Abstract
STUDY QUESTION
Is blastocyst biopsy and quantitative real-time PCR based comprehensive chromosome screening a consistent and reproducible approach across different biopsy practitioners?
SUMMARY ANSWER
The blastocyst biopsy approach provides highly consistent and reproducible laboratory and clinical outcomes across multiple practitioners from different IVF centres when all of the embryologists received identical training and use similar equipment.
WHAT IS KNOWN ALREADY
Recently there has been a trend towards trophectoderm (TE) biopsy in preimplantation genetic screening (PGS)/preimplantation genetic diagnosis (PGD) programmes. However, there is still a lack of knowledge about the reproducibility that can be obtained from multiple biopsy practitioners in different IVF centres in relation also to blastocysts of different morphology. Although it has been demonstrated that biopsy at the blastocyst stage has no impact on embryo viability, it remains a possibility that less experienced individual biopsy practitioners or laboratories performing TE biopsy may affect certain outcomes. We investigated whether TE biopsy practitioners can have an impact on the quality of the genetic test and the subsequent clinical outcomes.
STUDY DESIGN, SIZE, DURATION
This longitudinal cohort study, between April 2013 and December 2014, involved 2586 consecutive blastocyst biopsies performed at three different IVF centres and the analysis of 494 single frozen euploid embryo transfer cycles (FEET).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Seven biopsy practitioners performed the blastocyst biopsies in the study period and quantitative PCR was used for comprehensive chromosome screening (CCS). The same practitioner performed both the biopsy and tubing procedures for each blastocyst they biopsied. To investigate the quality of the biopsied samples, the diagnostic rate, sample-specific concurrence and the cell number retrieved in the biopsy were evaluated for each biopsy operator. Clinical outcomes following FEET cycles were stratified by biopsy operator and compared. Cellularity of the biopsy sample was also correlated with clinical outcomes.
MAIN RESULTS AND THE ROLE OF CHANCE
The seven practitioners performed 2586 biopsies, five in centre IVF-1 and one in each of the other two IVF centres (IVF-2 and IVF-3). Overall, 2437 out of 2586 (94.2%) blastocyst biopsies resulted in a conclusive diagnosis, 119 (4.6%) showed a nonconcurrent result and 30 (1.2%) failed to amplify, suggesting the absence of TE cells in the test tube or presence of degenerated/lysed cells only. Among the samples producing a conclusive diagnosis, a mean concurrence value of 0.253 (95% CI = 0.250–0.257) was observed. Logistic regression analysis adjusted for confounding factors showed no differences in the diagnosis rate and in the concurrence of the genetic analysis between different biopsy practitioners. An overall mean number of 7.32 cells (95% CI = 6.82–7.81; range 2–15) were predicted from all biopsies. Higher cellularity was significantly associated with a better quality of the CCS diagnosis (P < 0.01) and with the conclusive diagnosis rate, with nonconcurrent samples showing significantly lower numbers of cells (2.1; 95% CI=1.5–2.7) compared with samples resulting in a conclusive diagnosis (mean cells number 7.5; 95% CI = 7.1–7.9, P < 0.01). However, no differences were recorded between different biopsy practitioners regarding cellularity of the biopsy. Finally, logistic analysis showed no impact of the biopsy practitioners on the observed ongoing rates of implantation, biochemical pregnancy loss and miscarriage after the FEET cycles.
LIMITATIONS, REASONS FOR CAUTION
These data come from a restricted set of laboratories where all of the embryologists received identical training and use identical equipment. A single TE biopsy method and CCS technology was used and these data particularly apply to PGS programmes using blastocyst biopsy without zona opening at the cleavage stage and using qPCR-based CCS. To make firm conclusions on the potential impact of biopsy on biochemical pregnancy loss and miscarriages according to practitioner and biopsy cellularity, a larger sample size is needed.
WIDER IMPLICATIONS OF THE FINDINGS
We reported a very high consistency and reproducibility of the blastocyst biopsy approach coupled with qPCR-based CSS for both genetic and clinical outcomes across different practitioners working in different IVF centres when appropriate training is provided and when the same laboratory setting is used. These data are important considering the trend towards the use of blastocyst biopsy worldwide for PGD/PGS applications.
STUDY FUNDING/COMPETING INTEREST(S)
None.
Keywords: blastocyst biopsy, preimplantation genetic screening, aneuploidies, embryo selection, blastocyst evaluation
Introduction
Blastocyst biopsy is now widely used for both preimplantation genetic screening (PGS) for aneuploidies and preimplantation genetic diagnosis (PGD) of single gene defects. In particular, trophectoderm biopsy coupled with comprehensive chromosome screening (CCS) now represents the most promising approach for PGS to detect aneuploidies coming from both male and female meiosis, as well as clinically relevant mitotic errors fixed during preimplantation embryo development (Schoolcraft et al., 2010; van Echten-Arends et al., 2011; Forman et al., 2012a,b; Capalbo et al., 2013a,b). Biological and clinical evidence of the high effectiveness of blastocyst stage PGS to improve embryo selection are being reported, leading to a growing clinical application of this strategy worldwide (Fragouli et al., 2008; Schoolcraft et al., 2010; Forman et al., 2012a,b; Yang et al., 2012; Capalbo et al., 2013a,b). Several randomized controlled trials have been published, enrolling patients from different populations and using different CCS technologies, with all pointing out its clinical value for improving embryo selection in IVF cycles (Yang et al., 2012; Forman et al., 2013a,b; Scott et al., 2013a,b). In fact, the ESHRE PGD consortium data collection XII (data for the years 2009–2010) reported that <0.1% of PGS cycles performed in European centres were based on blastocyst stage biopsies (Moutou et al., 2014), but this rate has grown impressively up to 24% in 2012–2013 (Synodinos, 2014, data reporting from ESHRE PGD consortium) to the detriment of the polar bodies (PBs) biopsy strategy application that dramatically decreased to 2%. However, blastomere biopsy, whose application has been constantly around 74% from 2009 to 2014, remains the most widely used technique. This is despite all the published evidence of its drawbacks, ranging from the impact of mosaicism and the technical issues related to single cell genetic analysis, to the risk of compromising embryo viability (Los et al., 2004; Munnè et al., 2005; Johnson et al., 2010; Scott et al., 2013a,b; Van der Aa et al., 2013). This may be due to cleavage stage biopsy being easier to perform and standardize when compared with blastocyst biopsy, where the rate of embryo development is asynchronous and often different morphological qualities and degrees of expansion that may impact on the time and quality of the biopsy. Indeed, whereas most normally developing embryos reach the 6- to 10-cell stage on the morning of Day-3 post insemination, allowing a reproducible biopsy approach of a single cell, the timing of blastocyst expansion can vary by over 24 h and occur on Day 5 or 6, or even Day 7 in ∼5% of embryos (Capalbo et al., 2014a,b). Furthermore, trophectoderm (TE) biopsy involves removing a small segment of trophectoderm where the range of cells obtained is not precisely defined. Also, the TE layer may differ in elasticity between blastocysts of different quality.
Thus, although this approach yields good clinical results, variability in embryo quality, particularly in the trophectoderm layer, and the rate of development to the fully expanded blastocyst stage, remains a challenge. Indeed, as a consequence of the recent introduction of TE biopsy in PGS/PGD programmes on a large scale, there is still a lack of knowledge about the reproducibility and consistency that can be obtained from multiple biopsy practitioners in different IVF centres in relation to blastocysts of different morphological quality. It has been demonstrated by Scott et al. (2013a,b) that there is little, if any, impact of embryo biopsy at the blastocyst stage in their elegant prospective non-selection study. However, it still remains a possibility that less experienced or skilled biopsy practitioners may have different impacts on one or more laboratory or clinical aspects of the blastocyst biopsy approach.
In this study, we aimed to determine whether the operator performing the TE biopsy procedure can influence the quality of the TE biopsy by looking at genetic data obtained from a large cohort of human blastocysts from multiple centres, as well as whether the biopsy operator might impact the reproductive potential of euploid blastocysts following single frozen embryo transfer cycles.
Materials and Methods
Study design
This was a longitudinal cohort study performed between April 2013 and December 2014 involving 2586 consecutive blastocyst biopsies performed at three different GENERA IVF centres in Italy, namely Rome (hereafter referred as IVF centre 1), Marostica (hereafter referred as IVF centre 2) and Naples (hereafter referred as IVF centre 3) and the analysis of 494 single frozen euploid embryo transfer cycles (FEET).
From 2013 in these three IVF centres, blastocyst biopsy and quantitative PCR (qPCR) based CCS was increasingly explored to improve embryo selection. PGS was offered to patients of advanced female age (>35 years) or to younger patients with a history of unsuccessful IVF treatments (more than two failed IVF cycles) or previous spontaneous abortion (more than two miscarriages). In IVF centre 1, where most of the IVF-PGS cycles were performed, five different biopsy practitioners were gradually enrolled in the blastocyst biopsy programme over the study period while for IVF centres 2 and 3, only one resident practitioner at each centre was involved in the biopsy programme. Before performing clinical biopsies, all practitioners had to complete a training consisting of 20 supervised embryo biopsy and tubing procedures. The ability and competence of each practitioner were assessed on three parameters that defined the key performance indicators for blastocyst biopsy: (i) time length to perform the biopsy; (ii) percentage of blastocyst re-expansion after biopsy and (iii) genetic laboratory outcomes. In particular normal values were considered as <3 min to perform a single biopsy, >90% re-expansion within 3 h after biopsy and successful amplification and diagnosis in >90% of samples. Accordingly, a practitioner was considered completely trained when at least 9 out of 10 consecutive biopsies met these criteria (with a minimum of 20 full procedures completed). All embryologists in this study completed their training within the 20 trials.
All CCS procedures were performed at a single genetic centre (GENETYX srl, Marostica, Italy) using a single 24-chromosome aneuploidy screening technology based on qPCR.
The infertility treatment protocols, including hormonal stimulation, oocyte retrieval, in vitro fertilization, embryo culture and transfer methods, applied in these clinics have been previously described by Rienzi et al. (2010).
The Institutional Review Boards of the Clinics approved the study.
Outcome measures
The main outcome measures to determine the laboratory and clinical consistency of blastocyst biopsy were based on the quality of the genetic analysis and on the clinical outcomes achieved by each single operator, controlling for possible confounding factors.
To investigate the quality of the genetic test, the CCS diagnostic rate, sample-specific concurrence and cellularity of the TE biopsy were evaluated for each biopsy practitioner and corrected for embryo quality and batches of reagents used for the qPCR-based aneuploidy screening over the study period. The main laboratory outcome measure was the successful diagnostic rate.
To evaluate the cellular quality of the TE biopsies, the overall concurrence of the chromosome analysis was calculated for each sample. In particular, qPCR-based aneuploidy screening entails the analysis of four different assays per chromosome that are then normalized on a control set of normal male references. In this analysis, it is first assumed that the qPCR assay can assess only whole-chromosome aneuploidy, such that the four copy number assignments within each chromosome should always agree. Therefore, the standard deviation of the four measurements of copy number for each chromosome was calculated. The standard deviations of each of the 24 chromosomes are then averaged for each sample giving a precise estimation of the general quality of the analysis. The output is a numerical variable with values ranging from 0.1 for the best reactions to 0.4 for the poorest quality. Biopsies consisting of few, lysed or degenerated cells obtained higher scores of concurrence. Outliers (nonconcurrent samples with no diagnosis) are defined as samples found outside an interquartile range of 1.5 from the overall distribution of average sample-specific standard deviations for each sample type and, in general, samples resulting in concurrence values higher than 0.5.
Unamplified samples show failed amplification for all assays included for the 24-chromosome analysis, suggesting the absence of cells in the test tube that can be related to a failure during the tubing procedure.
Finally, a standardized curve based upon quantitative real-time PCR amplification of single fibroblast cell data was used to estimate the cellularity of the TE biopsies, which was then analysed as a continuous variable or in quartiles from least (1) to most (4) cellular.
To evaluate the possible impact of the biopsy operator on embryonic reproductive competence, clinical outcomes following single FEET cycles were stratified per biopsy operator and compared. Biochemical pregnancy loss (absence of an identifiable pregnancy on ultrasound examination after a positive pregnancy test), miscarriage (spontaneous termination of pregnancy between Week 7 and 20) and ongoing implantation rate (number of fetuses with heart activity beyond 20 weeks of gestation per transferred embryo) were compared between practitioners following adjustment for confounding factors (Farquharson et al., 2005). Cellularity of the biopsy sample was also correlated with clinical outcomes of FEET cycles. The main clinical outcome measure was the ongoing implantation rate.
Blastocyst classification and biopsy
The blastocysts were evaluated according to the degree of expansion and the quality of the inner cell mass and of the trophectoderm cells (Gardner and Schoolcraft, 1999). Details about embryo classification have been described previously (Capalbo et al., 2014a).
At 120–160 h from insemination, all expanded blastocysts, independently of ICM and TE morphology and regardless the presence of herniating cells or not, underwent TE biopsy as previously described (Capalbo et al., 2014a). In brief, the main difference in the biopsy method implemented in our clinical practice with the one initially described by McArthur in 2005 (McArthur et al., 2005) and then adopted in most IVF centres performing TE biopsy (Schoolcraft et al., 2010), resides in the moment of zona pellucida opening. According to our method, zona opening and TE cells retrieval are consequently performed on Day 5, 6 or 7 of preimplantation development at the time of biopsy. All blastocysts with a visible blastocoel where an inner cell mass could be identified and with at least a few cells forming the trophectoderm epithelium, were included. All biopsy procedures were conducted on a heated stage of a Nikon IX-70 microscope, equipped with micromanipulation tools in a dish prepared with three droplets of 10 µl of HEPES buffered medium (Quinn's Advantage®, Cooper Surgical) overlaid with pre-equilibrated mineral oil. The blastocyst is positioned on the holding pipette and oriented in a way that the ICM is clearly visible and opposite with respect to the biopsy pipette (Research Instruments, Cornwall TR11 4TA, UK). A diode laser (Research Instruments) is used to assist the opening of a 10–20 µm hole in the zona pellucida. A series of laser pulses are fired being careful not to damage the blastocyst. When the hole is sufficiently large to allow the passage of 3–10 TE cells, some media is blown from the biopsy pipette through it so that the TE detaches from the inner surface of the zona and starts collapsing. By entering the zona with the pipette, 3–10 TE cells are aspirated with moderate suction, and by synchronously firing several laser pulses aiming at the thinnest junctions between cells and stretching them with a continuous gentle suction until the sample separates from the body of the embryo. Following this, the retrieved TE fragment is expelled from the biopsy pipette to be collected during the tubing procedure. According to our practice, the same embryologist always performs both the TE biopsy and tubing procedures for each individual procedure. Soon after biopsy, the blastocyst is moved to a post-biopsy dish and put back into the incubator until vitrification. The TE fragment is extensively washed through 10 µl drops of hypotonic solution with a stripper pipette mounting 140 µm tips, in order to remove any possible fragmented or degenerated cells and, in presence of a witness, transferred to a PCR tube properly labelled with the couple univocal ID and embryo number. Samples are stored at −20°C, shipped to the referring genetic centre and then processed for qPCR analysis.
Aneuploidy screening of trophectoderm biopsies and cellularity assessment
Trophectoderm biopsies were sent to a reference genetic laboratory for the analysis (GENETYX srl, Marostica, Italy). All samples were processed for CCS by placing them in an alkaline lysis buffer and performing real-time polymerase chain reaction protocol of 24-chromosome analysis as previously described by Treff et al. (2012). In brief, multiplex amplification of 96 loci (four for each chromosome) was carried out, and a method of relative quantification (Schmittgen and Livak, 2008) was applied to predict the copy number status of each chromosome. This methodology was designed to specifically identify whole chromosome, not segmental, aneuploidy, and was validated in preclinical (Treff et al., 2012) and clinical studies (Scott et al., 2013b) as well as extensively validated in our laboratory (Capalbo et al., 2014b). A karyotype prediction was made for each embryo by a certified cytogeneticist.
A standard curve of quantitative real-time (q)PCR-based mean threshold cycles (CT) from a 96-plex reaction was established by analysing the results of assays on known numbers of cells (1, 2, 3, 4, 5, 10, 15 and 20) from cell lines that are easily counted and loaded. The curve was then used to interpolate cell numbers using the CT data from analysis of TE biopsies undergoing aneuploidy screening. This analysis may result in a little overestimation of the cell number predicted in TE biopsies due to the potential presence of tetraploid cells in the human blastocysts. However, this aspect will not affect the comparisons and analysis performed in the study since tetraploid cells will be evenly distributed in TE samples from different embryos and similarly represented in all biopsy practitioner's results.
Statistical analysis
Continuous data are presented as absolute, mean with 95% confidence interval (CI). Categorical variables are presented as absolute, percentage frequency with 95% CI. Fisher's exact test and ANOVA test with Bonferroni's correction were used to assess differences between categorical and continuous variables, respectively. Forward logistic regression analysis was used to control for potential confounding factors for the main comparisons. In particular, genetic data were controlled for preamplification lot number, gene expression master mix lot number, 24-chromosome qPCR primer pool number, blastocyst quality and timings of development to the blastocyst stage. Clinical outcomes of FEET cycles were controlled for female age, indication for PGS, blastocyst quality and day of blastocyst biopsy following fertilization.
Alpha was set at 0.05 for single comparisons and to 0.002 when Bonferroni's correction for multiplicity of testing was applied. All analyses were carried out using the statistical software R version 2.14.2 (Free Software Foundation, Inc., Boston, MA, USA).
Results
This study included the analysis of 2586 trophectoderm biopsies from 906 IVF-PGS cycles in three IVF centres using similar laboratory and clinical protocols and the evaluation of 494 single FEET cycles. The mean female age at oocyte retrieval was 39.4 (SD = 3.2; range 23–44) and the main indication for aneuploidy screening was advanced female age (Table I). No differences were observed between the three IVF centres regarding embryological, genetic and clinical outcomes (Table I). All IVF centres experienced consistent rates of ongoing implantation, biochemical pregnancy loss and miscarriage during the study period (Table I).
Table I.
Clinical and embryological data across the three IVF centres participating to the study.
| Total | Centre 1 | Centre 2 | Centre 3 | P-value | |
|---|---|---|---|---|---|
| Number of patients | 812 | 626 | 117 | 69 | |
| Number of cycles | 906 | 707 | 118 | 81 | |
| Mean female age (range) |
39.4 ± 3.2 (23.0–44.0) |
39.4 ± 3.2 (23.0–44.0) |
39.4 ± 3.1 (28.0–43.9) |
38.8 ± 3.7 (31.0–44.0) |
NS |
| Indication to PGS | |||||
| AMA (%) | 628 (69.3) | 486 (68.7) | 88 (74.6) | 54 (66.7) | NS |
| RPL (%) | 8 (0.9) | 6 (0.8) | 1 (0.8) | 1 (1.2) | NS |
| RIF (%) | 52 (5.7) | 38 (5.4) | 9 (7.6) | 5 (6.2) | NS |
| AMA + RPL (%) | 51 (5.6) | 38 (5.4) | 6 (5.1) | 7 (8.6) | NS |
| AMA + RIF (%) | 92 (10.2) | 80 (11.3) | 6 (5.1) | 6 (7.4) | NS |
| Other (%) | 75 (8.3) | 59 (8.3) | 8 (6.8) | 8 (9.9) | NS |
| Biopsied blastocysts | 2586 | 2179 | 200 | 207 | |
| Mean number of biopsied blastocysts per cycle (range) | 2.8 ± 1.9 (1–14) | 2.9 ± 1.9 (1–14) | 2.4 ± 1.6 (1–10) | 1.5 ± 0.7 (1–10) | NS |
| Morphology | |||||
| Excellent (%) | 1157 (44.8) | 993 (45.6) | 77 (38.7) | 87 (41.9) | NS |
| Good (%) | 387 (15.0) | 328 (15.0) | 28 (13.8) | 31 (15.2) | NS |
| Average (%) | 442 (17.1) | 350 (16.1) | 51 (25.4) | 41 (19.9) | NS |
| Poor (%) | 600 (23.2) | 508 (23.3) | 44 (22.1) | 48 (23.0) | NS |
| Day of biopsy | |||||
| Day 5 (%) | 955 (36.9) | 816 (37.4) | 63 (31.5) | 76 (36.7) | NS |
| Day 6 (%) | 1396 (54.0) | 1159 (53.2) | 127 (63.5) | 110 (53.1) | NS |
| Day 7 (%) | 236 (9.1) | 205 (9.4) | 10 (5.0) | 21 (10.1) | NS |
| DIAGNOSIS | |||||
| Euploid (%) | 1025 (39.6) | 864 (39.6) | 94 (47.2) | 70 (33.8) | NS |
| Single/Double aneuploid (%) | 1178 (45.5) | 986 (45.2) | 89 (44.3) | 103 (49.7) | |
| Complex aneuploid (%) | 234 (9.1) | 207 (9.5) | 7 (3.5) | 18 (8.5) | |
| Nonconcurrent analysis (%) | 119 (4.6) | 102 (4.7) | 8 (4.0) | 9 (4.3) | |
| No amplification (%) | 30 (1.2) | 21 (1.0) | 2 (1.0) | 7 (3.4) | |
| Transferred blastocysts | 494 | 432 | 34 | 28 | |
| Clinical outcomes | |||||
| Positive pregnancy tests (%) | 264 (53.4) | 225 (52.1) | 20 (58.8) | 19 (67.9) | NS |
| Biochemical pregnancies (%) | 18 (6.8) | 14 (6.2) | 2 (10.0) | 2 (10.5) | NS |
| Miscarriages (%) | 23 (9.3) | 21 (9.9) | 2 (11.0) | 0 (0) | NS |
| Ongoing implanted blastocysts (>12 weeks of gestation)(%) | 223 (45.1) | 190 (44.0) | 16 (47.1) | 17 (60.7) | NS |
The data highlight no relevant differences between the three IVF centres in terms of patient population, embryological data and clinical outcomes. In bold are reported the overall values from the 3 IVF centers.
AMA, advanced maternal age; RPL, recurrent pregnancy loss; RIF, recurrent implantation failure.
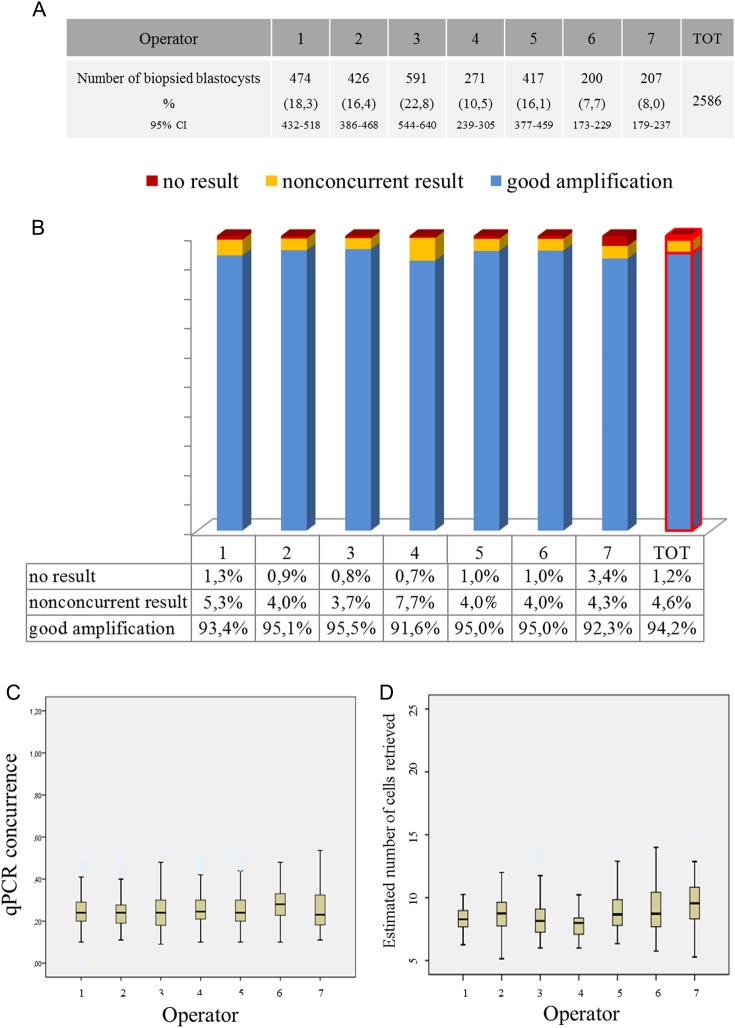
Seven biopsy practitioners performed the biopsy procedures, five in IVF centre 1 and one in each of the other two IVF centres. In total, five embryologists performed 2179 blastocyst biopsies in IVF centre 1 (84.3%; 95% CI = 83.5–86.3), while one embryologist for each centre performed 200 (7.7%; 95% CI = 6.7–8.8) and 207 (8.0%; 95% CI = 7.0–9.1) biopsy procedures for IVF centres 2 and 3, respectively (Fig. 1A). Embryo biopsies and FEET cycles were evenly distributed between embryologists of IVF centre 1 (Fig. 1A). There were 494 FEET performed, 432 in IVF centre 1, 34 and 28 in IVF centres 2 and 3, respectively (Table I).
Figure 1.
Comprehensive chromosome screening (CCS) data across different biopsy operators. (A) Number and percentage frequencies of biopsied blastocysts from each operator. (B) No significant differences were shown at the logistic regression analysis among different biopsy operators in terms of absence of amplification, nonconcurrent results and good quality CCS data, underlining the high reliability and reproducibility of CCS-based aneuploidy screening on trophectoderm (TE) fragments. Although not significant, Operator 7 showed a relatively higher no result rate that might highlight a difficulty in coordinating the tubing procedure as the most critical step in the blastocyst biopsy and processing procedure. (C) Boxplots displaying that qPCR data concurrence between different biopsy operators. (D) Boxplots displaying the estimated number of cells retrieved from different biopsy operators.
Genetic data per biopsy operator
Overall, 2437 out of 2586 (94.2%; 95% CI = 93.3–95.1) blastocyst biopsies resulted in a conclusive diagnosis at the first attempt, 119 (4.6%; 95% CI = 3.8–5.5) showed a nonconcurrent result and 30 (1.2%; 95% CI = 0.8–1.6) failed to amplify, suggesting the absence of TE cells in the test tube. Following preamplification excess reanalysis of nonconcurrent samples, the overall conclusive diagnosis rates increased to 96%. No differences were observed in CCS outcomes when stratified per biopsy operator (Fig. 1B). Logistic regression analysis confirmed that biopsy operator was not related to the outcome of the CCS diagnosis (conclusive versus nonconclusive result) following adjustment for possible confounding factors (P = 0.62; Supplementary Table SI). Although not statistically significant, practitioner 7 showed a notably higher no result rate relative to all of the others, highlighting a specific difficulty in co-ordinating the tubing procedure.
Among the samples producing a conclusive diagnosis, a mean concurrence value of 0.253 (95% CI = 0.250–0.257) was observed. ANOVA testing showed no differences in the concordance values between the seven embryologists, suggesting similar cellular quality of the biopsied samples (Fig. 1C).
The development of a standard curve using samples with increasing concentration of cells allowed a detailed estimation of the cell number in clinical TE biopsies. An overall mean number of 7.32 (95% CI = 6.82–7.81; range 2–15) was predicted from all biopsies.
Higher cellularity was significantly associated with a better quality of the genetic test, with lower concurrence levels observed with increasing number of cells estimated in the biopsy (P < 0.01). In particular, concurrence values of 0.28 (95% CI = 0.26–0.3), 0.24 (95% CI = 0.23–0.26), 0.21 (95% CI = 0.20–0.23) and 0.19 (95% CI = 0.18–0.20) were observed for TE biopsies containing high, medium high, medium low and low cellularity. The diagnosis rate was also correlated with TE biopsy cellularity, with nonconcurrent samples showing significant lower numbers of cells (2.1; 95% CI = 1.5–2.7) compared with samples resulting in a conclusive diagnosis (7.5; 95% CI = 7.1–7.9, P > 0.01; Supplementary Table SII).
The day of biopsy was also mildly associated with cellularity, with embryos biopsied on Day 5 showing a slightly lower cellular concentration compared with Day 6 and 7 blastocysts (6.6, 8.2 and 7.7 cells for Day 5, 6 and 7, respectively; P = 0.01; Supplementary Table SII).
No differences were also observed between embryos of different morphology and the outcomes of CCS diagnosis (P = 0.58), with good and poor quality embryos showing similar conclusive diagnostic rates and concordance values (Fig. 2).
Figure 2.
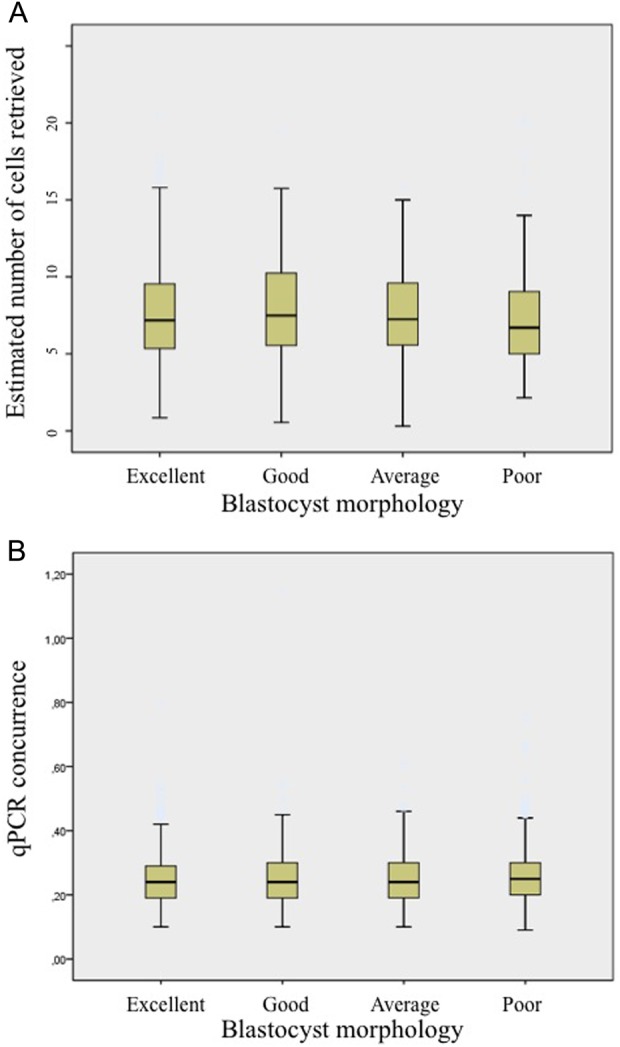
The number of cells retrieved and the CCS results do not correlate with blastocyst morphology. (A) Boxplot showing the estimated number of cells retrieved from blastocysts belonging to different morphological classes. Lower quality blastocysts did not require bigger TE fragments to obtain a proper diagnosis. (B) Boxplot relating qPCR data concurrence with blastocyst morphological evaluation. No significant differences were revealed among different classes, underlining the absence of influence of morphology upon CCS data quality.
Finally, no impact of biopsy practitioners on cellularity was observed. In particular, ANOVA analysis with Bonferroni's correction failed to show differences in cells number in clinical biopsies from different practitioners (Fig. 1D) as well as the logistic regression model adjusted for confounding factors (Supplementary Table SII).
Clinical data according to biopsy operator
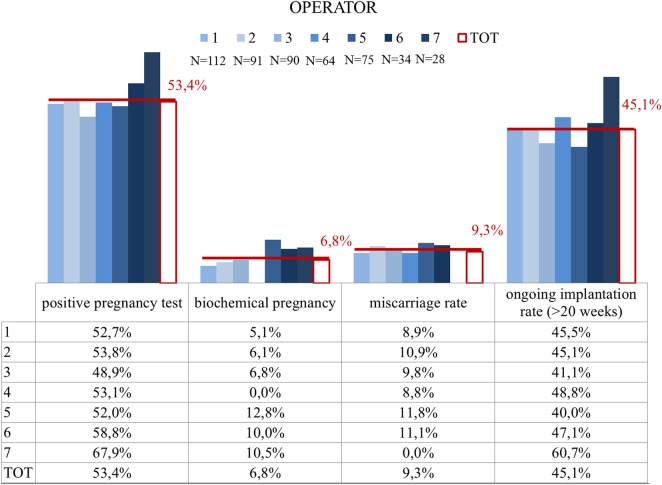
All of the 2586 blastocysts underwent vitrification soon after trophectoderm biopsy. None of the embryo showed degeneration immediately after biopsy although a prolonged observation of this phenomenon was not possible in accordance with our clinical settings where biopsied blastocysts are immediately vitrified. In the observational period, 501 euploid blastocysts were warmed and 6 did not survive (1.2%; 95% CI = 0.4–2.6). Within two hours after thawing, 494 single embryo transfers of euploid blastocysts were performed into the course of natural cycles. There were 264 embryos that implanted and 223 resulted in an ongoing pregnancy or delivered. There were biochemical pregnancy losses and 23 clinical miscarriages were recorded (Fig. 3). No differences in clinical outcomes were recorded between the different IVF centres (Table I).
Figure 3.
IVF clinical outcomes across the biopsy operators. Each operator is represented by a different grade of blue, while the total data are shown through a white red-edged column. Absolute numbers of biopsied blastocysts diagnosed as euploid and transferred per each operator are reported under the legend. Data are shown for positive pregnancy tests, biochemical pregnancy loss, miscarriages and ongoing (>20 gestational weeks) implantation. No significant differences in any of these variables among the seven biopsy operators were shown, subtending a comparable efficiency in performing the procedure without affecting blastocyst viability and implantation potential.
FEET cycles were evenly distributed among biopsy practitioners for IVF centre 1. Logistic regression analysis adjusted for possible confounders showed no impact of biopsy practitioners on the observed ongoing implantation rate of FEET cycles (Fig. 3; Supplementary Table SIII). Only extremely poor quality blastocysts showed a lower implantation rate (P < 0.01) compared with average, good and excellent quality blastocysts. Female age, indications for treatment, day of biopsy and IVF centres were all unrelated variables to the clinical outcomes.
Biochemical pregnancy losses and miscarriages were also evenly distributed among different embryologists (Fig. 3) although the small sample size did not allow a proper comparison for these outcomes.
Cellularity of the TE biopsy was also unrelated with clinical outcomes. The number of TE cells estimated for implanted and unimplanted blastocysts was 7.2 (3–14; 95% CI = 6.4–8.0) and 7.9 (4–15; 95% CI = 7.02–8.77), respectively (P = 0.32).
Discussion
In this study, we report for the first time a comprehensive analysis of the laboratory and clinical consistency of the blastocyst biopsy approach for aneuploidy screening in relation to the biopsy practitioners. These data are extremely important in the IVF field considering the impressive growth in application of the TE biopsy procedure in recent years as blastocyst biopsy can be seen as difficult to standardize compared with the biopsy of PBs or of a single cell at the cleavage stage of embryo development. The combination of different embryologists performing TE biopsy on blastocysts of different developmental stages and morphology can be thought to result in variable outcomes. On the contrary, in this study, when appropriate training was provided and working in a standardized IVF setting, very high consistency and reproducibility of the qPCR-based CSS blastocyst biopsy approach, for both the genetic and clinical outcomes, was observed.
First, to compare the quality of the biopsy samples obtained from different biopsy practitioners, we investigated the genetic laboratory outcomes of the qPCR-based CCS defining analytical indicators of cellular quality and quantity. The assessment of sample concurrence served as a measure of the cellular quality of the biopsy sample while cellular count was performed using a unique method based on the interpolation to a reference standard curve developed using samples with increasing cell number (Tao et al., 2013). All embryologists performed similarly in terms of conclusive CCS diagnosis, sample concurrence and number of TE cells sampled from the different blastocysts. One practitioner obtained a notably higher no result rate (3.4%) that was on average 4 to 5 times higher when compared with the others. This could reflect a lower ability in coordinating the tubing procedure for that operator. These data suggest performance of an extensive validation of the tubing step that can be the most critical step with the highest inter-operator variability observed. However, no differences were observed when comparing performances for the overall success rate, suggesting that the final laboratory outcome measure (conclusive diagnosis rate) was consistent for all practitioners. This evidence suggests that blastocysts biopsy is very reproducible from a technical perspective when adequate training is completed before starting the clinical application and when a robust genetic technology for CCS is used.
In this study, when all related laboratory procedures such us extended culture and vitrification were already optimized, a minimum training period of 20 procedures was sufficient for training experienced embryologists in TE biopsy, following which enrolling them in the biopsy programme was straightforward. The biopsy training for each of the embryologists was based on three main key performance indicators; (1) the time that the embryologist took to perform the biopsy, (2) the blastocysts re-expansion after biopsy and, (3) the successful amplification and diagnosis after the genetic analysis. Regardless of the varying degree of experience of the embryologists in the study (ranging from 4 years to immediate enrolment in the biopsy programme after training as for operators 4, 5, 6 and 7), the results were shown to be very consistent between practitioners when experienced embryologists were involved.
The analysis of genetic data in relation to the TE biopsy sampled also revealed a wide range of cellularity that composed different biopsy samples, with a range between 1 and 15 cells collected. Regardless, the quality of qPCR-based CCS analysis was mildly affected by this procedural variability. Although samples with higher cellularity resulted in better sample concurrence, diagnosis was still possible in more than 96% of samples with a very low cellularity (≤3 cells). Another interesting observation was that the blastocyst quality per se did not severely affect the CCS results, with poor quality blastocysts showing mildly lower cellularity in the biopsy but a similar rate of conclusive diagnosis when compared with good and excellent quality blastocysts. Combined with previous observations showing that even poor quality blastocysts, when euploid, retain a significant implantation potential (Capalbo et al., 2014a,b), these data further demonstrate the importance of considering all blastocysts for the biopsy and CCS regardless of their morphological appearance, provided that the expanded stage is reached.
Next, we compared clinical outcomes of single FEET cycles between different biopsy practitioners to investigate the clinical reproducibility of the procedure. First, although the blastocysts were cryopreserved soon after the biopsy, we did not observe any degeneration following trophectoderm biopsy. This observation is consistent with previous studies on the same matter performed by McArthur and Schoolcraft (McArthur et al., 2005, 2008; Schoolcraft et al., 2010). In particular, McArthur and colleagues reported 1050 biopsied blastocysts with no degeneration in their first paper in 2005, and confirmed the same result in a paper published in 2008 on a further 609 biopsy procedures. In 2009, Schoolcraft and colleagues also reported 100% of survival after biopsy of 287 blastocysts.
After warming, 98.8% of the biopsied blastocysts survived. Unfortunately, due to our clinical setting, where the embryo transfer is performed within two hours from warming, it was not possible to assess the blastocyst re-expansion rate following warming and long-term survival. Accordingly, the clinical FEET cycle data in this analysis have to be mainly considered as ongoing implantation rate per thawed euploid blastocyst, since almost all embryos included in this study were transferred immediately after warming, without waiting for re-expansion.
Our clinical data showed very high consistency between different practitioners performing the blastocyst biopsy procedure in terms of ongoing implantation rates, biochemical pregnancy losses and miscarriage rates, suggesting that the different embryologists had no impact at all on the reproductive potential of the embryos. Furthermore, a wide range of cellularity was noted between the different biopsy samples, however the number of trophectoderm cells removed from the blastocyst seemed to be unrelated to the clinical outcomes of FEET cycles in this dataset, although a higher sample size will be needed to corroborate these findings, particularly for biochemical losses and miscarriages. Furthermore, it should be acknowledged that the method used to assess cellularity provides an estimation of the actual number of TE cells removed from the embryo that can be partially overestimated due to the sporadic presence of tetraploid cells in the TE biopsies.
Another potential limitation of the study relies on the use of a single TE biopsy method and CCS technology. Even if it is expected that similar outcomes can be achieved with different blastocyst biopsy approaches and CCS technologies, these data particularly apply to PGS programmes using blastocyst biopsies without zona opening at the cleavage stage (Capalbo et al., 2014a) and a qPCR-based CCS technology (Treff et al., 2012). It should be also acknowledged that these data come from a small set of laboratories where all of the embryologists received identical training and used identical equipment.
All together these data provided evidence for a high reproducibility and consistency of the blastocyst biopsy approach in the clinical settings. The biopsy of a single cell as either a polar body or blastomere at the cleavage stage has been advocated in the past as the main approaches for PGS and PGD, due to the relatively easy technical approach and the lack of effective blastocyst culture systems and cryopreservation protocols. Growing preclinical and clinical evidence about blastocyst biopsy accuracy, safety and effectiveness indicate trophectoderm analysis as the most advantageous stage for all applications of preimplantation genetics although it was only recently introduced on a large scale into the clinical practice of IVF (Yang et al, 2012; Chang et al., 2013; Forman et al., 2013a,b; Scott et al., 2013a,b). The success of any new procedure is based on consistency and reproducibility in different settings and across different practitioners to reach a widespread consensus and application. In this study, we have demonstrated blastocyst biopsy and CCS to be a consistent and reproducible procedure across multiple practitioners and in different IVF centres for both laboratory and clinical outcomes when appropriate training is provided and when the same laboratory setting is used.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
A.C. designed the study, performed CCS data analysis and wrote the manuscript. D.C. and C.P. collected the data and performed the lysis, preamplification and plate loading procedures. R.M., F.S. and L.D. performed the biopsies. F.M.U., L.B. and R.V. provided clinical support for the study. F.M.U. and L.R. provided the direction of the study and critical revision of the manuscript.
Funding
No external funding was used for this study. Funding to pay the Open Access publication charges for this article was provided by GENERA centers for reproductive medicine.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
The authors thank GENERA and GENETYX team for their passion and dedicated work toward the improvement of the qPCR-based blastocyst biopsy programme during the years since its implementation in the clinical practice.
References
- Capalbo A, Bono S, Spizzichino L, Biricik A, Baldi M, Colamaria S, Ubaldi FM, Rienzi L, Fiorentino F. Sequential comprehensive chromosome analysis on polar bodies, blastomeres and trophoblast: insights into female meiotic errors and chromosomal segregation in the preimplantation window of embryo development. Hum Reprod 2013a;28:509–518. [DOI] [PubMed] [Google Scholar]
- Capalbo A, Wright G, Elliott T, Ubaldi FM, Rienzi L, Nagy ZP. FISH reanalysis of inner cell mass and trophectoderm samples of previously array-CGH screened blastocysts shows high accuracy of diagnosis and no major diagnostic impact of mosaicism at the blastocyst stage. Hum Reprod 2013b;28:2298–2307. [DOI] [PubMed] [Google Scholar]
- Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Nagy ZP, Ubaldi FM. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod 2014a;29:1173–1181. [DOI] [PubMed] [Google Scholar]
- Capalbo A, Treff NR, Cimadomo D, Tao X, Upham K, Ubaldi FM, Rienzi L, Scott RT Jr. Comparison of array comparative genomic hybridization and quantitative real-time PCR-based aneuploidy screening of blastocyst biopsies. Eur J Hum Genet 2014b;23:901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Huang CC, Tsai YY, Hung CC, Fang MY, Lin YC, Su YN, Chen SU, Yang YS. Blastocyst biopsy and vitrification are effective for preimplantation genetic diagnosis of monogenic diseases. Hum Reprod 2013;28:1435–1444. [DOI] [PubMed] [Google Scholar]
- Farquharson RG, Jauniaux E, Exalto N; ESHRE Special Interest Group for Early Pregnancy (SIGEP) . Updated and revised nomenclature for description of early pregnancy events. Hum Reprod 2005;20:3008–3011. [DOI] [PubMed] [Google Scholar]
- Forman EJ, Hong KH, Treff NR, Scott RT. Comprehensive chromosome screening and embryo selection: moving toward single euploid blastocyst transfer. Semin Reprod Med 2012a;30:236–242. [DOI] [PubMed] [Google Scholar]
- Forman EJ, Tao X, Ferry KM, Taylor D, Treff NR, Scott RT Jr. Single embryo transfer with comprehensive chromosome screening results in improved ongoing pregnancy rates and decreased miscarriage rates. Hum Reprod 2012b;27:1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman EJ, Treff NR, Stevens JM, Garnsey HM, Katz-Jaffe MG, Scott RT Jr, Schoolcraft WB. Embryos whose polar bodies contain isolated reciprocal chromosome aneuploidy are almost always euploid. Hum Reprod 2013a;28:502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, Treff NR, Scott RT Jr. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril 2013b;100:100–107. [DOI] [PubMed] [Google Scholar]
- Fragouli E, Lenzi M, Ross R, Katz-Jaffe M, Schoolcraft WB, Wells D. Comprehensive molecular cytogenetic analysis of the human blastocyst stage. Hum Reprod 2008;23:2596–2608. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D (eds). Toward Reproductive Certainty: Fertility and Genetics Beyond. Carnforth, UK: Parthenon Publishing, 1999, 378–388. [Google Scholar]
- Johnson DS, Cinnioglu C, Ross R, Filby A, Gemelos G, Hill M, Ryan A, Smotrich D, Rabinowitz M, Murray MJ. Comprehensive analysis of karyotypic mosaicism between trophectoderm and inner cell mass. Mol Hum Reprod 2010;16:944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los FJ, Van Opstal D, van den Berg C. The development of cytogenetically normal, abnormal and mosaic embryos: a theoretical model. Hum Reprod Update 2004;10:79–94. [DOI] [PubMed] [Google Scholar]
- McArthur SJ, Leigh D, Marshall JT, de Boer KA, Jansen RP. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertil Steril 2005;84:1628–1636. [DOI] [PubMed] [Google Scholar]
- McArthur SJ, Leigh D, Marshall JT, Gee AJ, De Boer KA, Jansen RP. Blastocyst trophectoderm biopsy and preimplantation genetic diagnosis for familial monogenic disorders and chromosomal translocations. Prenat Diagn 2008;28:434–442. [DOI] [PubMed] [Google Scholar]
- Moutou C, Goossens V, Coonen E, De Rycke M, Kokkali G, Renwick P, SenGupta SB, Vesela K, Traeger-Synodinos J. ESHRE PGD Consortium data collection XII: cycles from January to December 2009 with pregnancy follow-up to October 2010. Hum Reprod 2014;29:880–903. [DOI] [PubMed] [Google Scholar]
- Munné S, Velilla E, Colls P, Garcia Bermudez M, Vemuri MC, Steuerwald N, Garrisi J, Cohen J. Self-correction of chromosomally abnormal embryos in culture and implications for stem cell production. Fertil Steril 2005;84:1328–1334. [DOI] [PubMed] [Google Scholar]
- Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, Colamaria S, Sapienza F, Ubaldi F. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod 2010;25:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- Schoolcraft WB, Fragouli E, Stevens J, Munne S, Katz-Jaffe MG, Wells D. Clinical application of comprehensive chromosomal screening at the blastocyst stage. Fertil Steril 2010;94:1700–1706. [DOI] [PubMed] [Google Scholar]
- Scott RT Jr, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, Tao X, Treff NR. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril 2013a;100:697–703. [DOI] [PubMed] [Google Scholar]
- Scott RT Jr, Upham KM, Forman EJ, Zhao T, Treff NR. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril 2013b;100:624–630. [DOI] [PubMed] [Google Scholar]
- Synodinos, data from ESHRE PGD consortium, ESHRE annual meeting, Munich: 2014. [Google Scholar]
- Tao X, Devkota B, Lonczak A, Lebiedzinski M, Scott RT, Treff NR. Characterization of trophectoderm (TE) biopsy cell number. Fertil Steril 2013;100:S196–S197P-169. [Google Scholar]
- Treff NR, Tao X, Ferry KM, Su J, Taylor D, Scott RT Jr. Development and validation of an accurate quantitative real-time polymerase chain reaction-based assay for human blastocyst comprehensive chromosomal aneuploidy screening. Fertil Steril 2012;97:819–824. [DOI] [PubMed] [Google Scholar]
- Van der Aa N, Cheng J, Mateiu L, Zamani Esteki M, Kumar P, Dimitriadou E, Vanneste E, Moreau Y, Vermeesch JR, Voet T. Genome-wide copy number profiling of single cells in S-phase reveals DNA-replication domains. Nucleic Acids Res 2013;41:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Echten-Arends J, Mastenbroek S, Sikkema-Raddatz B, Korevaar JC, Heineman MJ, van der Veen F, Repping S. Chromosomal mosaicism in human preimplantation embryos: a systematic review. Hum Reprod Update 2011;17:620–627. [DOI] [PubMed] [Google Scholar]
- Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, Peck AC, Sills ES, Salem RD. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet 2012;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.