Abstract
Intratumoral hypoxia is a common finding in breast cancer and is associated with a significantly increased risk of metastasis and patient mortality. Hypoxia-inducible factors activate the transcription of a large battery of genes encoding proteins that promote primary tumor vascularization and growth, stromal cell recruitment, extracellular matrix remodeling, premetastatic niche formation, cell motility, local tissue invasion, extravasation at sites of metastasis, and maintenance of the cancer stem cell phenotype that is required to generate secondary tumors. Recent preclinical studies suggest that the combination of cytotoxic chemotherapy with drugs that inhibit hypoxia-inducible factors may improve outcome for women with triple-negative breast cancer.
Keywords: bone metastasis, lung metastasis, lymph node metastasis, mesenchymal stem cells, microvesicles, myeloid-derived suppressor cells, tumor-associated macrophages
1. Introduction
1.1. The tumor microenvironment has a major impact on cancer progression
Three general processes have major effects on cancer initiation and progression. First, discrete somatic mutations lead to tumor suppressor loss-of-function and oncoprotein gain-of-function [1]; second, epigenetic alterations change patterns of gene expression [2]; and third, alterations in the tumor microenvironment also lead to broad changes in gene expression [3]. Considerable attention has been paid to somatic mutations and epigenetic alterations, which are easily interrogated by high-throughput molecular techniques [4]. In contrast, changes in the tumor microenvironment are less easily assayed but have profound effects on cancer progression.
The tumor microenvironment can be subdivided into: the chemical microenvironment, which encompasses pH, PO2 and the concentration of other small molecules (e.g. NO) and metabolites (e.g. glucose, glutamine, lactate); and the cellular microenvironment, which includes tumor cells, stromal cells, and the extracellular matrix (ECM) produced by these cells. Among the stromal cell types are vascular endothelial cells (ECs) and pericytes, lymphatic ECs, fibroblasts, myofibroblasts, and various bone marrow-derived cells such as macrophages, neutrophils, mast cells, myeloid-derived suppressor cells (MDSCs), and mesenchymal stem cells (MSCs). Many of the stromal cells recruited to the primary tumor promote primary tumor growth or metastasis [5].
Metastases are found in only 6% of women with breast cancer at the time of initial presentation, yet 30% of women with early stage disease at diagnosis will eventually progress to metastatic disease, which is responsible for 40,000 deaths annually [6]. This review will focus on recent advances in understanding the pathogenesis of breast cancer that demonstrate how one critical aspect of the chemical microenvironment, reduced O2 availability (hypoxia), exerts major effects on the cellular microenvironment with profound consequences for cancer progression, i.e. acquisition of the invasive and metastatic properties that lead to patient mortality. Indeed, intratumoral hypoxia is a pathological stimulus that drives changes in gene expression, leading to alterations in cell signaling that in many cancers are comparable to those caused by somatic mutations or epigenetic changes.
1.2. Intratumoral hypoxia is commonly observed in breast and other cancers
Groundbreaking clinical studies, in which PO2 measurements of breast cancers were obtained in situ by use of Eppendorf microelectrodes, revealed a median of 65 mmHg (with all measurements > 10 mmHg) in normal breast tissue; in contrast, a meta-analysis of 10 studies involving over 200 patients revealed that the median PO2 in breast cancers prior to therapy was 10 mmHg [7]. Remarkably similar results were obtained in meta-analyses of studies involving over 700 patients with cervical cancer and over 500 patients with head and neck cancer; in these cancers, multiple studies demonstrated an association between intratumoral PO2 < 10 mmHg and decreased disease-free survival [7]. The severe intratumoral hypoxia detected in these studies is a result of diffusion-limited O2 delivery, in which rapid cancer cell proliferation leads to cells that are too far away from a blood vessel, and perfusion-limited O2 delivery, in which structurally and functionally abnormal tumor vessels do not maintain constant blood flow, so that cancer cells even immediately adjacent to a blood vessel may be hypoxic [8]. Regions of necrosis, which are commonly observed in advanced solid tumors, reflect the consequences of prolonged periods at O2 levels that are insufficient to maintain cell viability [9].
1.3. Expression and activity of hypoxia-inducible factors are increased in breast cancer
Cells respond to reduced O2 availability through changes in gene expression that are mediated by hypoxia-inducible factors, which are composed of an O2-regulated HIF-α subunit (HIF-1α, HIF-2α, or HIF-3α) and a constitutively expressed HIF-1β subunit [10, 11]. The HIF-α subunits are subjected to O2-dependent prolyl hydroxylation, ubiquitination, and proteasomal degradation, which are inhibited under hypoxic conditions [12], leading to stabilization and rapid accumulation of the proteins and transcriptional activity. Over 1500 HIF target genes have been identified thus far, although in any given cell, transcription of several hundred of these will increase significantly in response to hypoxia [13].
Immunohistochemical studies of tumor biopsies have linked increased HIF-1α protein levels with increased risk of metastasis [14] and mortality in lymph node-positive [15], lymph node-negative [16], HER2-positive [17], estrogen receptor-positive [18], and unselected [14, 19] breast cancer patients. Increased expression in primary breast cancers of a panel of 99 [20] or 16 [21] mRNAs encoded by HIF target genes is also associated with increased patient mortality.
2. Consequences of HIF activity in mouse models of breast cancer
2.1. HIF-1α promotes primary breast tumor growth and vascularization
Studies in many cancer cell types have established that HIF-1α promotes tumor xenograft growth and vascularization [22–24], which was also observed in an autochthonous model of breast cancer driven by expression of polyoma middle T antigen (PyMT) from the mouse mammary tumor virus (MMTV) promoter [25]. HIF-1 activates transcription of the VEGF gene encoding vascular endothelial growth factor [26] and HIF-1α levels in breast cancers are associated with VEGF expression and with microvessel density, even in ductal carcinoma in situ, the pre-invasive stage of breast cancer pathogenesis [27]. HIF mediates expression by cancer cells of angiogenic factors, such as VEGF, stromal-derived factor 1 (also known as CXCL12), and stem cell factor (also known as kit ligand), which induce the mobilization into the circulation of bone marrow-derived angiogenic cells that express the cognate receptors (VEGFR2, CXCR4, and CKIT, respectively) and home to the tumor where they promote vascularization [28].
2.2. HIF-1α promotes metastasis of breast cancer to axillary lymph nodes
In breast cancer, the most important clinical finding that predicts distant metastasis is the degree of axillary lymph node involvement; conversely, most women with distant metastases have lymph node involvement and it is likely that, at least in some cases, breast cancers access the peripheral circulation via the lymphatic system [29]. In an orthotopic mouse model, in which human MDA-MB-231 [30] breast cancer cells (BCCs) were implanted into the mammary fat pad of immunodeficient mice, expression of short hairpin RNA (shRNA) to inhibit HIF-1α, HIF-2α, or both led to decreased lymphatic vessel density in the primary tumor and decreased metastasis to the ipsilateral axillary lymph node [31]. Conditioned medium, which was collected from BCCs cultured under hypoxic conditions, increased the migration and proliferation of lymphatic ECs, whereas the effects of hypoxia were lost when HIF-1α and HIF-2α shRNA was expressed [31].
HIF-dependent expression of platelet-derived growth factor B (PDGF-B) was required for, and PDGF-B knockdown eliminated, the effects of hypoxia on lymphatic EC migration and proliferation in vitro; PDGF-B knockdown also decreased lymphatic vessel density and lymph node metastasis in vivo [31]. Hypoxia induced the binding of HIF-1 to a hypoxia response element located within intron 3 of the PDGFB gene. Remarkably, immunohistochemistry revealed that there was no association between HIF-1α and PDGF-B levels in biopsies from grade 1 breast cancers but an association was observed in grade 2 and grade 3 cancers, suggesting that HIF-1α was necessary but not sufficient for PDGFB gene expression during breast cancer progression [31].
2.3. HIF-1α promotes bone colonization by breast cancer cells
Injection of MDA-MB-231 cells into the left ventricle of immunodeficient mice results in the formation of osteolytic bone metastases. Compared to an empty vector subclone, expression of a constitutively active or dominant negative form of HIF-1α increased or decreased, respectively, the tumor area and blood vessel density in long bone sections [32]. HIF-1α knockdown by shRNA also reduced the radiographic area of osteolytic lesions, decreased vessel density in bone metastases, and increased survival time after injection of BCCs [33]. The bone metastases that formed after injection of MDA-MB-231 cells contained hypoxic regions and exposure of spleen cells to hypoxia in vitro inhibited osteoblast differentiation and stimulated osteoclast differentiation [32]. Because BCCs were injected directly into the circulation, these studies do not provide compelling evidence that HIF-1 is required for spontaneous metastasis from breast to bone as opposed to a more limited requirement for bone colonization by circulating tumor cells.
2.4. HIF-1α is required for metastasis of breast cancer to the lungs
Exposing cancer cells to hypoxia ex vivo is known to increase lung colonization after intravenous injection [34]. Conditional knockout of HIF-1α in mammary epithelial cells increased survival of MMTV-PyMT mice and decreased the number of spontaneous lung metastases by ~50% [25]. Knockdown of HIF-1α, HIF-2α, or both in human MDA-MB-231 BCCs significantly decreased the number of spontaneous lung metastases and total lung metastatic burden in immunodeficient mice after mammary fat pad injection [35]. In this orthotopic transplantation model, a large battery of HIF target genes that mediate specific steps in the metastatic process have been delineated, as described in the following section, providing a detailed molecular basis for the effect of intratumoral hypoxia on breast cancer metastasis.
3. Deconvoluting the multistep process of lung metastasis
3.1. Breast cancer cells recruit stromal cells to the primary tumor that promote metastasis
The molecular mechanisms by which stromal cells are recruited to, and communicate with, BCCs have not been well characterized. The production of colony stimulating factor 1 (CSF-1; also known as macrophage colony stimulating factor) by BCCs, has been reported to stimulate the recruitment of macrophages to primary breast tumors, leading to the secretion by tumor-associated macrophages (TAMs) of epidermal growth factor, which binds to its cognate receptor on BCCs to stimulate invasion and metastasis [36–38]. However, the mechanisms responsible for CSF-1 expression were not determined.
After subcutaneous co-injection of BCCs and MSCs, BCCs were reported to induce MSCs to secrete the chemokine CCL5, which bound to its cognate receptor CCR5 on BCCs to stimulate metastasis [39]. Remarkably, co-culture of human MDA-MB-231 BCCs with human bone marrow-derived MSCs for 48 hours prior to mammary fat pad injection significantly increased metastasis to axillary lymph nodes and lungs without having any effect on primary tumor growth [40].
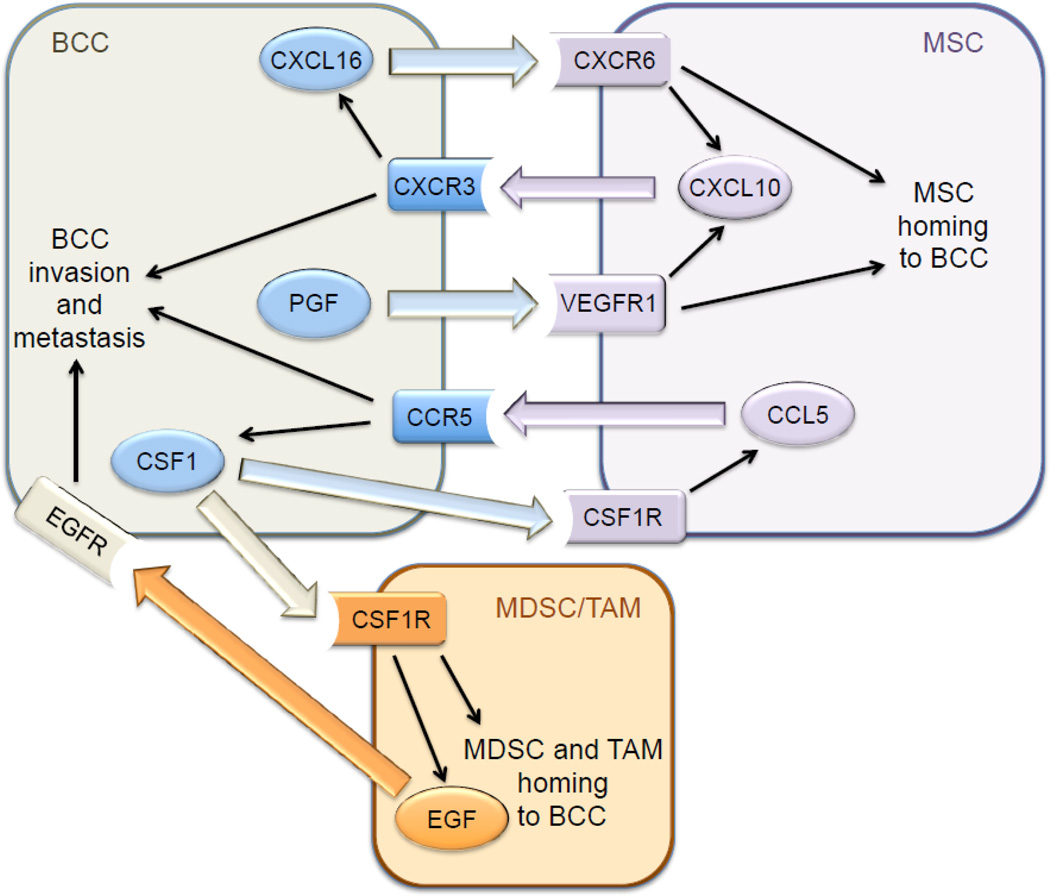
Hypoxia induces the HIF-dependent expression of placental growth factor (PGF) by BCCs, which binds to VEGF receptor 1 on MSCs and promotes their recruitment to primary breast tumors [40]. MSCs express the chemokine CXCL10, which binds to its cognate receptor CXCR3 on BCCs, and BCCs produce the chemokine CXCL16, which binds to its cognate receptor CXCR6 on MSCs and promotes their recruitment to the primary tumor [41]. Expression of PGF, CXCR3, and CXCL16 by BCCs is induced by hypoxia in a HIF-dependent manner [40, 41]. In addition, CXCL10MSC → CXCR3BCC signaling stimulates CXCL16BCC → CXCR6MSC signaling and vice versa [41], providing a molecular basis for the stimulatory effect of co-culture. Thus, hypoxia induces a powerful feed-forward loop that amplifies signals for MSC homing (Fig. 1). In addition, CXCL10 was shown to enhance BCC migration and invasion [40]. The stimulatory effect of MSC co-culture on breast cancer metastasis was lost when HIF-1α/HIF-2α, CXCR3, or CXCL16 expression was knocked down by shRNA in BCCs [40, 41].
Fig. 1.
Signaling between breast cancer cells (BCCs), mesenchymal stem cells (MSCs), myeloid-derived suppressor cells (MDSCs), and tumor associated macrophages (TAMs) stimulates metastasis. Large colored arrows indicate intercellular signaling through interaction of ligand with its cognate receptor. Thin black arrows indicate intracellular signaling activated by ligand-receptor interaction. Hypoxia-inducible factors directly activate transcription of the genes encoding the three ligands (CXCL16, PGF, CSF1) and two receptors (CCR5, CXCR3) in hypoxic BCCs.
The co-culture of BCCs with MSCs prior to mammary fat pad injection increased the recruitment of CSF1R+ F4/80+ TAMs and CD11b+ Ly6C+ MDSCs to the primary tumor in a HIF-dependent manner [41]. MSCs produce CCL5, which binds to its cognate receptor CCR5 on BCCs, and stimulates the production of CSF-1, which binds to its cognate receptor CSF-1R on MSCs, as well as on TAMs, thereby facilitating recruitment of TAMs to the tumor [38]. Hypoxia induces the HIF-dependent expression by BCCs of CCR5 and CSF-1, due to the direct binding of HIF-1 to two sites in the 5’-flanking region of the CSF1 gene and sites in the 5’-flanking region and exon 3 of the CCR5 gene [41]. CCL5MSC → CCR5BCC signaling stimulates CSF-1BCC → CSF-1RMSC signaling and vice versa, representing another feed-forward loop. Knockdown of HIF-1α/HIF-2α, CCR5, or CSF-1 expression in BCCs significantly impaired recruitment of TAMs and MDSCs to the primary tumor, and BCC metastasis to lymph nodes and lungs, without affecting primary tumor growth [41]. Thus, the recruitment of MSCs facilitates the subsequent recruitment of MDSCs and TAMs, with hypoxia stimulating homing of MSCs as well as MDSCs and TAMs to the primary tumor, leading to increased metastasis (Fig. 1).
All of the experiments described above were performed using human MDA-MB-231 BCCs implanted into immunodeficient mice. To interrogate these pathways in the context of an intact immune system, 4T1 mouse mammary carcinoma cells were implanted into syngeneic (and immunocompetent) Balb/c mice. HIF-1α/HIF-2α double knockdown in 4T1 cells inhibited the recruitment of TAMs and MDSCs to primary tumors and the metastasis of BCCs to lymph nodes and lungs after mammary fat pad injection [41].
The in vitro and in vivo data described above indicated that CXCL10MSC → CXCR3BCC signaling induces CXCL16BCC → CXCR6MSC signaling and vice versa and that both CXCR3 and CXCL16 gene expression is induced by hypoxia in BCCs. Analysis of gene expression data from over 500 primary human breast cancers revealed that CXCR3 and CXCL16 mRNA levels were very highly correlated (P = 10-13, Pearson’s test) in the clinical specimens [41], suggesting that the hypoxia-induced signaling pathways delineated in BCC lines and mice are clinically relevant.
3.2. Hypoxia induces collagen hydroxylation that stiffens the extracellular matrix
There is an extensive body of experimental data indicating that remodeling of the ECM promotes cancer invasion and metastasis [42, 43]. The histopathological detection of fibrosis, or increased fibrillar collagen content, in a primary breast tumor is associated with increased risk of cancer recurrence and patient mortality [44]. Type I collagen, which is the major constituent of ECM and the most abundant protein in the human body, forms when three procollagen polypeptides [two COL1(α1) subunits and one COL1(α2) subunit] assemble into a triple helix, as originally proposed by Pauling and Corey [45].
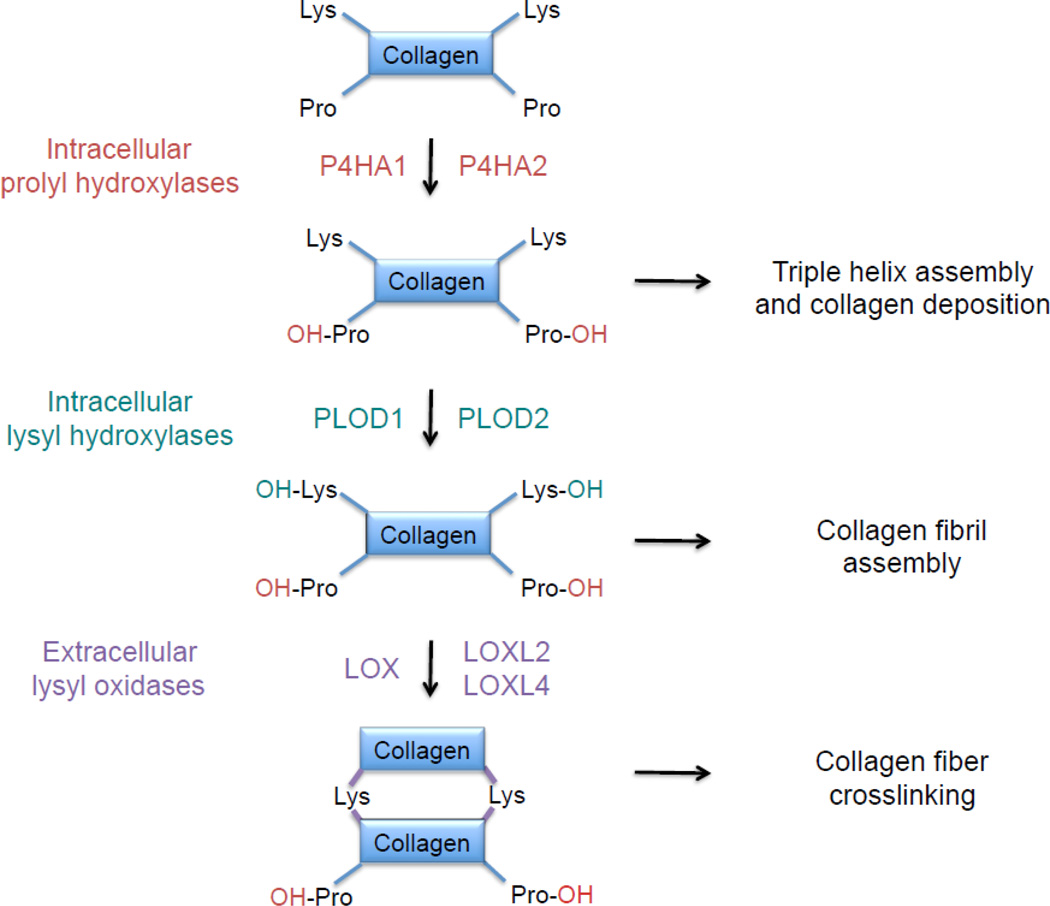
Type I collagen contains 338 X-Y-Gly triplets, with proline most commonly in the X position and hydroxyproline in the Y position preceding glycine [46]. The post-translational modification of proline residues in procollagen polypeptide chains to hydroxyproline is catalyzed by prolyl-4-hydroxylase (P4H) enzymes, which are α2β2 tetramers. In humans, there are three P4Hα isoforms and a single P4Hβ isoform, which are encoded by the P4HA1, P4HA2, P4HA3, and P4HB genes. Prolyl-4-hydroxylation is required for triple helix formation and extracellular deposition (Fig. 2). Expression of P4HA1 and P4HA2 was induced by hypoxia in several cell types [47].
Fig. 2.
Collagen biogenesis. The formation of mature collagen fibers involves three different types of post-translational modification that are mediated by prolyl hydroxylase, lysyl hydroxylase, and lysyl oxidase family members. All of the enzymes shown are products of genes that are transactivated by hypoxia-inducible factors in hypoxic breast cancer cells.
X-Lys-Gly sequences in helical domains and X-Lys-Ala/Ser sequences in the telopeptides of procollagens are also subject to lysine hydroxylation by procollagen-lysine, 2-oxoglutarate 5-dioxgenase (PLOD) enzymes, which are encoded by the PLOD1, PLOD2, and PLOD3 genes (Fig. 2). PLOD1 primarily hydroxylates lysine residues in helical domains and PLOD2 modifies the telopeptides, whereas PLOD3 hydroxylates type IV and V, but not type I, collagen [48]. Like prolyl hydroxylation, lysyl hydroxylation occurs prior to collagen deposition but, unlike prolyl hydroxylation, it is not required for extracellular deposition of collagen; however, it is required for collagen fibril assembly. Expression of PLOD1 and PLOD2 is induced by hypoxia [47].
After secretion, lysyl and hydroxylysyl residues are oxidatively deaminated by lysine oxidase (LOX) and LOX-like proteins (LOXL1, LOXL2, LOXL3, LOXL4), which is a modification that initiates the formation of intra-molecular as well as inter-molecular crosslinks, which may involve other amino acids, including histidinyl residues (Fig. 2). Collagen crosslinking is a major mechanism by which ECM stiffness increases during cancer progression [49, 50]. Expression of LOX, LOXL2, and LOXL4 was induced by hypoxia in several cell types [51–53].
Increased expression of P4HA1 and P4HA2 mRNA and protein was induced by exposure of MDA-MB-231 cells to 1% O2 and knockdown of HIF-1α, HIF-2α, or both significantly impaired the induction [54]. In breast tumors that arose after MDA-MB-231 cells were implanted into the mammary fat pad of immunodeficient mice, P4HA1 and P4HA2 co-localized with HIF-1α in perinecrotic (i.e. hypoxic) regions. Knockdown of P4HA1 or P4HA2 expression significantly impaired primary tumor growth and lymph node metastasis as well as completely eliminating lung metastasis. The reduction in hematogenous metastasis was associated with impaired tissue invasion and intravasation (invasion into blood vessels), as measured by the number of circulating tumor cells [54]. In contrast, the number of lung foci that developed after intravenous injection of P4HA1- or P4HA2-knockdown cells was not significantly decreased, indicating that the pro-metastatic effect of P4HA1 and P4HA2 reflects their roles in tissue invasion and intravasation.
Collagen deposition and fiber formation, hydroxyproline content, and stiffness were decreased in primary tumors derived from mammary fat pad implantation of P4HA1- or P4HA2-knockdown cells. Although cancer-associated fibroblasts are generally considered to be the major source of stromal collagen, these results suggest that BCCs are also major contributors to ECM remodeling [54]. Treatment of tumor-bearing mice with ethyl-3,4-dihydroxybenzoate, a P4H inhibitor, modestly decreased primary tumor growth but dramatically reduced collagen content in the primary tumor and metastatic burden in the lungs. Finally, analysis of data from The Cancer Genome Atlas (TCGA) revealed that human breast cancers with expression of P4HA1 or P4HA2 mRNA greater than the mean were associated with significantly decreased patient survival [54], suggesting that the preclinical studies described above have clinical relevance.
Expression of PLOD1 and PLOD2 mRNA and protein were induced by hypoxia in MDA-MB-231 cells and induction was lost in cells with HIF-1α knockdown, whereas HIF-2α knockdown had no significant effect [55]. Analysis of TCGA data revealed that increased expression of PLOD2, but not PLOD1, in primary breast cancers was associated with decreased patient survival. PLOD2 knockdown had no effect on the growth or total collagen content of primary tumors, but decreased fibrillar (crosslinked) collagen content and stiffness of primary tumors, and dramatically reduced local tissue invasion as well as metastasis to lymph nodes and lungs [55].
Analysis of LOX and LOXL1-4 expression in breast cancer cell lines revealed that hypoxia induced LOX and LOXL4 expression in MDA-MB-231 cells, whereas LOXL2 was induced in MDAMB-435 BCCs [56], and expression of LOX/LOXL proteins was not detected in non-metastatic MCF-7 BCCs [53]. The hypoxic induction of LOX, LOXL2, and LOXL4 was HIF-dependent. Analysis of primary breast cancers revealed a heterogeneous pattern of expression with different tumors overexpressing LOX only; LOX and LOXL2; LOXL2 and LOXL4; or LOX, LOXL2, and LOXL4 [53].
LOX family members are secreted proteins and conditioned medium from hypoxic BCCs stimulated collagen crosslinking, which was lost when HIF-1α and HIF-2α expression was knocked down [53]. Similar effects were observed when LOX or LOXL4 was knocked down in MDA-MB-231 cells or LOXL2 was knocked down in MDA-MB-435 cells [53]. Knockdown of LOX or LOXL4 in MDA-MB-231 cells or LOXL2 in MDA-MB-435 cells inhibited lung metastasis [51, 53]. While the effect of LOX or LOXL knockdown on metastasis is due in part to ECM remodeling of the primary tumor, it is also due to remote ECM remodeling in the lungs, as described below.
3.3. The pre-metastatic niche is established by signals from the primary tumor
Sites of metastatic colonization in the lung are preconditioned for the arrival of BCCs through modifications of the ECM and recruitment of bone marrow-derived cells, a process known as pre-metastatic niche formation [57]. Formation of the pre-metastatic niche is stimulated by factors that are secreted by the primary tumor, including VEGF, PGF, tumor necrosis factor-α, transforming growth factor-β (TGF-β), granulocyte colony stimulating factor, versican and LOX family members, which stimulate pulmonary production of fibronectin and proinflammatory proteins (S100A8 and S100A9) and recruitment macrophages, mast cells, and neutrophils [58].
As described above, LOX and LOXL proteins deaminate the ε-amino groups of lysine residues in collagen polypeptides, resulting in their intramolecular and intermolecular crosslinking (Fig. 2). Since this process occurs after extracellular deposition of collagens, LOX and LOXLs are secreted proteins. In addition to modifying collagen in the primary tumor, LOX produced in the primary tumor modifies collagen in the lungs, which facilitates the recruitment of bone marrow-derived cells, which in turn facilitates the recruitment of metastatic BCCs [59]. In mice implanted with MDA-MB-435 BCCs in the mammary fat pad, increased collagen cross-linking was observed in the lungs on day 8, whereas increased CD11b+ bone marrow-derived cells were first detected in the lungs on day 16, and BCCs were first detected in the lungs on day 24, demonstrating the sequential process of pre-metastatic niche formation [53].
Knockdown of LOX or LOXL4 in MDA-MB-231 or LOXL2 in MDA-MB-435 cells inhibited pre-metastatic niche formation [53, 59]. Whereas anti-LOX antibodies or chemical inhibitors of LOX (such as β-aminoproprionitrile) do not block the activity of all LOXL proteins, the HIF inhibitors digoxin and acriflavine blocked hypoxia-induced expression of all LOX and LOXL proteins, inhibited pre-metastatic niche formation, and significantly reduced lung metastasis [60].
Taken together, these studies demonstrate that intratumoral hypoxia is an important driving force for ECM remodeling that promotes BCC invasion and metastasis through the HIFdependent activation of genes encoding collagen prolyl hydroxylase, lysyl hydroxylase, and lysyl oxidase family members. MSCs were reported to stimulate the production of LOX by BCCs [40, 61], providing another example of crosstalk between these cell types that stimulates metastasis. Finally, although we have focused on the role of LOX family members, it should be noted that expression of other tumor-derived secreted factors that have been implicated in pre-metastatic niche formation (e.g. PGF, VEGF, TGF-β) are also induced by hypoxia in a HIF-dependent manner.
3.4. Activation of RhoA/ROCK1 signaling increases breast cancer cell motility
Whereas cancer progression is associated with remodeling that stiffens the ECM, the cytoskeleton undergoes remodeling that transforms rigid, immobile, and polarized epithelial cells with strong cell-to-cell adhesive contacts into motile and invasive cancer cells with decreased cell-to-cell contacts and increased mesenchymal properties, a process that is known as the epithelial-mesenchymal transition [62]. Members of the Rho family of small GTPases play a major role in this process by mediating the polymerization of actin (F-actin formation) to create stress fibers and the phosphorylation of myosin to trigger contractility that is the molecular basis for cell movement. Active (GTP-bound) Rho interacts with, and activates, Rho-associated coiled-coil-forming kinase (ROCK), which mediates the phosphorylation of myosin light chain (MLC), either directly or indirectly by inactivating myosin phosphatase, leading to actin-myosin contraction [63, 64]. The force that is generated by actin-myosin contraction is used to pull on the ECM at focal adhesions, leading to the activation of focal adhesion kinase (FAK), with a positive auto-regulatory loop between RhoA and FAK signaling [65]. RHOA and ROCK1 gene expression is coordinately induced within the invasive cancer cells of a breast tumor [66] and increased levels of RhoA and ROCK1 protein in biopsy specimens are associated with breast cancer progression [67, 68]. Somatic mutations are not the cause of RhoA or ROCK1 expression in most breast cancers, leaving the underlying molecular mechanism undefined.
Many studies have demonstrated the effect of hypoxia on cell movement and invasion in Boyden chambers [69, 70], which have many limitations including the confounding effects of gravity and pore size and the inability to perform dynamic or single cell analysis. Other studies have used video microscopy to study breast cancer cells that were exposed to hypoxia, then replated and analyzed for only short periods of time [71]. More recent studies have utilized microfluidic approaches to image cell motility in three dimensional cell culture [72].
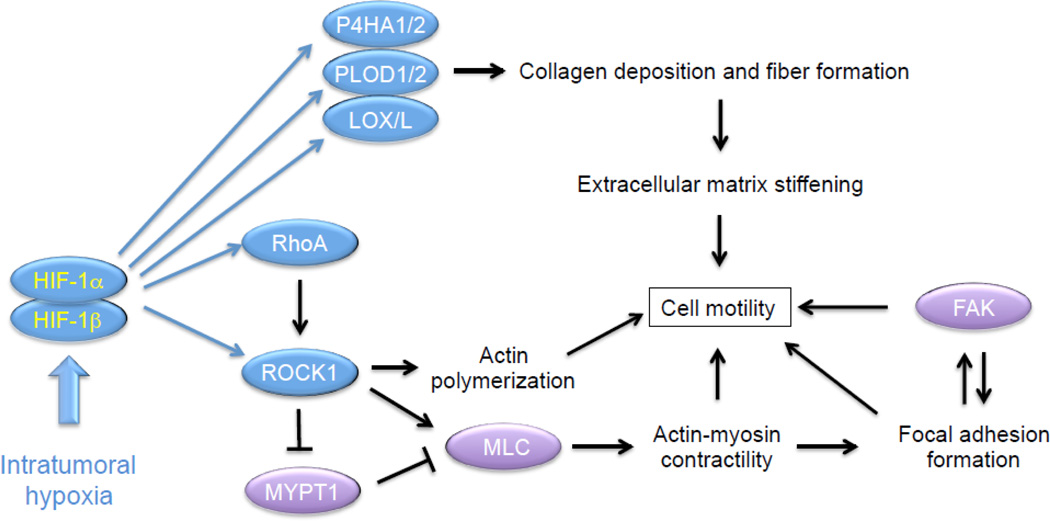
MDA-MB-231 subclones, which were stably transfected with empty vector (shEV) or expression vectors encoding shRNAs targeting HIF-1α and HIF-2α (sh1/2α), were plated on collagen-coated surfaces, exposed to 20% or 1% O2, and random motility of single cells was dynamically monitored over 22 hours. Mean velocity and total displacement of sh1/2α, but not shEV, cells increased significantly in response to hypoxia [73]. Hypoxia induced stress fiber formation in shEV cells, which was markedly impaired in sh1/2α cells. Molecular analyses revealed that RHOA and ROCK1 mRNA expression was increased in metastatic (MDA-MB-231 and MDA-MB-435) as compared to non-metastatic (MCF7 and T47D) BCC lines; hypoxia induced RHOA and ROCK1 mRNA and protein expression in all BCC lines analyzed. Hypoxia also induced phosphorylation of MLC and FAK in the metastatic cell lines, which was blocked by treatment with the ROCK inhibitor Y-27632 [73]. Focal adhesion formation was induced by hypoxia in shEV, but not sh1/2α, cells. The hypoxia-induced increase in cell motility was lost in MDA-MB-231 cells with knockdown of HIF-1α and HIF-2α or FAK [73]. Thus, hypoxia is sufficient to activate RhoA → ROCK1 → MLC → FAK signaling, which is the basis for BCC motility. Remarkably, the effect of hypoxia on motility was lost when cells were plated on a soft substratum [73], thereby providing a direct connection to the ECM remodeling (described in sections 3.2 and 3.3 above), which is also induced by hypoxia (Fig. 3).
Fig. 3.
Hypoxia induces collagen deposition and fiber formation that stiffen the extracellular matrix (ECM). Hypoxia also induces RhoA/ROCK1-mediated myosin light chain (MLC) phosphorylation and focal adhesion kinase (FAK) activity that are required for actin-myosin contractility and cell motility on stiff ECM. Blue oval, protein encoded by a HIF target gene; purple oval, protein that is regulated by HIF-dependent RhoA/ROCK1 signaling.
Hepatocyte growth factor (HGF) stimulates cell migration by binding to its cognate receptor MET, which is encoded by a HIF target gene [74]. Protein tyrosine kinase 6 (PTK6) is a downstream effector of MET that is also encoded by a HIF target gene and promotes breast cancer metastasis [75]. It would be interesting to determine whether there is crosstalk between the HGF → MET → PTK6 and RhoA → ROCK1 → MLC → FAK signaling pathways in hypoxic BCCs.
3.5. Hypoxia promotes margination and extravasation of circulating tumor cells
Once BCCs have intravasated into a blood vessel, they are carried through the bloodstream until their circulation is arrested by interaction with the luminal surface of a vascular EC, a process that is known as margination. Exposure of MDA-MB-231 cells to 1% O2 for 48 hours increased their adherence to vascular ECs, due to the HIF-dependent activation of L1 cell adhesion molecule (L1CAM) expression [35]. Knockdown of L1CAM markedly impaired adherence of BCCs to vascular ECs ex vivo, reduced lung colonization after intravenous injection, and decreased the number of spontaneous lung metastases after mammary fat pad implantation [35].
BCC margination is followed by extravasation, which is the process of moving from inside to outside of the blood vessel. In order for BCCs to migrate between adjacent ECs, they secrete angiopoietin-like 4 (ANGPTL4), which binds to receptors on ECs and inhibits EC-EC interactions [35, 76]. ANGPTL4 expression is induced by hypoxia in a HIF-dependent manner [35]. Knockdown of ANGPTL4 expression in MDA-MB-231 cells had no effect on primary tumor growth but dramatically impaired hypoxia-induced extravasation after intravenous injection and lung metastasis after mammary fat pad implantation [35, 76].
3.6. Hypoxia induces the breast cancer stem cell phenotype
Once cells have invaded, intravasated, circulated, marginated, extravasated, and homed to a metastatic niche, they must be capable of initiating a secondary tumor. Only a small percentage of cancer cells within a primary tumor, designated tumor-initiating cells or cancer stem cells (CSCs), have the capacity to undergo asymmetric divisions in which a CSC divides, yielding one CSC and one cell that has the ability to undergo a limited number of cell divisions that will contribute to the exponential growth of the bulk tumor. CSC self-renewal ensures that there will always be a source of cancer cells with proliferative potential. Hypoxia was first shown to induce the CSC phenotype in glioma [77] and, subsequently, in breast cancer [78, 79], in a HIF-dependent manner.
As for any cell type, the CSC phenotype is determined by patterns of gene expression that are controlled by transcription factors. In the case of breast CSCs, the co-activator TAZ (transcriptional activator with PDZ-binding motif) has been shown to promote breast CSC self-renewal and tumor-initiation capacity [80]. TAZ is an essential component of the Hippo pathway that regulates organ mass in both invertebrates and vertebrates [81]. Phosphorylation by LATS1 or LATS2 kinase maintains TAZ in the cytosol and prevents it from interacting with DNA-binding proteins of the TEAD family to activate transcription. TAZ expression is increased in 80% of high-grade breast cancers, which is associated with amplification of the WWTR1 locus encoding TAZ in approximately 10% of breast cancers [80], but the molecular basis for increased TAZ expression and activity in the majority of TAZ-overexpressing breast cancers was not known.
Analysis of TAZ mRNA and protein expression revealed that that basal expression was increased in the metastatic BCC lines MDA-MB-231 and MDA-MB-435 as compared to non-metastatic BCCs, but TAZ expression was induced by hypoxia in a HIF-1α-dependent and HIF-2α-independent manner in all BCC lines [82]. Chromatin immunoprecipitation (ChIP) assays demonstrated hypoxia-induced binding of HIF-1α and HIF-1β, but not HIF-2α, to a site in Intron 2 of the WWTR1 gene located 26 kb 3’ to the transcription start site. A 50-bp sequence containing the HIF-1 binding site functioned as a hypoxia response element in luciferase reporter assays, demonstrating that WWTR1 is a direct HIF-1 target gene and implicating intratumoral hypoxia as a mechanism for increased TAZ expression in high-grade breast cancers. Hypoxia increased the expression of connective tissue growth factor (CTGF) mRNA and increased the binding of TAZ to a region of the CTGF gene promoter containing multiple TAZ/TEAD-binding sites (but no HIF-1 sites) in a HIF-1α-dependent (and HIF-2α-independent) manner, indicating that HIF-1 amplifies TAZ target gene transcriptional activation in hypoxic BCCs by increasing TAZ expression [82].
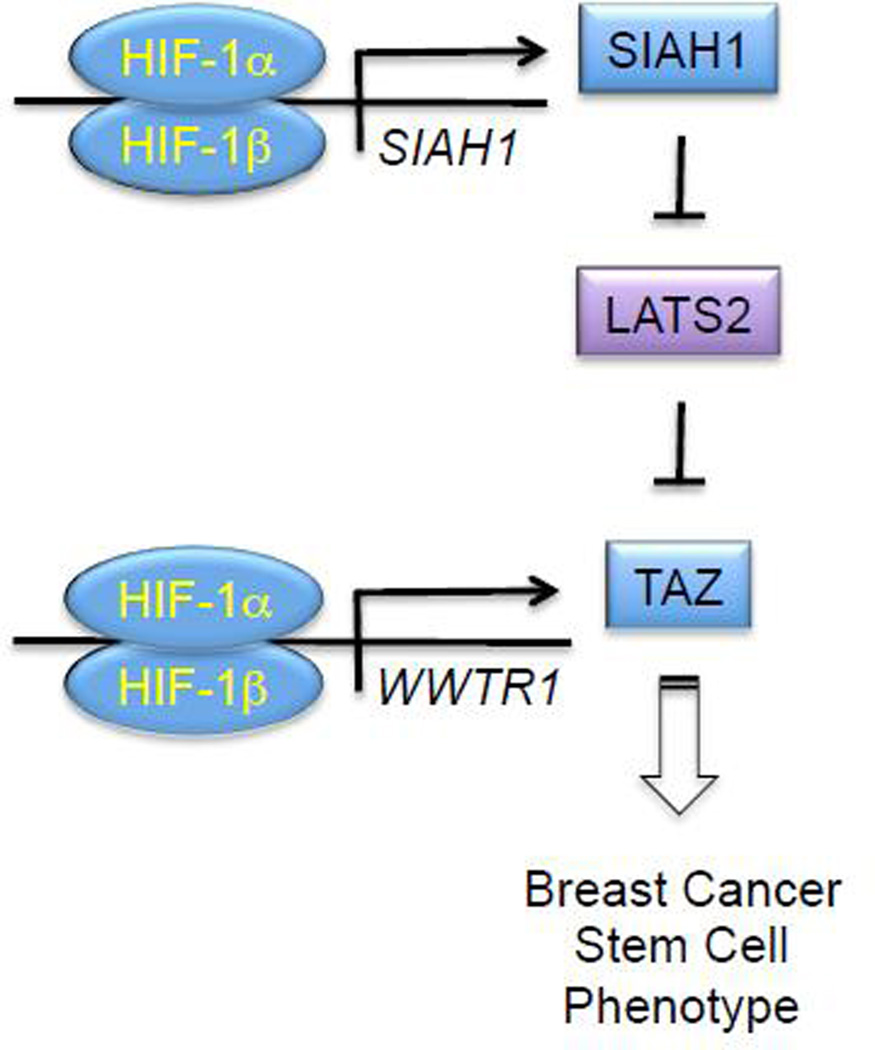
Analysis of TAZ by immunofluorescence or subcellular fractionation revealed that hypoxia induced the nuclear localization of TAZ in MDA-MB-231 subclones expressing a control shRNA or an shRNA targeting HIF-2α, but not in cells expressing an shRNA targeting HIF-1α [82]. Immunoblot assays revealed constitutive, low-level expression of LATS1, whereas LATS2 was abundantly expressed in non-hypoxic BCCs [82]. Hypoxia induced the proteasomal degradation of LATS2 through the HIF-1α-dependent expression of the ubiquitin protein ligase SIAH1, which was shown to be a direct HIF-1 target gene (Fig. 4). Remarkably, another study reported that the activity of YAP (Yes-associated protein), which is a homolog of TAZ, was induced by HIF-1α-dependent expression of SIAH2, which is a homolog of SIAH1, leading to LATS2 degradation [83].
Fig. 4.
HIF-1 induces transcription of the WWTR1 and SIAH1 genes to increase TAZ expression and activity in hypoxic breast cancer cells. WWTR1 encodes TAZ, whereas SIAH1 encodes a ubiquitin protein ligase that targets LATS2 for proteasomal degradation, thereby eliminating a negative regulator of TAZ nuclear localization and transcriptional activity.
Breast CSCs are enriched among BCCs that express aldehyde dehydrogenase (ALDH+) and among BCCs that form mammospheres when cultured on ultra-low adherence plates [84, 85]. Hypoxia increased the number of ALDH+ and mammosphere-forming CSCs, which was inhibited by shRNA knockdown of HIF-1α or SIAH1, but not HIF-2α, whereas TAZ knockdown reduced breast CSCs under both hypoxic and non-hypoxic conditions [82].
To analyze the effect of HIF or TAZ loss-of-function on tumor-initiating cells, 1000 cells of each MDA-MB-231 subclone were injected into the mammary fat pad of immunodeficient female mice, which were scored for the presence of mammary tumors 6 weeks later. Whereas 7 out of 7 mice injected with BCCs expressing the non-targeting control shRNA developed tumors, tumors formed in only 3/7, 2/7, and 0/7 mice injected with cells expressing shRNA targeting TAZ, HIF-2α, or HIF-1α, respectively. These results are consistent with the role of TAZ and HIF-1 in promoting the breast CSC phenotype, whereas HIF-2 may play either a role in CSCs that is not reflected in the ALDH and mammosphere assays or a non-CSC-related role that is required for early tumor formation, such as promoting vascularization.
Analysis of gene expression and survival data from over 1,000 breast cancer patients revealed that patients with expression of both HIF and TAZ target genes in their primary tumor that was greater than the median had significantly reduced survival compared to patients with low expression of HIF and/or TAZ target genes [82]. These clinical results are consistent with a role for HIF-1 in amplifying TAZ target gene expression as well as the other (TAZ-independent) molecular mechanisms described in previous sections by which HIF-1 promotes breast cancer metastasis. Finally, in addition to the role of HIF-1 in mediating increased TAZ activity, TAZ interacts with HIF-1α and serves as a co-activator for HIF-1-dependent gene transcription in hypoxic BCCs [86], indicating that TAZ and HIF amplify the activity of each other.
3.7. Microvesicles transfer the hypoxic phenotype to non-hypoxic cells and stimulate their invasive and metastatic properties
Sections 3.1 to 3.6 have delineated molecular mechanisms by which hypoxia induces the expression of genes that promote the invasive and metastatic properties of BCCs. Extracellular vesicles are a novel cell-derived component of the tumor microenvironment and recent studies suggest that hypoxic BCCs are able to transfer their phenotype to non-hypoxic cells through the production of microvesicles and exosomes, which are extracellular vesicles that contain proteins, mRNAs, and microRNAs. Microvesicles are particles of 0.1- to 1-µm diameter that form by outward budding and fission of the plasma membrane, which is mediated by contraction of the actin cytoskeleton, whereas exosomes are 50- to 200-nm diameter particles that form from inward budding of the limiting membrane of multivesicular bodies and are released from the cell by fusion with the plasma membrane [87–89]. In different cancers, extracellular vesicles have been reported to promote angiogenesis, cancer stem cells, epithelial-mesenchymal transition, or metastasis by effects on a variety of cell types in the tumor microenvironment [90].
The small GTPases RAB27A and RAB27B have been implicated in exosome biogenesis in melanoma and HeLa cells [91, 92], but the molecular mechanisms regulating extracellular vesicle formation have not been delineated. Hypoxia stimulates the release of extracellular vesicles that promote angiogenesis [93–97] and increased exosome formation by hypoxic cancer cells requires HIF activity [96, 98], but the relevant HIF target genes have not been identified.
Analysis of peripheral blood from breast cancer patients revealed increased numbers of microvesicles compared to controls [99]. Microvesicle formation was increased in metastatic MDA-MB-231 and MDA-MB-435 cells as compared to non-metastatic MCF-7 BCCs, whereas hypoxia induced microvesicle formation by two-fold in all three BCC lines, but this effect was lost in subclones expressing shRNAs targeting HIF-1α and HIF-2α [100]. Expression of RAB22A was correlated with the expression of HIF target genes in primary breast cancers and RAB22A expression greater than the median was associated with increased overall survival and distant metastasis-free survival. ChIP assays revealed hypoxia-induced binding of HIF-1 to a site in the 5’-untranslated sequence within exon 1 of the RAB22A gene in BCCs [100].
Immunocytochemistry suggested that RAB22A was a component of the microvesicles that formed in hypoxic cells and expression of shRNA targeting RAB22A eliminated hypoxia-induced microvesicle formation as well as hypoxia-induced invasion of BCCs through Matrigel [100]. Microvesicles collected from hypoxic BCCs and incubated with non-hypoxic cells induced focal adhesion formation and invasion in vitro, as well as lung colonization after intravenous injection, and this effect was lost in cells expressing shRNA that targeted RAB22A. Knockdown of RAB22A expression in MDA-MB-231 cells had no effect on primary tumor growth after mammary fat pad injection but significantly reduced the number of metastases from breast to lungs [100].
Previous studies implicated hypoxia-induced extracellular vesicle formation as a means by which cancer cells influence the phenotype of vascular and other stromal cells [93–96], whereas HIF- and RAB22A-dependent microvesicle formation increases the invasive and metastatic properties of a primary breast tumor by transferring the phenotype of hypoxic BCCs to non-hypoxic BCCs [100]. Many questions remain to be answered about the role of extracellular vesicles in cancer progression. Are tumor-derived factors, such as LOX family members, that act to promote pre-metastatic niche formation delivered to the lungs in extracellular vesicles? It would be interesting to test whether RAB22A loss-of-function in BCCs affects collagen crosslinking in the lungs of tumor-bearing mice. What are the molecular mechanisms by which hypoxia-induced microvesicles affect focal adhesion formation, invasion, and metastasis? One appealing mechanism is that the microvesicle cargo may include inducers of HIF-1 activity, such as microRNAs targeting negative regulators [97], or even HIF-1 itself [101].
3.8. Summary
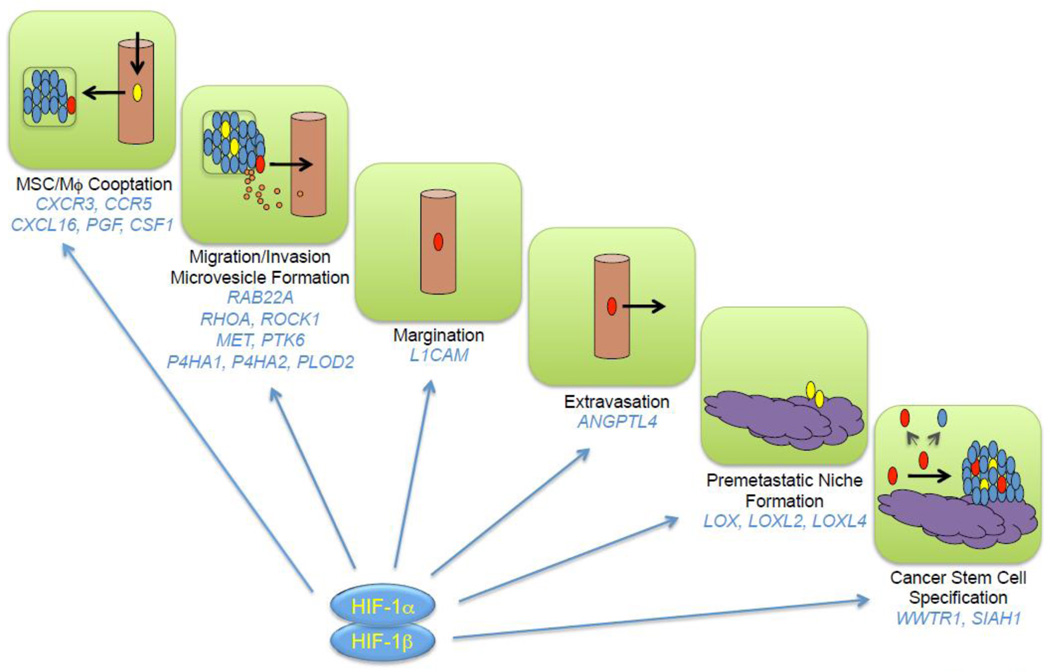
HIFs function as master regulators of breast cancer metastasis by activating the transcription of genes encoding proteins that participate in multiple steps of the metastatic process, only a subset of which have been reviewed here (Fig. 5). In addition to HIF target genes encoding proteins that directly participate in metastasis, HIF-1 encodes transcriptional regulatory proteins such as JMJD2C, which is required for breast cancer metastasis and encodes a histone demethylase that is recruited by HIF-1 to target genes to further amplify their expression [102].
Fig. 5.
HIF-1 activates the transcription of genes that control multiple steps in the metastatic process. Blue, red, yellow, and orange ovals denote bulk cancer cells, cancer stem cells, bone marrow-derived cells (mesenchymal stem cells, myeloid-derived suppressor cells and tumor associated macrophages), and extracellular vesicles (exosomes and microvesicles) respectively. Cancer stem cells give rise to both stem cells and non-stem cells and can initiate secondary (metastatic) tumors.
4. Implications for cancer therapy
Breast cancers are stratified into three major groups for therapy. Cancers that express the estrogen receptor (ER) or progesterone receptor (PR) or both are treated with an ER antagonist, such as tamoxifen, or an aromatase inhibitor, such as letrozole. Cancers that overexpress human EGF receptor 2 (HER2) are treated with an anti-HER2 antibody, such as trastuzumab, or a tyrosine kinase inhibitor, such as lapatinib. Cancers that lack expression of ER, PR, or HER2, which constitute approximately 15% of all breast cancers (30% among African-American women), are designated “triple negative” and, in the absence of any targeted therapy, are treated with cytotoxic chemotherapy, with greatly increased rates of relapse, metastasis, and death compared to the other breast cancer types [103]. Many of the studies described in this review were performed with the MDA-MB-231 cell line, which was derived from a metastatic triple-negative breast cancer [30].
There are now several drugs (acriflavine, digoxin, and ganetespib) that have been shown to inhibit HIF-1 and shown to block breast cancer primary tumor growth, angiogenesis, pre-metastatic niche formation, and local invasion as well as metastasis to lymph nodes and lungs in orthotopic mouse models [31, 35, 60, 104]. Recent studies in mice indicate that combining digoxin with cytotoxic chemotherapy (paclitaxel or gemcitabine) leads to tumor regression by blocking HIF-dependent transcriptional responses that promote the resistance of breast CSCs to chemotherapy [21]. HIF inhibitors may also induce immunogenic cell death [105].
Analysis of hundreds of triple-negative breast cancers revealed a wide diversity of somatic mutations none of which were found at high frequency [4]. Given the remarkable overexpression of HIF target genes in triple-negative breast cancer [4, 21], the master regulatory role of HIFs in metastasis (Fig. 5), and their involvement in chemotherapy resistance, HIF inhibitors may represent a targeted therapy for these breast cancers.
Acknowledgments
G.L.S. is an American Cancer Society Research Professor and the C. Michael Armstrong Professor at the Johns Hopkins University School of Medicine. Cancer research in his lab is supported by the American Cancer Society and the Department of Defense Breast Cancer Research Program (Impact Award W81XWH-12-1-0464).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat. Rev. Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Cancer Genome Atlas Network. Comprehensive molecular portrait of human breast tumors. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 6.O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(Suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 7.Vaupel P, Höckel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid. Redox Signal. 2007;9:1221–1235. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 8.Vaupel P, Mayer A, Höckel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004;381:335–354. doi: 10.1016/S0076-6879(04)81023-1. [DOI] [PubMed] [Google Scholar]
- 9.Harris AL. Hypoxia—a key regulatory factor in tumor growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 10.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu. Rev. Physiol. 2014;76:39–56. doi: 10.1146/annurev-physiol-021113-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dales JP, Garcia S, Meunier-Carpentier S, Andrac-Meyer L, Haddad O, Lavaut MN, Allasia C, Bonnier P, Charpin C. Overexpression of hypoxia-inducible factor HIF-1α predicts early relapse in breast cancer: retrospective study in a series of 745 patients. Int. J. Cancer. 2005;116:734–739. doi: 10.1002/ijc.20984. [DOI] [PubMed] [Google Scholar]
- 15.Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M. R. associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin. Cancer Res. 2002;8:1831–1837. [PubMed] [Google Scholar]
- 16.Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1α independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97:1573–1581. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- 17.Giatromanolaki A, Koukourakis MI, Simopoulos C, Polychronidis A, Gatter KC, Harris AL, Sivridis E. c-erbB-2 related aggressiveness in breast cancer is hypoxia-inducible factor-1α dependent. Clin. Cancer Res. 2004;10:7972–7977. doi: 10.1158/1078-0432.CCR-04-1068. [DOI] [PubMed] [Google Scholar]
- 18.Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield S, Bersiga A, Allevi G, Milani M, Aguggini S, Gandolfi V, Dogliotti L, Bottini A, Harris AL, Fox SB. Hypoxia-inducible factor-1α expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin. Cancer Res. 2006;12:4562–4568. doi: 10.1158/1078-0432.CCR-05-2690. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Ibusuki M, Okumura Y, Kawasoe T, Kai K, Ivama K, Iwase H. Hypoxia-inducible factor 1α is closely linked to an aggressive phenotype in breast cancer. Breast Cancer Res. Treat. 2008;110:465–475. doi: 10.1007/s10549-007-9742-1. [DOI] [PubMed] [Google Scholar]
- 20.Winter SC, Buffa FM, Silva P, Miller C, Valentine HR, Turley H, Shah KA, Cox GJ, Corbridge RJ, Homer JJ, Musgrove B, Slevin N, Sloan P, Price P, West CM, Harris AL. Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer Res. 2007;67:3441–3449. doi: 10.1158/0008-5472.CAN-06-3322. [DOI] [PubMed] [Google Scholar]
- 21.Samanta D, Gilkes DM, Chaturvedi P, Xiang L, Semenza GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E5429–E5438. doi: 10.1073/pnas.1421438111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 23.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 24.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1α is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 2007;67:563–572. doi: 10.1158/0008-5472.CAN-06-2701. [DOI] [PubMed] [Google Scholar]
- 26.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1α during breast carcinogenesis. J. Natl. Cancer Inst. 2001;93:309–314. doi: 10.1093/jnci/93.4.309. [DOI] [PubMed] [Google Scholar]
- 28.Lee K, Qian DZ, Rey S, Wei H, Liu JO, Semenza GL. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2353–2358. doi: 10.1073/pnas.0812801106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Ran S, Volk L, Hall K, Flister MJ. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. 2010;17:229–251. doi: 10.1016/j.pathophys.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cailleau R, Young R, Olivé M, Reeves WJ., Jr Breast tumor cell lines from pleural effusions. J. Natl. Cancer Inst. 1978;53:661–674. doi: 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schito L, Rey S, Tafani M, Zhang H, Wong CC, Russo A, Russo MA, Semenza GL. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2707–E2716. doi: 10.1073/pnas.1214019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiraga T, Kizaka-Kondoh S, Hirota K, Hiraoka M, Yoneda T. Hypoxia and hypoxia-inducible factor 1 expression enhance osteolytic bone metastases of breast cancer. Cancer Res. 2007;67:4157–4163. doi: 10.1158/0008-5472.CAN-06-2355. [DOI] [PubMed] [Google Scholar]
- 33.Dunn LK, Mohammed KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, Peng XH, Chirgwin JM, Guise TA. Hypoxia and TGF-β drive breast cancer metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subarsky P, Hill RP. The hypoxic tumor microenvironment and metastatic progression. Clin. Exp. Metastasis. 2003;20:237–250. doi: 10.1023/a:1022939318102. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Wong CC, Wei H, Gilkes DM, Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B, Winnard Jr PT, Raman V, Zhen L, Mitzner WA, Sukumar S, Semenza GL. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 37.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 38.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumor stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 40.Chaturvedi P, Gilkes DM, Wong CC, Kshitiz, Luo W, Zhang H, Wei H, Takano N, Schito L, Levchenko A, Semenza GL. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J. Clin. Invest. 2013;123:189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia-inducible factor- dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E2120–E2129. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boudreau A, van’t Veer LJ, Bissell MJ. An “elite hacker”: breast tumors exploit the normal microenvironment program to instruct their progression and biological diversity. Cell Adh. Migr. 2012;6:236–2489. doi: 10.4161/cam.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasebe T, Tsuda H, Tsubono Y, Imoto S, Mukai K. Fibrotic focus in invasive ductal carcinoma of the breast: a histopathological prognostic parameter for tumor recurrence and tumor death within three years after the initial operation. Jpn. J. Cancer Res. 1997;88:590–599. doi: 10.1111/j.1349-7006.1997.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pauling L, Corey RB. The structure of fibrous proteins of the collagen-gelatin group. Proc. Natl. Acad. Sci. U. S. A. 1951;37:272–281. doi: 10.1073/pnas.37.5.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorres KL, Raines RT. Prolyl-4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010;45:106–204. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hofbauer KH, Gess B, Lohaus C, Meyer HE, Katschinski D, Kurtz A. Oxygen tension regulates the expression of a group of procollagen hydroxylases. Eur. J. Biochem. 2003;270:4515–4522. doi: 10.1046/j.1432-1033.2003.03846.x. [DOI] [PubMed] [Google Scholar]
- 48.Yamauchi M, Sricholpech M. Lysine post-translational modifications of collagen. Essays Biochem. 2012;52:113–133. doi: 10.1042/bse0520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumor progression. Nat. Rev. Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumor metastasis. Nat. Rev. Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 52.Schietke R, Warnecke C, Wacker I, Schödel J, Mole DR, Campean V, Amann K, Goppelt-Struebe M, Behrens J, Eckardt KU, Wiesener MS. The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in hypoxia: insights into cellular transformation processes mediated by HIF-1. J. Biol. Chem. 2010;285:6658–6669. doi: 10.1074/jbc.M109.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong CC, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, Fraley SJ, Wong CM, Khoo US, Ng IO, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilkes DM, Chaturvedi P, Bajpai S, Wong CC, Wei H, Pitcairn S, Hubbi ME, Wirtz D, Semenza GL. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013;73:3285–3296. doi: 10.1158/0008-5472.CAN-12-3963. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Gilkes DM, Bajpai S, Wong CC, Chaturvedi P, Hubbi ME, Wirtz D, Semenza GL. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol. Cancer Res. 2013;11:456–466. doi: 10.1158/1541-7786.MCR-12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009;69:5292–5293. doi: 10.1158/0008-5472.CAN-09-1528. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive hematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sceneay J, Smyth MJ, Möller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 59.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong CC, Zhang H, Gilkes DM, Chen J, Wei H, Chaturvedi P, Hubbi ME, Semenza GL. Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J. Mol. Med. 2012;90:803–815. doi: 10.1007/s00109-011-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Haibi CP, Bell GW, Zhang J, Collmann AY, Wood D, Scherber CM, Csizmadia E, Mariani O, zhu C, Campagne A, Toner M, Bhatia SN, Irimia D, Vincent-Salomon A, Karnoub AE. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17460–17465. doi: 10.1073/pnas.1206653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindsey S, Langhans SA. Crosstalk of oncogenic signaling pathways during epithelial-mesenchymal transition. Front. Oncol. 2014;4:358. doi: 10.3389/fonc.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK, and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 64.Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis. 2009;26:273–287. doi: 10.1007/s10585-008-9174-2. [DOI] [PubMed] [Google Scholar]
- 65.Plotnikov SV, Waterman CM. Guiding cell migration by tugging. Curr. Opin. Cell Biol. 2013;25:619–626. doi: 10.1016/j.ceb.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 67.Belizzi A, Mangia A, Chiriatti A, Petroni S, Quaranta M, Schittulli F, Malfettone A, Cardone RA, Paradiso A, Reshkin SJ. RhoA protein expression in primary breast cancers and matched lymphocytes is associated with progression of the disease. Int. J. Mol. Med. 2008;22:25–31. [PubMed] [Google Scholar]
- 68.Lane J, Martin TA, Watkins G, Mansel RE, Jiang WG. The expression and prognostic value of ROCK I and ROCK II and their role in human breast cancer. Int. J. Oncol. 2008;33:585–593. [PubMed] [Google Scholar]
- 69.Graham CH, Fitzpatrick TE, McCrae KR. Hypoxia stimulates urokinase receptor expression through a heme protein-dependent pathway. Blood. 1998;91:3300–3307. [PubMed] [Google Scholar]
- 70.Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. [PubMed] [Google Scholar]
- 71.Ginis I, Faller DV. Hypoxia affects tumor cell invasiveness in vitro: the role of hypoxia-activated ligand HAL1/13 (Ku86 autoantigen) Cancer Lett. 2000;154:163–174. doi: 10.1016/s0304-3835(00)00388-8. [DOI] [PubMed] [Google Scholar]
- 72.Funamoto K, Zervantonakis IK, Liu Y, Ochs CJ, Kim C, Kamm RD. A novel microfluidic platform for high-resolution imaging of a three-dimensional cell culture under a controlled hypoxic environment. Lab Chip. 2012;12:4855–4863. doi: 10.1039/c2lc40306d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilkes DM, Xiang L, Lee SJ, Chaturvedi P, Hubbi ME, Wirtz D, Semenza GL. Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E384–E393. doi: 10.1073/pnas.1321510111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 75.Regan Anderson TM, Peacock DL, Daniel AR, Hubbard GK, Lofgren KA, Girard BJ, Schörg A, Hoogewijs D, Wenger RH, Seagroves TN, Lange CA. Breast tumor kinase (Brk/PTK6) is a mediator of hypoxia-associated breast cancer progression. Cancer Res. 2013;73:5810–5820. doi: 10.1158/0008-5472.CAN-13-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massagué J. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:759–772. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conley SJ, Gheordunescu E, Kakarala P, Newman B, Korkaya H, Heath AN, Clouthier SG, Wicha MS. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwab LP, Peacock DL, Majumdar D, Ingels JF, Jensen LC, Smith KD, Cushing RC, Seagroves TN. Hypoxia-inducible factor 1α promotes primary tumor growth and tumorinitiating cell activity in breast cancer. Breast Cancer Res. 2012;14:R6. doi: 10.1186/bcr3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, Daidone MG, Dupont S, Basso G, Bicciato S, Piccolo S. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 81.Pan D. The Hippo signaling pathway in development and cancer. Dev. Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiang L, Gilkes DM, Hu H, Takano N, Luo W, Lu H, Bullen JW, Samanta D, Liang H, Semenza GL. Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget. 2014;5:12509–12527. doi: 10.18632/oncotarget.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma B, Chen Y, Chen L, Cheng H, Mu C, Li J, Gao R, Zhou C, Cao L, Liu J, Zhu Y, Chen Q, Wu S. Hypoxia regulates Hippo signaling through the SIAH2 ubiquitin E3 ligase. Nat. Cell Biol. 2005;17:95–103. doi: 10.1038/ncb3073. [DOI] [PubMed] [Google Scholar]
- 84.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum B, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 86.Bendinelli P, Maroni P, Matteucci E, Luzatti A, Perrucchini G, Desiderio MA. Hypoxia inducible factor-1 is activated by transcriptional co-activator with PDZ-binding motif (TAZ) versus WW domain-containing oxidoreductase (WWOX) in hypoxic microenvironment of bone metastasis from breast cancer. Eur. J. Cancer. 2013;49:2608–2618. doi: 10.1016/j.ejca.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 87.D’Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–1299. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. 2013;91:431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberts CT, Jr, Kurre P. Vesicle trafficking and RNA transfer add complexity and connectivity to cell-cell communication. Cancer Res. 2013;73:3200–3205. doi: 10.1158/0008-5472.CAN-13-0265. [DOI] [PubMed] [Google Scholar]
- 90.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Gould B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 92.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanaoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wysoczynski M, Ratajczak MZ. Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int. J. Cancer. 2009;125:1595–1603. doi: 10.1002/ijc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol. Cell. Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Svensson KJ, Kucharzewska P, Christianson HC, Sköld S, Löfstedt T, Johansson MC, Mörgelin M, Bengzon J, Ruf W, Belting M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain F, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Umezu T, Tadokoro H, Azuma K, Yoshikawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor inhibiting HIF-1. Blood. 2014;124:3748–3757. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Galindo-Hernandez O, Villegas-Comonfort S, Candanedo F, González-Vázquez MC, Chavez-Ocaña S, Jimenez-Villanueva X, Sierra-Martinez M, Salazar EP. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch. Med. Res. 2013;44:208–214. doi: 10.1016/j.arcmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 100.Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza GL. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E3234–E3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, Yoshizaki T, Pagano JS, Shackelford J. Exosomal HIF-1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33:4613–4622. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luo W, Chang R, Zhong J, Pandey A, Semenza GL. Histone demethylase JMJD2C is a coactivator for hypoxia-inducible factor 1 that is required for breast cancer progression. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E3367–E3376. doi: 10.1073/pnas.1217394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.O'Toole SA, Beith JM, Millar EK, West R, McLean A, Cazet A, Swarbrick A, Oakes SR. Therapeutic targets in triple negative breast cancer. J. Clin. Pathol. 2013;66:530–542. doi: 10.1136/jclinpath-2012-201361. [DOI] [PubMed] [Google Scholar]
- 104.Xiang L, Gilkes DM, Chaturvedi P, Luo W, Hu H, Takano N, Liang H, Semenza GL. Ganetespib blocks HIF-1 activity and inhibits tumor growth, vascularization, stem cell maintenance, invasion, and metastasis in orthotopic mouse models of triple-negative breast cancer. J. Mol. Med. 2014;92:151–164. doi: 10.1007/s00109-013-1102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao T, Ren H, Jia L, Chen J, Xin W, Yan F, Li J, Wang X, Gao S, Qian D, Huang C, Hao J. Inhibition of HIF-1α by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma. Oncotarget. 2015;6:2250–2262. doi: 10.18632/oncotarget.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]