Abstract
Background
Paradoxical tuberculosis (TB)-associated Immune Reconstitution Inflammatory Syndrome (IRIS) is a common complication of combination antiretroviral treatment (cART) initiation in adults residing in resource-limited regions. Little is known about the burden and presentation of TB-IRIS in children initiating cART while receiving TB treatment.
Methods
Prospective cohort study of South African children initiating cART while on TB treatment. Children were assessed clinically and by chest x-ray before starting cART and at 2, 4, 6, and 12 weeks post cART initiation. All children who presented with any signs or symptoms suggestive of paradoxical TB-IRIS were classified according to the consensus adult TB-IRIS case definition developed by the International Network for Study of HIV-associated IRIS (INSHI) and reviewed by an independent expert panel.
Results
In 7 of the 104 children enrolled in the cohort, symptoms and/or clinical or radiological signs suggestive of paradoxical TB-IRIS developed after a median of 14 days of cART. In two of these cases there was agreement between the INSHI case definition and the expert panel. In an additional 3 cases, the INSHI criteria were fulfilled but the expert panel made an alternative diagnosis of pneumonia (n=2) and poor adherence to cART (n=1).
Conclusions
The burden of paradoxical TB-IRIS in children with underlying TB initiating cART is low. Including response to antibiotic treatment for pneumonia as a criterion for an alternative diagnosis may improve the specificity of the INSHI case definition.
Keywords: TB, HIV, IRIS, antiretroviral treatment, Africa
INTRODUCTION
Immune reconstitution inflammatory syndrome (IRIS) is a complication of combination antiretroviral treatment (cART) characterized by clinical deterioration in the first months of cART.1 IRIS is believed to be due to rapid restoration of pathogen-specific immune responses, resulting in an exuberant inflammatory response to a partially treated infection (“paradoxical” IRIS) or progression of a latent or sub-clinical infection (“unmasking” IRIS).2 Although several pathogens are associated with IRIS, Mycobacterium tuberculosis accounts for nearly half of all IRIS cases.3,4
TB-IRIS in adults has been studied extensively, but little is known about TB-IRIS in children.4,5 Extrapolating data from adults to children may be problematic as the immunological and pathophysiological response to M. tuberculosis differs between adults and children.6 With 1.2 million children expected to receive cART by 2015,7,8 a better understanding of pediatric IRIS is important to ensure optimal clinical management of children initiating cART.
In the absence of a biomarker, the IRIS diagnosis relies on case definitions. These are typically developed by experts and incorporate clinical and laboratory data. To promote standardization and comparability of data, the International Network for the Study of HIV-associated IRIS (INSHI) published consensus case definitions for paradoxical TB-IRIS, ART-associated TB, and unmasking TB-IRIS in adults.9 These definitions built upon existing IRIS case definitions with three important changes: (1) adaptation of general IRIS case definitions to be specific for TB-IRIS; (2) omission of laboratory criteria of response to cART (rise in CD4 count, decrease in viral load) for use in resource-limited settings; and (3) a three month timeframe for the temporal association with initiation of cART. This INSHI definition has been successfully used in the identification of paradoxical TB-IRIS cases among adults in two retrospective cohort studies, 10,11 three prospective cohorts, 12-14 and three randomized controlled trials. 15-17
We aimed to determine the burden of paradoxical TB-IRIS and evaluate the INSHI definition for paradoxical TB-IRIS in a prospective cohort of South African children initiating cART while on TB treatment.
METHODS
Study population
The TB HIV IRIS and Nutrition in Kids (THINK) study was a prospective cohort study of cART-naive HIV-infected children aged 0 to 8 years initiating cART according to South African treatment guidelines.18,19 Children were enrolled if presenting to the outpatient Harriet Shezi Children's HIV Clinic or the Chris Hani Baragwanath Hospital in Soweto, South Africa between September 2009 and March 2012.
Participating children had been diagnosed with TB prior to enrollment by their routine care provider, based on a combination of clinical signs, contact with an adult with active TB, positive tuberculin skin test, suggestive chest X-ray, or positive sputum smear microscopy or culture. All diagnostic and treatment decisions were made by the routine care provider in accordance with the national guidelines for TB and HIV.20,21 Children generally received isoniazid, rifampicin, pyrazinamide, and ethambutol for two months followed by isoniazid and rifampicin for four months. Prior to April 2010, initiation of cART in children receiving TB treatment was delayed for at least two months and children receiving a cART regimen consisting of lopinavir/ritonavir (LPV/r) received additional ritonavir at a 1:1 dosage with lopinavir (super-boosted LPV/r).18 Starting in April 2010, initiation of cART was recommended 2-4 weeks after starting TB treatment, consisting of super-boosted LPV/r for children ≤8 years of age children.19 Children were eligible for study participation independent of duration of TB treatment at the start of cART.
Data collection
The THINK study included a pre-cART visit, cART initiation visit, follow-up visits at 2, 4, 8 and 12 weeks, and visits every three months thereafter until 24 months post cART initiation. On the day of cART initiation, the child's health status was assessed by questionnaire and physical examination. Children were assessed by a frontal and lateral chest x-ray and a tuberculin skin test unless these were performed within two months preceding cART initiation. Blood was drawn for CD4 percentage and viral load. During follow-up visits, children were assessed for the development of paradoxical TB-IRIS using a standardized symptom screen, clinical exam and chest X-ray. The symptom screen inquired about new or worsening constitutional, respiratory or abdominal symptoms, new or worsening lymph nodes, headaches or drowsiness. A physical exam was performed for signs of paradoxical TB-IRIS including enlarged lymph nodes, focal tissue involvement, central nervous system involvement, abdominal involvement, presence of serositis, and tuberculin hypersensitivity. A chest x-ray was performed at 2, 4, 8 and 12 weeks following cART initiation, results were communicated to the clinic clinician. A pediatric radiologist (NM) reviewed all chest x-rays using a standardized recording form to classify the evolution on chest X-ray findings as improved, stable or worsening.
Classification of initial TB diagnosis
We used the consensus clinical case definitions for pediatric TB to, retrospectively based on available data, classify TB as confirmed TB, probable TB, possible TB, unlikely TB and not TB.22
Classification of clinical events by expert opinion
An independent panel of 3 South African clinicians experienced in pediatric HIV and TB (SM, LF, CV) reviewed the data of all children in whom any clinical event, symptoms or signs suggestive of paradoxical IRIS was recorded during the first 3 months of cART. The panel had access to clinical information, laboratory data, chest X-rays, sputum smear microscopy and culture results. Study investigators had access to all study documents as well as the routine clinic files to answer questions raised by the panel. Experts blinded from each other classified each case. In case of disagreement, the classification reached by the majority of experts was used.
Classification of clinical events by INSHI criteria
All children presenting with symptoms or signs compatible with paradoxical TB-IRIS were classified according to the INSHI criteria. We used the adult criteria because (1) pediatric definitions23 have not been validated, (2) viral load monitoring is rarely performed in resource limited settings, and (3) it is unclear whether tuberculin skin test or interferon gamma release assay (IGRA) conversion as “supportive information” should count as a major or minor criterion, making it difficult to use this information. For the adult INSHI criteria, a diagnosis of paradoxical TB-IRIS requires (1) a diagnosis of TB that fulfills the WHO criteria for diagnosis of TB, (2) an initial response to TB treatment, (3) presence of at least one major or two minor clinical criteria, and exclusion of alternative explanations such as drug resistant TB, poor adherence, other infections, neoplasm, drug toxicity or drug reaction. When alternative explanations cannot be excluded, a case of probable paradoxical IRIS is reclassified as paradoxical IRIS if resolution of clinical or radiological findings occurs without change in TB or cART treatment (Figure 1). We classified any child with definite, probable or possible TB as a case of TB given that there is no strict WHO case definition for diagnosis of childhood TB.
FIGURE 1.
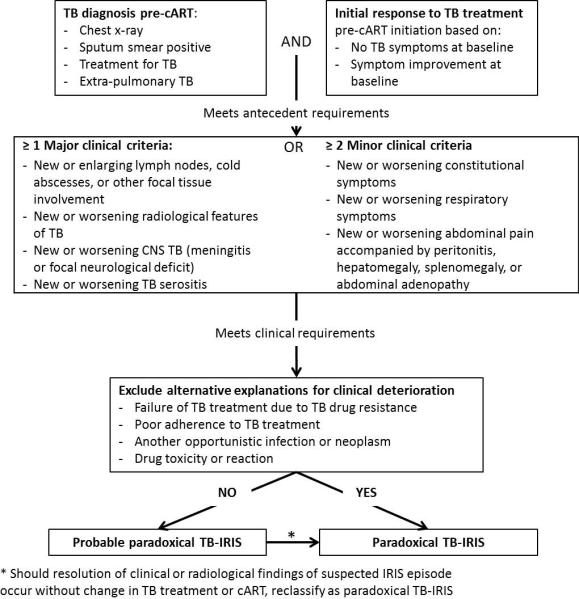
Diagnostic criteria for paradoxical TB-IRIS established by the International Network for the Study of HIV-associated IRIS (INSHI) 9.
Ethics statement
This study was approved by the Institutional Review Boards of the University of North Carolina at Chapel Hill and the University of the Witwatersrand, Johannesburg, South Africa. All caregivers gave written permission, all children ≥7 years gave assent.
RESULTS
In total, 104 children receiving TB treatment at time of cART initiation were enrolled. At baseline, median age was 30 months (IQR: 18-55), 61% were male and 41% were malnourished (weight for age z score < −2), median CD4% was 15.5 (IQR 9.7 – 21.7), median viral load 310,000 copies/ml (IQR 64,747 - 1,059,113) and median hemoglobin 9.6 mg/dl (9.0 – 10.8). The median time between start of TB treatment and cART initiation was 25 days (IQR 15 - 42) (Table 1). During the first 12 weeks of cART, none of the children died, 5 were hospitalized, 4 with symptoms or signs compatible with TB-IRIS and one for marasmic kwashiorkor.
Table 1.
Characteristics of 104 children receiving treatment for TB at time of initiation of combination antiretroviral treatment
| N or median | % or IQR | |
|---|---|---|
| Age (months) | 30 | 18.7 - 53.9 |
| Male sex | 63 | 60.6 |
| Weight for age z-score ≤ −2 | 43 | 41.4 |
| Time between TB treatment and start cART (days) | 25 | 15 - 42 |
| Baseline CD4%a | 15.5 | 9.7 - 21.7 |
| Baseline viral loadb | 310,000 | 64,437 - 1,059,113 |
| Baseline hemoglobinc | 9.6 | 9.0 - 10.8 |
CD4 % not available for 1 child with paradoxical TB-IRIS
Viral load not available for 11 children
Hemoglobin not available for 3 children
In 7 (6.7%) of the 104 children, the symptoms or signs compatible with paradoxical TB-IRIS were recorded during the first 3 months of cART (Tables 2-4). In only two cases were the INSHI case definition for paradoxical TB-IRIS fulfilled and the expert panel diagnosed TB-IRIS. In 3 children, the INSHI criteria were fulfilled but the expert panel made an alternative diagnosis. Two children were classified as not TB-IRIS by both INSHI criteria and expert panel.
Table 2.
Baseline [before initiation of combination antiretroviral treatment (ART)] characteristics of seven children presenting with signs or symptoms suggestive of paradoxical tuberculosis (TB) immune reconstitution inflammatory syndrome (IRIS)
| Case | Age (months) | Chest X-ray findings | Smear microscopy | Culture | Type of TB | Diagnostic category | Pre-ART CD4 % | Pre-ART viral load |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | Opacification, perihilar streakiness, perihilar nodes | Neg | Neg | Extrapulmonary (abdominal) + pulmonary TB | Probable TB | 11.0 | 1,700,000 |
| 2 | 8 | Perihilar streakiness, perihilar nodes | Pos | Neg | Pulmonary TB | Probable TB | 16.0 | 9,900,000 |
| 3 | 24 | Enlargement of hilar nodes | Neg | Neg | Extrapulmonary (abdominal) + pulmonary TB | Probable TB | 7.9 | 63,000 |
| 4 | 81 | Not available | Pos | Pos | Extrapulmonary (abdominal and lymph node) TB | Confirmed TB | 29.1 | 481,202 |
| 5 | 8 | Opacification, multiple cavities, collapse right upper lobe, perihilar streakiness, perihilar and mediastinal nodes, bronchial compression | Neg | Neg | pulmonary TB | Probable TB | 50.5 | 690,000 |
| 6 | 53 | Perihilar nodes | Pos | Neg | pulmonary TB | Probable TB | 20.6 | 230,000 |
| 7 | 30 | Opacification, reticulonodular infiltrate, perihilar streakiness, perihilar nodes, bronchial compression | Neg | Neg | pulmonary TB | Probable TB | 24.1 | 380,000 |
Table 4.
Classification of possible tuberculosis (TB) associated immune reconstitution inflammatory syndrome (IRIS) event by expert panel and the consensus definition developed by the International Network for the Study of HIV-associated IRIS (INSHI)
| Case | EXPERT PANEL | INSHI CRITERIA |
|||||
|---|---|---|---|---|---|---|---|
| Meets antecedent criteria | Meets clinical criteria (≥1 major or ≥2 minor) | Alternative explanation | Resolution without change in treatment for TB or HIV | Classification | |||
| TB diagnosis according to guidelines | Condition stabilizes or improves on TB treatment | ||||||
| 1 | Not TB-IRIS | Yes | Yes Radiological improvement but persistence of night sweats |
No | No | Yes | Not TB-IRIS |
| 2 | Paradoxical TB-IRIS | Yes | Yes Weight gain, other symptoms stabilized |
Yes | No | Yes | Paradoxical TB-IRIS |
| 3 |
Not TB-IRIS Increase in VL suggests non-adherence to ART |
Yes | Yes | Yes | No | Yes | Paradoxical TB-IRIS |
| 4 | Not TB-IRIS | Yes | No Continued weight loss after 2.5 weeks of TB treatment |
Yes | Yes Mother stopped TB treatment at start antiretroviral treatment |
Yes | Not TB-IRIS |
| 5 | Paradoxical TB-IRIS | Yes | Yes | Yes | No | Yes | Paradoxical TB-IRIS |
| 6 |
Not TB-IRIS Pneumonia most likely diagnosis; cannot exclude paradoxical TB-IRIS |
Yes | Yes | Yes | Resolution without change in antiretroviral or TB treatment regimen | Yes | Paradoxical TB-IRIS |
| 7 |
Not TB-IRIS Pneumonia most likely diagnosis; cannot exclude paradoxical TB-IRIS |
Yes | Yes | Yes | Resolution without change in antiretroviral or TB treatment regimen | Yes | Paradoxical TB-IRIS |
Description of two cases classified as TB-IRIS by INSHI criteria and expert panel
An 8-month old child (case 2) started TB treatment based on clinical symptoms, chest X-ray suggestive of TB, and a smear positive gastric aspirate. Two months later, at time of cART initiation, the child had gained weight but still complained of cough, night sweats, and difficulty breathing. Two weeks after cART initiation, the child presented with new weight loss and haemoptysis. The chest X-ray showed a new opacification and reticulonodular infiltrates. During hospital admission for 8 days, treatment consisted of fluconazole, amikacin, piperacillin/tazobactam and nebulization. Four weeks later, without change in TB or cART treatment regimen, the chest X-ray improved and symptoms resolved.
Another 8-month old child (case 5) started TB treatment based on clinical symptoms and highly suggestive chest X-ray findings of opacification, multiple cavities, right upper lobe collapse, and mediastinal nodes with bronchial compression. Radiological symptoms initially improved but four weeks after cART initiation, worsening hilar nodes and worsening right upper lobe collapse were noted. The child also developed a fever, worsening weight loss, and shortness of breath. In hospital, the child received 5 days of ampicillin, gentamycin, and oxygen. A chest X-ray 2 months after cART initiation indicated improvement under the same TB treatment and cART regimen.
Description of three cases classified as TB-IRIS by INSHI criteria but not by the expert panel
Two children (case 6 and 7) were classified as paradoxical TB-IRIS by INSHI criteria but the expert panel favored a diagnosis of pneumonia, acknowledging that paradoxical IRIS could not be ruled out. Case 6 was a 53-month-old child who had started TB treatment based on symptoms, suggestive X-ray and positive smear microscopy. Two weeks later, symptoms had resolved and cART was started. One month later, the child presented with night sweats and cough. X-ray findings were stable. The child became asymptomatic and remained free of symptoms following completion of an ambulatory course of antibiotics. Case 7 was a 30-month old child who started TB treatment based on symptoms and chest x-ray findings of opacification, reticulonodular infiltrate, perihilar streakiness, perihilar nodes and bronchial compression. Two weeks after start of TB treatment, symptoms had resolved and cART was initiated. Two weeks later, the child presented with fever and respiratory distress. The child was hospitalized, responded well to a course of antibiotics and remained symptom free after completion of the antibiotics course.
One child (case 3) was classified as paradoxical TB-IRIS by INSHI criteria but the expert panel judged non-adherence to cART and HIV progression to be the most likely cause of clinical deterioration. The 2-year old boy was diagnosed with TB based on clinical symptoms, chest X-ray findings of hilar nodes and collapse of the right middle lobe, and splenic micro-abscesses on sonar. At time of cART initiation, the child had improved and was asymptomatic. After 2 months of cART, the X-ray showed worsening of hilar nodes and middle lobe collapse. CD4 count had increased from 7.9 to 16.6%. Viral load had risen sharply, from 63,000 to 400,000 copies/ml. One month later, under the same ambulatory TB treatment and cART regimen, the X-ray showed substantial improvement. While meeting INSHI criteria, the expert panel incorrectly decided that the viral load increase on cART indicated poor adherence and excluded a diagnosis of TB-IRIS. A later interview revealed that the mother stopped administering cART because she was worried about the clinical deterioration. This highlights the complexity of interpreting temporality of events, especially when reviews are performed by independent experts who may not have access to all qualitative details and case stories.
Description of two cases classified as not TB-IRIS by INSHI criteria and expert panel
A 10-month old child (case 1) showed radiological improvement on TB treatment prior to cART initiation despite continued presence of night sweats. Two weeks after starting cART, the child presented with weight loss. Chest x-ray was stable. As such, only 1 minor clinical criterion was present and the child did not fulfill the INSHI criteria for a TB-IRIS diagnosis. A 6-year old child (case 4) had culture confirmed TB. He continued to lose weight after 2.5 weeks of TB treatment, when cART was initiated. As such, the antecedent criterion of improved or stabilized condition on TB treatment was not met. Two weeks later, the child continued to lose weight and developed breathing difficulties. Upon investigation, the mother reported that she had misunderstood instructions and had stopped TB treatment when starting cART.
DISCUSSION
The occurrence of paradoxical TB-IRIS complicates the management of individuals initiating cART because its clinical presentation can resemble that of another infection, drug toxicity, non-adherence with HIV disease progression, or infection with drug-resistant tuberculosis. In this prospective cohort study, we observed a relatively low burden of paradoxical TB-IRIS in children initiating cART, with 5/104 (4.8%) children meeting the INSHI criteria and 2/104 (1.9%) children diagnosed with paradoxical TB-IRIS according to an expert panel.
In adult populations, paradoxical TB-IRIS develops typically after a median of 14 to 22 days and the most common clinical criteria are new or increasing lymphadenopathy and onset of fever.10-12,14,15,17 The lower incidence in children could be due to the fact that a proportion of the children diagnosed clinically may not have TB and are thus not be at risk of developing paradoxical TB-IRIS. Furthermore, the paucibacilly nature of TB in children may result in a lower risk of paradoxical TB-IRIS as the pathogen load is a risk factor for TB-IRIS.
Adults who develop paradoxical TB-IRIS are more likely to present with severe immunosuppression (median CD4 count range 26 to 64 cells/mm3),10,11,14-17 have extrapulmonary TB,12,14,15 and initiate cART after a shorter duration of TB treatment.11,15,17 Knowledge of paradoxical TB-IRIS in children is limited to the publication of 6 cases. Narendran et al described a severely immunosuppressed (CD4 count 51 cells/mm3) 12-year-old Indian boy with smear positive pulmonary TB who developed fever and mediastinal nodes three days after starting cART.24 Rabie at al described a 3-year-old severely immunosuppressed (CD4 count 4 cells/mm3) South African boy with pulmonary, abdominal and pericardial TB who developed fever, chylous ascites and chylothorax 11 days after cART initiation.25 Zampoli et al described 4 cases of paradoxical TB-IRIS.26 Children were between 3 months and 8 years of age, had a diagnosis of pulmonary (n=2) or disseminated TB (pulmonary plus abdominal, n=2), a baseline CD4 count ranging between 16 and 744, and started cART 6 days to 3 months after the start of TB treatment. Symptoms or signs of paradoxical TB consisted of worsening respiratory symptoms with radiological deterioration (n=2), development of ascites (n=1) and worsening axillary lymph nodes and cold abscess. Similar to the six previously reported pediatric cases and experience in adults, paradoxical TB-IRIS developed soon (median 15 days, range 14- 60 days) after initiation of cART.24-27 In contrast to the reported pediatric and adult cases, only 1 child in our cohort who developed paradoxical TB (according to INSHI criteria) had severe immunodeficiency (CD4% <15) and extrapulmonary TB.
Agreement between the expert panel and INSHI consensus case definition was low, with discordance in three cases and only 2 of the 5 cases fulfilling the INSHI case definition being classified as cases of paradoxical TB-IRIS by the expert panel. In one case an alternative diagnosis of non-adherence to cART was made based on an increase in viral load on cART. In two cases, the clinical deterioration was attributed to pneumonia. It is interesting to note that spontaneous resolution of disease under the IRIS case definition proposed by French et al. includes the phrase “without specific antimicrobial therapy”, which would have tipped the diagnosis away from paradoxical TB-IRIS in the two cases presenting with pneumonia.28 The low rate of agreement observed in our study highlights the difficulty in diagnosing paradoxical TB-IRIS in children in the absence of a biomarker. Two adult studies have attempted to validate the INSHI case definition and found relatively high agreement. Manosuthi et al. compared the INSHI case definitions to the non-TB specific IRIS definition proposed by French et al (which formed the basis for the development of the INSHI definition), and found that 18 of 20 cases fulfilled both the French and INSHI definition.12,28 Haddow et al compared the INSHI classification to the opinion of an expert panel, and found that 13 of the 18 cases identified by experts fulfilled the INSHI criteria.13
While this is the first prospective cohort study of paradoxical TB-IRIS in children, several limitations should be noted. First, common to many studies of pediatric TB, most children did not have confirmation of TB. Misclassification of TB status may have resulted in an underestimation of the true burden of paradoxical TB-IRIS. Second, we evaluated the adult instead of the pediatric INSHI case definition,23 because the pediatric definition has not been validated and uses evidence of virologic response to cART, tuberculin skin test conversion, or 5-fold increase in IGRA as supportive observations. While inclusion of virologic response may have correctly classified one of the cases (case 4), viral load is rarely available soon after start cART. The observation that paradoxical TB-IRIS often occurs in the first two weeks after start cART thus complicates the use of virologic response. Similarly, the use of tuberculin skin test or IGRA conversion at time of IRIS is not practical in most settings.
Conclusion
In this prospective study using standardized clinical and radiological assessment for pediatric paradoxical TB-IRIS, we observed a low burden of IRIS, with only 5 children of 104 children fulfilling the INSHI consensus case definition for paradoxical TB-IRIS. The validity of the adult INSHI criteria for diagnosis of paradoxical TB-IRIS in children is suboptimal, with only 2 children classified as paradoxical TB-IRIS by both the INSHI criteria and the expert panel. Including response to antibiotic treatment for lower respiratory tract infection as a criterion for an alternative diagnosis may improve the specificity to the INSHI case definition.
Table 3.
Clinical, antiretroviral treatment (ART), radiological and laboratory characteristics of six children at time of presentation with symptoms or signs suggestive of paradoxical tuberculosis (TB)- associated immune reconstitution inflammatory syndrome (IRIS)
| Case | Time start TB treatment to start ART (days) | Response to TB treatment | Duration of ART at IRIS (days) | Paradoxical reaction | Change between pre-ART and time of IRIS | |
|---|---|---|---|---|---|---|
| CD4% | Viral load | |||||
| 1 | 36 | Radiological improvement Persistence of night sweats |
14 |
Clinical: worsening weight loss Radiological: no abnormal findings |
Not done | Not done |
| 2 | 68 | Weight gain Persistence of cough, night sweats and difficulty breathing |
15 |
Clinical: - New weight loss - New respiratory symptom: haemoptysis Radiological: - New opacification - New reticulonodular infiltrates |
Not done | Not done |
| 3 | 17 | Resolution of symptoms | 60 |
Clinical: - New weight loss Radiological: - Worsening of enlargement of hilar nodes - Worsening of right middle lobe collapse |
+7.7 | +337,000 |
| 4 | 19 | Child continued to lose weight after 2.5 weeks of TB treatment | 14 |
Clinical: - Worsening weight loss - New respiratory symptom (difficulty breathing) Radiological: Chest X-ray not done |
+4.5 | −475,594 |
| 5 | 58 | Radiological improvement | 28 |
Clinical: - New fever - Worsening weight loss - New respiratory symptom (difficulty breathing) Radiological: - Worsening of hilar nodes - Worsening of bronchial compression |
Not done | Not done |
| 6 | 18 | Resolution of symptoms | 32 |
Clinical: - Worsening night sweats - New respiratory symptoms (cough) Radiological: - Stable chest X-ray findings |
Not done | Not done |
| 7 | 15 | Resolution of symptoms | 14 |
Clinical: - New respiratory symptoms (respiratory distress) - New fever Radiological: - New left pleural effusion - New right mid lobe collapse |
Not done | Not done |
Acknowledgements
The authors thank the participating children and their parents for the time they devoted to the study. We also thank the THINK study staff and Shezi clinic staff for their valuable contribution to the study.
Funding Source: All phases of this study were supported by an NIH grant, R01 HD058972; DM is supported by the Fogarty Internal Center (K01TW008005). The use of REDCap was partially funded by the Clinical and Translational Science Award program of the Division of Research Resources, National Institutes of Health (grant number 1UL1TR001111). The United States Agency for International Development's United States President's Emergency Plan for AIDS Relief Project at Wits Reproductive Health Institute provided partial funding for staff, equipment and technical support at the Harriet Shezi Children's Clinic.
Footnotes
Financial Disclosure: The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.French MA, Lenzo N, John M, Mallal SA, McKinnon EJ, James IR, Price P, Flexman JP, Tay-Kearney ML. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Medicine. 2000;1(2):107–115. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 2.Stone SF, Price P, French MA. Immune restoration disease: a consequence of dysregulated immune responses after HAART. Current HIV Research. 2004;2(3):235–242. doi: 10.2174/1570162043351345. [DOI] [PubMed] [Google Scholar]
- 3.Murdoch DM, Venter WD, Van Rie A, Feldman C. Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Research and Therapy. 2007;4:9. doi: 10.1186/1742-6405-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meintjes G, Rabie H, Wilkinson RJ, Cotton MF. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clinics Chest Medicine. 2009;30(4):797–810. doi: 10.1016/j.ccm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Link-Gelles R, Moultrie H, Sawry S, Murdoch D, Van Rie A. Tuberculosis Immune Reconstitution Inflammatory Syndrome in Children Initiating Antiretroviral Therapy for HIV Infection: A Systematic Literature Review. Pediatric Infectious Disease Journal. 2014;33(5):499–503. doi: 10.1097/INF.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stop TB Partnership Childhood TB Subgroup WHO. Guidance for National Tuberculosis Programmes on the management of tuberculosis in children. Chapter 1: introduction and diagnosis of tuberculosis in children. Int J of Tuberc and Lung Disease. 2006;10(10):1091–1097. [PubMed] [Google Scholar]
- 7.World Health Organization . Antiretroviral Medicines in Low- and Middle-Income Countries: Forecasts of Global and Regional Demand for 2012-2015. Geneva, Switzerland: May, 2013. 2013. [Google Scholar]
- 8.World Health Organization . Treatment of children living with HIV. Geneva, Switzerland: 2013. [Google Scholar]
- 9.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, Elliott JH, Murdoch D, Wilkinson RJ, Seyler C, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infectious Diseases. 2008;8(8):516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eshun-Wilson I, Havers F, Nachega JB, Prozesky HW, Taljaard JJ, Zeier MD, Cotton M, Simon G, Soentjens P. Evaluation of paradoxical TB-associated IRIS with the use of standardized case definitions for resource-limited settings. J Int Assoc Physicians AIDS Care. 2010;9(2):104–108. doi: 10.1177/1545109710361537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma SK, Dhooria S, Barwad P, Kadhiravan T, Ranjan S, Miglani S, Gupta D. A study of TB-associated immune reconstitution inflammatory syndrome using the consensus case-definition. Indian Journal of Medical Research. 2010;131:804–808. [PubMed] [Google Scholar]
- 12.Manosuthi W, Van Tieu H, Mankatitham W, Lueangniyomkul A, Ananworanich J, Avihingsanon A, Siangphoe U, Klongugkara S, Likanonsakul S, Thawornwan U, et al. Clinical case definition and manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2009;23(18):2467–2471. doi: 10.1097/QAD.0b013e32832f7b59. [DOI] [PubMed] [Google Scholar]
- 13.Haddow LJ, Moosa MY, Mosam A, Moodley P, Parboosing R, Easterbrook PJ. Incidence, clinical spectrum, risk factors and impact of HIV-associated immune reconstitution inflammatory syndrome in South Africa. PLoS One. 2012;7(11):e40623. doi: 10.1371/journal.pone.0040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worodria W, Massinga-Loembe M, Mayanja-Kizza H, Namaganda J, Kambugu A, Manabe YC, Kestens L, Colebunders R. Antiretroviral treatment-associated tuberculosis in a prospective cohort of HIV-infected patients starting ART. Clin Dev Immunol. 2011;2011:758350. doi: 10.1155/2011/758350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laureillard D, Marcy O, Madec Y, Chea S, Chan S, Borand L, Fernandez M, Prak N, Kim C, Dim B, et al. Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome after early initiation of antiretroviral therapy in a randomized clinical trial. AIDS. 2013;27(16):2577–2586. doi: 10.1097/01.aids.0000432456.14099.c7. [DOI] [PubMed] [Google Scholar]
- 16.Naidoo K, Yende-Zuma N, Padayatchi N, Naidoo K, Jithoo N, Nair G, Bamber S, Gengiah S, El-Sadr WM, Friedland G, et al. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Annals of Internal Medicine. 2012;157(5):313–324. doi: 10.7326/0003-4819-157-5-201209040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luetkemeyer AF, Kendall MA, Nyirenda M, Wu X, Ive P, Benson CA, Andersen JW, Swindells S, Sanne IM, Havlir DV, et al. Tuberculosis Immune Reconstitution Inflammatory Syndrome in A5221 STRIDE: Timing, Severity and Implications for HIV-TB programs. J Acquir Immune Defic Syndr. 2013 doi: 10.1097/QAI.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidelines for the management of HIV in children. National Department of Health, Republic of South Africa; 2004. [Google Scholar]
- 19.Guidelines for the management of HIV in children. 2nd edition National Department of Health, Republic of South Africa; 2010. [Google Scholar]
- 20.Department of Health Republic of South Africa. The South African Antiretroviral Treatment Guidelines. 2008 [Google Scholar]
- 21.Department of Health Republic of South Africa. The South African Antiretroviral Treatment Guidelines. 2010 [Google Scholar]
- 22.Graham SM, Ahmed T, Amanullah F, Browning R, Cardenas V, Casenghi M, Cuevas LE, Gale M, Gie RP, Grzemska M, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. Journal of Infectious Diseases. 2012;205(Suppl 2):S199–208. doi: 10.1093/infdis/jis008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulware DR, Callens S, Pahwa S. Pediatric HIV immune reconstitution inflammatory syndrome. Current Opinion in HIV and AIDS. 2008;3(4):461–467. doi: 10.1097/COH.0b013e3282fe9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narendran G, Swaminathan S, Sathish S, Rajasekaran S. Immune reconstitution syndrome in a child with TB and HIV. Indian journal of Pediatrics. 2006;73(7):627–629. doi: 10.1007/BF02759931. [DOI] [PubMed] [Google Scholar]
- 25.Rabie H, Lomp A, Goussard P, Nel E, Cotton M. Paradoxical tuberculosis associated immune reconstitution inflammatory syndrome presenting with chylous ascites and chylothorax in a HIV-1 infected child. Journal of Tropical Pediatrics. 2010;56(5):355–358. doi: 10.1093/tropej/fmp141. [DOI] [PubMed] [Google Scholar]
- 26.Zampoli M, Kilborn T, Eley B. Tuberculosis during early antiretroviral-induced immune reconstitution in HIV-infected children. Int J Tuberc Lung Dis. 2007;11(4):417–423. [PubMed] [Google Scholar]
- 27.Wang ME, Castillo ME, Montano SM, Zunt JR. Immune reconstitution inflammatory syndrome in human immunodeficiency virus-infected children in Peru. Pediatr Infect Dis J. 2009;28(10):900–903. doi: 10.1097/INF.0b013e3181a4b7fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18(12):1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]


