduced Fmrp expression, thereby (partly) resembling the effect of the FMR1 PM in the brain. Lu et al. (2012) used a transgenic model carrying a human yeast artificial chromosome (YAC) with the human PM allele with 90 CGG repeats (YAC-TG296) on a FVB background, homozygous for the wild-type Fmr1 allele (Peier and Nelson, 2002). Since this YAC mouse contains the human FMR1 sequence it has the potential to form FMRpolyG. However, to our knowledge, there is no report yet on the presence or absence of inclusions containing FMRpolyG in the ovaries of these YAC mice. Hoffman et al. (2012) used the ‘NIH knock-in’ mouse on a C57Bl/6 background in which a stable 130 CGG-CCG repeat tract was used to replace the endogenous Fmr1 gene (Entezam et al., 2007). This NIH mouse contains a stopcodon 5′ of the CGG repeat and thus does not produce FMRpolyG (Todd et al., 2013). It has been suggested that this might be the reason the NIH mice show fewer ubiquitin-positive inclusions in the brain than the exCGG-KI mouse used in this study. This might also explain why ovaries of the NIH mice lack ubiquitin inclusions, in contrast to what we show in this study with the Dutch exCGG-KI mice. Our results suggest that FMRpolyG from RAN translation may at least partially play a role in FXPOI since we find its presence in the inclusions. Although, the exact role of the inclusions remain to be determined, the sequestration of the different components in the inclusions might lead to cellular dysfunction (Sellier et al., 2010). However, a role for the free expanded CGG mRNA cannot be ruled out, since the NIH KI mouse also shows defects in ovulation despite the fact that no inclusions were found. In addition, since the inclusions are found in the stromal cells that do not directly influence ovulation, it might very well be that the free soluble FMRpolyG affects ovulation. For example, the potential toxic effect of free soluble FMRpolyG could take place in the granulosa cells, cells known to affect ovulation and known to express FMR1 mRNA and its product. Indeed, the expression of an expanded CGG repeat can also have deleterious effects without the formation of inclusions (Hukema et al., 2014). Future studies are needed to provide insight into the role of the different entities (RNA, FMRpolyG and/or inclusions) in the development of FXPOI, and mouse models will be a very useful tool in such studies.
In summary, this study provides the first clear evidence for FMRpolyG translation in FXPOI. Since we find FMRpolyG-positive inclusions in ovaries and pituitary in both human samples and mouse samples with CGG repeat in the PM range, this suggests that there is, at least partly, a role for FMRpolyG in FXPOI. In addition, our results from the exCGG-KI mouse model suggest that the loss of fertility in FXPOI occurs at the level of ovulation, and that this may be due to a problem in the HPG axis. This has important implications for women carrying a PM and further research should focus on trying to unravel the mechanism underlying reduced ovulation in these women.
Authors’ roles
J.A.V., R.W. and R.K.H.: study design; R.A.M.B, P.K., E.A.W.F.M.S., M.G., N.C.B., S.L.S. and R.F.B.: sample collection; R.A.M.B, P.K. and E.A.W.F.M.S.: laboratory work and data analysis under supervision of J.A.V., R.W., R.K.H., R.A.M.B., J.A.V., S.L.S. and R.K.H.: manuscript drafting; R.A.M.B., J.A.V., P.K., E.A.W.F.M., M.G., N.C.B., S.L.S., R.F.B., R.W. and R.K.H.: critically revision and final approval of the manuscript.
Funding
This work was supported by NFXF to R.K.H.; ZonMW (project 113301201 to R.K.H. and project 113301401 to R.W.), under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; the Netherlands Brain Foundation (F2012(1)-101 to R.W. and F2015(1)-02 to R.K.H.) and NIH (NINDS NS079775 to R.W. and R.F.B.).
Conflict of interest
None declared.
Acknowledgements
The authors wish to acknowledge the contribution of Chantal Sellier, Max Kros, Santoso Jaeri and Ingeborg Nieuwenhuizen-Bakker to this work.
References
- Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, Gorwill H, Nolin SL, Glicksman A et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study—preliminary data. Am J Med Genet 1999;83:322–325. [PMC free article] [PubMed] [Google Scholar]
- Ascano M, Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, Langlois C, Munschauer M, Dewell S, Hafner M et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 2012;492:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW 3rd, Rademakers R et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 2013;77:639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker CE, Kooy RF, D'Hooge R, Tamanini F, Willemsen R, Nieuwenhuizen I, De Vries BBA, Reyniers E, Hoogeveen AT, Willems PJ et al. Introduction of a FMR1 transgene in the fragile X knockout mouse. Neurosci Res Commun 2000;26:265–277. [Google Scholar]
- Berman RF, Buijsen RA, Usdin K, Pintado E, Kooy F, Pretto D, Pessah IN, Nelson DL, Zalewski Z, Charlet-Bergeurand N et al. Mouse models of the fragile X premutation and fragile X-associated tremor/ataxia syndrome. J Neurodev Disord 2014;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontekoe CJ, Bakker CE, Nieuwenhuizen IM, van Der Linde H, Lans H, de Lange D, Hirst MC, Oostra BA. Instability of a (CGG)(98) repeat in the Fmr1 promoter. Hum Mol Genet 2001;10:1693–1699. [DOI] [PubMed] [Google Scholar]
- Brouwer JR, Mientjes EJ, Bakker CE, Nieuwenhuizen IM, Severijnen LA, Van der Linde HC, Nelson DL, Oostra BA, Willemsen R. Elevated Fmr1 mRNA levels and reduced protein expression in a mouse model with an unmethylated Fragile X full mutation. Exp Cell Res 2007;313:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Severijnen E, de Jong FH, Hessl D, Hagerman RJ, Oostra BA, Willemsen R. Altered hypothalamus-pituitary-adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology 2008;33:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijsen RA, Sellier C, Severijnen LA, Oulad-Abdelghani M, Verhagen RF, Berman RF, Charlet-Berguerand N, Willemsen R, Hukema RK. FMRpolyG-positive inclusions in CNS and non-CNS organs of a fragile X premutation carrier with fragile X-associated tremor/ataxia syndrome. Acta Neuropathol Commun 2014;2:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byskov AG. Cell kinetic studies of follicular atresia in the mouse ovary. J Reprod Fertil 1974;37:277–285. [DOI] [PubMed] [Google Scholar]
- Chang MC, Decaro JJ, Zheng M, Gearing M, Shubeck L, Sherman SL, Welt CK. Ovarian histopathological and ubiquitin-immunophenotypic features in fragile X-associated primary ovarian insufficiency: a study of five cases and selected controls. Histopathology 2011;59:1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol 1986;67:604–606. [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 1999;140:5789–5796. [DOI] [PubMed] [Google Scholar]
- Entezam A, Biacsi R, Orrison B, Saha T, Hoffman GE, Grabczyk E, Nussbaum RL, Usdin K. Regional FMRP deficits and large repeat expansions into the full mutation range in a new Fragile X premutation mouse model. Gene 2007;395:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferder I, Parborell F, Sundblad V, Chiauzzi V, Gomez K, Charreau E, Tesone M, Dain L. Expression of fragile X mental retardation protein (FMRP) and Fmr1 mRNA during folliculogenesis in the rat. Reproduction 2013;154:335–343. [DOI] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain 2006;129:243–255. [DOI] [PubMed] [Google Scholar]
- Greco CM, Soontrapornchai K, Wirojanan J, Gould JE, Hagerman PJ, Hagerman RJ. Testicular and pituitary inclusion formation in fragile X associated tremor/ataxia syndrome. J Urol 2007;177:1434–1437. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 2001;57:127–130. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Le WW, Entezam A, Otsuka N, Tong ZB, Nelson L, Flaws JA, McDonald JH, Jafar S, Usdin K. Ovarian Abnormalities in a Mouse Model of Fragile X Primary Ovarian Insufficiency. J Histochem Cytochem 2012;60:439–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukema RK, Oostra BA. The CGG repeat and the FMR1 gene. Methods Mol Biol 2013;1010:155–176. [DOI] [PubMed] [Google Scholar]
- Hukema RK, Buijsen RA, Raske C, Severijnen LA, Nieuwenhuizen-Bakker I, Minneboo M, Maas A, de Crom R, Kros JM, Hagerman PJ et al. Induced expression of expanded CGG RNA causes mitochondrial dysfunction in vivo. Cell Cycle 2014;13:2600–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukema RK, Buijsen RA, Schonewille M, Raske C, Severijnen LA, Nieuwenhuizen-Bakker I, Verhagen RF, van Dessel L, Maas A, Charlet-Berguerand N et al. Reversibility of neuropathology and motor deficits in an inducible mouse model for FXTAS. Hum Mol Genet 2015;24:4948–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Greco CM, Spath MA, Smits AP, Navarro CS, Tassone F, Kros JM, Severijnen LA, Berry-Kravis EM, Berman RF et al. Widespread non-central nervous system organ pathology in fragile X premutation carriers with fragile X-associated tremor/ataxia syndrome and CGG knock-in mice. Acta Neuropathol 2011;122:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, Leal J. Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am J Med Genet A 2014;164A:1648–1658. [DOI] [PubMed] [Google Scholar]
- Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, Themmen AP, Visser JA. Serum anti–Mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 2006;147:3228–3234. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- Lu C, Lin L, Tan H, Wu H, Sherman SL, Gao F, Jin P, Chen D. Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum Mol Genet 2012;21:5039–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 2013;339:1335–1338. [DOI] [PubMed] [Google Scholar]
- Oh SY, He F, Krans A, Frazer M, Taylor JP, Paulson HL, Todd PK. RAN translation at CGG repeats induces ubiquitin proteasome system impairment in models of fragile X-associated tremor ataxia syndrome. Hum Mol Genet 2015;24:4317–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman P. Rate and course of atresia during follicular development in the adult cyclic rat. J Reprod Fertil 1985;73:261–270. [DOI] [PubMed] [Google Scholar]
- Peier A, Nelson D. Instability of a premutation-sized CGG repeat in FMR1 YAC transgenic mice. Genomics 2002;80:423–432. [DOI] [PubMed] [Google Scholar]
- Sellier C, Rau F, Liu Y, Tassone F, Hukema RK, Gattoni R, Schneider A, Richard S, Willemsen R, Elliott DJ et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J 2010;29:1248–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet 2000;97:189–194. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Welt C, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med 2011;29:299–307. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: A new mechanism of involvement in the Fragile-X syndrome. Am J Hum Genet 2000;66:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd PK, Oh SY, Krans A, He F, Sellier C, Frazer M, Renoux AJ, Chen KC, Scaglione KM, Basrur V et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron 2013;78:440–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JA, Durlinger AL, Peters IJ, van den Heuvel ER, Rose UM, Kramer P, de Jong FH, Themmen AP. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Mullerian hormone null mice. Endocrinology 2007;148:2301–2308. [DOI] [PubMed] [Google Scholar]
- Visser JA, Schipper I, Laven JS, Themmen AP. Anti-Mullerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol 2012;8:331–341. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen L, Nieuwenhuizen I, Schrier M, VanUnen L, Tassone F, Hoogeveen A et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet 2003;12:949–959. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Levenga J, Oostra B. CGG repeat in the FMR1 gene: size matters. Clin Genet 2011;80:214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MA et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci U S A 2011;108:260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]