Abstract
Adaptive choice behavior depends critically on identifying and learning from outcome-predicting cues. We hypothesized that attention may be preferentially directed toward certain outcome-predicting cues. We studied this possibility by analyzing event-related potential (ERP) responses in humans during a probabilistic decision-making task. Participants viewed pairs of outcome-predicting visual cues and then chose to wager either a small (i.e., loss-minimizing) or large (i.e., gain-maximizing) amount of money. The cues were bilaterally presented, which allowed us to extract the relative neural responses to each cue by using a contralateral-versus-ipsilateral ERP contrast. We found an early lateralized ERP response, whose features matched the attention-shift-related N2pc component and whose amplitude scaled with the learned reward-predicting value of the cues as predicted by an attention-for-reward model. Consistently, we found a double dissociation involving the N2pc. Across participants, gain-maximization positively correlated with the N2pc amplitude to the most reliable gain-predicting cue, suggesting an attentional bias toward such cues. Conversely, loss-minimization was negatively correlated with the N2pc amplitude to the most reliable loss-predicting cue, suggesting an attentional avoidance toward such stimuli. These results indicate that learned stimulus–reward associations can influence rapid attention allocation, and that differences in this process are associated with individual differences in economic decision-making performance.
Keywords: EEG, ERP, learning, N2pc, reward
Introduction
Maximizing gains and minimizing losses are hallmarks of successful economic decision-making. Behavioral studies have found that individuals' ability to make decisions that maximize gains and minimize losses can affect life outcomes. For example, previous behavioral studies have found that deficits in gain-maximization and loss-minimization are associated with negative life outcomes in gamblers (Siler 2010), in depressed patients (Maddox et al. 2012), and across the overall population (Knutson et al. 2011). Accordingly, understanding the brain mechanisms underlying gain-maximization and loss-minimization is an important pursuit in decision neuroscience. We recently reported that individuals' tendencies to maximize gains and minimize losses were associated with their event-related potential (ERP) responses to feedback concerning those gains and losses (San Martín et al. 2013), adding to a rapidly developing human electrophysiological literature demonstrating the role of feedback-related brain activity in shaping choice behavior (Frank et al. 2005; Cohen and Ranganath 2007; Hewig et al. 2007; Goyer et al. 2008; San Martín et al. 2010; Billeke et al. 2012; for a review, see San Martín 2012).
Rapid attentional biases toward sensory cues that provide information about potential outcomes for different courses of action are another important, but much less-studied, neurocognitive mechanism that may underlie differences in choice behavior. Attentional biasing may play a key role when several simultaneously available cues provide either complementary or contradictory information about the best course of action. Here, we studied this possibility by analyzing ERP responses in humans during a probabilistic decision-making task, in which participants chose between a large and a small wager after observing a pair of probabilistic cues that defined the likelihood of positive or negative outcomes. Critically, these 2 cues were presented bilaterally, allowing us to compute the relative attentional allocation toward each cue by using a contralateral-versus-ipsilateral ERP contrast.
Several lines of research have suggested that the deployment of attention toward a stimulus is modulated by the stimulus's reinforcement history. For example, reward-associated targets are less likely to be missed in the attentional blink (Raymond and Brien 2009), and visual search can be both facilitated (Hickey et al. 2010a) or disrupted (Anderson et al. 2011; Hickey and van Zoest 2012) depending on whether targets or distractors have been associated with rewards. Recent studies have also shown amplified brain responses to reward-predicting visual stimuli by measuring the attentional-shift-related N2pc ERP component (Kiss et al. 2009; Hickey et al. 2010b). This component is an enhanced negative-polarity response over parietal–occipital scalp sites contralateral to the hemifield of a lateralized visual target stimulus; it typically emerges around 200–300 ms poststimulus and is thought to reflect attentional focusing processes toward that target (Luck and Hillyard 1994; Woodman and Luck 1999; Hopf et al. 2000). In multidistractor visual search tasks, the N2pc has been shown to be larger for targets that are associated with high, versus low, reward (Kiss et al. 2009). Moreover, if a distractor in the array has recently been associated with reward, a larger N2pc is elicited by that distractor than by the target, suggesting enhanced attentional capture (Hickey et al. 2010b). Interestingly, the N2pc has also been shown to be modulated by emotional valence in a 2-stimulus (single target and single-distractor) visual detection task (Kiss et al. 2007), as well as by contextual, task-irrelevant, emotional stimuli in a luminance-change detection task (Buodo et al. 2010). These studies suggest the strong, but as yet untested, prediction that the attentional-orienting processes indexed by the N2pc may shape visually-cued economic choices. Here, we evaluated whether the N2pc activity may reflect the attention drawn toward visual cues by virtue of their association with reward—such that individual differences in cued economic choices may be associated with individual differences in the N2pc responses to those cues.
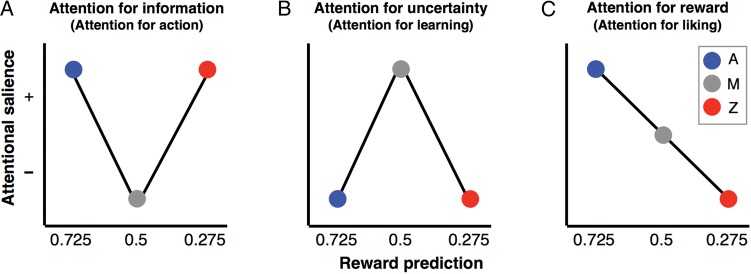
That reinforcement history can alter the degree to which a particular stimulus attracts attention (i.e., salience) has extensively been demonstrated in the context of animal conditioning (Gottlieb 2012). This literature describes 3 possible mechanisms by which stimuli might change in salience as a consequence of learning. According to the proposal of an “attention for action” mechanism (Mackintosh 1975), organisms will attend more to stimuli that are reliable predictors of subsequent positive or negative events—and thus that provide information that can help inform actions (i.e., thus a useful alternative name would be “attention for information”). A second “attention for learning” model (Pearce and Hall 1980) suggests the reverse, namely that animals will attend more to cues that have been associated with uncertainty in order to learn more about stimuli for which the animal does not yet possess an adequate predictive mental model (i.e., thus a useful alternative name being “attention for uncertainty”). Finally, a more recently proposed “attention for liking” model (Hogarth et al. 2010) suggests that attention will be captured by stimuli that predict positive outcomes and will be shifted away from aversive cues (i.e., an alternative name thus being “attention for reward”). We hypothesized that the reinforcement history may influence stimulus salience, and thus cued economic choices, through any of these 3 mechanisms (i.e., attention for information, uncertainty, or reward, using the alternative names proposed above). Here, we evaluated which one of these models provides a better characterization of the attentional responses to outcome-predicting visual cues in an economic decision-making task.
Materials and Methods
Participants
Forty-five healthy, right-handed, adult volunteers (22 males) participated in this study (aged 18–31 years; mean = 23.0). Participants gave written informed consent and were financially compensated at $15 per hour, with an extra bonus (mean = $12.20, standard deviation [SD] = $7.75) based on their performance during the experimental session. All procedures were approved by the Duke University Health System Institutional Review Board. We excluded 4 participants from further data analysis due to technical difficulties during their experimental sessions, leaving a final sample of 41 participants (20 males). A different subset of the electroencephalogram (EEG) data of this subject sample, collected under the same experimental sessions, was already reported in San Martín et al. (2013). That earlier published work presented analyses on the feedback-locked ERP, while here we report on the stimulus-locked ERP activity elicited by the outcome-predicting cues (Fig. 1A).
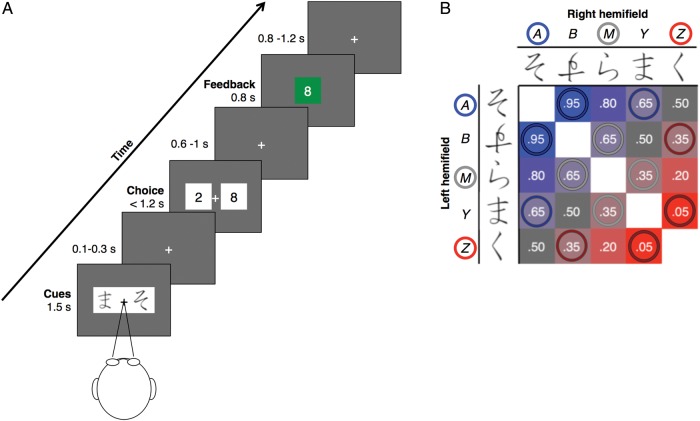
Figure 1.
Experimental design. (A) In each trial, participants covertly attended to a bilaterally presented cue stimulus pair providing information about the probability of winning on that trial. They then chose between making a large bet (8 points) and a small bet (2 points) by pressing a button corresponding to the side of the screen containing their preference. Feedback was provided as a green box surrounding the wager amount if the participants won the bet and as a red box if the participant lost. (B) The stimulus pair to be presented on each trial was randomly selected from a set of 20 possible pairs that were formed from 5 different novel symbols. These cue symbols, labeled A, B, M, Y, and Z here, were each associated with a relative likelihood of gain versus loss. Each stimulus pair was thus associated with a total probability of winning [P(win)] for that trial, derived from the combination of the gain/loss probabilities of each of the individual cues, as annotated and color-coded in the figure [ranging from blue to red as P(win) decreases]. ERP responses and participants' behavior were independently evaluated for the most reliable gain-predicting cue (i.e., A), the neutral cue (i.e., M), and the most reliable loss-predicting cue (i.e., Z), based on trial-types circled in blue, gray and red, respectively, inside the matrix figure. This blue-gray-red color code is consistently used throughout the figures.
Stimuli and Task
We designed a probabilistic decision-making task in which participants made betting decisions aimed to maximize the magnitude of gains and minimize the magnitude of losses (San Martín et al. 2013). Participants sat in front of a 19-inch CRT monitor located 60 cm from their eyes. They performed 800 trials over the course of a single experimental session that was divided into 40 blocks. Subjects were told that each trial would start with the presentation of 2 symbols, and that some symbols would tend to be followed by losses and some symbols by gains across trials. Participants were instructed to try to learn the association between symbols and outcomes and to use that information to bet either 2 points or 8 points on each trial. Participants were also informed that they would receive a monetary bonus proportional to the total points earned during the session. They completed a brief 20-trial practice session before data collection, using a set of symbols different from those used during the experiment.
The temporal sequence of the task as it unfolded over a single trial is shown in Figure 1A. All stimuli appeared on a dark gray background. Each trial started with the simultaneous presentation of a centralized fixation cross (1° × 1°) and the bilateral presentation of a pair of cues (Hiragana characters) that were displayed for 1500 ms. Participants were instructed to maintain fixation on the fixation cross throughout the experimental runs. The cues were presented laterally equidistant (3°) from the center of the fixation cross, with the size of each cue such that it fits into a square frame subtending 2.5° × 2.5° of visual angle. The 2 cues presented on each trial were randomly selected, without replacement, from a set of 20 possible pairings of 5 unique symbols (Fig. 1B), which for referencing purposes are tagged as A, B, M, Y, and Z. No cue was ever paired with itself, and all the cues were paired with each one of the other cues the same number of times within each block of trial and throughout the experiment. As a result, each cue was presented 8 times per block: 4 times in the left hemifield and 4 times in the right hemifield.
After a within-trial inter-stimulus interval (ISI) jittered between 100 and 300 ms following the offset of the cue pair, 2 white squares with the numerals “8” and “2” that depicted the wager amount choices appeared randomly in the right and left hemifield, equidistant from the fixation cross. Participants chose their wager amount for the trial by pressing a button with the hand corresponding to the side of the screen containing their wager preference. Note that because the bet values 8 and 2 were presented randomly in the right or left hemifield, there was no relationship between the sidedness of the wager amount choice and the sidedness of the previously presented outcome-predicting cues, and thus there could be no planning of choosing the numeral to the right versus left of fixation until they appeared on the screen. The outcome of the trial (duration 800 ms) was subsequently presented after another within-trial ISI jittered between 600 and 1000 ms after the choice response, appearing as a centrally presented green box around the chosen number if the participant gained the points bet on that trial or as a red box around the chosen number if the participant lost such points. If no response was made within 1200 ms following the onset of the wager screen, the words “no response” were presented, along with a red box corresponding to losing 8 points. The next trial started after an intertrial interval jittered between 800 and 1200 ms.
Each pair of cues had a prediction probability of winning versus losing [P(win)] associated with it, which was calculated as an adjustment from 50% determined by each of the 2 cues: P(win) = 0.5 + PL + PR, where PL and PR are the winning probability adjustments associated with the cue presented on the left and right hemifield, respectively (+0.3, +0.15, 0, −0.15, and −0.3; for cues labeled A, B, M, Y, and Z, respectively, in Fig. 1B). The assignment between labels (and thus the probabilities) and the actual symbols was randomized across participants. A key feature of the paradigm was that, although participants had no control over the probably of winning versus losing on a trial (i.e., the valence of the outcome), they could make a choice that would influence the “magnitude” of the outcome. In order to optimize their choices (i.e., maximizing gains and minimizing losses), the participants had to learn, by trial-and-error, the reward-predicting value of the different cues, thereby enabling them to choose the large wager on trials likely to lead to winning outcomes and to choose the small wager on trials likely to lead to losing outcomes.
We structured the stimulus set so that outcome probability and uncertainty were orthogonally related. The probability of a positive outcome was mapped linearly across the cues: A = 0.725, B = 0.6125, M = 0.5, Y = 0.3875, and Z = 0.275. Uncertainty varied with a different structure, however, being lowest for the extreme cues (i.e., A predicting a gain with 72.5% of certainty and Z predicting a loss with 72.5% of certainty) and highest for the middle cue (i.e., M being equally likely to predict a gain or a loss).
EEG Recording and Preprocessing
The EEG was recorded continuously from 64 channels mounted in a customized extended-coverage elastic cap (Duke64 design, Electro-Cap International, www.electro-cap.com) using a recording bandpass of 0.01–100 Hz at a sampling rate of 500 Hz (SynAmps, Neuroscan). All channels were referenced to the right mastoid during recording. The positions of all 64 channels were equally spaced across the customized cap and covered the whole head from slightly above the eyebrows in front to below the inion posteriorly (Woldorff et al. 2002). Impedances of all channels were kept below 5 kΩ, and fixation was monitored with electrooculogram recordings. Recordings took place in an electrically shielded, sound-attenuated, dimly lit, experimental chamber.
Offline, EEG data were exported to MATLAB (MathWorks) and processed using custom scripts and the EEGLAB software suite (Delorme and Makeig 2004). The data were then low-pass filtered below 40 Hz and re-referenced to the algebraic average of the left and right mastoid electrodes. Artifacts caused by eye movements and muscular activity were removed using independent component analysis (a similar approach can be found in Debener et al. 2005; Eichele et al. 2005; Scheibe et al. 2010; San Martín et al. 2013). To analyze cue-locked ERP responses, the continuous EEG data were divided into 900-ms epochs, spanning from 400 ms before to 500 ms after the onset of the cue-pair stimulus. Voltages were calculated relative to a 200-ms prestimulus baseline period.
ERP Extraction Procedure
Through our analyses, we wished to characterize the neural activity associated with the processing of the visual cues by virtue of their reward-predicting value. Specifically, we were interested in the possibility that the different outcome-predicting visual cues would elicit differential early lateralized ERP responses over posterior scalp sites (i.e., N2pc activity) that would reflect relative levels of attentional orienting toward those cues. To identify such effects of attention, while controlling for all other aspects of the task, we extracted the contralateral and ipsilateral cue-triggered ERP activity for the presentation of 3 of the 5 cues: A (the most reliable gain-predicting cue), M (the neutral cue, with no relative contribution of gain-versus-loss prediction), and Z (the most reliable loss-predicting cue), using exclusively the trials in which B or Y was presented in the opposite hemifield. We made this choice in order to compute behavioral performance and ERPs for cues A, M, and Z independently of each other (Fig. 1B). Therefore, the ERP extracted for each A cue, for example, was not conflated with any of responses associated with the other 2 cues being analyzed (i.e., M and Z), so that we could obtain a more selective measure of these value-based responses with no overlapping assignment of trials. Corresponding behavioral data were analyzed according to the same scheme.
ERPs were calculated using standard signal-averaging procedures for extracting contralateralized attentional effects (Luck 2005). First, for each subject, EEG waveforms for all the electrode sites were averaged separately for each combination of cue (A, M, and Z) and cue position (left hemifield vs. right hemifield). Then we identified all the pairs of corresponding left and right electrodes of equal distance from the scalp midline (e.g., P3/P4 and O1/O2) and extracted the contralateral and ipsilateral ERP activity for each cue for each of these pairs of electrodes. More specifically, the contralateral ERP for each cue was extracted from the left electrode sites when the cue was presented in the right hemifield, and averaged with the activity at the corresponding right electrode sites when the cue was presented in the left hemifield. Similarly, the ipsilateral ERP for each cue was calculated by averaging the signal recorded at the left electrode sites when the cue was presented on the left hemifield with the signal at the equivalent right electrode sites when the cue was presented on the right hemifield.
On the basis of previous studies (e.g., Kiss et al. 2009; Hickey et al. 2010b) and in line with visual inspection of grand average ERP waveforms, the elicited N2pc brain activity was quantified for statistical purposes as the average potential from a 200- to 400-ms window in the contralateral-minus-ipsilateral difference wave at electrode sites PO7/PO8. For topographic maps, the relative lateralized activity for each of the relevant conditions is plotted across the scalp.
Behavioral Data Analysis
Our main behavioral and ERP data analyses assumed that the participants were aware of the differences between the probabilities of winning associated with the visual cues. Thus, we wanted to exclude from analysis blocks of trials in which the participants, on average, may have not yet learned the probability of winning for the different cues. In order to detect those blocks, we ran an ANOVA on the probability of high bets per cue on each experimental block up to the block in which participants' choices significatively distinguished between A and Z cues. (Tukey's range test was used for these within-blocks post hoc comparisons.) In particular, we wanted to determine the first block in which participants' behavior indicated that they were aware of the differences between the cues, so that we could exclude from analysis any possible early blocks in which they were not yet aware of those differences.
Subsequently, we defined a gain-maximization score for each subject as his/her observed probability to bet the larger amount on trials presenting cue A [P(high bet)A]. Conversely, a loss-minimization score was determined for each subject as his/her observed probability to bet the smaller amount on trials presenting cue Z [1 − P(high bet)Z]. As explained above, the trials presenting both A and Z together in the same trial were excluded from this and all our other data analyses (i.e., because only trials with cues B or Y on the other side were included in the analyses). We also defined a metric of overall decision performance as the ability to behaviorally distinguish between cues A and Z [i.e., P(high bet)A − P(high bet)Z].
ERP Data Analysis
In our main ERP data analysis, we evaluated differences between the N2pc responses to the different outcome-predicting visual cues. We computed 3 contralateral-minus-ipsilateral responses for each participant, corresponding to the averaged EEG activity time locked to the presentation of each of the 3 cue types included in this analysis (A, M, and Z). These 3 cues are color-coded blue, gray, and red, respectively, throughout the presented figures. On average across the participants, 151 trials went into the contralateral-minus-ipsilateral ERP wave for cue A (SD, 8.45), 152 into the ERP for M (SD, 8.54) and 151 trials into the ERP for Z (SD, 8.08). Repeated-measures ANOVAs were performed on the N2pc amplitude with “cues” as the explanatory factor. Tukey's range test was used for post hoc comparisons.
Secondly, we directly tested whether the N2pc amplitude fits the “attention for information”, “attention for uncertainty”, or “attention for reward” models (Fig. 2). The amplitude of the N2pc component to A, M, and Z for each participant was used as a dependent measure to fit a multiple linear model according to the following equation:
| (1) |
where Linfo/uncertainty was a categorical variable representing both the attention for information and the attention for uncertainty models, with levels equal to −1, 0, and −1 for cues A, M, and Z, respectively. Note that the same regressor variable was used to represent these 2 models since they were exact opposites of each other (Fig. 2A,B), with their distinction being the sign of the derived β1. A positive β1 estimate would support the attention for information model (i.e., enhanced negativity contralateral versus ipsilateral to cues A and Z compared with M) and a negative β1 estimate would support the attention for uncertainty model (i.e., a larger N2pc response to M compared with both A and Z). In contrast, Lreward was a categorical variable with levels equal to −1, 0, and +1 for A, M, and Z, respectively, such that a positive β2 estimate would support the attention-for-reward model (Fig. 2C; a larger N2pc for A, followed by M, followed by Z). Given that the fit of the different models might vary between participants depending on how well they learned the cue/p(win) contingencies, we included the covariate P representing the overall decision-performance score for each participant [P(high bet)A− P(high bet)Z]. The ϵ parameter was a vector of error terms. These parameters were determined using the MATLAB function “glmfit” after demeaning the regressors.
Figure 2.
Models of attention toward outcome-predicting cues. Three proposed mechanisms by which stimuli might change in attentional salience as a consequence of their associated reward probability (modified from Gottlieb 2012). In this figure, the values on the x-axis (labeled “reward prediction”) correspond to the mean win-probability associated with cues A, M, and Z throughout the experimental session (represented by blue, gray and red dots, respectively). The y-axis represents predictions about the salience of A, M, and Z derived from each of these attentional models. (A) According to the attention for information model, attention would be prioritized toward stimuli that reliably predict upcoming outcomes, regardless of whether that outcome was likely to be positive or negative (i.e., cues A and Z). (B) According to the attention for uncertainty model, attention would be prioritized toward stimuli that are associated with high uncertainty about the upcoming outcomes (i.e., cue M). (C) According to the attention-for-reward model, attention would be prioritized towards stimuli that predict positive outcomes (i.e., A) and relatively shifted away from stimuli predicting negative outcomes (i.e., Z).
Finally, we explored participant-wise correlations between individual differences in gain-maximization and loss-minimization behavioral scores and the amplitude of the N2pc response to the gain-predicting and loss-predicting cues (i.e., for A and Z, respectively). As such, we performed a multiple linear regression using the amplitude of the N2pc response to the A and Z cues as explanatory variables for the gain-maximization and loss-minimization behavioral scores across participants, according to the following equations:
| (2) |
where the βA-gain-max and βZ-gain-max estimates indicate the participant-wise correlation between gain-maximization behavioral scores and the amplitude of the N2pc to the A cue and the N2pc to the Z cue, respectively. For example, a negative βA-gain-max estimate would indicate that the greater (i.e., the more negative) the N2pc response to the A cue, the better the participant's gain-maximization score.
| (3) |
where the βA-loss-min and βZ-loss-min estimates indicate the participant-wise correlation between loss-minimization behavioral scores and the amplitude of the N2pc to the A cue and the N2pc to the Z cue, respectively. For example, a positive βZ-loss-min estimate would indicate that the smaller (i.e., the less negative) the N2pc response to the Z cue, the better the participant's loss-minimization score. Again, we performed this analysis using the MATLAB function glmfit.
Results
Behavioral Results: Initial Learning of Symbols
Since we were interested in characterizing neural activity associated with the processing of visual cues by virtue of their learned associations with reward, we wished to analyze only trials in which the participants had already learned the cue/P(win) contingencies. As illustrated in Figure 3, participants quickly learned the contingencies of the task. Indeed, an ANOVA on the probability of high bets per cue on the first block revealed that, on average, participants' choices already distinguished between cues within this block (F2,80 = 3.3061, P < 0.05), with a greater proportion of high bets on trials presenting the gain-predicting cue A, compared with trials presenting the loss-predicting cue Z (P < 0.05, Tukey's range post hoc tests). Given this result we decided to include all the trials of the session in our behavioral and ERP data analyses.
Figure 3.
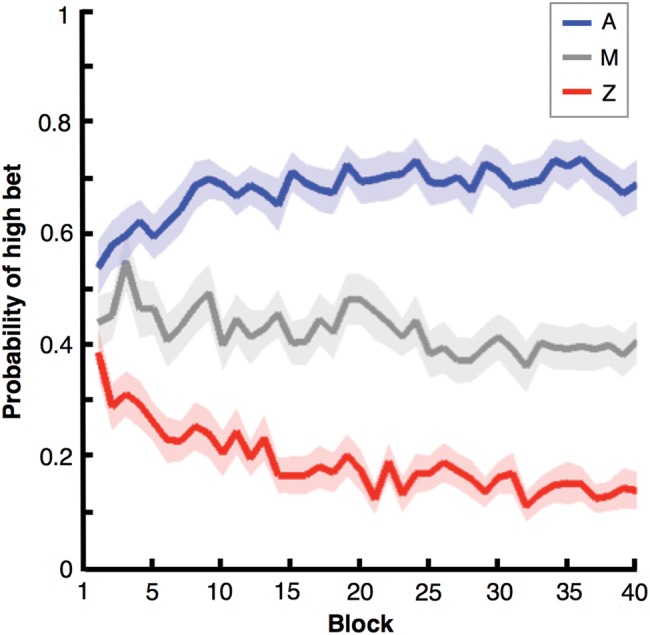
Choice behavior across time. Participants' bet choices distinguished between cues A, M, and Z early and consistently throughout the experimental session. Specifically, differences in choice behavior for A and Z were already significant during the first block of the session (shaded areas indicate SEM for each trace).
Behavioral Results: Gain-Maximization and Loss-Minimization
In order to evaluate the relationship between individual differences in choice behavior and ERP responses, we extracted a gain-maximization score and a loss-minimization score for each participant defined, respectively, as the observed probability across the experimental session to bet the larger amount on trials with an A cue and the observed probability to bet the smaller amount on trials with a Z cue. Overall, participants' were better at minimizing their losses (mean = 0.81, SD = 0.16) than maximizing their gains (mean = 0.68, SD = 0.16) [t(80) = 3.76, P < 0.0005]. We did not find a significant correlation between the bias towards loss-minimization (i.e., the difference between loss-minimization and gain-maximization scores for each participant) with respect to either age (r = 0.13, P = 0.43) or gender (t = −0.45, P = 0.66). The average decision-performance score was 0.48 (SD = 0.26).
ERP Results
The Attention-Reflecting N2pc Is Selectively Elicited by the Gain-Predicting Cue
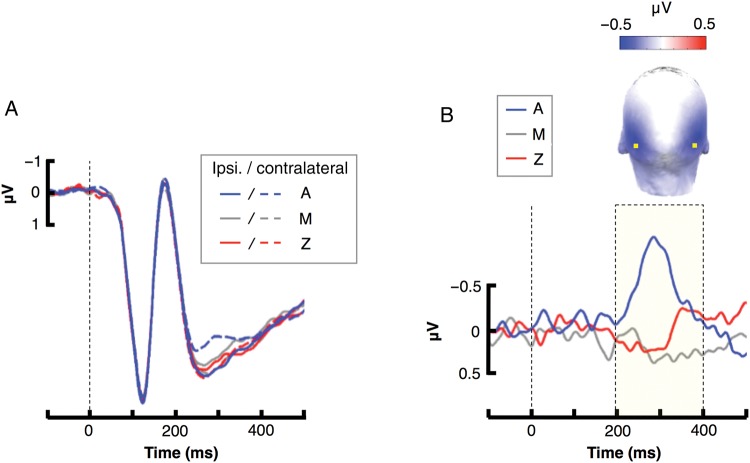
Figure 4 shows ERP waveforms elicited at parietal–occipital scalp sites PO7/PO8 by the cues A, M, and Z. The attention-sensitive N2pc component (enhanced negativity contralateral vs. ipsilateral to the cue) appeared as a larger for cue A than for the other 2 cues. Indeed, an ANOVA on the amplitude of the N2pc revealed a significant difference between the 3 cue types (F2,80 = 6.4311, P < 0.005), with a larger N2pc amplitude for cue A compared with both M (P < 0.005) and Z (P < 0.05). Moreover, the amplitude of the N2pc was different from zero when elicited by the presence of cue A [t(40) = −3.48, P < 0.005], but it was statistically indistinguishable from zero for M [t(40) = 1.77, P = 0.08] and Z [t(40) = 0.37, P = 0.72)]. In summary, when collapsed across the 41 participants, the N2pc response was selectively elicited by the cue that was a positive-reward predictor.
Figure 4.
N2pc evoked to the 3 cue types A, M, and Z. (A) Visual ERP at channel PO7/PO8 for contralateral (dashed) and ipsilateral (solid) to the gain-predicting cue (A, in blue), the neutral cue (M, in gray), and the loss-predicting cue (Z, in red). (B) Contralateral minus ipsilateral subtraction, yielding the attention-sensitive N2pc component to the gain-predicting cue (A, in blue), the neutral cue (M, in gray), and the loss-predicting cue (Z, in red). Mean amplitudes were computed in the latency range shown by the dashed box. The scalp topography shows the distribution of the N2pc for cue type A in that latency range. Yellow dots show the positions of the PO7/PO8 electrode sites on the scalp.
This result is inconsistent both with the attention-for-information model (Fig. 2A), which predicts an enhanced N2pc for both the A and Z cues (given that they were equally informative), and with the attention-for-uncertainty model (Fig. 2B), which predicts a maximum N2pc for M (i.e., the cue offering the least prediction value). Finally, this result is only partially consistent with the attention-for-reward model (Fig. 2C), which indeed predicts a maximum N2pc amplitude in response to A, as was the case, but also predicts a continued decrease in the N2pc amplitude from A to M to Z, which was not seen in this grand average over all participants.
N2pc Responses Were Consistent with Attention-for-Reward Model in High Decision-Performance Participants
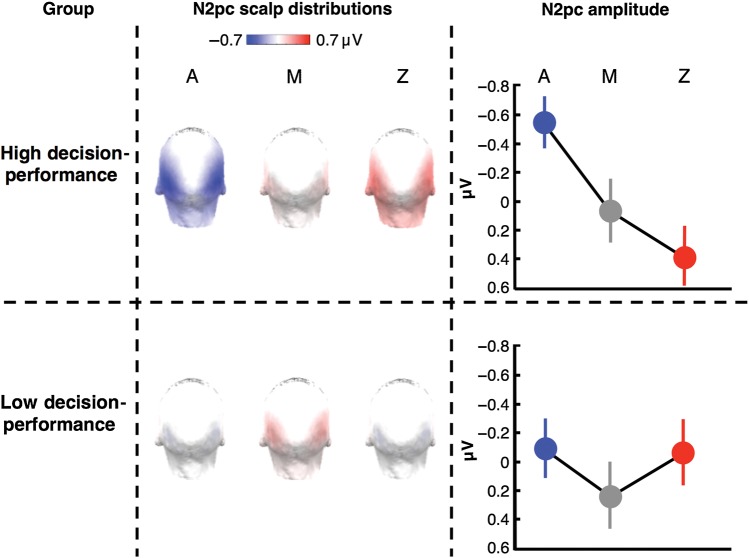
A limitation of the ERP data analysis described above is that it does not take into account differences in reward learning among the participants. Even though our behavioral analyses showed that, on average, participants already distinguished between A and Z during the first block of the task, some may have not learned this distinction very well even across the whole session. As a result, it was possible that differences between the amplitude of the N2pc response to the various cues may have been diluted, at the group level, by the inclusion of participants that underperformed in learning the contingencies between cues and the probability of winning.
In order to overcome the limitations of the previous analysis, we used a multiple linear regression procedure that evaluated the relationship between the N2pc and the alternative attentional models described in Figure 2, while taking into consideration individual differences in learning-guided choice behavior (see Eq. 1 in the Materials and Methods section). We found that none of our original attentional models, by themselves, predicted the N2pc amplitude for cues A, M, and Z (see Table 1 for statistics). However, we did find a significant association between the N2pc amplitude and the regressor representing the interaction between the attention-for-reward model and decision performance (β5 = 0.94, P < 0.01). In particular, the fit between the attention-for-reward model and the N2pc responses increased, across participants, as decision-performance scores improved.
Table 1.
Association between N2pc responses and models of attention toward outcome-predicting cues
| Model predictors | Model estimates |
|
|---|---|---|
| β | P | |
| Constants | 0.01 | 0.93 |
| Information/uncertainty | −0.20 | 0.21 |
| Positive-reward predictors | 0.20 | 0.27 |
| Performance | −0.02 | 0.92 |
| Information/uncertainty × performance | 0.02 | 0.95 |
| Positive-reward predictors × performance | 0.94 | 0.006a |
| R-squared | 0.17 | |
aSignificant with a Bonferroni-corrected alpha level set at 0.05 (for 6 tests).
We also performed additional analyses as a function of decision performance. In particular, we selected the group of highest-performing subjects (n = 15, decision-performance scores at least 0.5 SD above the mean) and the group of lowest-performing participants (n = 11, decision-performance scores at least 0.5 SD below the mean), and examined the patterns of the N2pc activity elicited by the cues. As can be seen in Figure 5, the high-performing group had an N2pc pattern that closely followed the attention-for-reward model, whereas the low-performing group had a much less differentiated pattern. To analyze these patterns statistically, we tested the fit of the attention-for-information/uncertainty and the attention-for-reward models separately in these 2 groups [N2pc amplitude = Constant + β1(Linfo/uncertainty) + β2(Lreward) + ϵ]. Consistent with the result across the whole sample described above, we found: (1) that the regressor representing the attention-for-information/uncertainty models showed no significant association with the N2pc responses in either of these 2 groups (P-values >0.1); and (2) that the attention-for-reward model significantly predicted the amplitude of the N2pc responses in high decision-performance participants (β2 = 0.47, P < 0.005), while showing no significant association with the N2pc in the low decision-performance participants (β2 = 0.05, P = 0.71). In summary, the attention-related N2pc response to the cues linearly increased with the probability of winning associated with each cue, as predicted by the attention-for-reward model, but only in participants that successfully learned the contingencies between cues and probability of winning.
Figure 5.
N2pc responses are consistent with the attention-for-reward model in high decision-performance participants. Displayed are the scalp distribution and mean N2pc amplitudes for cues A, M, and Z in a group of high decision-performance participants (n = 15, decision-performance scores at least 0.5 SD above the mean) and a group of low decision-performance participants (n = 11, decision-performance scores at least 0.5 SD below the mean). In high performers, but not in low performers, the N2pc responses were consistent with the attention-for-reward model. (Error bars indicate SEM.)
Finally, a visual inspection of Figure 5 shows that, on average, high decision-performance participants presented a reversed, positive-polarity N2pc for cue Z. However, according to a two-tailed t-test, this positive-polarity N2pc did not reach statistical significance [t(14) = 1.71, P = 0.11].
N2pc Responses to Gain-Predicting and Loss-Predicting Cues Independently Predict Gain-Maximization and Loss-Minimization Across Participants
If the attention-for-reward model provides a good characterization of the amplitude of the N2pc responses in our task, as indicated by the previous analysis, we should also observe a double dissociation in which the N2pc responses to A and Z independently predict gain-maximization and loss-minimization scores across participants. We used a multiple linear regression procedure to evaluate this possibility (Eqs 2 and 3 in the Materials and Methods section). As shown in Figure 6, we found that across participants the larger the N2pc for cue A (i.e., the larger the relative contralateral parietal–occipital negativity) the higher the gain-maximization score (βA-gain-max = −0.06, P < 0.05), independent of the N2pc for Z (βZ-gain-max = 0.01, P = 0.75). Critically, we also found that the larger the N2pc for Z, the lower (i.e., the worse) the loss-minimization score (βZ-loss-min = 0.08, P < 0.05), independent of the N2pc for A (βA-loss-min = −0.02, P = 0.47). This result thus provides strong support for the interpretation of the N2pc as reflecting the focusing on attention toward positive-reward predictors. Indeed, a large N2pc for A was associated with betting high on trials presenting A (i.e., a good gain-maximization performance), and a large N2pc for Z was associated with betting high on trials presenting Z (i.e., a poor loss-minimization performance).
Figure 6.
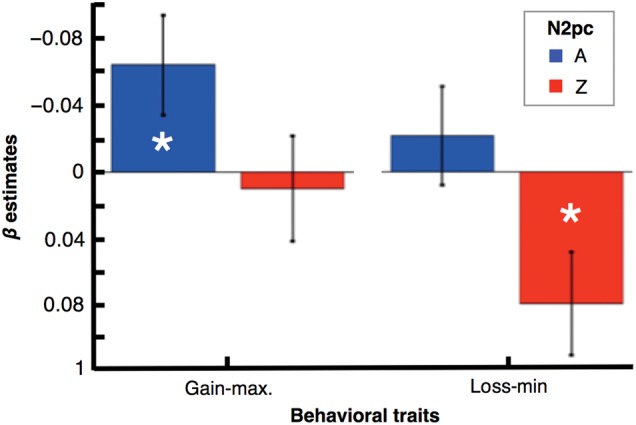
Double dissociation between gain-maximization and loss-minimization. Across participants, gain-maximization scores correlated with the N2pc amplitude for cue A (the larger the N2pc for A, the better the gain-maximizing performance), but showed no significant association with the N2pc amplitude for cue Z. In contrast, loss-minimization scores were negatively correlated with the N2pc amplitude for cue Z (the larger the N2pc for Z, the worst the loss-minimizing performance), but showed no significant association with the N2pc amplitude for cue A. (Error bars indicate SEM.)
To further support the existence of a double dissociation, we performed a stepwise regression procedure (Table 2). First, in order to evaluate the selective association between the N2pc elicited by cue A and the gain-maximization scores, we removed the N2pc to Z as a predictor from our original gain-maximization model [i.e., βZ-gain-max(N2pcZ) given by Eq. 2 in the Materials and Methods section]. The performance of this new model was not statistically different from the performance of the original one (F1,38 = 0.11, P = 0.74). However, when we removed the N2pc to cue A as a predictor from the original gain-maximization model, the new model performed significantly worse than the original one (F1,38 = 4.58, P < 0.05). We then performed an analogous procedure to evaluate the selective association between the N2pc to Z and the loss-minimization scores. As expected, we found exactly the inverse set of results. The performance of a model that excluded the N2pc to A was not statistically different from that of the original loss-minimization model (F1,38 = 0.54, P = 0.47), but a model that excluded the N2pc to Z performed significantly worse than that original model (F1,38= 6.47, P < 0.05).
Table 2.
Gain-maximization and loss-minimization are, respectively, associated with N2pc responses to gain-predicting and loss-predicting cues
| Model predictors | R-squared | Model estimates |
|
|---|---|---|---|
| β | P | ||
| Predicting gain-maximization | |||
| Original model | 0.12 | ||
| Constant | 0.65 | <0.001 | |
| N2pc A | −0.06 | 0.03 | |
| N2pc Z | 0.01 | 0.75 | |
| Constant + N2pc A | 0.11 | ||
| Constants | 0.65 | <0.001 | |
| N2pc A | −0.07 | 0.03 | |
| Constant + N2pc Z | 0.01 | ||
| Constants | 0.68 | <0.001 | |
| N2pc Z | 0.02 | 0.55 | |
| Predicting loss-minimization | |||
| Original model | 0.17 | ||
| Constant | 0.79 | <0.001 | |
| N2pc A | −0.02 | 0.47 | |
| N2pc Z | 0.08 | 0.02 | |
| Constant + N2pc Z | 0.16 | ||
| Constants | 0.80 | <0.001 | |
| N2pc Z | 0.08 | 0.01 | |
| Constant + N2pc A | 0.03 | ||
| Constants | 0.79 | <0.001 | |
| N2pc A | −0.03 | 0.31 | |
In summary, we found a double dissociation that was consistent with the interpretation of the N2pc as reflecting an attention-for-reward mechanism in cued economic choices: across participants, improvements in gain-maximization scores were positively correlated with the N2pc amplitude elicited by the gain-predicting cue (i.e., A), independently of the N2pc response to the loss-predicting cue (i.e., Z) and, conversely, improvements in loss-minimization scores were negatively correlated with the N2pc amplitude elicited by the loss-predicting cue, independently of the N2pc response to the gain-predicting cue.
Discussion
To promote survival and well-being, the brain must control behavior in a way that maximizes gains and minimizes potential losses. Previous research has indicated that gain-maximizing and loss-minimizing choices are associated with the functioning of different brain mechanisms (Venkatraman et al. 2009), and recently we reported that individual differences in gain-maximizing and loss-minimizing behaviors are associated with individual differences in the neural responses to feedback stimuli, indicating the receipt of those gains and losses (San Martín et al. 2013). Here we expand our understanding of the neural mechanisms underlying these types of decisions by analyzing the attention-related brain responses to outcome-predicting cues. In particular, we show that individual differences in gain-maximization and loss-minimization covary with that in cortical brain activity that reflects attentional bias toward those cues by virtue of their reward-predicting values.
Our design allowed us to differentiate rapid, lateralized, brain responses to cues representing distinct reward probabilities. In particular, we found that outcome-predicting cues elicited a contralaterally distributed, negative-polarity, ERP deflection that, based on its latency and scalp distribution, closely resembled the N2pc ERP component commonly associated with lateralized shifting and focusing of visual spatial attention (Luck and Hillyard 1994; Eimer 1996; Woodman and Luck 1999). The results indicate that, in the context of cued economic choice tasks, the N2pc activity reflects lateralized bias in the amount of attention drawn toward visual cues by virtue of their predictive association with positive outcomes, and that individual differences in cued economic choices are associated with that in the N2pc responses to those cues. Interestingly, and consistent with the attention-for-reward model, the results further indicate that efficient loss-minimization is associated with a significant reduction in the N2pc for the loss-predicting cue Z. Thus, the present electrophysiological results provide insights into how the brain allocates attention to environmental cues during the formation of economic decisions.
Gain-Predicting Cues Command More Attentional Orienting Than Neutral Cues and Loss-Predicting Cues
The current findings fit well with results from studies using reaction-time visual search tasks that have shown that the N2pc component is larger for targets (Kiss et al. 2009) or distractors (Hickey et al. 2010b) that have been associated with a large-magnitude versus low-magnitude reward. At the same time, our findings further extend our knowledge of the significance of attention-related ERP neural responses to economic decision-making by showing a modulation of attentional orienting in a task in which participants needed to passively observe a pair of outcome-predicting cues in order to later, after the outcome-predicting cues had already been removed from the screen, make an adaptive economic choice. We found this effect despite the fact that there was no obvious reason for the participants to rapidly allocate more attention to one or the other cue in the screen; participants' had plenty of time to look at the cues (1.5 s), and even though A and Z cues where the most informative ones, other cues (B and Y) were still relevant to make appropriate decisions throughout the task. Moreover, we found that the amplitude of the N2pc attentional-orienting component was larger for a visual cue that predicted the occurrence of a monetary gain compared with both a neutral cue (i.e., associated with a 50% chance of winning vs. losing) and a loss-predicting cue. Critically, this result indicates that reward associations modulate the N2pc to environmental cues, even when the delivery of reward depends on a cue-informed subsequent choice, rather than on the detection of the cue stimulus itself.
A large literature suggests that the N2pc reflects spatial attentional selection and/or the suppression of surrounding distractor information (Eimer 1996; Woodman and Luck 1999; Kiss et al. 2007; Buodo et al. 2010; Hickey et al. 2010b) within extrastriate visual regions in parietal and occipital cortex (Hopf et al. 2000). Indeed, a series of neuroimaging results has demonstrated a relative boosting of the neural representation of attended stimuli at the expense of competing stimuli within these and other visual regions (Kastner and Ungerleider 2000; Driver 2001). In line with this previous literature, we interpret our results as indicating that reward-prediction value influences visual processing in extrastriate visual cortex in a spatially selective fashion within 200–400 ms after the onset of outcome-predicting visual stimuli, such that gain-predicting cues capture more attention than neutral and loss-predicting cues. This idea is also consistent with a series of studies in non-human animals, showing that cues consistently preceding large quantities of food command attentional orienting (Morris and Bouton 2006) and that enforcing deprivation increases the attention drawn toward food (Mogg et al. 1998) or drugs of abuse (Field et al. 2004).
Loss-Minimization Is Associated with Decreased Attentional Allocation for Loss-Predicting Cues
Previous studies on animal conditioning have supported an early claim (Mackintosh 1975) that organisms learn to attend to relevant stimuli that predict outcomes and to ignore irrelevant stimuli (Pearce and Mackintosh 2010). We used a task in which participants could not alter the probabilities of outcomes (i.e., of winning vs. losing), but could maximize their gains by learning that certain cues (here, in particular, cue A) predicted the likelihood of gains and could minimize their losses by learning that other cues (cue Z) predicted the likelihood of losses. From this, we expected to find that improvements in the ability to maximize gains, across participants, would be associated with an increase in the N2pc amplitude for A cues, reflecting an increase of the attention drawn toward cues predicting a positive-reward likelihood and thus indicating that a high bet would be appropriate. This prediction was also motivated by results from a previous behavioral study showing an association between reward-seeking personality traits and the allocation of attention toward objects characterized by reward-associated features (Hickey et al. 2010a).
Conversely, we further hypothesized that improvements in the ability to minimize losses might also be associated with an increase in the N2pc amplitude for Z cues, reflecting more attention drawn toward cues predicting a loss likelihood and thus that a low bet was appropriate. We did indeed find a double dissociation according to which gain-maximization scaled with the N2pc amplitude for A cues independently of the N2pc for Z and loss-minimization covaried with the N2pc for Z independently of the N2pc for A. For loss-minimization, however, we found that the directionality of the results was the opposite of what we hypothesized. Rather than loss-minimization improving with the increase in the N2pc amplitude for Z, it was negatively correlated with the N2pc amplitude to Z.
This intriguing result thus suggests that the effective minimization of economic losses in our task may be associated either with an active attentional aversion away from the cue that informed the participant that a loss-minimizing decision was warranted, or with a relative decrease in the attention allocated toward Z as result of more attention being deployed toward the cue on the other side of the screen (B or Y). This second possible interpretation is consistent with the data reflecting relative attentional responses between whatever 2 cues are present at a time. Alternatively, another possible interpretation of the results derives from the N2pc being mainly elicited by the A cues, with much less differentiation between the N2pc's for the other cues. This could imply that the attentional responses are dominated by fairly selective biasing for the clearly reward-predicting A's, an explanation that would be consistent with previous evidence suggesting a dichotomous way of categorizing visual stimuli in economic decision-making tasks (Hajcak et al. 2006).
To our knowledge, this is the first study reporting effects on the attention-sensitive N2pc for stimuli predicting monetary losses in the context of a cued economic choice task, and future studies may help clarify the observed N2pc effects for loss-predicting stimuli. Some initial constraints for such an explanation can be found in studies linking attention and emotion. Specifically, by analyzing eye movements, some studies have reported that aversive cues (e.g., spiders) can induce attentional avoidance (Pflugshaupt et al. 2005; Weierich et al. 2008). Moreover, the attentional avoidance of aversive cues has been observed to scale with individual differences in negative mood (Beevers and Carver 2003; Calvo and Eysenck 2010). It could be the case that our results reflect a general property of emotional attention in which attention is shifted away from threatening or loss-predicting stimuli. Future studies will be required, however, to more fully support or refute this interpretation. Such studies could, for example, evaluate the participant-wise association between this N2pc reversal for cues predicting monetary losses and self-reported or physiologically measured negative mood or association.
These results also raise the question of whether the observed N2pc reversal for loss-predicting cues reflects the focusing of attention toward the stimuli in the hemifield opposite to the loss-predicting stimuli, the active suppression of attentional priority at the location of the loss-predicting stimuli, or a combination of these 2 mechanisms. Interestingly, recent studies indicate that activity during the N2pc latency may actually reflect not just an attentional focusing process toward a target stimulus, but also an active process of attentional suppression of the processing of the concurrent distractors that is indexed by a “distractor positivity” (Pd) effect (Hickey et al. 2009; Sawaki and Luck 2010; Sawaki et al. 2012). Although our experimental design does not allow isolation of the relative contribution of these 2 possible effects, follow-up studies could shed light into this issue by manipulating physical parameters of the stimuli (Hickey et al. 2009), or by affecting the relative latency between the 2 effects (Sawaki et al. 2012). Regardless, however, the present results indicate that effective learning about environmental cue stimuli predicting likely losses is associated with a decrease in attention allocation toward such cue stimuli.
The N2pc Reflects the Functioning of an Attention-for-Reward Mechanism in Cued-Choice Behavior
The results discussed so far characterize the attention-sensitive N2pc activity in the current context as reflecting an attention-for-reward mechanism [akin to what has also been called attention for liking (Vuilleumier 2005; Hogarth et al. 2010)], whereby subjects preferentially direct attention toward reward-predicting cues and shift their attention away from aversive or loss-predicting cues. This observed N2pc effect parallels reward prediction error responses that have been observed in the brain as phasic increases in the activity of midbrain dopamine (DA) for positive-reward-predicting cues and phasic decreases for negative-reward-predicting cues (Schultz et al. 1997; Schultz 2002). Indeed, several lines of evidence suggest that value-driven attentional orienting may arise through a modulation of visual activity triggered by such dopaminergic responses. First, a recent study in the rat has shown that the presence of DA-encoded prediction-error signals in the nucleus accumbens is necessary to orient behavior towards reward-predicting cues (Flagel et al. 2011). Secondly, it has also been shown in the rat that reward expectations modulate the activity of individual cells in the visual cortex (Shuler and Bear 2006). In humans, reward-dependent improvements in the allocation of attention have been found to be associated with increased neural activity both within the nucleus accumbens and within visual object-representation areas (Krebs et al. 2011). Finally, and also in humans, the facilitatory effect that reward has on the detection of visual targets has been shown to scale, across subjects, with measures of the feedback-related negativity (Hickey et al. 2010b), a midline ERP component that is thought to be generated when a reward prediction error signal is conveyed to the anterior cingulate cortex via the mesencephalic DA system (Holroyd and Coles 2002).
Within economic decision-making, our current electrophysiological results indicate that attention is drawn toward probabilistic outcome-predicting visual cues by virtue of their learned reward-predicting value. Moreover, they show that individual differences in this rapid attentional-orienting brain activity are associated with that in gain-maximizing and loss-minimizing economic choices: the stronger the attentional bias toward gain-predicting cues, the better the participants' ability to maximize those gains, and the stronger the attentional avoidance of loss-predicting cues, the better the participants' ability to minimize those losses. Although the correlational nature of this evidence does not allow us to claim a causal role of the reported ERP effects in the participants' decision-making, an attentional bias toward reward-predicting stimuli may have benefits when foraging for rewards, as suggested previously (Hickey et al. 2010b). It seems quite plausible that our N2pc results may reflect the functioning of an evolutionarily conserved mechanism to approach rewards and avoid threats, but implemented as attentional biasing toward environmental cues that predict positive or negative upcoming outcomes.
Although future studies are required in order to extract specific conclusions about the causal role of the processes reflected by the N2pc in cued-choice behavior, previous literature suggests that once established, preferential-coding mechanisms trigger processing cascades that ultimately orient or modulate behavior (Small et al. 2005; Engelmann et al. 2009) by interacting with higher-order cognitive functions (Locke and Braver 2008). Indeed, our results suggest that the role of visual processing in decision-making is not just to represent the visual features of the objects in the environment, but also to weigh their sensory representations according to the reward-predicting value of the represented objects and to orient attention accordingly.
Funding
This work was supported by a Fulbright scholarship and a CONICYT grant to R.S.M., and by NIH grants (R01-MH060415 and R01-NS051048) to M.G.W.
Notes
We thank Kenneth C. Roberts for helpful assistance during the preparation of the experiment and Stevan Budi for assistance with data collection. Conflict of Interest: None declared.
References
- Anderson BA, Laurent PA, Yantis S. 2011. Value-driven attentional capture. Proc Natl Acad Sci USA. 108:10367–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Carver CS. 2003. Attentional bias and mood persistence as prospective predictors of dysphoria. Cogn Ther Res. 27:619–637. [Google Scholar]
- Billeke P, Zamorano F, Cosmelli D, Aboitiz F. 2012. Oscillatory brain activity correlates with risk perception and predicts social decisions. Cereb Cortex. 23:2872–2883. [DOI] [PubMed] [Google Scholar]
- Buodo G, Sarlo M, Munafò M. 2010. The neural correlates of attentional bias in blood phobia as revealed by the N2pc. Soc Cogn Affect Neurosci. 5:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MG, Eysenck MW. 2010. Early vigilance and late avoidance of threat processing: repressive coping versus low/high anxiety. Cogn Emot. 14:763–787. [Google Scholar]
- Cohen MX, Ranganath C. 2007. Reinforcement learning signals predict future decisions. J Neurosci. 27:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. 2005. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 25:11730–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 134:9–21. [DOI] [PubMed] [Google Scholar]
- Driver J. 2001. A selective review of selective attention research from the past century. Br J Psychol. 92(Part 1):53–78. [PubMed] [Google Scholar]
- Eichele T, Specht K, Moosmann M, Jongsma MLA, Quiroga RQ, Nordby H, Hugdahl K. 2005. Assessing the spatiotemporal evolution of neuronal activation with single-trial event-related potentials and functional MRI. Proc Natl Acad Sci USA. 102:17798–17803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M. 1996. The N2pc component as an indicator of attentional selectivity. Electroencephalogr Clin Neurophysiol. 99:225–234. [DOI] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L. 2009. Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Front Hum Neurosci. 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. 2004. Eye movements to smoking-related cues: effects of nicotine deprivation. Psychopharmacology (Berl). 173:116–123. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H. 2011. A selective role for dopamine in stimulus-reward learning. Nature. 469:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Woroch BS, Curran T. 2005. Error-related negativity predicts reinforcement learning and conflict biases. Neuron. 47:495–501. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. 2012. Attention, learning, and the value of information. Neuron. 76:281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer JP, Woldorff MG, Huettel SA. 2008. Rapid electrophysiological brain responses are influenced by both valence and magnitude of monetary rewards. J Cogn Neurosci. 20:2058–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. 2006. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol Psychol. 71:148–154. [DOI] [PubMed] [Google Scholar]
- Hewig J, Trippe R, Hecht H, Coles MGH, Holroyd CB, Miltner WHR. 2007. Decision-making in Blackjack: an electrophysiological analysis. Cereb Cortex. 17:865–877. [DOI] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. 2010a. Reward guides vision when it's your thing: trait reward-seeking in reward-mediated visual priming. PLoS ONE. 5:e14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. 2010b. Reward changes salience in human vision via the anterior cingulate. J Neurosci. 30:11096–11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Di Lollo V, McDonald JJ. 2009. Electrophysiological indices of target and distractor processing in visual search. J Cogn Neurosci. 21:760–775. [DOI] [PubMed] [Google Scholar]
- Hickey C, van Zoest W. 2012. Reward creates oculomotor salience. Curr Biol. 22:R219–R220. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Dickinson A, Duka T. 2010. Selective attention to conditioned stimuli in human discrimination learning: untangling the effects of outcome prediction, valence, arousal, and uncertainty. In: Mitchell CJ, Le Pelley ME, editors. Attention and associative learning. Oxford, UK: Oxford University Press; p. 71–97. [Google Scholar]
- Holroyd CB, Coles MGH. 2002. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 109:679–709. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Luck SJ, Girelli M, Hagner T, Mangun GR, Scheich H, Heinze HJ. 2000. Neural sources of focused attention in visual search. Cereb Cortex. 10:1233–1241. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. 2000. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 23:315–341. [DOI] [PubMed] [Google Scholar]
- Kiss M, Driver J, Eimer M. 2009. Reward priority of visual target singletons modulates event-related potential signatures of attentional selection. Psychol Sci. 20:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss M, Goolsby BA, Raymond JE, Shapiro KL, Silvert L, Nobre AC, Fragopanagos N, Taylor JG, Eimer M. 2007. Efficient attentional selection predicts distractor devaluation: event-related potential evidence for a direct link between attention and emotion. J Cogn Neurosci. 19:1316–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Samanez-Larkin GR, Kuhnen CM. 2011. Gain and loss learning differentially contribute to life financial outcomes. PLoS ONE. 6:e24390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Egner T, Woldorff MG. 2011. The neural underpinnings of how reward associations can both guide and misguide attention. J Neurosci. 31:9752–9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke HS, Braver TS. 2008. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cogn Affect Behav Neurosci. 8:99–112. [DOI] [PubMed] [Google Scholar]
- Luck S. 2005. An introduction to the event-related potential technique. Cambridge, MA: MIT Press. [Google Scholar]
- Luck SJ, Hillyard SA. 1994. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 31:291–308. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. 1975. A theory of attention: variations in the associability of stimuli with reinforcement. Psychol Rev. 82:276–298. [Google Scholar]
- Maddox WT, Gorlick MA, Worthy DA, Beevers CG. 2012. Depressive symptoms enhance loss-minimization, but attenuate gain-maximization in history-dependent decision-making. Cognition. 125:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Hyare H, Lee S. 1998. Selective attention to food-related stimuli in hunger: are attentional biases specific to emotional and psychopathological states, or are they also found in normal drive states? Behav Res Ther. 36:227–237. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. 2006. Effect of unconditioned stimulus magnitude on the emergence of conditioned responding. J Exp Psychol Anim Behav Process. 32:371–385. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G. 1980. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 87:532–552. [PubMed] [Google Scholar]
- Pearce JM, Mackintosh NJ. 2010. Two theories of attention: a review and a possible integration. In: Mitchell CJ, Le Pelley ME, editors. Attention and associative learning. Oxford, UK: Oxford University Press; p. 11–39. [Google Scholar]
- Pflugshaupt T, Mosimann UP, von Wartburg R, Schmitt W, Nyffeler T, Müri RM. 2005. Hypervigilance-avoidance pattern in spider phobia. J Anxiety Disord. 19:105–116. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Brien JLO. 2009. Selective visual attention and motivation. Psychol Sci. 20:981–989. [DOI] [PubMed] [Google Scholar]
- San Martín R. 2012. Event-related potential studies of outcome processing and feedback-guided learning. Front Hum Neurosci. 6:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martín R, Appelbaum LG, Pearson JM, Huettel SA, Woldorff MG. 2013. Rapid brain responses independently predict gain maximization and loss minimization during economic decision making. J Neurosci. 33:7011–7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martín R, Manes F, Hurtado E, Isla P, Ibañez A. 2010. Size and probability of rewards modulate the feedback error-related negativity associated with wins but not losses in a monetarily rewarded gambling task. Neuroimage. 51:1194–1204. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Geng JJ, Luck SJ. 2012. A common neural mechanism for preventing and terminating the allocation of attention. J Neurosci. 32:10725–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki R, Luck SJ. 2010. Capture versus suppression of attention by salient singletons: electrophysiological evidence for an automatic attend-to-me signal. Atten Percep Psychophys. 72:1455–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe C, Ullsperger M, Sommer W, Heekeren HR. 2010. Effects of parametrical and trial-to-trial variation in prior probability processing revealed by simultaneous electroencephalogram/functional magnetic resonance imaging. J Neurosci. 30:16709–16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. 2002. Getting formal with dopamine and reward. Neuron. 36:241–263. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. 1997. A neural substrate of prediction and reward. Science. 275:1593–1599. [DOI] [PubMed] [Google Scholar]
- Shuler MG, Bear MF. 2006. Krebs. Science. 311:1606–1609. [DOI] [PubMed] [Google Scholar]
- Siler K. 2010. Social and psychological challenges of poker. J Gambl Stud. 26:401–420. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulam M-M. 2005. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cereb Cortex. 15:1855–1865. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Payne JW, Bettman JR, Luce MF, Huettel SA. 2009. Separate neural mechanisms underlie choices and strategic preferences in risky decision making. Neuron. 62:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. 2005. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 9:585–594. [DOI] [PubMed] [Google Scholar]
- Weierich MR, Treat TA, Hollingworth A. 2008. Theories and measurement of visual attentional processing in anxiety. Cogn Emot. 22:985–1018. [Google Scholar]
- Woldorff MG, Liotti M, Seabolt M, Busse L, Lancaster JL, Fox PT. 2002. The temporal dynamics of the effects in occipital cortex of visual-spatial selective attention. Brain Res Cogn Brain Res. 15:1–15. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. 1999. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 400:867–869. [DOI] [PubMed] [Google Scholar]