Abstract
Cellular studies showed that disinhibition, evoked pharmacologically or by a suitably timed priming stimulus, can augment long-term plasticity (LTP) induction. We demonstrated previously that transcranial magnetic stimulation evokes a period of presumably GABABergic late cortical disinhibition (LCD) in human primary motor cortex (M1). Here, we hypothesized that, in keeping with cellular studies, LCD can augment LTP-like plasticity in humans. In Experiment 1, patterned repetitive TMS was applied to left M1, consisting of 6 trains (intertrain interval, 8 s) of 4 doublets (interpulse interval equal to individual peak I-wave facilitation, 1.3–1.5 ms) spaced by the individual peak LCD (interdoublet interval (IDI), 200–250 ms). This intervention (total of 48 pulses applied over ∼45 s) increased motor-evoked potential amplitude, a marker of corticospinal excitability, in a right hand muscle by 147% ± 4%. Control experiments showed that IDIs shorter or longer than LCD did not result in LTP-like plasticity. Experiment 2 indicated topographic specificity to the M1 hand region stimulated by TMS and duration of the LTP-like plasticity of 60 min. In conclusion, GABABergic LCD offers a powerful new approach for augmenting LTP-like plasticity induction in human cortex. We refer to this protocol as disinhibition stimulation (DIS).
Keywords: I-waves, late cortical disinhibition, LTP-like plasticity, motor cortex, transcranial magnetic stimulation
Introduction
There are a number of in vitro stimulation protocols that can induce long-term plasticity (LTP) of excitatory synapses across a variety of experimental preparations (Thickbroom 2007). However, in vivo excitatory synapses are typically components of more complex networks that include excitatory and inhibitory connections, and there is evidence that the level of inhibitory tone in the neuronal network can modulate the efficacy of LTP. Theta-burst stimulation of superficial layers of rat primary motor cortex (M1) typically fails to induce LTP unless there is simultaneous blockade of intracortical inhibition either by a pharmacological agent such as the GABAA receptor antagonist bicuculline (Wigstrom and Gustafsson 1983a, 1983b, 1985; Hess and Donoghue 1994; Castro-Alamancos et al. 1995; Hess et al. 1996; Hess and Donoghue 1996) or by tetanization of vertical afferents, which reduce the efficacy of inhibitory transmission (Iriki et al. 1989; Hess et al. 1996). Likewise, induction of associative LTP fails in piriform cortex unless GABAA receptor activity is blocked (Kanter and Haberly 1993).
Network considerations are also relevant to human studies of LTP-like plasticity that employ noninvasive brain stimulation to M1, and in keeping with experimental observations, there is evidence that the level of cortical inhibition can be important in the human also. The induction of an LTP-like increase in corticospinal excitability with paired-associative stimulation is reduced by diazepam and tiagabine, which increase neurotransmission through the GABAA receptor (Heidegger et al. 2010) and by the GABAB receptor agonist baclofen (McDonnell et al. 2007). In contrast, ischemic nerve block, which decreases GABA-related cortical inhibition (i.e., disinhibition, Levy et al. 2002), enhances the LTP-like effects of repetitive transcranial magnetic stimulation (rTMS) and practice-dependent plasticity in healthy participants (Ziemann, Corwell et al. 1998; Ziemann, Hallett et al. 1998; Ziemann et al. 2001) and in people who have suffered a stroke (Muellbacher et al. 2002). Pretreatment with lorazepam, a positive allosteric modulator of the GABAA receptor, reduces these effects (Ziemann, Hallett et al. 1998; Bütefisch et al. 2000; Ziemann et al. 2001; Teo et al. 2009).
While the level of inhibition can be manipulated pharmacologically, disinhibition can also be evoked electrophysiologically by the application of a suitably timed priming stimulus (PS). In cellular preparations, a PS to an inhibitory neuron leads to a reduction in the IPSP evoked by a test stimulus given a few hundred milliseconds later (paired-pulse depression; Diamond et al. 1988; Davies et al. 1990; Davies et al. 1991; Mott and Lewis 1991; Nathan and Lambert 1991; Otis et al. 1993; Deisz et al. 1997; Deisz 1999). The PS is thought to activate pre- and post-synaptic GABAB receptors with different effects: Postsynaptic activation brings about inhibition, whereas presynaptic activation inhibits further GABA release and results in disinhibition. With paired-pulse depression, the time-course of presynaptic disinhibition outlasts that of postsynaptic inhibition resulting in a late period during which disinhibition dominates (Otis et al. 1993; Deisz 1999). Postsynaptic depolarization (Larson and Lynch 1986; Pacelli et al. 1989) and NMDA receptor activation (Davies and Collingridge 1996) at excitatory synapses are facilitated during this period of disinhibition, and these effects have been harnessed to accelerate the induction of synaptic plasticity (Larson and Lynch 1986; Larson et al. 1986; Rose and Dunwiddie 1986; Staubli and Lynch 1987; Diamond et al. 1988; Greenstein et al. 1988; Pacelli et al. 1989; Davies et al. 1991; Mott and Lewis 1991).
A similar period of late cortical disinhibition (LCD) has recently been described in human M1 (Cash et al. 2010, 2011). If a PS is delivered by TMS, it evokes release of GABA from inhibitory interneurons that activates postsynaptic GABAA receptors leading to short-lasting (<20 ms) postsynaptic inhibition in the form of short-interval intracortical inhibition (SICI) (Kujirai et al. 1993; Ziemann et al. 1996; Hanajima et al. 1998; Di Lazzaro et al. 2000, 2006), and GABAB receptors leading to long-lasting (100–200 ms) postsynaptic inhibition in the form of long-interval intracortical inhibition (LICI) (Valls-Sole et al. 1992; McDonnell et al. 2006; Müller-Dahlhaus et al. 2008). Disinhibition occurs during the LICI phase (Werhahn et al. 1999; Sanger et al. 2001; McDonnell et al. 2006; Chu et al. 2008; Ni et al. 2011) but has been shown to outlast LICI and result in a period in which disinhibition dominates (LCD, Cash et al. 2010). Disinhibition is demonstrated by the reduction in SICI that occurs around 100–250 ms following PS, and LCD is the period of disinhibition that continues beyond LICI (i.e., from ∼190 to 250 ms). As with cellular studies, LCD is associated with increased net excitability, as reflected by increased motor-evoked potential (MEP) amplitude evoked by a single test-pulse delivered between 190 and 250 ms following PS (long-interval cortical facilitation; LICF). Short-interval intracortical facilitation (SICF) is likewise increased during LCD, and indicative of increased excitability at the cortical level (Cash et al. 2008, 2010, 2011). In summary, although this has not been directly proven, for example, by pharmacological studies, LCD most likely reflects presynaptic GABABergic disinhibition (cf. Supplementary Figure), similar to paired-pulse depression at the cellular level.
We postulated that LCD can be harnessed to improve the efficacy of LTP-like plasticity induction in human cortex. In the present study, we have tested this by combining LCD and a plasticity-inducing protocol known as I-wave TMS (ITMS; Thickbroom et al. 2006). ITMS targets facilitatory I-wave networks and increases excitability by repeatedly delivering paired-pulses at an interpulse interval (IPI) corresponding to the periodicity of I-waves (∼1.5 ms) (Cash et al. 2009, 2013).
In the present study, a series of trains consisting of 4 ITMS doublets was delivered such that each doublet in the train (after the first) was delivered during the LCD evoked by its preceding doublet. We show that this intervention is topographically specific and highly effective in inducing LTP-like plasticity when the doublets are presented at LCD periodicity, but not at intervals shorter or longer than LCD. We refer to this protocol as disinhibition stimulation (DIS).
Methods
Participants
In Experiment 1, 11 healthy volunteers were screened for the presence of LICF and SICF. Of these, 8 [3 female, aged 22–35 years; right hand dominant according to the Edinburgh Inventory (Oldfield 1971)], were recruited to participate in the study. A further 11 healthy volunteers were screened in the same manner for Experiment 2 (see also Section Experiment 2: duration of effects and topographic specificity) to investigate the duration of effects and topographic specificity, 9 of whom were then recruited (4 female, ages 22–44). All participants gave informed written consent and completed a safety questionnaire prior to the study, which had the approval of the institution's ethics board (Goethe-University of Frankfurt am Main or University Health Network, Toronto) and conformed to the Declaration of Helsinki. Participants were seated comfortably and were asked to remain at rest but alert with eyes open for the duration of the study.
Electromyography
MEPs were recorded using surface electrodes placed in a belly-tendon arrangement over the first dorsal interosseous (FDI) target muscle of the right hand. In Experiment 2, surface electromyography (EMG) was additionally recorded from abductor pollicis brevis (APB), extensor carpi radialis (ECR), and biceps brachii (BB) muscles. The peak-to-peak amplitude of the FDI MEP was the primary outcome measure. The electromyographic signal was amplified and filtered (20 Hz–2 kHz), digitized (sample rate 4 kHz) using a CED Micro 1401 (Cambridge Electronic Design), monitored, and stored on a computer.
TMS: Setup Measurements
Stimulus parameters were investigated and optimized for each individual. TMS was delivered using 3 linked magnetic stimulators (Magstim) with a monophasic current waveform connected to a 70 mm figure-of-eight coil placed over the optimal scalp position (determined from initial exploration) for the hand area of the left M1, and orientated 45° to the sagittal plane so that the induced current in the brain was directed from posterior to anterior (PA). This coil orientation is considered optimal for activating corticospinal neurons trans-synaptically (Di Lazzaro et al. 2008). Resting motor threshold (RMT) at the optimal scalp position was tested by the relative frequency method (Groppa et al. 2012) and defined as the lowest stimulator intensity eliciting MEPs from the right FDI of >50 µV peak-to-peak amplitude in at least 5 of 10 trials. The test stimulus intensity that generated a MEP of 1 mV (SI1mV) was also determined.
Determining IPI for Maximum SICF
Paired-pulse TMS (each pulse 110% RMT) were delivered at IPIs in the range 1.1–2.3 ms in 0.2 ms steps. All IPIs, together with single-pulse TMS (at 110% RMT) were delivered in blocks of 8 stimuli (blocks in a pseudo-random order). Averaging peak-to-peak FDI MEP amplitude for each IPI, and expressing this as a percentage of the mean test MEP amplitude, generated a SICF curve. For the remainder of the study, ITMS doublets were delivered at the IPI for which each participant's SICF curve was maximal.
Determining Interdoublet Interval for Maximum LICF
This measurement was undertaken to confirm that LICF was present after an ITMS doublet, as it is after single-pulse (SP) TMS (Cash et al. 2010, 2011), and to optimize the IDI for maximum LICF. A triple pulse was delivered consisting of an ITMS doublet (110% RMT) followed by a test stimulus (TS, SP TMS, SI1mV) at intervals in the range 150–300 ms (150, 190, 200, 210, 220, 250 and 300 ms; blocks of 10 triplets at each interval delivered in pseudo-randomized order, see Fig. 2b insert). The mean TS MEP amplitude at each interval was expressed as a percentage of mean TS MEP alone to generate a LICF curve. The optimal LICF interval (OPT) was determined from the maximum of this curve for each individual and was used as the IDI for the primary intervention.
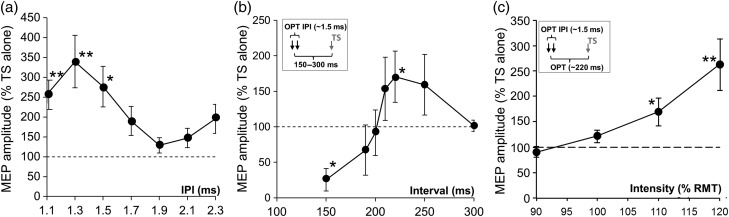
Figure 2.
(a) IPI curve for short-interval intracortical facilitation (SICF). The effect of IPI on SICF was significant (LMM: F6,14 = 3.8; P < 0.05). On average, SICF was maximal at an IPI of 1.3 ms, and SICF was significantly greater than SP baseline at IPIs of 1.1, 1.3, and 1.5 ms (256% ± 37%, 339% ± 66%, and 276% ± 51% of baseline, respectively; P < 0.05). Individually, the maximum SICF occurred at an IPI of either 1.3 ms (n = 6 participants) or 1.5 ms (n = 2), and subsequently ITMS doublets were delivered at these individually optimal IPIs. (b) Interstimulus interval for maximum LICF. Triple-pulse TMS (see inset) was used to determine the temporal pattern of inhibition (equivalent to LICI) and LICF (it was previously shown that this MEP facilitation correlates with LCD) evoked by TMS doublets. Doublets were delivered at individual I-wave frequency of ∼1.5 ms (optimal interpulse interval, IPI-OPT) followed by a test stimulus (TS) at 150–300 ms. On average, the greatest reduction in TS MEP amplitude occurred 150 ms post-ITMS consistent with LICI (25% ± 16% of baseline, P < 0.05), and the greatest increase occurred at 220 ms (164% ± 30% of baseline; P < 0.05). The optimal IDI of LCD for each subject occurred at either 220 ms (n = 4) or 250 ms (n = 4) post-ITMS, and these values were used to set the IDI for each participant. In all cases, TS amplitude returned to baseline by 300 ms. (c) Effects of ITMS doublet intensity on LICF. At the individual optimal IDI (OPT, see inset), increasing the intensity of the pulses making up the ITMS doublet, from 90% to 120% RMT (in 10% steps), led to a progressive increase in TS MEP (90% ± 11%, 122% ± 13%, 175% ± 31%, and 263% ± 51%, respectively, of TS baseline; LMM: F3,8.5 = 5.47, P < 0.05). Post hoc t-tests revealed that the minimum intensity for a significant increase in TS MEP amplitude (indicating LICF) was 110% RMT. *P < 0.05; **P < 0.01; results expressed as mean ± SEM.
Influence of ITMS Doublet Intensity on LICF
The intensity of ITMS doublets (each pulse in the doublet was always of equal intensity) was varied (pseudo-randomly, blocks of 10 doublets per intensity) between 90% and 120% RMT in 10% steps. Single-pulse TS of SI1mV were delivered at OPT after each doublet (see Fig. 2c insert), and the doublet intensity that gave maximal LICF was determined for each individual.
TMS: Intervention Protocol and Corticomotor Excitability
This phase of the study required the use of a MagPro X100 magnetic stimulator (MagVenture) to deliver the necessary pattern and frequency of stimulation. A 75 mm figure-of-eight coil (biphasic pulse, AP-PA-induced current direction in the brain, 0.28 ms duration pulse), connected to the MagPro, was held over the optimal scalp position for activating the right FDI (as determined by initial exploration), and orientated 45° lateral to the midline.
Single-pulse RMT was determined according to the criteria described above. A train of 4 ITMS doublets was then delivered at an IDI given by OPT, and stimulator intensity was adjusted (in the range 100%–120% RMT) to determine the minimum intensity that resulted in an increase in doublet MEP amplitude across the train. For safety reasons, this minimum intensity was used for all subsequent doublet protocols.
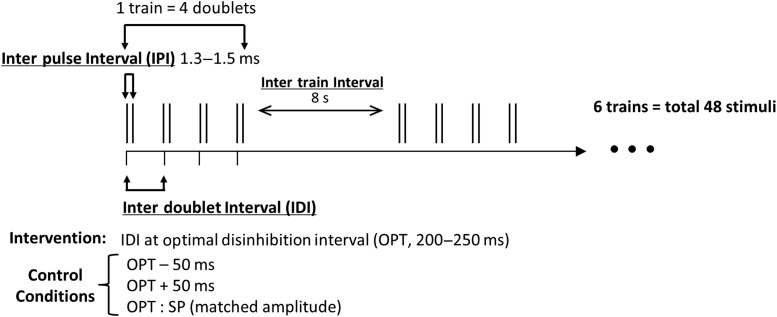
The primary TMS intervention consisted of 6 of these trains delivered at an intertrain interval of 8 s (Fig. 1). The intervention thus consisted of 48 stimuli delivered over ∼45 s (6 trains of 4 ITMS doublets, each train 8 s apart).
Figure 1.
Intervention protocol: The primary TMS intervention consisted of 6 trains (intertrain interval, 8 s) of 4 ITMS doublets (interpulse interval equal to individual peak I-wave facilitation, 1.3–1.5 ms) spaced by the individual optimal disinhibition interval (OPT, 200–250 ms). A total of 48 pulses were applied over ∼45 s. The OPT interval was determined using triple-pulse TMS (see Fig. 2a). Control conditions consisted of decreasing (OPT − 50 ms) or increasing (OPT + 50 ms) the IDI, and replacing ITMS with amplitude-matched SP TMS. SP MEPs were recorded pre- and post-intervention to investigate effects on cortical excitability.
At baseline, 4 blocks of 10 SP TS (at SI1mV and 5 ± 1.25 s intervals, ∼1-min acquisition time) were delivered, each block 1 min apart, and the mean MEP amplitude of each block was calculated. Postintervention, blocks of 10 TS were delivered at 1, 3, 5, 7, 9, 11, 16, 21, 26, and 31 min, and the mean MEP amplitude for each block calculated.
TMS: Control Protocols
Control conditions used the same protocol, except that either the IDI was (1) decreased by 50 ms (OPT − 50; n = 8 participants), (2) increased by 50 ms (OPT + 50; n = 8), or (3) IDI was retained at OPT, but doublets were replaced by SP stimuli at an intensity that matched the ITMS doublet MEP amplitude (OPT − SP; n = 7).
We used a pseudo-randomized double-blinded crossover design. This was achieved by 2 experimenters: One experimenter performed the intervention, whereas the main experimenter who was blinded toward interventional condition performed all other TMS measurements and was responsible for analyzing the data. Pseudo-randomization followed a preset table of the order of interventional conditions that was fully balanced across participants. This table was sealed away from the main experimenter who was responsible for all TMS measurements and data analysis. In a given subject, experiments were separated by several days but carried out at the same time of day for each subject to control for individual chronotype and diurnal variations in cortisol levels (Sale et al. 2007, 2008) and GABAergic inhibition in motor cortex (Lang et al. 2011), and female participants were asked to avoid bookings that were likely to take place in the first days of the menstrual cycle to minimize potential hormonal confounding effects on neuroplasticity between sessions (Inghilleri et al. 2004). Participants were instructed not to contract hand muscles during or following intervention as this may interact with plastic effects (Gentner et al. 2008; Huang et al. 2008).
Experiment 2: Duration of Effects and Topographic Specificity
For this additional experiment, all procedures in Sections Participants; Electromyography; TMS: setup measurements; Determining IPI for maximum SICF; Determining interdoublet interval for maximum LICF were adhered to and the intervention was delivered at optimal intervals as described in Section TMS: intervention protocol and corticomotor excitability. To investigate the topographic specificity of the plasticity protocol, EMG was recorded from FDI as before and additionally from APB, ECR, and BB. To investigate duration of effects, baseline excitability was recorded in the same manner and time-points as described in Section TMS: intervention protocol and corticomotor excitability, and additionally at 45, 60, 90, and 120 min postintervention as well as 14 h later on the following morning. The intervention was administered at approximately 6 PM in all cases.
Safety
Participants were instructed to remain alert throughout the interventions and were warned that if they perceived a spread of movement from hand into arm muscles, they should move away from the coil and report this to the investigator (Pascual-Leone et al. 1994). The investigator closely monitored the arm for any indication of a spread of activation. A neurologist was on standby for all intervention experiments. No exact precedent exists for this intervention protocol, and we followed the safety criteria for rTMS guidelines (cf. Fig. 5 in Pascual-Leone et al. 1993; cf. Table 3 in Chen et al. 1997; cf. Table 3 in Wassermann 1998; cf. Table 4 in Rossi et al. 2009) and the minimum intensity of stimulation was chosen that produced an effect across doublets in a train (see above) and was capped at 120% RMT. No participants reported any spread in excitability or moved away from the coil, and there were no adverse effects. Participants were asked for feedback after the studies and none raised concerns about their experience.
Data Analysis
Effect of IPI
Normalized MEP amplitude versus IPI curves were calculated from mean MEP amplitude (as a percentage of baseline) for each subject, averaged across subjects. A linear mixed model (LMM) with random effect of individual was used to examine the effect of the fixed factor IPI and muscle (where relevant) on normalized MEP amplitude. Restricted maximum likelihood estimation was used in all modeling.
Effect of Interventions
To confirm that preintervention baseline MEP amplitude did not differ between interventions, an LMM analysis was performed with intervention as the fixed effect. To determine whether there was an effect of intervention on MEP amplitude over time and a difference across interventions or muscles, LMM analysis was performed with fixed factors “intervention” or “muscle”, and “time” (0 [i.e., preintervention], 1, 3, 5, 7, 9, 11, 16, 21, 26, and 31 min postintervention), and the dependent variable MEP amplitude expressed as a percentage of preintervention baseline. In Experiment 2, additional time-points 45, 60, 90, 120 min, and 14 h were included in the analyses. Random effect of individual was included in all LMM analyses.
Results are expressed as mean ± SEM. Datasets for each measure (e.g., SICF, LICF in Section Effect of IPI, and plasticity in Section Effect of interventions) were assessed for normality of data distribution using the Kolmogorov–Smirnov test and log-transformed if necessary to generate normality as required for valid LMM statistical assessment. When significance of condition main effects was indicated by LMM, post hoc two-tailed t-tests (critical P-value 0.05) were performed using Fisher's LSD. Correlations were tested using the permutation (exact) test (Ludbrook and Dudley 1998). Statistical analysis was performed using SPSS version 16.0.
Results
Experiment 1
IPI for Maximum SICF
Group mean RMT was 52% ± 3% of maximum stimulator output. Mean single-pulse MEP amplitude at 110% RMT was 0.54 ± 0.09 mV. The effect of IPI on SICF was significant (LMM: F6,14 = 3.8; P < 0.05). On average, SICF was maximal at an IPI of 1.3 ms, and SICF was significantly greater than SP baseline at IPIs of 1.1, 1.3, and 1.5 ms (256% ± 37%, 339% ± 66%, and 276% ± 51% of baseline, respectively; P < 0.05; Fig. 2a). Individually, the maximum SICF occurred at an IPI of either 1.3 ms (n = 6 participants) or 1.5 ms (n = 2), and subsequently ITMS doublets were delivered at these individually optimal IPIs.
IDI for Maximum LICF
There was a significant effect of IDI on LICF (LMM: F6,8.4 = 4.6, P < 0.05). Figure 2b shows that when the TS was delivered after an ITMS doublet, TS MEP amplitude was initially reduced (in keeping with LICI) and then transiently increased (in keeping with LICF)—a pattern similar to that reported after an SP PS (Cash et al. 2010, 2011). On average, the greatest reduction in TS amplitude (i.e., LICI) occurred 150 ms post-ITMS (25% ± 16% of baseline, P < 0.05), and the greatest increase (i.e., LICF) occurred at 220 ms (164% ± 30% of baseline; P < 0.05). The optimal IDI of LICF for each subject occurred at either 220 ms (n = 4) or 250 ms (n = 4) post-ITMS, and these values were used to set the IDI for each participant. In all cases, TS amplitude returned to baseline by 300 ms.
ITMS Doublet Intensity and LICF
At the optimal IDI, increasing the intensity of the pulses making up the ITMS doublet, from 90% to 120% RMT (in 10% steps), led to a progressive increase in the amplitude of TS delivered at OPT (90% ± 11%, 122% ± 13%, 175% ± 31%, and 263% ± 51%, respectively, of TS baseline; LMM: F3,8.5 = 5.47, P < 0.05). Post hoc t-tests revealed that the minimum intensity for a significant increase in TS amplitude (LICF) was 110% RMT (Fig. 2c).
Determination of Stimulus Intensity for Intervention
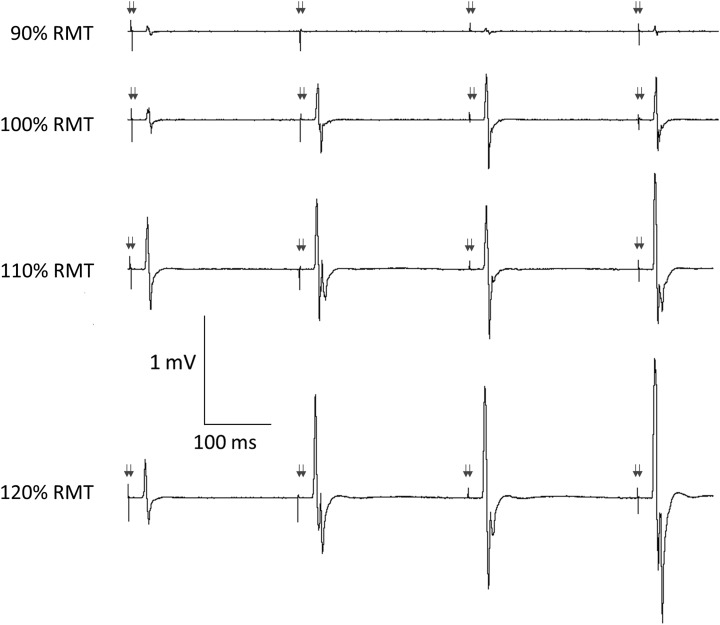
The MEP amplitude of ITMS doublets increased within a train (at OPT IDI) and increased with ITMS intensity (Fig. 3). The minimum ITMS intensity that resulted in an increase in MEP amplitude during a train of 4 doublets (delivered at OPT IDI) was on average 111% ± 3% RMT.
Figure 3.
Influence of ITMS doublet intensity on MEP amplitude during train at OPT. MEPs during a 4-doublet train in 1 representative participant at the individual OPT (250 ms, arrows indicate approximate time of TMS doublets). When TMS doublets were delivered at 100% RMT, an intensity too low to generate LICF, there was no continuous increase in MEP amplitude throughout the train. However, MEP amplitude increased throughout the train at higher TMS doublet intensities of 110%–120% RMT, in line with the increase in LICF with TMS doublet intensity in Figure 2c.
Baseline MEP Measurements
Baseline TS MEP amplitude (i.e., SI1mV) did not differ for the 4 interventions (1.08 ± 0.03, 0.97 ± 0.07, 0.92 ± 0.05, and 0.97 ± 0.1 mV for OPT − SP, OPT − 50, OPT, and OPT + 50, respectively; LMM: F3,13.4 = 2.0; P = 0.168).
Changes in MEP Amplitude during Intervention
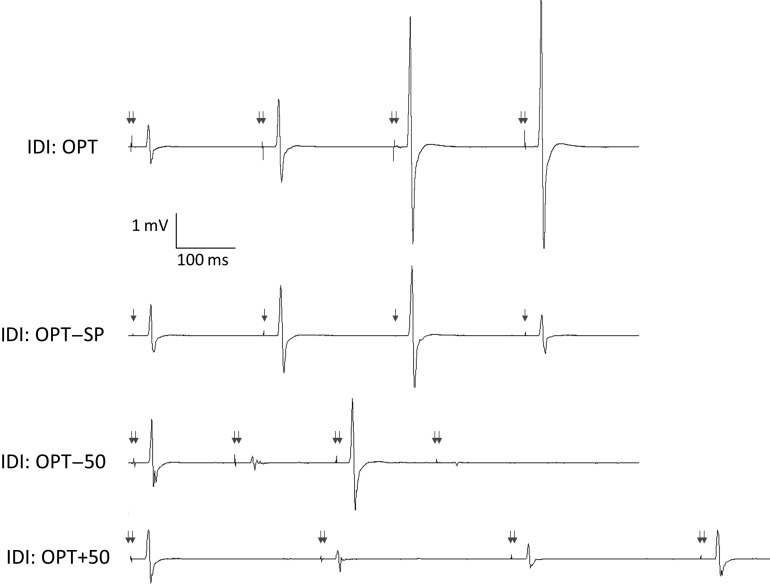
There was no significant difference within or across intervention in the MEP amplitude elicited by the first ITMS doublet of each of the 6 trains (LMM: effect of train: F5,37.2 = 0.427, P = 0.83; effect of intervention: F3,55.5 = 0.35, P = 0.79; interaction train × intervention: F15,28.46 = 0.24, P = 1.0). SP MEP amplitude was successfully matched to MEP amplitude elicited by ITMS doublets (1.40 ± 0.35 mV vs. 1.31 ± 0.25 mV, respectively). The ratio of MEP amplitude elicited by the fourth to the first ITMS doublet did not differ significantly for each train within any intervention but did differ between interventions (LMM: effect of train: F5,33.9 = 0.4, P = 0.85; effect of intervention: F3,55.6 = 4.9, P < 0.01; interaction train × intervention: F15,22.2 = 0.76, P = 0.71). Post hoc analyses indicated that this ratio was significantly greater for OPT (2.19 ± 0.18) compared with the other interventions (1.03 ± 0.08, 1.34 ± 0.05, 1.16 ± 0.05 for OPT − SP, OPT − 50, and OPT + 50, respectively; P < 0.01 for each comparison with OPT, comparisons between control conditions not significant; Fig. 4).
Figure 4.
Influence of IDI on MEP amplitude during train. MEPs during a 4-doublet train in 1 individual. Doublets at the individualized OPT (220 ms) resulted in a continuous increase in MEP amplitude throughout the train that was not observed at other intervals (OPT − 50 ms, OPT + 50 ms) or when using matched amplitude single-pulse stimuli (OPT − SP). TMS intensity was 110% RMT; arrows indicate approximate time of TMS.
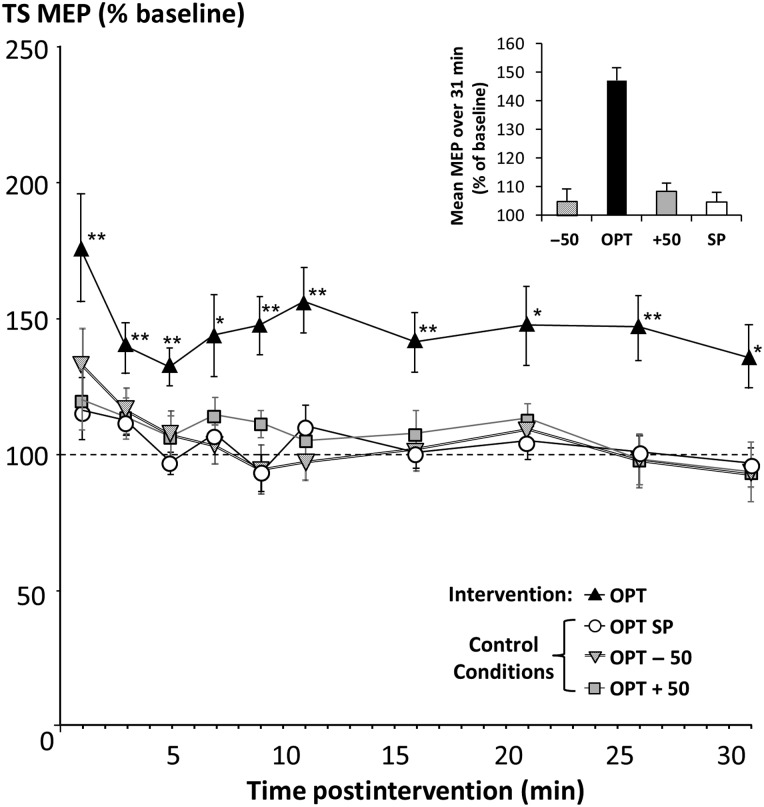
Changes in MEP Amplitude Postintervention
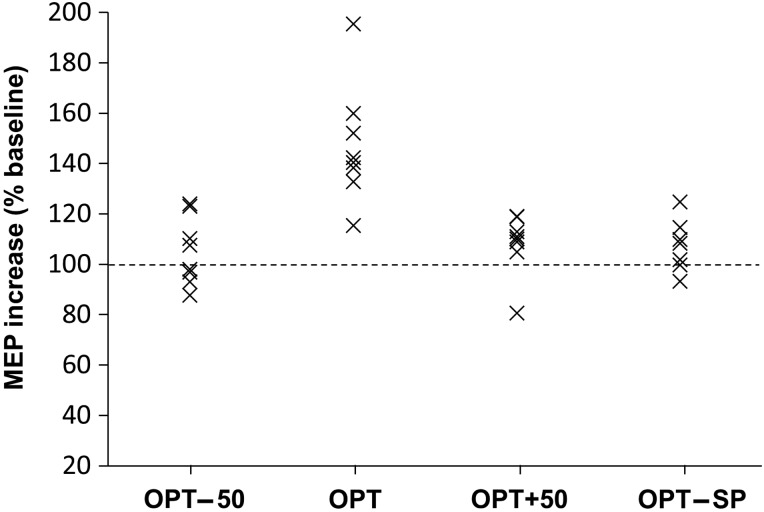
Figure 5 illustrates the time-course of MEP amplitude changes postintervention. There was a significant effect of time, intervention, and their interaction (LMM: effect of time: F10,19.4 = 14.1, P < 0.001); effect of intervention: F3,97.1 = 27.6, P < 0.001); interaction: F29,24.8 = 26.5, P < 0.001). Pairwise comparisons indicated that OPT differed significantly to the other interventional treatments (all comparisons P < 0.001), whereas no differences between the other interventions were detected. Post hoc t-tests showed that MEP amplitude was increased at all time-points postintervention (1–31 min) for the OPT intervention, whereas no other intervention showed a significant MEP change (Fig. 5). The average MEP (derived from the group average) over 31 min postintervention relative to preintervention baseline was 147% ± 4% at OPT, 105% ± 4% at OPT − 50, 108% ± 3% at OPT + 50, and 105% ± 5% in the SP condition (inset, Fig. 5). There was no significant correlation between stimulus intensity and intervention efficacy at OPT (r = 0.48, P = 0.23). The interindividual variability of this MEP increase for the 4 interventional conditions is displayed in Figure 6, indicating consistent MEP increase across individuals at OPT and no change in the other conditions.
Figure 5.
Time-course of MEP amplitude changes postintervention. The primary TMS intervention at OPT resulted in a significant increase in MEP amplitude postintervention (P < 0.001), whereas control experiments showed that IDIs shorter or longer than LCD did not result in LTP-like plasticity, indicating that LCD facilitates synaptic plasticity in human M1. The average MEP over 31 min postintervention relative to preintervention baseline was 147% ± 4% at OPT, 105% ± 4% at OPT − 50, 108% ± 3% at OPT + 50, and 105% ± 5% in the OPT − SP condition (see inset). *P < 0.05, **P < 0.01 (post hoc t-tests, significant difference of TS MEP amplitude from baseline).
Figure 6.
Interindividual variability. The average increase in excitability pre- to post-intervention (1–30 min, % baseline) is indicated for each individual and each interventional protocol. The increase in postintervention excitability was consistent across participants at OPT with little change at other intervals.
Experiment 2
SICF
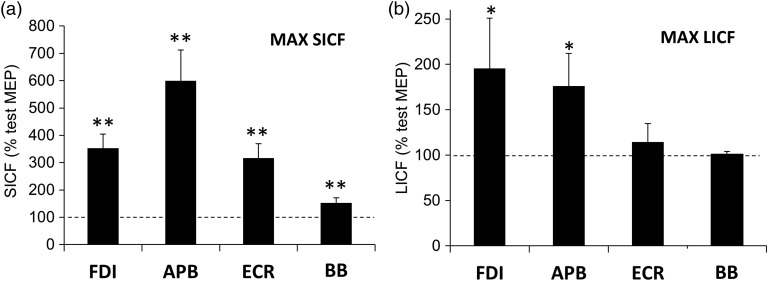
The effects of IPI and muscle on SICF were significant (LMM: effect of IPI: F6,40.7 = 22.0, P < 0.01; effect of muscle: F3,113.2 = 32.9, P < 0.01). Peak SICF in the FDI target muscle was 353% ± 52% of TS alone, and in APB, ECR, and BB, it was 600% ± 112%, 316% ± 53%, and 153% ± 19%, respectively, of TS alone (Fig. 7a). There was a trend for greater SICF in APB compared with FDI, but this difference was not significant. SICF was maximal at IPI 1.3 ms in all muscles (averaged across individuals). Mean test MEP amplitude (at 110% RMT) was 0.60 ± 0.12 mV for FDI, and in APB, ECR, and BB, it was 0.55 ± 0.18, 0.25 ± 0.06 and 0.1 ± 0.02 mV, respectively.
Figure 7.
Topographic differences in SICF and LICF. (a) SICF and (b) LICF were calculated at the optimal IPI for FDI, APB, ECR, and BB muscles. Both measures were greatest in hand muscles. SICF was greatest in APB and FDI but was still significant in ECR and BB, whereas LICF was greatest in the FDI target muscle but not significant in ECR and BB. Results expressed as means ± SEM. *P < 0.05; **P < 0.01 (post hoc t-tests, significant difference from test MEP amplitude).
LICF
There were significant effects of IDI and muscle on LICF (LMM: effect of IDI: F6,47.9 = 7.9, P < 0.01; effect of muscle: F3,39.3 = 9.4, P < 0.01). LICF at the OPT IDI was greatest for the FDI target muscle (196% ± 55% of TS baseline, P < 0.05) and decreased with increasing distance from the target muscle: APB: 176% ± 36% (P < 0.05), ECR: 114% ± 21% (ns), and BB: 101% ± 3% (ns) of TS baseline (Fig. 7b). The MEP amplitude elicited by TS alone was 1.15 ± 0.15 mV in FDI, and in APB, ECR, and BB, it was 1.31 ± 0.3, 0.41 ± 0.01, and 0.15 ± 0.03 mV, respectively. OPT IDI (maximum LICF) varied between 200 and 250 ms.
Baseline MEP Measurements
Prior to intervention, baseline MEP amplitudes were 0.81 ± 0.08 mV in FDI, 0.78 ± 0.24 mV in APB, 0.36 ± 0.09 mV in ECR, and 0.14 ± 0.04 in BB.
Changes in MEP Amplitude during Intervention
During intervention, the ratio of MEP amplitude elicited by the fourth to the first ITMS doublet differed between muscles (LMM: effect of muscle: F3,14.6 = 5.4; P < 0.05). Post hoc analyses indicated that the ratio was not significantly different between FDI (2.15 ± 0.28) and APB (1.93 ± 0.32) but was significantly greater in FDI (but not APB) compared with ECR (1.72 ± 0.32) and BB (1.15 ± 0.12), both comparisons P < 0.05. Average MEP amplitude of the first pulse in each train differed between muscles (P < 0.01) but not across trains (LMM: effect of muscle: F3,46.7 = 46.3, P < 0.01). This amplitude was 1.83 ± 0.10 mV for FDI, and in APB, ECR, and BB, it was 1.63 ± 0.2, 0.61 ± 0.01, and 0.17 ± 0.02 mV, respectively.
Changes in MEP Amplitude Postintervention
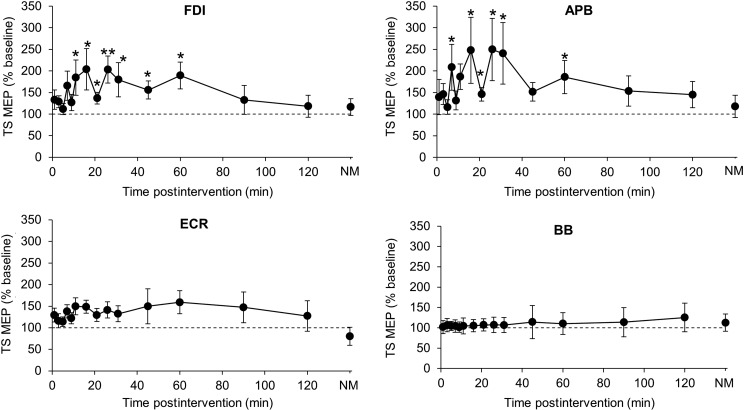
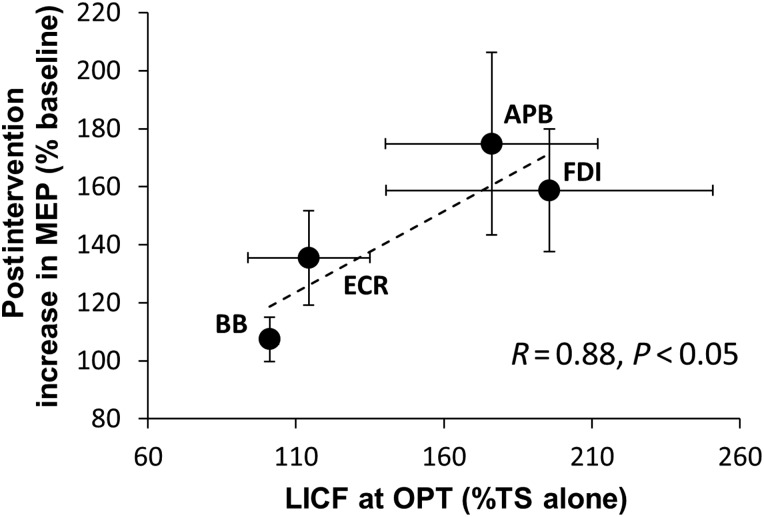
There was a significant effect of time, muscle, and their interaction (LMM: effect of time: F15,18.0 = 419.6, P < 0.001; effect of muscle: F3,76.3 = 24.4, P < 0.001); interaction: F43, 12.3 = 3.3, P = 0.05) (Fig. 8). Pairwise comparisons revealed that the response in both FDI and APB differed compared with the other muscles (all comparisons P < 0.05) but did not differ significantly between FDI and APB. Post hoc t-tests revealed significant postinterventional increases in TS MEP amplitude compared with baseline for up to 60 min in FDI and APB (Fig. 8). The increase in MEP amplitude averaged over 120 min postintervention correlated with LICF across muscles (r = 0.88, P < 0.05; Fig. 9). Taken together, these results indicate topographic specificity of the intervention to the M1 hand representation stimulated by TMS.
Figure 8.
Topographic specificity of MEP amplitude changes postintervention. Comparison of the percentage change (compared with baseline) in single-pulse MEP amplitude 1–120 min and the next morning (NM) after intervention delivered at OPT to FDI hotspot, shown for FDI, APB, ECR, and BB muscles. LMM indicated a significant effect of time in FDI and APB, but not ECR or BB. Data are means ± SEM. *P < 0.05; **P < 0.01 (post hoc t-tests, significant difference of TS MEP amplitude from baseline).
Figure 9.
Correlation between LICF and mean postintervention increase in MEP amplitude. Correlation of LICF (x-axis) and the mean increase in MEP amplitude 1–120 min postintervention over MEP amplitude at baseline (y-axis) for each muscle (r = 0.88, P < 0.05). Horizontal and vertical error bars indicate SEM for LICF and increase in MEP amplitude postintervention, respectively.
Discussion
When paired with LCD, just 48 TMS pulses delivered over less than a minute were sufficient to induce a long-lasting (up to 60 min) period of increased corticomotor excitability. There was no such effect at intervals not corresponding to LCD or when the ITMS doublets were replaced with SP TMS (adjusted in intensity to match MEP amplitude). The intervention was topographically specific, and its effectiveness was proportional to the level of LICF for each muscle. The results provide novel evidence that targeting disinhibition can be highly effective at inducing LTP-like effects in human motor cortex.
The period that corresponds to LCD is thought to arise from a disparity between the time-course of pre- and post-synaptic GABABergic inhibition (Otis et al. 1993; Deisz 1999). In the human, LCD can be identified in a modified LICI protocol in which the interstimulus interval is extended beyond the time-course of LICI. In this post-LICI period, the conditioned MEP amplitude is greater than the unconditioned MEP, resulting in the phenomenon of LICF. This late-interval MEP facilitation is comparable with a postsynaptic depolarization that occurs during the late disinhibitory period in cellular studies (Larson and Lynch 1986; Pacelli et al. 1989). We have shown that during (but not after) LICF, inhibition (as determined by SICI) is decreased as would be expected from disinhibition (Cash et al. 2011). We therefore identified this period, which follows on from LICI and lasts ∼50 ms, with LCD, and it is this period that we have targeted for the delivery of ITMS.
The intervention we chose to incorporate with LCD was repetitive ITMS. SICF circuitry underpins ITMS (Sewerin et al. 2011; Cash et al. 2013) and is enhanced during LCD (Cash et al. 2011), which provides a rationale for this combination. We also established here that SICF doublets evoke the same pattern of disinhibition (i.e., LICI followed by LICF, Fig. 2b) as was previously observed following a single stimulus (Cash et al. 2010, 2011). We decided to deliver the protocol at high frequency (i.e., trains of 4 doublets at individual LCD interval of 200–250 ms) to shorten the protocol duration to <1 min, and because previous protocols of 5 Hz paired-associative stimulation (Quartarone et al. 2006) and theta-burst stimulation, that is, burst stimulation at 5 Hz (Huang et al. 2005), demonstrated the feasibility to induce long-term changes in corticospinal excitability. We inserted an intertrain interval of 8 s for safety reasons as well as to avoid a potential buildup of inhibitory effects that can arise from continuous stimulation (Huang et al. 2005, 2011; Rothkegel et al. 2010).
The DIS protocol contained a number of optimizations. While ITMS originally used a fixed IPI of 1.5 ms [corresponding to the first SICF peak (Thickbroom et al. 2006)], it has recently been shown that this interval is critical and that increasing the IPI to 2 ms (corresponding to the next SICF trough) results in LTD-like behavior (Cash et al. 2013). A better effect size has also been reported when the IPI is optimized for each individual according to peak SICF (Sewerin et al. 2011). It therefore appears that ITMS effects closely follow SICF phase dynamics (Cash et al. 2013). Given this sensitivity to IPI, we tailored IPI to each individual SICF peak. Likewise, as LCD covers a relatively short-time window that could be missed according to individual variation, we determined the optimal IDI according to the LCD peak in each individual. Intensity of stimulation was set based on a compromise between the intensity that gave higher LCD (i.e., higher intensity) and safety considerations. Finally, as the technique depended on both disinhibition and I-wave facilitation, the presence of LICF and SICF were inclusion criteria for the study, resulting in a small number of participants (5/22 = 22.7%) not proceeding past screening. For comparison, 15%–35% of healthy subjects do not show SICI or intracortical facilitation (Wassermann 2002; Orth et al. 2003). All of these considerations were included in the design to maximize the efficacy of the DIS protocol and minimize variability by accounting for individual physiological parameters.
Of note, SP TMS adjusted in intensity to match MEP amplitude elicited by TMS doublets did not result in LTP-like MEP increase when given at the optimal individual interstimulus interval to target LCD. This is an important control experiment that suggests that SICF, reflecting activation of glutamatergic excitatory cortical interneurons that are also responsible for the generation of I-waves (Ilic et al. 2002), and LCD, likely reflecting GABABergic disinhibition (Cash et al. 2011), cooperate for induction of LTP-like plasticity.
There is increasing interest in the basis for variation in the efficacy of most TMS interventions, and a number of factors are being considered including genetic polymorphisms (for review, Ridding and Ziemann 2010). Physiological parameters may be directly linked to genetic variation, for example, in the mouse, genetic deletion of the GABA transporter (GAT1), which is responsible for reuptake of GABA from the synaptic cleft, prolongs the decay kinetics of GABAergic transmission, decreasing theta oscillation frequency and modulating the frequency range in which theta-burst stimulation can induce LTP (Gong et al. 2009). Furthermore, genetic deletion of GABAB autoreceptors in Gabbr1−/− mice leads to a failure to exhibit LTP (Vigot et al. 2006). It is conceivable that interindividual temporal variation in the latency or presence of LCD or variation in the efficacy of TMS plasticity protocols is linked to GAT activity or GABA receptor polymorphisms. Determining physiological variations between individuals and modifying individual parameters accordingly, where possible, or considering such factors in the choice of a suitable intervention for a given individual may enhance efficacy of plasticity induction. Other factors such as SICF IPI (Sewerin et al. 2011; Cash et al. 2013) and stimulus intensity also may be important. We observed that stimulus intensity influenced the progressive increase in MEP amplitude during a train (Fig. 3), in line with intensity driven during- and post-train effects in animal studies (Timofeev et al. 2002). It has also been observed that reducing stimulus intensity can result in a switch from LTP- to LTD-like effects with human regular rTMS and theta-burst stimulation protocols (Berger et al. 2011; Doeltgen and Ridding 2011). It remains to be seen whether stimulation during LCD can enhance LTD-like plasticity; cellular studies have demonstrated that pharmacological GABAergic disinhibition resulted in abolition of LTD (Watanabe et al. 2007) or even a switch toward induction of LTP (Caballero et al. 2014). The protocol could be modified in a number of ways to induce LTD-like decreases in excitability such as by stimulation during the LICI phase, continuous stimulation akin to continuous theta-burst stimulation, lower intensity, or targeting the SICF trough of 2 ms. Examining this broad range of possibilities will be of interest in future studies.
The extent to which the approach might generalize to other excitatory TMS interventions would also be of interest. In principle, theta-burst stimulation (Huang et al. 2005), paired-associative stimulation (Stefan et al. 2000), and quadro-pulse stimulation (Hamada et al. 2007) could be adapted to conform to LCD dynamics. Indeed, it has been previously suggested that the effectiveness of intermittent theta-burst stimulation may be due to a serendipitous similarity between theta rhythm periodicity (200 ms) and LCD (Thickbroom 2007), and such a connection has been proposed in cellular studies (Larson et al. 1986; Davies et al. 1991; Mott and Lewis 1991). In the present study, a ±50-ms adjustment in periodicity resulted in a significant loss of effect size, suggesting that tuning theta-burst stimulation frequency to match LCD periodicity may be an option and could potentially help to reduce variability between individuals, which has recently become a topic of considerable interest (Hamada et al. 2013). We also observed a relatively low level of variability in plasticity response (Fig. 6), which may be attributable to adjusting for individual variation in physiological parameters, controlling for factors such as time of day, which can influence cortisol levels (Sale et al. 2007, 2008) and GABAergic inhibition in motor cortex (Lang et al. 2011), avoiding testing at certain timing during the menstrual cycle (Inghilleri et al. 2004), which can affect plasticity, and asking participants to avoid contraction during- and post-intervention, which has been associated with depotentiation (Gentner et al. 2008; Huang et al. 2008).
Increases in excitability postintervention were specific to the M1 hand representation stimulated by TMS. The postintervention MEP amplitude increase in each muscle was proportional to the degree of LICF (Fig. 9). Similar topographic specificity has been shown in other studies using noninvasive brain stimulation for LTP-like plasticity induction (Stefan et al. 2000; Ridding and Taylor 2001; Rosenkranz and Rothwell 2006; Cheeran et al. 2008; Pötter-Nerger et al. 2009; Dileone et al. 2010; Ni et al. 2014), and for interneuronal circuitry (Shimizu et al. 1999; Chen and Garg 2000; Saisanen et al. 2011).
The aftereffect we observed by targeting disinhibition was long-lasting with respect to the duration of the intervention. It is known that disinhibition can have a powerful effect on plasticity induction, even to the extent of being permissive (Davies et al. 1991; Mott and Lewis 1991; Hess and Donoghue 1994), or evoking LTP (∼30 min) from a single primed burst (Diamond et al. 1988) or single non-primed burst in the presence of a GABAA receptor antagonist (Wigstrom and Gustafsson 1983a, 1985). Despite this strong aftereffect at OPT, during the intervention, there was no statistical increase in MEP amplitude for successive trains, although MEP amplitude within each train increased. It is possible that LTP-like effects take some time to manifest and that they may not become apparent until after the intervention, given that the duration of the intervention was <1 min. One advantage to the absence of a within-intervention effect is that it may reduce the likelihood of a spreading increase in corticomotor excitability that may develop rapidly during high-frequency regular rTMS and would constitute a safety concern (Pascual-Leone et al. 1993). Disinhibition at the cellular level has been shown to permit the depolarization necessary to adequately unmask the NMDAR ionophore from Mg2+ blockade, thus facilitating NMDAR-mediated Ca2+ current during subsequent stimulation and resulting in greater plasticity induction (Larson and Lynch 1986; Mott and Lewis 1992; Davies and Collingridge 1996), with NMDAR currents accounting for ∼35% of the increase in response amplitude during a disinhibited burst train (Larson and Lynch 1988). Indeed, one of the proposed physiological effects for presynaptic disinhibition is to facilitate the synaptic activation of NMDA receptors during trains (Collingridge et al. 1988; Mangan and Lothman 1996). Similar mechanisms may contribute to the increase in MEP amplitude during the trains and induction of LTP-like plasticity in the present OPT protocol. As local Ca2+ transients decay over several hundred milliseconds, NMDAR activity may be progressively more easily evoked during a disinhibited train but may decline between trains if the intertrain interval is several seconds long (Maeda et al. 1999; Koester and Sakmann 2000; Wittenberg and Wang 2006). Such properties of Ca2+ transients could potentially account for the progressive increase in MEP amplitude during the train but not from one train to the next. The steady increase in MEP amplitude within a train could also suggest that successive ITMS pairs continue to evoke and progressively build up LCD. We have previously argued that disinhibition may have a role in entraining cortical rhythms (Cash et al. 2010, 2011), which could occur if successive phases of disinhibition were sustained within a target network, or if after-hyperpolarization rebound currents interact with disinhibition.
Pharmacological disinhibition is not available for use in humans because GABA receptor antagonists have proconvulsant properties. Motor cortical disinhibition evoked by transient deafferentation using limb ischemic nerve block (Brasil-Neto et al. 1993; Levy et al. 2002) or selective upper brachial plexus anesthesia enhances LTP-like plasticity and motor learning in healthy subjects (Ziemann, Corwell et al. 1998, 2001) and in chronic stroke patients (Muellbacher et al. 2002), but these techniques are painful and too complex so that they have not gained wide application. The present method of electrophysiologically evoking disinhibition in M1 offers an elegant alternative requiring only 1 IPI curve to identify LCD and could be considered for incorporation into other plasticity protocols.
Finally, as a limitation of the current study, we have not proven the cortical origin of the LTP-like MEP increase. However, we have shown previously that ITMS results in an increase in SICF (Cash et al. 2009), which reflects excitability of neural elements in motor cortex responsible for I-wave generation (Ilic et al. 2002). Plasticity induction was also dependent on delivery during a time window (LCD) that we have previously shown involves cortical interneuronal SICI and SICF circuitry. The facilitation of SICF during LCD also suggests that the cortical circuitry targeted by ITMS is facilitated during this period. Therefore, we consider it very likely that the LTP-like MEP increase in the present modified ITMS experiment also originated in motor cortical I-wave circuitry. Another limitation is that we have not proven that a doublet PS is necessary for induction of LTP-like plasticity. This would require substitution of the doublet PS by an SP PS paired with a doublet TS at the OPT interval. However, testing this in the high-frequency trains of the present experiment is not feasible because subsequent alternating SP PS and doublet TS in the train interact with each other. Therefore, repeated application of this SP PS + doublet TS pair at low frequency (e.g., 0.2 Hz for 15 min, as in standard ITMS protocols) would be mandatory. As we aimed here at creating a brief protocol (<1 min) for highly effective LTP-like plasticity induction, such an additional experiment would be beyond the intent and scope of the present study. Finally, the OPT − SP condition was matched to the OPT condition with respect to initial MEP amplitude in the trains. While it could be argued that a perfect match would be one in which the OPT − SP condition also resulted in a within-train increase in MEP amplitude, this would have been impractical to implement given the variability between trains and individuals. The current experiments suggest that a within-train MEP increase during intervention may be important for induction of LTP-like plasticity. Lastly, although we refer to the changes as “LTP-like” due to several shared cardinal characteristics including duration of ≥30 min, topographic specificity, and associativity, it is not possible to confirm that LTP has occurred using noninvasive methods at the systems level in human subjects.
In conclusion, the results indicate that by applying patterned stimulation that evokes both disinhibition and synaptic plasticity (DIS), a long-lasting aftereffect can be induced by a remarkably short period of stimulation. The ability to evoke disinhibition in human motor cortex offers a powerful new approach for modulating corticomotor excitability and plasticity induction with noninvasive brain stimulation. By harnessing this physiological effect, it may be possible to further improve the delivery and efficacy of neuromodulatory protocols in clinical applications.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by Deutscher Akademischer Austausch Dienst (DAAD), the University of Western Australia (Grant for research student training), and the Neurotrauma Research Program of Western Australia and Canadian Institutes of Health Research.
Supplementary Material
Notes
We are grateful to Dr. J. Downer for lending us the equipment necessary to perform the second main experiment and thank all participants. Conflict of Interest: None declared.
References
- Berger U, Korngreen A, Bar-Gad I, Friedman A, Wolfus S, Yeshurun Y, Lavidor M. 2011. Magnetic stimulation intensity modulates motor inhibition. Neurosci Lett. 504:93–97. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, Maccabee P, Cracco J, Hallett M, Cohen LG. 1993. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 116(Pt 3):511–525. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. 2000. Mechanisms of use-dependent plasticity in the human motor cortex. Proceedings of the National Academy of Sciences of the United States of America. 97:3661–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Thomases DR, Flores-Barrera E, Cass DK, Tseng KY. 2014. Emergence of GABAergic-dependent regulation of input-specific plasticity in the adult rat prefrontal cortex during adolescence. Psychopharmacology. 231:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash RF, Benwell NM, Murray K, Mastaglia FL, Thickbroom GW. 2009. Neuromodulation by paired-pulse TMS at an I-wave interval facilitates multiple I-waves. Exp Brain Res. 193:1–7. [DOI] [PubMed] [Google Scholar]
- Cash RF, Mastaglia FL, Thickbroom GW. 2013. Evidence for high-fidelity timing-dependent synaptic plasticity of human motor cortex. J Neurophysiol. 109:106–112. [DOI] [PubMed] [Google Scholar]
- Cash RF, Ziemann U, Mastaglia F, Thickbroom GW. 2008. Evidence for long-interval cortical facilitation (LICF) in human motor cortex. Brain Stimul. 1:296–296. [Google Scholar]
- Cash RF, Ziemann U, Murray K, Thickbroom GW. 2010. Late cortical disinhibition in human motor cortex: a triple-pulse transcranial magnetic stimulation study. J Neurophysiol. 103:511–518. [DOI] [PubMed] [Google Scholar]
- Cash RF, Ziemann U, Thickbroom GW. 2011. Inhibitory and disinhibitory effects on I-wave facilitation in motor cortex. J Neurophysiol. 105:100–106. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Donoghue JP, Connors BW. 1995. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 15:5324–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC. 2008. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 586:5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Garg R. 2000. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. J Neurophysiol. 83:1426–1434. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Classen J, Wassermann EM, Hallett M, Cohen LG. 1997. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol. 105:415–421. [DOI] [PubMed] [Google Scholar]
- Chu J, Gunraj C, Chen R. 2008. Possible differences between the time courses of presynaptic and postsynaptic GABAB mediated inhibition in the human motor cortex. Exp Brain Res. 184:571–577. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Herron CE, Lester RA. 1988. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 399:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Collingridge GL. 1996. Regulation of EPSPs by the synaptic activation of GABAB autoreceptors in rat hippocampus. J Physiol. 496(Pt 2):451–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Davies SN, Collingridge GL. 1990. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 424:513–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. 1991. GABA autoreceptors regulate the induction of LTP. Nature. 349:609–611. [DOI] [PubMed] [Google Scholar]
- Deisz RA. 1999. The GABA(B) receptor antagonist CGP 55845A reduces presynaptic GABA(B) actions in neocortical neurons of the rat in vitro. Neuroscience. 93:1241–1249. [DOI] [PubMed] [Google Scholar]
- Deisz RA, Billard JM, Zieglgansberger W. 1997. Presynaptic and postsynaptic GABAB receptors of neocortical neurons of the rat in vitro: differences in pharmacology and ionic mechanisms. Synapse. 25:62–72. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Dunwiddie TV, Rose GM. 1988. Characteristics of hippocampal primed burst potentiation in vitro and in the awake rat. J Neurosci. 8:4079–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. 2000. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 111:794–799. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA, Ziemann U. 2006. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 575:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Ziemann U, Lemon RN. 2008. State of the art: Physiology of transcranial motor cortex stimulation. Brain Stim. 1:345–362. [DOI] [PubMed] [Google Scholar]
- Dileone M, Profice P, Pilato F, Alfieri P, Cesarini L, Mercuri E, Leoni C, Tartaglia M, Di Iorio R, Zampino G, et al. 2010. Enhanced human brain associative plasticity in Costello syndrome. J Physiol. 588:3445–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeltgen SH, Ridding MC. 2011. Low-intensity, short-interval theta burst stimulation modulates excitatory but not inhibitory motor networks. Clin Neurophysiol. 122:1411–1416. [DOI] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. 2008. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 18:2046–2053. [DOI] [PubMed] [Google Scholar]
- Gong N, Li Y, Cai GQ, Niu RF, Fang Q, Wu K, Chen Z, Lin LN, Xu L, Fei J, et al. 2009. GABA transporter-1 activity modulates hippocampal theta oscillation and theta burst stimulation-induced long-term potentiation. J Neurosci. 29:15836–15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein YJ, Pavlides C, Winson J. 1988. Long-term potentiation in the dentate gyrus is preferentially induced at theta rhythm periodicity. Brain Res. 438:331–334. [DOI] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, Thickbroom GW, et al. 2012. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 123:858–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Hanajima R, Terao Y, Arai N, Furubayashi T, Inomata-Terada S, Yugeta A, Matsumoto H, Shirota Y, Ugawa Y. 2007. Quadro-pulse stimulation is more effective than paired-pulse stimulation for plasticity induction of the human motor cortex. Clin Neurophysiol. 118:2672–2682. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. 2013. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 23:1593–1605. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. 1998. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 509(Pt 2):607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidegger T, Krakow K, Ziemann U. 2010. Effects of antiepileptic drugs on associative LTP-like plasticity in human motor cortex. Eur J Neurosci. 32:1215–1222. [DOI] [PubMed] [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP. 1996. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 75:1765–1778. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. 1994. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol. 71:2543–2547. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. 1996. Long-term potentiation and long-term depression of horizontal connections in rat motor cortex. Acta Neurobiol Exp (Wars). 56:397–405. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. 2005. Theta burst stimulation of the human motor cortex. Neuron. 45:201–206. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Chen RS, Lu CS, Chuang WL. 2011. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin Neurophysiol. 122:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. 2008. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 18:563–570. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. 2002. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 545:153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Conte A, Curra A, Frasca V, Lorenzano C, Berardelli A. 2004. Ovarian hormones and cortical excitability. An rTMS study in humans. Clin Neurophysiol. 115:1063–1068. [DOI] [PubMed] [Google Scholar]
- Iriki A, Pavlides C, Keller A, Asanuma H. 1989. Long-term potentiation in the motor cortex. Science. 245:1385–1387. [DOI] [PubMed] [Google Scholar]
- Kanter ED, Haberly LB. 1993. Associative long-term potentiation in piriform cortex slices requires GABAA blockade. J Neurosci. 13:2477–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Sakmann B. 2000. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J Physiol. 529(Pt 3):625–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. 1993. Corticocortical inhibition in human motor cortex. J Physiol. 471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Rothkegel H, Reiber H, Hasan A, Sueske E, Tergau F, Ehrenreich H, Wuttke W, Paulus W. 2011. Circadian modulation of GABA-mediated cortical inhibition. Cereb Cortex. 21:2299–2306. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. 1986. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 232:985–988. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. 1988. Role of N-methyl-D-aspartate receptors in the induction of synaptic potentiation by burst stimulation patterned after the hippocampal theta-rhythm. Brain Res. 441:111–118. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. 1986. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 368:347–350. [DOI] [PubMed] [Google Scholar]
- Levy LM, Ziemann U, Chen R, Cohen LG. 2002. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann Neurol. 52:755–761. [DOI] [PubMed] [Google Scholar]
- Ludbrook J, Dudley H. 1998. Why permutation tests are superior to t and F tests in biomedical research. Am Statistic. 52:127–132. [Google Scholar]
- Maeda H, Ellis-Davies GC, Ito K, Miyashita Y, Kasai H. 1999. Supralinear Ca2+ signaling by cooperative and mobile Ca2+ buffering in Purkinje neurons. Neuron. 24:989–1002. [DOI] [PubMed] [Google Scholar]
- Mangan PS, Lothman EW. 1996. Profound disturbances of pre- and postsynaptic GABAB-receptor-mediated processes in region CA1 in a chronic model of temporal lobe epilepsy. J Neurophysiol. 76:1282–1296. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. 2006. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 173:86–93. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. 2007. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res. 180:181–186. [DOI] [PubMed] [Google Scholar]
- Mott DD, Lewis DV. 1991. Facilitation of the induction of long-term potentiation by GABAB receptors. Science. 252:1718–1720. [DOI] [PubMed] [Google Scholar]
- Mott DD, Lewis DV. 1992. GABAB receptors mediate disinhibition and facilitate long-term potentiation in the dentate gyrus. Epilepsy Res Suppl. 7:119–134. [PubMed] [Google Scholar]
- Muellbacher W, Richards C, Ziemann U, Wittenberg G, Weltz D, Boroojerdi B, Cohen L, Hallett M. 2002. Improving hand function in chronic stroke. Arch Neurol. 59:1278–1282. [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus JFM, Liu Y, Ziemann U. 2008. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 586:495–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan T, Lambert JD. 1991. Depression of the fast IPSP underlies paired-pulse facilitation in area CA1 of the rat hippocampus. J Neurophysiol. 66:1704–1715. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Kailey P, Cash RFH, Chen R. 2014. Heterosynaptic modulation of motor cortical plasticity in human. J Neurosci. 34:7314–7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Wagle-Shukla A, Udupa K, Mazzella F, Lozano AM, Chen R. 2011. Direct demonstration of inhibitory interactions between long interval intracortical inhibition and short interval intracortical inhibition. J Physiol. 589:2955–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9:97–113. [DOI] [PubMed] [Google Scholar]
- Orth M, Snijders AH, Rothwell JC. 2003. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 114:2362–2369. [DOI] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. 1993. Characterization of synaptically elicited GABAB responses using patch-clamp recordings in rat hippocampal slices. J Physiol. 463:391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacelli GJ, Su W, Kelso SR. 1989. Activity-induced depression of synaptic inhibition during LTP-inducing patterned stimulation. Brain Res. 486:26–32. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Houser CM, Reese K, Shotland LI, Grafman J, Sato S, Valls-Sole J, Brasil-Neto JP, Wassermann EM, Cohen LG, et al. 1993. Safety of rapid-rate transcranial magnetic stimulation in normal volunteers. Electroencephalogr Clin Neurophysiol. 89:120–130. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. 1994. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 117(Pt 4):847–858. [DOI] [PubMed] [Google Scholar]
- Pötter-Nerger M, Fischer S, Mastroeni C, Groppa S, Deuschl G, Volkmann J, Quartarone A, Munchau A, Siebner HR. 2009. Inducing homeostatic-like plasticity in human motor cortex through converging corticocortical inputs. J Neurophysiol. 102:3180–3190. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant'Angelo A, Girlanda P, Siebner HR. 2006. Rapid-rate paired associative stimulation of the median nerve and motor cortex can produce long-lasting changes in motor cortical excitability in humans. J Physiol. 575:657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL. 2001. Mechanisms of motor-evoked potential facilitation following prolonged dual peripheral and central stimulation in humans. J Physiol. 537:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Ziemann U. 2010. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 588:2291–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GM, Dunwiddie TV. 1986. Induction of hippocampal long-term potentiation using physiologically patterned stimulation. Neurosci Lett. 69:244–248. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. 2006. Differences between the effects of three plasticity inducing protocols on the organization of the human motor cortex. Eur J Neurosci. 23:822–829. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 120:2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkegel H, Sommer M, Paulus W. 2010. Breaks during 5 Hz rTMS are essential for facilitatory after effects. Clin Neurophysiol. 121:426–430. [DOI] [PubMed] [Google Scholar]
- Saisanen L, Julkunen P, Niskanen E, Hukkanen T, Mervaala E, Karhu J, Kononen M. 2011. Short- and intermediate-interval cortical inhibition and facilitation assessed by navigated transcranial magnetic stimulation. J Neurosci Methods. 195:241–248. [DOI] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. 2008. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci. 28:8285–8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. 2007. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res. 181:615–626. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. 2001. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 530:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewerin S, Taubert M, Vollmann H, Conde V, Villringer A, Ragert P. 2011. Enhancing the effect of repetitive I-wave paired-pulse TMS (iTMS) by adjusting for the individual I-wave periodicity. BMC Neurosci. 12:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Filippi MM, Palmieri MG, Oliveri M, Vernieri F, Pasqualetti P, Rossini PM. 1999. Modulation of intracortical excitability for different muscles in the upper extremity: paired magnetic stimulation study with focal versus non-focal coils. Clin Neurophysiol. 110:575–581. [DOI] [PubMed] [Google Scholar]
- Staubli U, Lynch G. 1987. Stable hippocampal long-term potentiation elicited by “theta” pattern stimulation. Brain Res. 435:227–234. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. 2000. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 123(Pt 3):572–584. [DOI] [PubMed] [Google Scholar]
- Teo JT, Terranova C, Swayne O, Greenwood RJ, Rothwell JC. 2009. Differing effects of intracortical circuits on plasticity. Exp Brain Res. 193:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW. 2007. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 180:583–593. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Edwards DJ, Mastaglia FL. 2006. Repetitive paired-pulse TMS at I-wave periodicity markedly increases corticospinal excitability: a new technique for modulating synaptic plasticity. Clin Neurophysiol. 117:61–66. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Bazhenov M, Houweling AR, Sejnowski TJ, Steriade M. 2002. Short- and medium-term plasticity associated with augmenting responses in cortical slabs and spindles in intact cortex of cats in vivo. J Physiol. 542:583–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. 1992. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 85:355–364. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, et al. 2006. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 50:589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. 1998. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 108:1–16. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. 2002. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 113:1165–1171. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kamatani D, Hishida R, Kudoh M, Shibuki K. 2007. Long-term depression induced by local tetanic stimulation in the rat auditory cortex. Brain Res. 1166:20–28. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. 1999. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 517(Pt 2):591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. 1983a. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 301:603–604. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. 1985. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta Physiol Scand. 125:159–172. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. 1983b. Large long-lasting potentiation in the dentate gyrus in vitro during blockade of inhibition. Brain Res. 275:153–158. [DOI] [PubMed] [Google Scholar]
- Wittenberg GM, Wang SS. 2006. Malleability of spike-timing-dependent plasticity at the CA3-CA1 synapse. J Neurosci. 26:6610–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. 1998. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 18:1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, Cohen LG. 1998. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci. 18:7000–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. 1996. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 109:127–135. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. 2001. Modulation of practice-dependent plasticity in human motor cortex. Brain. 124:1171–1181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.