Abstract
Background
Rickets and vitamin D deficiency appeared to increase in Alaskan children, starting in the 1990s. We evaluated the epidemiology of rickets and vitamin D deficiency in Alaska Native (AN) children in 2001-2010.
Methods
We analyzed 2001-2010 visits with rickets or vitamin D deficiency diagnosis for AN and American Indian children and the general U.S. population aged <10 years. We conducted a case-control study of AN rickets/vitamin D deficient cases and age- and region-matched controls.
Results
AN children annual rickets-associated hospitalization rate (2.23/100,000 children/year) was higher than general U.S. rate (1.23; 95% CI 1.08-1.39). Rickets incidence increased with latitude. Rickets/vitamin D deficiency cases were more likely to have malnutrition (OR 38.1; 95% CI 4.9-294), had similar breastfeeding prevalence, and were less likely to have received vitamin D supplementation (OR 0.23; 95% CI 0.1-0.87), than controls.
Conclusions
Our findings highlight the importance of latitude, malnutrition and lack of vitamin D supplementation as risk factors for rickets.
Keywords: pediatric, arctic, rickets, vitamin D
INTRODUCTION
Nutritional rickets from vitamin D deficiency was once thought to be nearly eliminated from the developed world, but reports of rickets have increased.1-5 Vitamin D deficiency is caused by lack of exposure to sunlight and insufficient dietary intake, and children at risk for developing vitamin D deficiency and nutritional rickets include exclusively breast-fed infants, those living in northern latitudes and more pigmented racial groups.1, 6 Given these risk factors, Alaska Native (AN) children may be at higher risk to develop vitamin D deficiency and rickets.
Clinicians in Alaska identified rickets in Alaskan children in the 1990s7 and one Alaskan study described high prevalence of vitamin D deficiency in a sample of low-income Alaskan children;8 however, the incidence of rickets in AN children living in the far north with limited sun exposure is unknown. We evaluated the epidemiology of both rickets and vitamin D deficiency in AN children to estimate the incidence of acute rickets in AN children and compare with that of other American Indian (AI)/AN children and the general U.S. child population; we conducted a case-control study to determine risk factors for development of rickets in AN children.
MATERIALS AND METHODS
This study was reviewed and approved by the Alaska Area tribal Institutional Review Board (IRB) and the Centers for Disease Control and Prevention (CDC) IRB, and by tribal organizations: the Alaska Native Tribal Health Consortium, Southcentral Foundation, Arctic Slope Native Association, Norton Sound Corporation, Yukon Kuskokwim Health Corporation, Bristol Bay Area Native Health Corporation, Tanana Chiefs Conference, and Southeast Alaska Regional Health Consortium. Requirement for informed consent was waived under OHRP guidelines [45 CFR 46.116(c)(2)]. Cases from Alaska regions from which approval was obtained were included in the case-control analysis.
For this retrospective analysis of both rickets and vitamin D deficiency morbidity among children <10 years of age, rickets-associated records were defined using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for rickets 268.1 (rickets, late effect) and 268.0 (rickets, active), and vitamin D deficiency was defined as ICD-9-CM 268.9 (unspecified vitamin D deficiency).9 A rickets- or vitamin D deficiency-associated hospitalization and outpatient visit was defined as any of up to 15 diagnoses on individual records listing these ICD-9-CM codes. Statistical significance for analyses was considered as P<0.05, and analyses were completed using SAS/STAT® software Version 9.3.
We analyzed rickets- and vitamin D deficiency-associated hospitalizations and outpatient visits for 2001-2010 for AI/AN children <10 years of age, who received care in Indian Health Service (IHS) or tribal facilities using the IHS direct and contract inpatient and outpatient visit data from the IHS National Patient Information Reporting System (NIPRS).10 These data consist of all hospital discharge and outpatient visit records from IHS-operated, tribally-operated and IHS-contracted hospitals and facilities for eligible AI/AN persons.10, 11,12 The 12 IHS administrative areas were classified into six regions: Alaska, Southwest, Northern Plains, Southern Plains, East, and West.12 The IHS West region did not have any IHS- or tribally-operated hospitals and was therefore excluded from only the hospitalization analysis, including the denominator. The corresponding IHS user population for fiscal years 2001-2010 were used as the annual denominator; the user population includes all registered AI/AN individuals who received any IHS-funded health care service at least once during the preceding 3 years.13 Average annual rickets- and vitamin D deficiency-associated hospitalization visits, outpatient visits and outpatient incidence rates were calculated per 100,000 AI/AN children using the number of occurrences with the corresponding denominator for the time period. For the incidence rate, outpatient visits within years were linked and analyzed by patient.
For the U.S. general population of children <10 years of age, we analyzed inpatient rickets- and vitamin D deficiency-associated visits for 2001-2010 using the Nationwide Inpatient Sample (NIS).14 The NIS is a nationally representative database of hospitalizations conducted by the Healthcare Cost and Utilization Project (HCUP) in collaboration with participating states which includes a 20% sample of participating U.S. community hospitals.14, 15 The NIS data do not include data from IHS or tribal facilities. Estimates of the number of hospitalizations with standard errors (SEs) were calculated using the HCUP weighting methodology for the NIS. The annual denominators were the National Center for Health Statistics annual bridged race population estimates.16 Annual and average annual hospitalization rates were calculated per 100,000 persons of the corresponding population. SUDAAN software (RTI International, Raleigh, North Carolina) was used to account for the HCUP NIS sampling design.17 The rates with 95% confidence intervals (CIs) were calculated as the weighted number of hospitalizations per 100,000 children for the corresponding groups. If the relative standard error (SE) of estimates exceeded 0.30 or if the unweighted number of visits in the strata was <30, the estimates were considered unreliable and not considered.14
Case Control Study
We identified potential AN children with a ICD-9-CM diagnosis code for rickets or vitamin D deficiency during 1999-2013 through: 1) the above retrospective database search for AN children using NIPRS IHS/Tribal data, 2) electronic medical records query at approving tribal facilities for AN children < 10 years. We also reviewed electronic medical records for children with a visit at Alaska Native Medical Center during 2001-2012 with 25-hydroxyvitamin D (25(OH)D) level <15 ng/mL (37 IU/mL).6
Medical records for potential cases were reviewed for demographic and medical data including date of birth, community of residence, gender, maternal age, birthweight, gestational age, vitamin supplements, date of diagnosis, presentation (e.g. seizures, tibial bowing), and underlying medical conditions (including malnutrition, failure to thrive, hepatic disease, etc.) in provider note or ICD-9-coded diagnosis, weight and height for age (Z scores from norm), vitamin D laboratory tests (25(OH)D, calcium, phosphorous, alkaline phosphatase, intact parathyroid hormone) radiographs, and feeding methods (breast, bottle, solids). Latitude was calculated from community of residence closest to diagnosis date. Two pediatric endocrinologists (RL, MB) evaluated all available clinical and lab findings, including response to treatment, and categorized children as 1.) nutritional rickets, 2.) nutritional vitamin D deficiency, 3.) rickets/vitamin D deficiency associated with underlying malabsorption or hepatic disease, or 4.) non-cases. Rickets cases had clinical/radiographic evidence of rickets; nutritional vitamin D deficiency cases were defined as a 25(OH)D level <15 ng/mL without recorded clinical or radiographic signs consistent with rickets. Children with non-nutritional rickets and non-rickets children with 25(OH)D level >15 ng/mL were excluded.
Rickets and vitamin D deficiency cases were matched to controls from the same region with closest birthdate. For each confirmed case and matched control we conducted chart reviews from birth to date of diagnosis for medical conditions, measurements, visit diagnoses and laboratory tests. Rickets and vitamin D deficiency cases were compared by chi-square, Fisher’s exact, t-test or Wilcoxon rank sum test as appropriate. Cases and matched controls were compared using a conditional logistic regression model to account for the matching. Analysis of healthcare visits is restricted to children with information on one or more visits during the first year of life.
RESULTS
Hospitalization, Outpatient Visit, and Incidence Rates
During 2001-2010, 6 AN children <10 years of age were hospitalized with rickets, for an average annual AN rickets-associated hospitalization rate of 2.23 per 100,000, which is higher than the rate for the general U.S. population <10 years of age of 1.23 (95% CI 1.08-1.39) and the rate for AI children from all other IHS regions combined (0.13). The average annual AN vitamin D deficiency-associated average annual hospitalization rate for children < 10 years was 1.86, compared with 0.73 (95% CI 0.60-0.85) for the general US child population <10 years of age, and 0.04 for AI children < 10 years of age from all other IHS regions combined.
The average annual rickets-associated outpatient incidence rate of 11.15 is higher than that for AI children from all other IHS regions, which ranged from 2.08-4.31 (p<0.05; Table 1). In contrast, the average annual vitamin D deficiency-associated outpatient incidence rate of 8.17 was similar to rates for AI children in the Southwest (7.62) and West (9.94), higher than the Northern Plains (4.86; p=0.008) and Southern Plains (2.93; p<0.001), and lower than the East (16.29; p=0.003) (Table 1).
Table 1.
Average annual Rickets-associated and vitamin D deficiency-associated outpatient visit rates/100,000 and incidence rates/100,000/year in AI/AN children population <10 years, by Indian Health Service (IHS) region, 2001-2010.
| IHS | Rickets | Vitamin D deficiency | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| region* | No. Visits | Visit rate/ 100,000 |
Incidence | Incidence rate/100,000 |
No. Visits | Visit rate/ 100,000 |
Incidence | Incidence rate/100,000 |
| Alaska | 89 | 33.06 | 30 | 11.15 | 50 | 18.58 | 22 | 8.17 |
| East ** | - | - | - | - | 15 | 18.79 | 13 | 16.29 |
| N. Plains | 25 | 4.34 | 12 | 2.08 | 38 | 6.59 | 28 | 4.86 |
| S. Plains | 32 | 5.86 | 15 | 2.75 | 43 | 7.88 | 16 | 2.93 |
| Southwest | 101 | 10.40 | 30 | 3.09 | 114 | 11.74 | 74 | 7.62 |
| West | 30 | 9.95 | 13 | 4.31 | 65 | 21.55 | 30 | 9.95 |
Rates are from the Indian Health Service direct and contract outpatient visit health care data.
Rates with ≤ 5 visits are not reported.
Alaska Native Case Control Study: Confirmed rickets and vitamin D deficiency cases
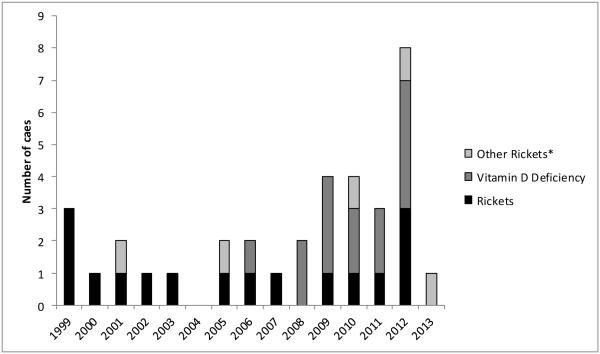
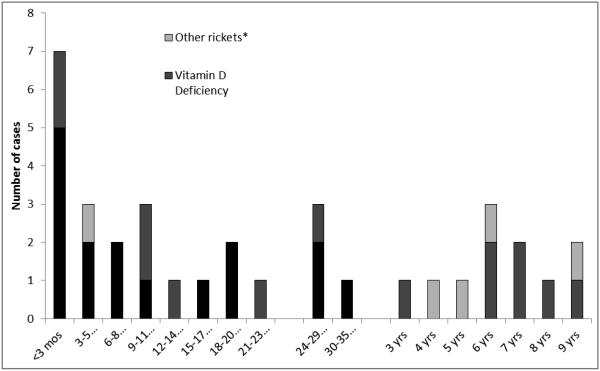
We reviewed 56 potential cases and confirmed 30 cases of nutritional rickets (n=16) or vitamin D deficiency (n=14) among AN children during 1999-2013. (Five additional cases diagnosed with nutritional rickets/vitamin D deficiency associated with malabsorption or liver disease are not included.) During 1999-2005, 8 rickets cases and no additional vitamin D deficiency cases were identified; while during 2006-2013, 14 vitamin D deficiency cases and 8 rickets cases were identified (Figure 1). Among the 16 confirmed rickets cases: the mean age of diagnosis was 0.98 years, 63% (n=10) were aged <1 year, and 75% (n=12) were male. Eleven of the rickets cases were diagnosed during November –April, compared with five diagnosed during May – October (p=0.210); however, only one rickets case was diagnosed during summer months (June-September; p=0.029). Among the 14 children with confirmed vitamin D deficiency, the mean age was 4.04 years and 43% were male (Table 2). Mean age of diagnosis in children with rickets was significantly younger than children with vitamin D deficiency (p=0.002; Table 3, Figure 2). Because these cases constituted the entire known cohort of AN rickets cases, we calculated the rickets average annual incidence as 4.2 per 100,000; the rickets annual incidence increased by latitude of residence from 0.0 in latitude 50-57 degrees (southeast Alaska), to 21.4 per 100,000 in latitude 70-73 degrees (northern Alaska; p<0.001) (Table 3).
Figure 1.
Alaska Native confirmed rickets and vitamin D deficiency cases < 10 years of age, by year of diagnosis, 1999-2013.
* Other rickets: Children with rickets/vitamin D deficiency associated with hepatic disease or malabsorption.
Table 2.
Clinical characteristics of Alaska Native children at diagnosis, with confirmed rickets diagnosed 1999-2012.
| No. | Age (months) |
Sex | Laboratory results at Diagnosis |
Presenting Reason | Radiographic findings |
Physical exam |
Weight for age percent |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 OHD (ng/mL) |
Calcium (mg/dl) |
Phos. (mg/dl) |
Alk. Phos. (IU/L) |
|||||||
| 1 | <3 | M | 5 | 6.2 | 2.6 | 927 | Pneumonia, FTT | Fracture, osteopenia |
Short Stature |
6.1 |
|
| ||||||||||
| 2 | <3 | M | 0 | 6.2 | 4.3 | 626 | FTT, hypocalcemic seizure |
-- | -- | 0.5 |
|
| ||||||||||
| 3 | <3 | M | 4 | 5.8 | 1057 | Hypocalcemic seizure |
-- | -- | -- | |
|
| ||||||||||
| 4 | <3 | F | 6 | 9.3 | 1240 | Rickets on x-ray | Rachitic rosary | -- | -- | |
|
| ||||||||||
| 5 | 3-5 | F | 7 | 8.5 | 3.3 | 953 | VSD | Negative | 22.0 | |
|
| ||||||||||
| 6 | 3-5 | M | 16 | 9.8 | 3.2 | 469 | FTT, rachitic rosary | -- | Short Stature |
<0.1 |
|
| ||||||||||
| 7 | 3-5 | M | 7 | 8.4 | 1192 | Other | -- | -- | 11.9 | |
|
| ||||||||||
| 8 | 6-11 | M | 7 | 7.7 | 3.9 | 124 | FTT | Fracture, cup/fray | -- | 5.7 |
|
| ||||||||||
| 9 | 6-11 | M | 5 | 6.9 | 3.5 | 504 | Seizure meds | Negative | Frontal bossing |
75.9 |
|
| ||||||||||
| 10 | 6-11 | F | 10 | 5.8 | 5.6 | 628 | Hypocalcemic seizure |
-- | -- | 91.4 |
|
| ||||||||||
| 11 | 12-23 | M | 8.1 | 2.6 | 977 | FTT, leg bowing | Metaphyseal changes, tibial bowing |
Short stature, tibial bowing |
0.5 | |
|
| ||||||||||
| 12 | 12-23 | M | 7 | 8.6 | 2.7 | 756 | Leg Bowing, hyponatremic seizure |
Negative | Tibial bowing |
8.9 |
|
| ||||||||||
| 13 | 12-23 | F | 4 | 9.2 | 2.8 | 531 | Leg bowing | Metaphyseal changes, rachitic rosary, tibial bowing |
Short stature, tibial bowing |
34.3 |
|
| ||||||||||
| 14 | 24-35 | M | 9.2 | 2 | 1273 | Leg bowing | Metaphyseal changes, radial/tibial bowing |
-- | 17.4 | |
|
| ||||||||||
| 15 | 24-35 | M | 10.3 | 5.2 | 288 | Leg bowing | Metaphyseal changes, tibial bowing |
Tibial bowing, fracture |
-- | |
|
| ||||||||||
| 16 | 24-35 | M | 9.9 | 597 | Leg bowing | Metaphyseal changes, femoral bowing |
Tibial bowing |
85.3 | ||
|
| ||||||||||
Table 3.
Characteristics of Alaska Native children < 10 years old with rickets or vitamin D deficiency diagnosed during 1999 through 2013.
| Rickets (A) | Vitamin D Deficiency (B) |
A+B | p-value A vs. B |
||
|---|---|---|---|---|---|
| 16 | 14 | 30 | |||
| Latitude of residence: | |||||
| 70-73.9 degrees | 3 (21.4)* | ||||
| 66-69.9 degrees | 5 (17.9) | ||||
| 62-65.9 degrees | 2 ( 2.8) | ||||
| 58-61.9 degrees | 6 (2.7) | ||||
| 54-57.9 degrees | 0 | ||||
| 50-53.9 degrees | 0 | ||||
| Age at Diagnosis | |||||
| Mean | 0.98 yrs | 4.04 yrs | 2.41 yrs | 0.002 | |
| Median | 0.67 yrs | 2.99 yrs | 1.14 yrs | 0.014 | |
| <1 year | 10 (63%) | 4 (25%) | 14 (47%) | 0.081 | |
| Sex | Male | 12 (75%) | 6 (43%) | 18 (60%) | 0.135 |
| Presenting Reason | |||||
| Medical conditions** | 1 (6%) | 6 (43%) | 7 (23%) | 0.031 | |
| Physical signsα, FTT, or Seizure | 13(81%) | 3 (21%) | 16 (53%) | 0.003 | |
| Underlying conditions | |||||
| Malnutrition | 8 (50%) | 6 (43%) | 14 (47%) | 0.730 | |
| Prematurity | 1 (6%) | 1 (7%) | 2 (7%) | 1.0 | |
| Seizures | 1 (6%) | 1 (7%) | 2 (7%) | 1.0 | |
| Chronic lung disease | 5 (31%) | 1 (7%) | 6 (20%) | 0.175 | |
| Gestational age <37 weeks | 3/15 (20%) | 1/10 (10%) | 4/25 (16%) | 0.626 | |
| Radiologic findings | |||||
| No x-ray | 5 | 10 | 15 | ||
| Normal/negative | 3 (19%) | 3 (21%) | 6 (20%) | 1.0 | |
| Any evidenceβ | 8 (50%) | 1 (7%) | 9 (30%) | 0.017 | |
| Exam Findingsα | 7 (44%) | 1 (7%) | 8 (27%) | 0.039 | |
| 25 (OH)D | (n=12) | (n=12) | (n=24) | ||
| Mean | 6.5 | 9.5 | 7.98 | 0.069 | |
| (Min, Max) | (0, 16) | (4, 15) | (0, 16) | ||
| Ever Breast-fed within 9 months | 8/13 (62%) | 8/9 (89%) | 16/22 (73%) | 0.333 | |
| Ever Breast-fed (more restrictive)€ | 8/10 (80%) | 8/9 (89%) | 16/19 (84%) | 1.0 | |
| Ever Exclusively breast-fed | 5/8 (63%) | 6/8 (75%) | 11/16 (69%) | 1.0 | |
| Vitamin supplement before diagnosis | 8 (50%) | 2 (14%) | 10 (33%) | 0.058 | |
Incidence of confirmed rickets by degrees latitude among children <10 years of age 2000-2012.
Group A (ventricular septal defect), group B (hypothyroid, congenital adrenal hypertrophy, intestinal disaccharidase def, brain malformation, renal disease, apnea).
Bowing, rachitic rosary, frontal bossing, wrist flaring.
Metaphyseal changes, bowing, fracture, osteopenia, rachitic rosary.
Information at birth to 6 months, or known ever breastfed <9 months.
Figure 2.
Age at diagnosis of Alaska Native children < 10 years of age with confirmed rickets or vitamin D deficiency 1999-2013.
* Other rickets: Children with rickets/vitamin D deficiency associated with hepatic disease or malabsorption
The 16 children with nutritional rickets are presented in Table 2. Seven of 10 children with rickets diagnosed in the first 12 months of life presented with hypocalcemic seizures (3) or failure to thrive (FTT) (4), while all 6 children diagnosed after 12 months of age presented with leg bowing (Table 2). We compared cases with vitamin D deficiency resulting in rickets to those with vitamin D deficiency alone (Table 3). Radiographic evidence of rickets was documented in half (8/16) of the rickets cases. As expected, children with rickets were more likely to have radiographic changes (p=0.017) and exam findings (leg bowing, rachitic rosary, frontal bossing, wrist flaring) (p=0.039), than children with vitamin D deficiency alone, who were more likely to have a 25(OH)D level drawn because of an underlying medical condition (p=0.031). Mean 25(OH)D concentrations were similar in rickets cases (6.5 ng/mL) and vitamin D deficiency cases (9.5 ng/mL) (95% CI -0.25, 6.2; p=0.069). Intact parathyroid hormone concentration was only available for 3 rickets cases.
Rickets and vitamin D deficiency cases had similar rates of breast-feeding documented in the medical record (80% and 89%, respectively). Vitamin D supplementation was documented in a low proportion of rickets and vitamin D deficiency cases (22% and 17%, respectively) (Table 3).
Case Control Study: Comparison of rickets and vitamin D deficiency cases with controls
The 26 cases were matched to 90 controls. All cases had between 2-5 controls. Sixty six (71%) of the controls were born within 1 day of the case, 79 (85%) within 5 days, and all within 21 days. Rickets and vitamin D deficiency cases were more likely to have a physician diagnosis of malnutrition/FTT than control children (Odds ratio [OR] 38.1; p=0.001; Table 4). This association remained when restricted to rickets cases (OR 20.5; 95% CI 2.5, 171; p=0.005). The rate of chronic lung disease among rickets cases (33%) was similar to controls (10%) (OR 4.9; p=0.076). Cases and controls also had similar rates of ever breast-feeding (75% vs. 76%) and of ever exclusively breastfeeding (OR 2.1; p=0.292). Similar proportions of cases and controls were exclusively formula-fed at birth. However, cases were less likely than controls to have any documentation of vitamin D supplementation in the first 6 months of life (OR 0.23; p=0.030). This difference remained significant when accounting for children not exclusively breast-fed in the first 6 months of life (OR 0.11; p=0.047).
Table 4.
Rickets/vitamin D deficiency cases in Alaska Native children <10 years of age, 1999-2013, compared with matched controls
| Case N=26 |
Control N=93 |
Matched OR*
(95% Confidence Interval) |
P value | ||
|---|---|---|---|---|---|
| Sex | Male | 14 (54%) | 39 (42%) | 1.69 (0.69, 4.1) | 0.249 |
| Medical conditions | |||||
| Malnutrition | 12 (47%) | 2 (2%) | 38.1 (4.9, 294) | 0.001 | |
| Prematurity | 2 (8%) | 5 (5%) | 1.62 (0.25, 10.5) | 0.614 | |
| Chronic Lung Disease | 5 (19%) | 11 (12%) | 1.70 (0.51, 5.7) | 0.387 | |
| Gestational age <37 weeks | 4/22 (18%) | 7/71 (10%) | 2.06 (0.37, 11.4) | 0.408 | |
| Ever breast-fed | 15/20 (75%) | 60/79 (76%) | 1.35 (0.36, 5.1) | 0.655 | |
| Ever breast-fed (restrictive)** | 15/18 (83%) | 60/76 (79%) | 2.56 (0.48, 13.9) | 0.277 | |
| Ever exclusively breast-fed | 10/15 (67%) | 39/75 (52%) | 2.13 (0.52, 8.6) | 0.292 | |
| Exclusively formula-fed at birth | 2/20 (10%) | 13/79 (16%) | 0.31 (0.04, 2.6) | 0.277 | |
| Vitamin D in the first 6 monthsβ | 3/18 (17%) | 32/67 (48%) | 0.23 (0.1, 0.87) | 0.030 | |
OR = Odds Ratio
Restrictive = Feeding information by 2 months of age or known breast-fed by 9 months of age.
Among cases diagnosed ≥ 6 months of age.
We compared rickets/vitamin D deficiency cases to controls for inpatient and outpatient visit rates during the first year of life for several conditions that might be associated with low vitamin D levels. For acute respiratory infections [ARI], asthma and otitis media there was no difference in the percent with any visit, mean or median number of visits or mean age at first visit. Rickets cases alone had a higher proportion with an ARI visit (OR 6.9; 95% CI: 1.1, 73) and lower mean age at ARI visit (diff=0.28 months, (95% CI: 0.07, 0.48) than control children. Seven of 10 rickets (86%) cases diagnosed before 1 year of age had an acute lower respiratory infection (ALRI) before diagnosis compared with 3/17 (18%) matched controls (OR10.9; 95% CI: 1.3, 100). Three of these 7 cases had a presenting diagnosis of FTT.
DISCUSSION
Several authors have described nutritional rickets in North America, primarily in breastfed, more pigmented children who received no supplemental vitamin D.1, 18 We found higher rickets- and vitamin D deficiency-associated hospitalization rates among AN children <10 years of age than in other similarly aged AI children living in other IHS regions and in the general U.S. child population. In addition, our calculated incidence of confirmed rickets in AN children <10 years of age from the case control study (4.2/100,000) is higher than incidence estimates in Canadian children except among aboriginal children in the far north.18 In our case control study the number of rickets cases were similar in the early (1999-2005) and late (2006-2013) periods, while vitamin D deficient cases increased in the late period. This may be due to increased provider awareness and recent focus on vitamin D deficiency in the scientific literature. Similar to Canadian children, AN children with rickets were young and presented with physical signs and symptoms of rickets (FTT, hypocalcemic seizures, leg bowing).18
Humans manufacture vitamin D from cholesterol following exposure to sunlight, and through diet and dietary supplements.5, 19 Among these sources, most humans depend on sun exposure to satisfy their requirements for vitamin D. These requirements may be modified by season, latitude, time of day, skin pigmentation, aging, sunscreen use, and glass barriers, which all influence the cutaneous production of vitamin D3. Above 37 degrees latitude during November – February, there is an 80-100% decrease in the number of ultraviolet B photons reaching the earth’s surface, making Alaskans and northern latitude populations at higher risk for vitamin D deficiency.18, 20, 21 Furthermore, the rickets incidence among AN children in our study increased with increased latitude, and fewer rickets cases were diagnosed during summer months. Risk factors for developing vitamin D deficiency and nutritional rickets in children besides low sun exposure, include exclusive breast-feeding (since breast milk is low in vitamin D), low maternal vitamin D stores, preterm birth, immigration to the U.S., darker skin pigmentation, and poor weight gain.1, 20
People throughout the United States, including AN people living in Alaska, can achieve adequate vitamin D stores through some combination of vitamin D supplementation, sunlight, and routine consumption of vitamin D rich foods. The latter mechanism may be particularly appropriate for residents of northern Alaskan villages where exposure to sunlight is limited, particularly in the winter. While store bought vitamin D rich foods may be expensive, or not available, many traditional subsistence foods provide an excellent and economical source of vitamin D.22 For example, salmon typically contains 500-1000 IU/3.5-ounce serving23 and consumption of high quantities of oily fish as part of a traditional indigenous diet is associated with higher vitamin D intake.21, 22, 24 Consumption of high quantities of oily fish with a traditional indigenous diet is associated with higher vitamin D concentration;21,22 however, Alaska Native people have had increasing dependency on store-bought foods since the 1950s. 21, 22, 25 Given the low traditional food consumption in many arctic populations, vitamin D supplementation is important. Consistent with this, the American Academy of Pediatrics (AAP) recommends that regardless of sunlight and food intake, all breastfed infants/children and those receiving < 1 Liter per day of infant formula receive 400 IU/day of vitamin D supplementation.26
Breastfeeding has been associated with increased risk of vitamin D deficiency and rickets since human milk typically contains a vitamin D concentration of 25 IU/L (10 ng/mL) or less;27 however, vitamin D deficiency has also been recognized in completely formula-fed Canadian infants.18, 28 The first reports of rickets in Alaska were in breast-fed infants,7 and breast-feeding without vitamin D supplementation was a risk factor for 25(OH)D concentration <15 ng/mL.8 In our study, a history of documented breastfeeding was not associated with an increased risk of rickets/vitamin D deficiency; however, lack of vitamin D supplementation was associated with development of rickets and vitamin D deficiency. This finding underscores the importance of supplementation, as recommended by the AAP.26 In Canada it is recommended that breast-fed infants residing above the 55th latitude receive 800 IU/d during the winter months;29 however, Ward found no reported cases of rickets among breast-fed children who received regular vitamin D (400 IU/d).18 This suggests that current AAP guidelines can be effective if consistently implemented.18 In addition to vitamin D supplementation, overall nutritional intake is likely important. In our study, AN children with confirmed rickets/vitamin D deficiency also had a dramatically higher rate of malnutrition than controls, and Ward identifies FTT in 5% of children with vitamin D deficient rickets in Canada.18
Although the IHS/Tribal and NIS data are useful for evaluating rates, these data have some previously described limitations.30 In addition, the analysis of visit rates and clinical information was dependent on documentation of ICD-9-CM codes, laboratory, clinical data, feeding and vitamin D supplementation in electronic medical records and charts. Lack of documentation of vitamin D supplementation does not necessarily correlate with it not occurring. We were unable to calculate reliable outpatient visit rates for the general U.S. population due to the low disease occurrence. Based on our finding of nearly identical hospital visit rate and incidence in the AI/AN population, we expect that the U.S. annual hospitalization rate is similar to the incidence. For the case/control study, some potential cases could not be confirmed because of missing data. Lastly, our case numbers are small, limiting the generalizability of results.
These findings highlight the importance of northern latitude and malnutrition as potential contributors to vitamin D deficiency and rickets and the importance of vitamin D supplementation in both breast-fed and formula-fed infants. Further studies should evaluate optimal vitamin D supplementation, the contribution of traditional AN diet to healthy vitamin D levels.
Acknowledgements
The authors thank the Alaska Native children from study regions, and the staff from Arctic Investigations Program – CDC, Yukon Kuskokwim Health Corporation, Southcentral Foundation, Arctic Slope Native Association, Norton Sound Corporation, Yukon Kuskokwim Health Corporation, Bristol Bay Area Native Health Corporation, and Tanana Chiefs Conference who facilitated the study. The authors thank Anthony Kretz, Gail Thompson and Jonathan Peterson from Arctic Investigations Program – CDC for their substantial contribution to study implementation and database management. The authors also thank Claudia Steiner MD, Healthcare Cost and Utilization Project, Center for Delivery, Organization and Markets, Agency for Healthcare Research and Quality, USDHHS, for her assistance with the Nationwide Inpatient Sample, and the state data organizations that voluntarily contribute their hospitalization data to the Healthcare Cost and Utilization Project to create the Nationwide Inpatient Sample.
Funding Source: No honorarium or grant or any other form of payment was given to anyone to produce the manuscript or conduct the study.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- ARI
acute respiratory infection
- ALRI
acute lower respiratory infection
- AN
Alaska Native
- AI/AN
American Indian/Alaska Native
- FTT
Failure to thrive
- HCUP
Healthcare Cost and Utilization Project
- IHS
Indian Health Service
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- NIPRS
National Patient Information Reporting System
- NIS
Nationwide Inpatient Sample
Footnotes
Financial Disclosure: No authors have any financial relationships relevant to this work to disclose.
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of CDC or the Indian Health Service.
REFERENCES
- 1.Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Annals Tropical Paediatr. 2006;26:1–16. doi: 10.1179/146532806X90556. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Robinson PD, Hogler W, Craig ME, Verge CF, Walker JL, et al. The re-emerging burden of rickets: a decade of experience from Sydney. Arch Dis Childhood. 2006;91:564–568. doi: 10.1136/adc.2004.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutrition. 2004;80(Suppl):1697S–1705S. doi: 10.1093/ajcn/80.6.1697S. [DOI] [PubMed] [Google Scholar]
- 5.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124:e362–370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 7.Gessner BD, deSchweinitz E, Petersen KM, Lewandowski C. Nutritional rickets among breast-fed black and Alaska Native children. Alaska Medicine. 1997;39:72–74. [PubMed] [Google Scholar]
- 8.Gessner BD, Plotnik J, Muth PT. 25-hydroxyvitamin D levels among healthy children in Alaska. J Pediatr. 2003;143:434–437. doi: 10.1067/S0022-3476(03)00410-4. [DOI] [PubMed] [Google Scholar]
- 9.International Classification of Disease, ninth Revision, Clinical Modification (CD-ROM) 2011. [cited June 24, 2014] Available at: http://cdc.gov/nchs/icd/icd9cm.htm.
- 10.Indian Health Service . Direct/CHS Inpatient and outpatient visit data, fiscal years 2001-2011. Indian Health Service; Albuquerque, NM: 2012. [Google Scholar]
- 11.Singleton RJ, Holman RC, Yorita KL, Holve S, Paisano EL, et al. Diarrhea-associated hospitalizations and outpatient visits among American Indian and Alaska Native children younger than five years of age, 2000-2004. Pediatr Infect Dis J. 2007;26:1006–1013. doi: 10.1097/INF.0b013e3181256595. [DOI] [PubMed] [Google Scholar]
- 12.Holman RC, Curns AT, Cheek JE, Singleton RJ, Anderson LJ, Pinner RW. Infectious disease hospitalizations among American Indian and Alaska Native infants. Pediatrics. 2003;111:176e–182e. doi: 10.1542/peds.111.2.e176. [DOI] [PubMed] [Google Scholar]
- 13.Indian Health Service . Trends in Indian Health 2002-2003. Indian Health Service; Rockville, MD: 2009. [Google Scholar]
- 14.Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality Nationwide Inpatient Sample (NIS) [cited December 14, 2013] Available at: http://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 15.Houchens REA. HCUP Method Series Report # 2003-02. U.S. Agency for Healthcare Research and Quality; 2001. Final Report on Calculating Nationwide Inpatient Sample (NIS) Variances, 2001. ONLINE June 3, 2005; cited Jun 24, 2014. [Google Scholar]
- 16.National Center for Health Statistics Intercensal estimates of the resident population of the United States for July 1, 2000-July 1, 2009, by year, county, single-year of age (0, 1, .., 85 years and over), bridged race, Hispanic origin, and sex. [updated June 24, 2014; cited June 24, 2014] Prepared under a collaborative arrangement with the U.S. Census Bureau. http://www.cdc.gov/nchs/nvss/bridged_race.htm.
- 17.Institute RT . SUDAAN (Version 10.0) Research Triangle Institute; Research Triangle Park, NC: 2008. [Google Scholar]
- 18.Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. Can Med Assoc J. 2007;177:161–166. doi: 10.1503/cmaj.061377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreiter SR, Schwartz RP, Kirkman HN, Jr., Charlton PA, Calikoglu AS, Davenport ML. Nutritional rickets in African American breast-fed infants. J Pediatr. 2000;137:153–157. doi: 10.1067/mpd.2000.109009. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Amer J Clin Nutrition. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 21.Sharma S, Barr AB, Macdonald HM, Sheehy T, Novotny R, Corriveau A. Vitamin D deficiency and disease risk among aboriginal Arctic populations. Nutr Rev. 2011;69:468–478. doi: 10.1111/j.1753-4887.2011.00406.x. [DOI] [PubMed] [Google Scholar]
- 22.Bersamin A, Zidenberg-Cherr S, Stern JS, Luick BR. Nutrient intakes are associated with adherence to a traditional diet among Yup'ik Eskimos living in remote Alaska Native communities: the CANHR Study. Int J Circumpolar Health. 2007;66:62–70. doi: 10.3402/ijch.v66i1.18228. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF. Vitamin D: a D-Lightful health perspective. Nutr Rev. 2008;66:S182–194. doi: 10.1111/j.1753-4887.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JS, Nobmann ED, Asay E, Lanier AP. Dietary intake of Alaska Native people in two regions and implications for health: the Alaska Native Dietary and Subsistence Food Assessment Project. Int J Circumpolar Health. 2009;68:109–122. doi: 10.3402/ijch.v68i2.18320. [DOI] [PubMed] [Google Scholar]
- 25.El Hayek J, Egeland G, Weiler H. Vitamin D status of Inuit preschoolers reflects season and vitamin D intake. J Nutrition. 2010:1839–1845. doi: 10.3945/jn.110.124644. [DOI] [PubMed] [Google Scholar]
- 26.Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 27.Gartner LM, Greer FR, Section on Breastfeeding, Committee on Nutrition. American Academy of Pediatrics Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2003;111:908–910. doi: 10.1542/peds.111.4.908. [DOI] [PubMed] [Google Scholar]
- 28.Gross ML, Tenenbein M, Sellers EA. Severe vitamin D deficiency in 6 Canadian First Nation formula-fed infants. Int J Circumpolar Health. 2013;72:20244. doi: 10.3402/ijch.v72i0.20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canadian Paediatric Society Vitamin D supplementation in northern Native communities. J Pediatr Child Health. 2002;7:459–465. doi: 10.1093/pch/7.7.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singleton RJ, Holman RC, Folkema AM, Wenger JD, Steiner CA, Redd JT. Trends in lower respiratory tract infection hospitalizations among American Indian/Alaska Native children and the general US child population. J Pediatrics. 2012;161:296–302. doi: 10.1016/j.jpeds.2012.02.004. [DOI] [PubMed] [Google Scholar]