Abstract
Leishmaniasis is a major tropical disease for which current chemotherapies, pentavalent antimonials, are inadequate and cause severe side effects. It has been reported that trifluralin, a microtubule-disrupting herbicide, is inhibitory to Leishmania amazonensis. In this study, the in vitro effect of trifluralin on different species of trypanosomatid protozoans was determined. In addition to L. amazonensis, trifluralin is effective against Leishmania major and Leishmania tropica, which cause cutaneous infections, Leishmania donovani, which causes visceral disease, Leishmania panamensis, which may cause mucocutaneous infection, and Trypanosoma brucei, an important human and veterinary pathogen. Moreover, most encouragingly, trifluralin is effective in vivo as a topical ointment against L. major and Leishmania mexicana murine cutaneous leishmaniasis. Thus, trifluralin is a promising lead drug for several related, prevalent tropical diseases: leishmaniasis, trypanosomiasis of animals, and, possibly, African trypanosomiasis in humans.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila J. L., Avila A. Correlation of sinefungin susceptibility and drug-affinity for protein carboxymethyltransferase activity in American Leishmania species. Mol Biochem Parasitol. 1987 Nov;26(1-2):69–75. doi: 10.1016/0166-6851(87)90131-9. [DOI] [PubMed] [Google Scholar]
- Bhaumik M., Das S., Adhya S. Evidence for translational control of beta-tubulin synthesis during differentiation of Leishmania donovani. Parasitology. 1991 Oct;103(Pt 2):197–205. doi: 10.1017/s0031182000059485. [DOI] [PubMed] [Google Scholar]
- Bienen E. J., Hill G. C., Shin K. O. Elaboration of mitochondrial function during Trypanosoma brucei differentiation. Mol Biochem Parasitol. 1983 Jan;7(1):75–86. doi: 10.1016/0166-6851(83)90118-4. [DOI] [PubMed] [Google Scholar]
- Birkett C. R., Parma A. E., Gerke-Bonet R., Woodward R., Gull K. Isolation of cDNA clones encoding proteins of complex structures: analysis of the Trypanosoma brucei cytoskeleton. Gene. 1992 Jan 2;110(1):65–70. doi: 10.1016/0378-1119(92)90445-u. [DOI] [PubMed] [Google Scholar]
- Bordier C., Garavito R. M., Armbruster B. Biochemical and structural analyses of microtubules in the pellicular membrane of Leishmania tropica. J Protozool. 1982 Nov;29(4):560–565. doi: 10.1111/j.1550-7408.1982.tb01335.x. [DOI] [PubMed] [Google Scholar]
- Chan M. M., Fong D. Inhibition of leishmanias but not host macrophages by the antitubulin herbicide trifluralin. Science. 1990 Aug 24;249(4971):924–926. doi: 10.1126/science.2392684. [DOI] [PubMed] [Google Scholar]
- Chan M. M., Triemer R. E., Fong D. Effect of the anti-microtubule drug oryzalin on growth and differentiation of the parasitic protozoan Leishmania mexicana. Differentiation. 1991 Feb;46(1):15–21. doi: 10.1111/j.1432-0436.1991.tb00861.x. [DOI] [PubMed] [Google Scholar]
- El-On J., Jacobs G. P., Witztum E., Greenblatt C. L. Development of topical treatment for cutaneous leishmaniasis caused by Leishmania major in experimental animals. Antimicrob Agents Chemother. 1984 Nov;26(5):745–751. doi: 10.1128/aac.26.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-On J., Livshin R., Even-Paz Z., Hamburger D., Weinrauch L. Topical treatment of cutaneous leishmaniasis. J Invest Dermatol. 1986 Aug;87(2):284–288. doi: 10.1111/1523-1747.ep12696697. [DOI] [PubMed] [Google Scholar]
- Fong D., Chang K. P. Tubulin biosynthesis in the developmental cycle of a parasitic protozoan, Leishmania mexicana: changes during differentiation of motile and nonmotile stages. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7624–7628. doi: 10.1073/pnas.78.12.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong D., Lee B. Beta tubulin gene of the parasitic protozoan Leishmania mexicana. Mol Biochem Parasitol. 1988 Oct;31(1):97–106. doi: 10.1016/0166-6851(88)90149-1. [DOI] [PubMed] [Google Scholar]
- Fong D., Wallach M., Keithly J., Melera P. W., Chang K. P. Differential expression of mRNAs for alpha- and beta-tubulin during differentiation of the parasitic protozoan Leishmania mexicana. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5782–5786. doi: 10.1073/pnas.81.18.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis P. C., Emmerson J. L., Adams E. R., Owen N. V. Oncogenicity study of trifluralin in B6C3F1 mice. Food Chem Toxicol. 1991 Aug;29(8):549–555. doi: 10.1016/0278-6915(91)90047-b. [DOI] [PubMed] [Google Scholar]
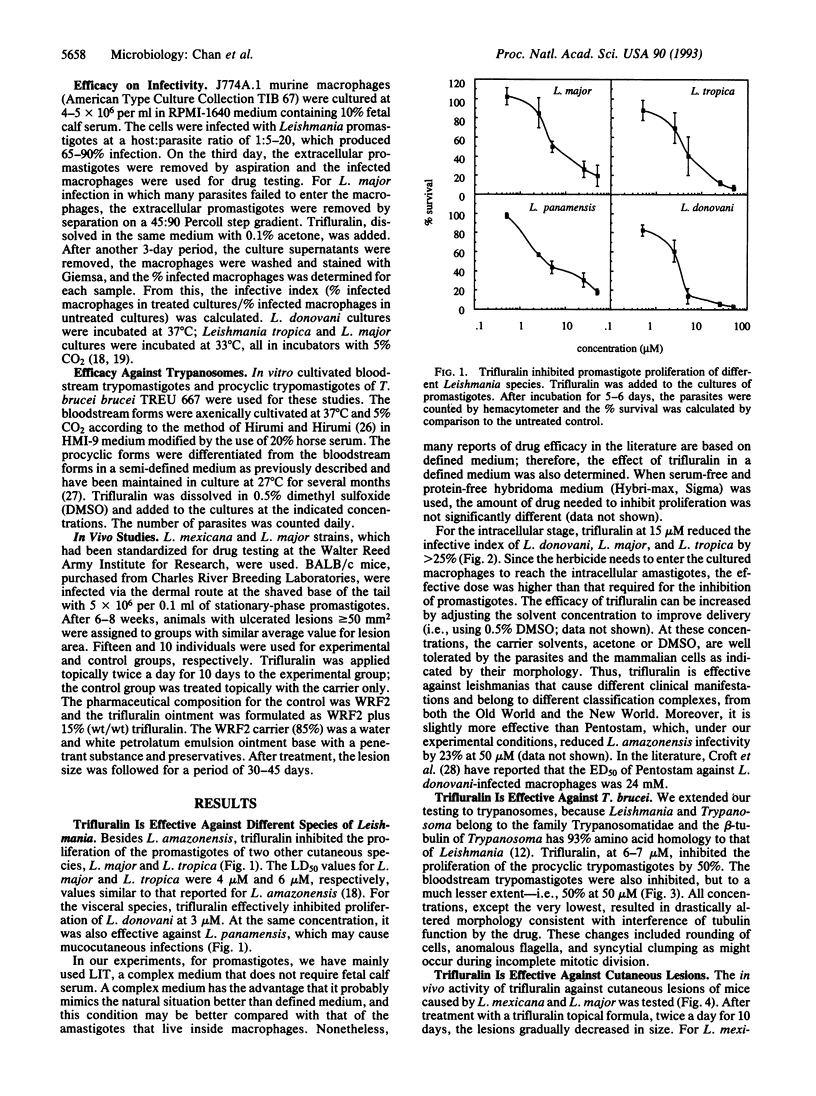
- Garriott M. L., Adams E. R., Probst G. S., Emmerson J. L., Oberly T. J., Kindig D. E., Neal S. B., Bewsey B. J., Rexroat M. A. Genotoxicity studies on the preemergence herbicide trifluralin. Mutat Res. 1991 Jun;260(2):187–193. doi: 10.1016/0165-1218(91)90007-9. [DOI] [PubMed] [Google Scholar]
- Grogl M., Thomason T. N., Franke E. D. Drug resistance in leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am J Trop Med Hyg. 1992 Jul;47(1):117–126. doi: 10.4269/ajtmh.1992.47.117. [DOI] [PubMed] [Google Scholar]
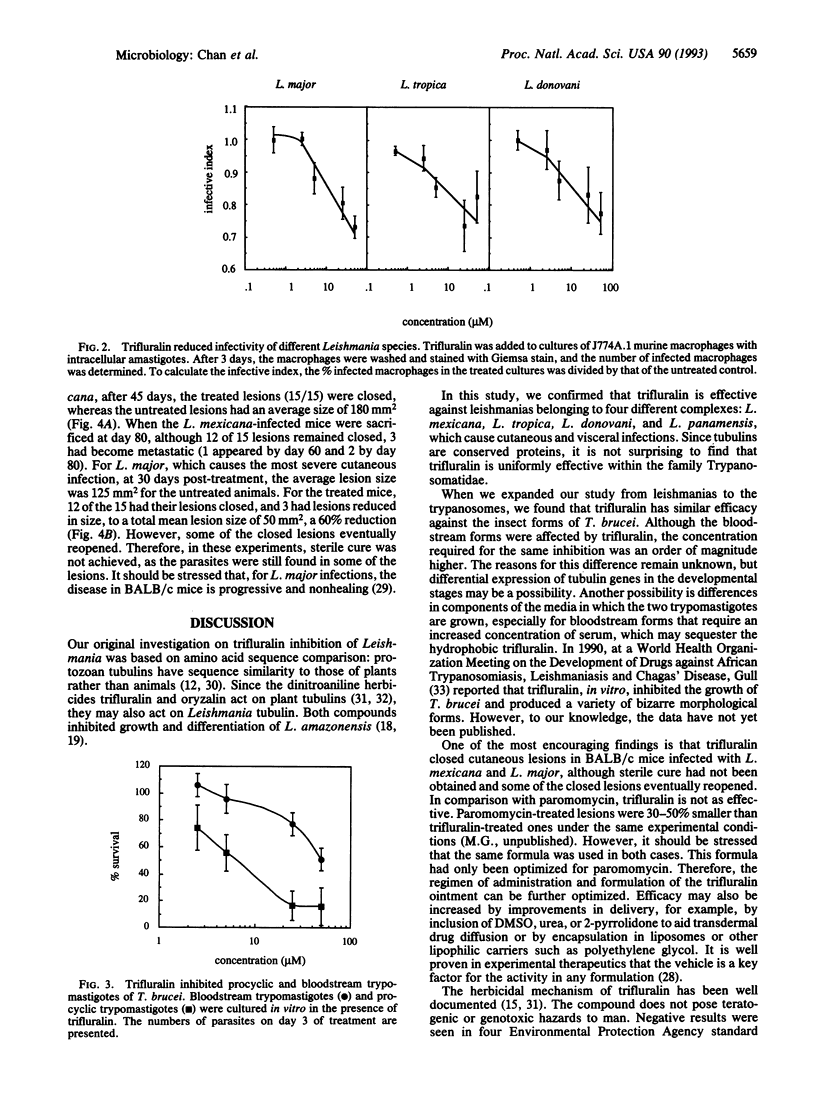
- Hirumi H., Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989 Dec;75(6):985–989. [PubMed] [Google Scholar]
- Kimmel B. E., Samson S., Wu J., Hirschberg R., Yarbrough L. R. Tubulin genes of the African trypanosome Trypanosoma brucei rhodesiense:nucleotide sequence of a 3.7-kb fragment containing genes for alpha and beta tubulins. Gene. 1985;35(3):237–248. doi: 10.1016/0378-1119(85)90002-2. [DOI] [PubMed] [Google Scholar]
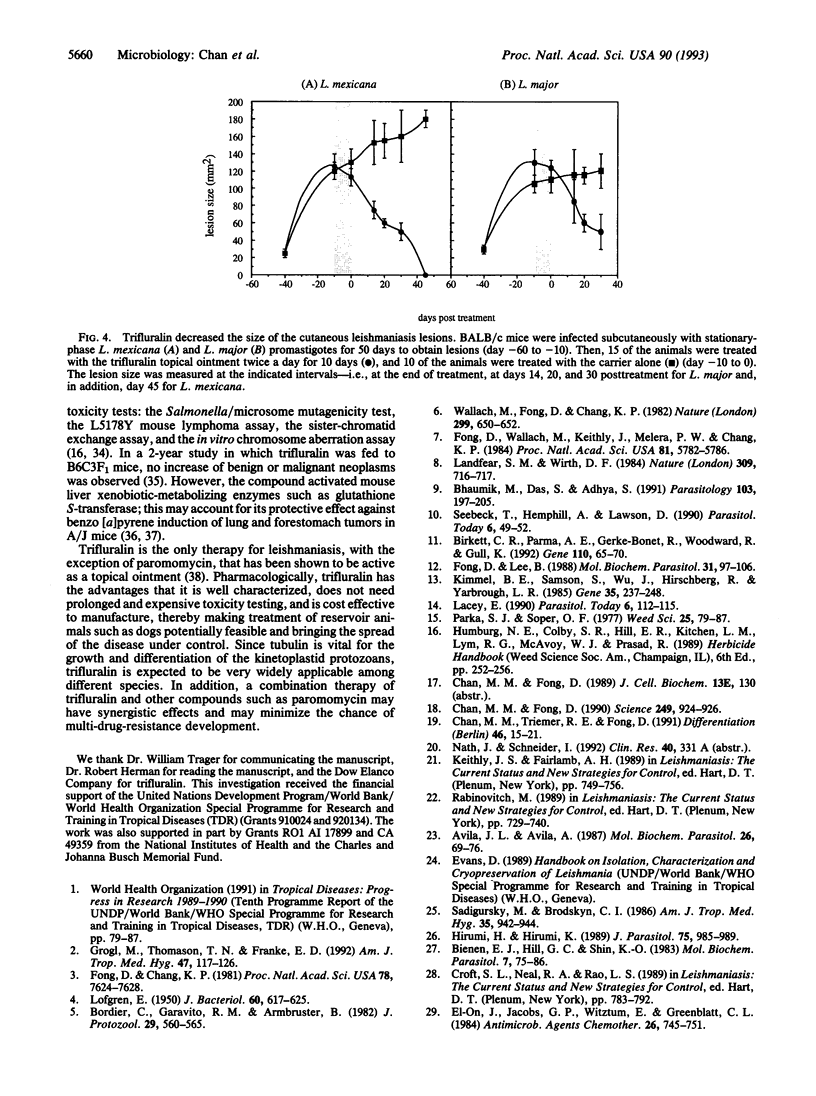
- LOFGREN R. The structure of Leishmania tropica as revealed by phase and electron microscopy. J Bacteriol. 1950 Nov;60(5):617–625. doi: 10.1128/jb.60.5.617-625.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey E. Mode of action of benzimidazoles. Parasitol Today. 1990 Apr;6(4):112–115. doi: 10.1016/0169-4758(90)90227-u. [DOI] [PubMed] [Google Scholar]
- Landfear S. M., Wirth D. F. Control of tubulin gene expression in the parasitic protozoan Leishmania enriettii. Nature. 1984 Jun 21;309(5970):716–717. doi: 10.1038/309716a0. [DOI] [PubMed] [Google Scholar]
- Moody D. E., Narloch B. A., Shull L. R., Hammock B. D. The effect of structurally divergent herbicides on mouse liver xenobiotic-metabolizing enzymes (P-450-dependent mono-oxygenases, epoxide hydrolases and glutathione S-transferases) and carnitine acetyltransferase. Toxicol Lett. 1991 Dec;59(1-3):175–185. doi: 10.1016/0378-4274(91)90070-m. [DOI] [PubMed] [Google Scholar]
- Morejohn L. C., Fosket D. E. The biochemistry of compounds with anti-microtubule activity in plant cells. Pharmacol Ther. 1991;51(2):217–230. doi: 10.1016/0163-7258(91)90078-z. [DOI] [PubMed] [Google Scholar]
- Sadigursky M., Brodskyn C. I. A new liquid medium without blood and serum for culture of hemoflagellates. Am J Trop Med Hyg. 1986 Sep;35(5):942–944. doi: 10.4269/ajtmh.1986.35.942. [DOI] [PubMed] [Google Scholar]
- Seeback T., Hemphill A., Lawson D. The Cytoskeleton of trypanosomes. Parasitol Today. 1990 Feb;6(2):49–52. doi: 10.1016/0169-4758(90)90069-g. [DOI] [PubMed] [Google Scholar]
- Triano E. A., Simpson J. B., Kratky M., Lang W. R., Triolo A. J. Protective effects of trifluralin on benzo(a)pyrene-induced tumors in A/J mice. Cancer Res. 1985 Feb;45(2):601–607. [PubMed] [Google Scholar]
- Wallach M., Fong D., Chang K. P. Post-transcriptional control of tubulin biosynthesis during leishmanial differentiation. Nature. 1982 Oct 14;299(5884):650–652. doi: 10.1038/299650a0. [DOI] [PubMed] [Google Scholar]