Abstract
BACKGROUND
To minimize the risk of pneumonia many anesthesiologists delay anesthesia-requiring procedures when patients exhibit signs of viral upper respiratory tract infection. Post-influenza secondary bacterial pneumonias (SBP) are a major cause of morbidity and mortality. Increased host susceptibility to SBP post-influenza has been attributed to physical damage to the pulmonary epithelium, but flu-induced effects on the immune system are being shown to also play an important role. We demonstrate that halothane mitigates risk of SBP post-flu through modulation of the effects of type I interferon (IFN).
METHODS
Mice (n=6–15) were exposed to halothane or ketamine and treated with influenza and S. pneumoniae. Bronchoalveolar lavage (BAL) and lung homogenate was procured for measurement of inflammatory cells, cytokines, chemokines, albumin, MPO, and bacterial load.
RESULTS
Halothane exposure resulted in decreased bacterial burden (7.9 ± 3.9 × 105 vs 3.4 ± 1.6 × 108 colony forming units, p<0.01), clinical score (0.6 ± 0.2 vs 2.3 ± 0.2, p<0.0001) and lung injury (as measured by BAL albumin, 1.5 ± 0.7 vs 6.8 ± 1.6 mg/ml, p<0.01) in CD-1 mice infected with flu for 7 days and challenged with S. pneumoniae on day 6 post-flu. IFN receptor A1 knockout mice similarly infected with flu and S. pneumoniae, but not exposed to halothane, demonstrated a reduction of lung bacterial burden equivalent to that achieved in halothane-exposed wild-type mice.
CONCLUSIONS
These findings indicate that the use of halogenated volatile anesthetics modulate the type I IFN response to influenza and enhance post-infection anti-bacterial immunity.
INTRODUCTION
A major complication of post-viral morbidity and mortality is secondary bacterial pneumonia (SBP) 1. In a previous prospective blinded cohort study, we observed that pediatric patients exposed to halothane that had viral upper respiratory tract infections, had significantly fewer new clinical symptoms, and their symptoms were of a shorter duration compared to patients that did not receive halothane 2. Although not isolated from patients in this study, one of the most common and potentially deadly viral pathogens is influenza, due to the unique relationship it has with pneumococcal SBP 1.
Influenza predisposes the host to pneumococcal infection in several ways, including hindrance of the mucocilliary escalator, denuding the epithelial barrier allowing bacterial adherence, and impairing antibacterial immunity due to factors of the host anti-viral response 1,3–6. Prior to the 2009 H1N1 pandemic, 40,000 to 200,000 hospitalizations occurred due to influenza or its complications, and 25% of mortality was attributable to SBP 7,8. These numbers vary with pandemics and flu season duration. The major cause of death with the 1918–1919 Spanish flu, was SBP, prior to the advent of antimicrobial compounds 9. However, given the rapid rate of gain-of-resistance by pathogens to these agents, novel therapies that do not target the pathogen life cycle, but modulate host immunity, are urgently needed. The 2009 H1N1 pandemic was fortunately milder than predicted, but seasonal flu with SBP is a perennial killer, and the next pandemic always looms on the horizon.
Volatile anesthetics have been in use for decades, yet their anesthetic mechanism of action is far from completely understood, as is their capacity to modulate immune responses 10–14. We hypothesized that the beneficial effect(s) of volatile anesthetics observed in our past study is due to immune modulation, as exposure to volatile anesthetics does not alter peak viral titers during influenza 14. We have also shown that halothane and isoflurane modulate the influence of type I interferons (IFN) on immune cells, and completely abrogate type I IFN-induced cytotoxic action of natural killer lymphocytes 15. From our past work we hypothesized halothane could decrease type I IFN mediated host susceptibility to SBP post-influenza by minimizing impairment of the host anti-bacterial immune response post-flu 3–6. To test this hypothesis we employed our H1N1 murine model of influenza-associated pneumococcal infection, whereby we challenged mice with S. pneumoniae 6 days post-flu. Our goals of this study were to determine parameters of the host anti-bacterial immune response post-influenza that are beneficially altered by halothane, if these alterations enhance resistance to SBP, and if modulation of the type I IFN signaling axis is central to the ability of halothane to minimize risk of SBP post-flu. Findings from this work may lead to novel immune-modulating therapies to combat SBP post-flu, such as low-dose halogenated volatile anesthetic usage to enhance pulmonary anti-bacterial immunity in flu patients. Additionally, this work may contribute to the creation of novel therapeutics that modulate the immune response through mechanisms similar to halothane.
MATERIALS AND METHODS
Use of animals
All procedures involving the mice in these studies were approved by the Institutional Animal Care and Use Committee of the Veterans Administration Western New York Healthcare System (Buffalo, NY) and conformed to the guidelines described in the Guide for the Care and Use of Laboratory Animals from the National Academy of Sciences.
Mouse models
Male, 3 weeks old, CD-1 (outbred strain) mice were obtained from Charles River Laboratories (Wilmington, MA) and housed for 1 week to acclimate prior to initiating experiments. Male, 4–6 weeks old, IFN-A1 receptor knockout (IFNAR KO; Ifnar1tm1Agt/Ifnar1tm1Agt) mice on a C57BL/6 background were obtained from the Mutant Mouse Regional Resource Center (c/o The Jackson Laboratory, Bar Harbor, ME). They were bred, along with C57BL/6 wild-type control mice, in the laboratory animal facility of the VAWNYHS. The knockout strain has no overt phenotypic defects 16. The number of animals used in this study was based on our previous work with influenza in mice 12–14,17,18. As a general rule, a specific experiment was repeated 3–4 times with a minimum of 3 mice in each group to account for inter- and intra-experiment variability, as well as potential experimental or assay failures. Our flu model has been established as a non-lethal challenge, wherein no more than 10% of any group succumbs to illness. Increasing the number of insults (i.e. flu and bacteria) increases the risk of animal mortality, and so additional animals were typically allocated to such groups. For humane reasons, in the interest of using as few animals as possible, fewer animals were used in subsequent experiments once it was determined that the double-infectious insult was a non-lethal model.
Halothane exposure
Mice were exposed to 1.5% halothane in 100% oxygen for two hours in a 37°C chamber infused at 1.5 L/min just prior to influenza infection (day 0), and again for two hours on day 4 post-influenza infection. An experimental timeline explaining groups and treatment/infection schedule is provided (Figure 1). Chamber concentrations of halothane, O2, and CO2 were continuously monitored with a Rascal II Ramen gas analyzer (Ohmeda Medical Equipment, Salt Lake City, UT). Our previous work demonstrated that day 0 exposure to anesthetic exerted the most clinical benefit (mortality, morbidity, lung damage, clinical score), and day 4 post-influenza was the last day post-influenza infection prior to sacrifice at which clinical benefit could be observed. The goal of this study was to identify the immune-modulating effects of halothane. To maximize the likelihood of identifying the specific parameters altered by this anesthetic, we wished to maximize the beneficial effects of the agent. To this end, we exposed all mice to halothane twice, as described above (first sentence of “Halothane exposure” section), on both day 0 (day of influenza infection) and day 4 (4 days post-influenza).
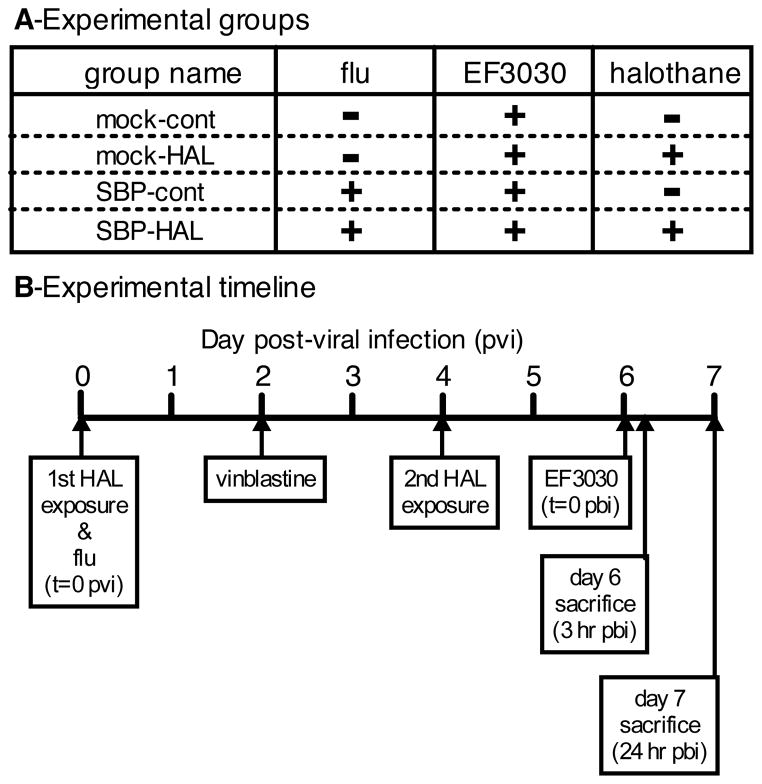
Figure 1. Experimental groups, and model timeline of anesthesia, treatment, and infections.
A) CD-1 mouse experimental group names used in the text and their respective treatments. SBP = secondary bacterial pneumonia; HAL = halothane; cont = control; EF3030 = Streptococcus pneumoniae. In the case of type I interferon knockout mice and their wild type controls, all mice were challenged with secondary bacterial pneumonia, and the only variable was +/− halothane exposure. B) Timeline of events in experimental model. Influenza inoculation (or mock infection) occurred on day 0 post-viral infection (pvi). Mice in the halothane groups were exposed for 2 hours on both days 0 and 4 pvi. EF3030 infection occurred on day 6 pvi. Mice were sacrificed on day 6 pvi, 3 hours post-EF3030, or day 7 pvi, 24 hours post-EF3030. In neutropenia studies, 5mg/kg vinblastine treatment was administered on day 2 pvi.
Influenza virus strain and infection procedure
The A/PR/8/34, mouse-adapted H1N1 strain of influenza virus was the generous gift of Dr. Hunein Massab (University of Michigan, Ann Arbor, MI). This strain was originally isolated from a patient in Puerto Rico in 1934. At the end of the halothane exposure, mice were inoculated with 40 plaque-forming units of influenza (determined by plaque formation in Madin-Darby Canine Kidney epithelial cell monolayers), intranasally. Non-halothane exposed control mice were sedated by intraperitoneal injection of 100 mg/kg ketamine in normal saline to facilitate virus inoculation.
Streptococcus pneumoniae strain and infection procedure
The EF3030 strain of Streptococcus pneumoniae, a clinical otitis media isolate with a 19F capsular serotype, was grown to mid-log phase in Todd-Hewitt broth with 0.2% yeast extract media, aliquoted, and stored at −80°C. At the time of bacterial infection (6 days post-viral infection), an aliquot was thawed and diluted in PBS, and either 2.5×106, or 120 colony-forming units (cfu, determined by titration on tryptic soy 5% sheep blood agar plates) was delivered to the lungs by oropharyngeal aspiration in a 50 μl volume during ketamine sedation.
Morbidity/clinical scoring
Mice were randomly assigned to the various groups, and weighed daily throughout the virus infection period. Just prior to sacrifice (24 hr post-bacterial infection, i.e., 7 days post-viral infection) the symptoms of illness were scored (0–6) daily by the same blinded researcher by awarding 1 point each for the following: piloerection, hunched posture, impaired gait, labored breathing, lethargy, and weight loss 10% of the body weight at time of infection 19.
Sacrifice, tissue harvest, and bronchoalveolar lavage (BAL) procedures
At 7 days post-viral infection (with or without 24 hr bacterial exposure), the mice were anesthetized with 2.75% isoflurane and exsanguinated by transecting the vena cava/abdominal aorta through a peritoneal incision. The diaphragm and rib cage were cut away to facilitate injection of 5 ml Hank’s Balanced Salt Solution with Ca2+, Mg2+ (37°C) into the right ventricle of the heart to flush the pulmonary vasculature of residual blood. Following exposure of the trachea a 22 ga stainless steel cannula was inserted into the trachea and secured with a suture. A 4-way stopcock with 2, 5 cc syringes attached was connected to the cannula and BAL performed by instilling 1 ml Hank’s Balanced Salt Solution with Ca2+, Mg2+ (37°C) into the lungs with one syringe and collecting the volume with the other. This procedure was repeated for a total of 5 instillations with the collected volume stored on ice. Lastly, the lungs were excised and stored on ice.
Sample processing
Collected bronchoalveolar lavage (BAL) fluid was centrifuged at 1,500 × g for 3 min at 4°C and the supernatant stored at −80°C. The recovered cells were enumerated with a MultiSizer III (Beckman Coulter, Fullerton, CA) Coulter counter, a cytoslide prepared with a CytoSpin 3 cytocentrifuge (Shandon Lipshaw, Pittsburg, PA), and stained with Diff-Quik (Dade Behring, Newark, NJ) for differential counting by light microscopy. The lungs were homogenized in buffer containing 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, pH=7.4 with protease inhibitor cocktail (Calbiochem, Gibbstown, NJ) containing 500 μM AEBSF, 150 nM aprotinin, 1 μM E-64, 0.5 mM EDTA, and 1 μM leupeptin (total weight of buffer+lungs = 3 g) using a Polytron PT-2000 tissue homogenizer (Brinkman Instruments, Westbury, NY). Myeloperoxidase (MPO) was extracted from the homogenate, as previously described 20.
Determination of lung bacterial load
EF3030 bacterial titer was determined in BAL fluid and lung homogenate by removing 100 μl aliquots prior to being centrifuged and plating 10-fold serial dilutions in phosphate buffered saline on tryptic soy 5% sheep blood agar plates (VWR, Rochester, NY), incubating at 37°C for 24 hr, and counting colonies, each representing a cfu. Lung bacterial load was calculated as: (BAL fluid cfu/ml × recovered BAL volume, ml) + (lung homogenate cfu/ml × 3 ml).
Cytokine/chemokine/albumin analysis by enzyme linked immuno-sorbent assay (ELISA)
BAL fluid cytokine, chemokine, and albumin concentrations were determined by ELISA, as described previously 21. Capture and detection antibodies and recombinant protein standards were acquired from R&D Systems (Minneapolis, MN) except for albumin (Bethyl Laboratories, Montgomery, TX) 20.
Myeloperoxidase activity assay
Myeloperoxidase activity was assessed by adding 10 μl of lung homogenate extract to 300μl of buffer containing 50 mM KH2PO4, 176 mM H2O2, 52.5 mM o-dianisidine dihydrochloride, pH=6.0 in a 96-well flat-bottom plate. Absorbance readings at 460 nm were taken for 90 sec every 2 sec using a SpectraMax 190 microplate reader with SoftMax Pro v.4.0 software (MDS Analytical Technologies, Sunnyvale, CA). MPO activity was expressed in arbitrary units expressed as absorbance change per minute (abs/min) as defined as the absorbance change per minute over the linear portion of the curve 20.
In vivo polymorphonuclear cell (PMN; neutrophil) depletion
Vinblastine (Sigma-Aldrich, St. Louis, MO), 5 mg/kg, was administered to mice intravenously on day 2 post-viral infection by retro-orbital injection 22. Successful depletion of PMN four days following vinblastine (day 6 post-viral infection), at the time of secondary pneumococcal challenge, was confirmed by obtaining a drop of blood from a tail snip, producing a blood smear slide, and staining with Diff-Quik. A leukocyte differential count was determined by light microscopy.
Statistical analysis
A two-way ANOVA was performed with influenza- and halothane-exposure as the factors to determine if there was a main effect due to halothane exposure (p<0.05 considered to be significant). A two-tailed Bonferroni post-hoc test (correcting for multiple comparisons) was performed to determine the source of that variability. Analyses were performed using Prism 5 for Mac OS X (GraphPad Software, San Diego, CA). Some data was lost during the study due to: animal mortality (minor contributor to loss due to use of non-lethal influenza inoculum), cytokine ELISA assay failure due to occasional hyper-reactive wells of assay plates, and contamination of blood agar plates that prevented bacterial quantification.
RESULTS
Halothane maintained anti-bacterial immune function post-flu and decreased morbidity and lung injury
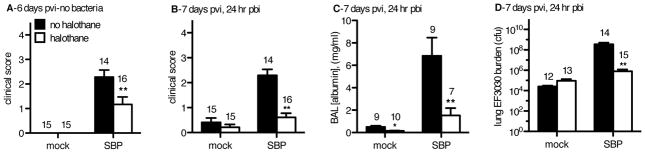
At day 6 post-viral infection (pvi), halothane exposure significantly minimized clinical symptoms following influenza (flu) infection (Figure 2A, p<0.05 by two-way ANOVA with Bonferroni post-hoc test). Upon pulmonary challenge with 2.5×106 cfu of the EF3030 strain of pneumococcus, mock flu-infected mice (mock-cont and mock-HAL groups; Figure 1) exhibited an equivalent level of clinical symptoms 24 hours post-bacterial infection (pbi), regardless of anesthetic exposure (Figure 2B). However, mice infected with influenza 6 days prior to the EF3030 challenge, our secondary bacterial pneumonia (SBP) groups (SBP-cont and SBP-HAL), had significantly less symptoms of illness 24 hours post-bacterial infection (pbi) if anesthetized with halothane. SBP-HAL mice exhibited symptoms comparable to mice that had never been infected with flu, indicating diminution of host morbidity upon SBP challenge due to halothane exposure (Figure 2B). Importantly, airway albumin levels, a reliable marker of pulmonary damage, were significantly decreased by over 4-fold in SBP-HAL mice compared to the SBP-cont group (Figure 2C). Assessment of bacterial burden at 24 hours pbi revealed that mock-cont and mock-HAL mice challenged only with EF3030 displayed no difference in bacterial burden due to halothane exposure indicating that halothane does not fundamentally enhance the anti-bacterial capacity of the host in the absence of prior flu infection (Figure 2D). By contrast, SBP-HAL mice challenged with pneumococci 6 days post-flu had almost 450-fold less viable bacteria burden compared to the SBP-cont group, and their bacterial burden was equivalent to mice that had never been infected with flu (Figure 2D). Of note, given the initial 2.5 × 106 cfu inoculum of EF3030, most of the SBP-HAL mice had bacterial burdens less than this initial inoculum at 24 hours pbi, indicating effective bacterial clearance, whereas SBP-cont mice harbored burdens in the 109 cfu range, indicating pneumococcal outgrowth, far larger than the initial inoculum (Figure 2D). In order to identify how halothane might maintain anti-bacterial immunity post-flu, we assessed levels of a panel of essential chemical and cellular elements essential for mediating pulmonary anti-bacterial immunity.
Figure 2. Halothane minimizes clinical symptoms, lung damage, and bacterial burden of secondary bacterial pneumonia (SBP) post-flu.
CD-1 mice were inoculated intranasally (i.n.) with 40 plaque-forming units A/PR/8/34 influenza virus (SBP) or mock-infected (mock). Mock-HAL and SBP-HAL mice (white bars) were exposed to 2% halothane for 2 hr just prior to infection (day 0) and again for 2 hr on day 4 post-viral infection (pvi). Mock-cont and SBP-cont mice (black bars) received ketamine sedation. A) On day 6 pvi, mice were assessed for clinical score on a 6-point scale then inoculated i.n. with 2.5×106 colony-forming units (cfu) Streptococcus pneumoniae (EF3030). B) 24 hr post-bacterial infection (pbi) mice were again assessed for clinical score. They were sacrificed, bronchoalveolar lavage (BAL) performed and lungs harvested. C) Cell-free BAL fluid was assessed for albumin concentration, as an indicator of lung injury. D) Non-clarified recovered BAL fluid and lung homogenates were titered for EF3030 cfu and a total EF3030 lung burden determined. Sample sizes are displayed above groups. *p<0.05, **p<0.01 SBP-HAL compared to SBP-cont. Data expressed as mean +/− SEM.
Halothane altered the expression of cytokines and chemokines that affect immune cell recruitment during an influenza infection and 24 hr secondary pneumococcal challenge
SBP-HAL mice exhibited significantly decreased levels of total airway infiltrates, airway macrophages and parenchymal polymorphonuclear cells/neutrophils (PMN) compared to SBP-cont mice at 24 hours pbi (data not shown). In accordance with this, KC, MIP-2 (neutrophilic chemokines) and MCP-1 (a monocytic chemokine) were also diminished in the SBP-HAL group compared to SBP-cont mice (data not shown). However, the significant differences of bacterial burden and lung injury between the SBP-cont and SBP-HAL mice at the 24 hour pbi time-point could significantly affect these immunological parameters so definitive conclusions regarding halothane-mediated immune-modulation post-flu cannot be made (Figure 2C and 2D). We therefore sought to determine how halothane might beneficially alter the ability of the host to immediately respond to a secondary bacterial challenge post-flu at 3 hours pbi, when bacterial burden and lung injury are equivalent for all groups.
Mice were again challenged with 2.5×106 cfu EF3030 six days pvi (or post mock-flu infection) and sacrificed at 3 hours pbi, to identify differences in their immediate immunological response to pneumococcal challenge. At 3 hours pbi, mice in all groups harbored more pneumococci than the original inoculum, indicating all groups experience an initial and equivalent out-growth of bacteria in the first few hours pbi, irrespective of prior flu infection or anesthetic exposure (Figure 3A). Importantly, tissue damage was also equivalent between the two mock-flu infected groups, and between the two SBP groups (Figure 3B). This eliminated the concern that differences in pneumococcal burden or tissue damage could affect the host immune response, and allowed for adequate assessment of the immunological parameters altered by halothane at this early time point post-pneumococcal challenge.
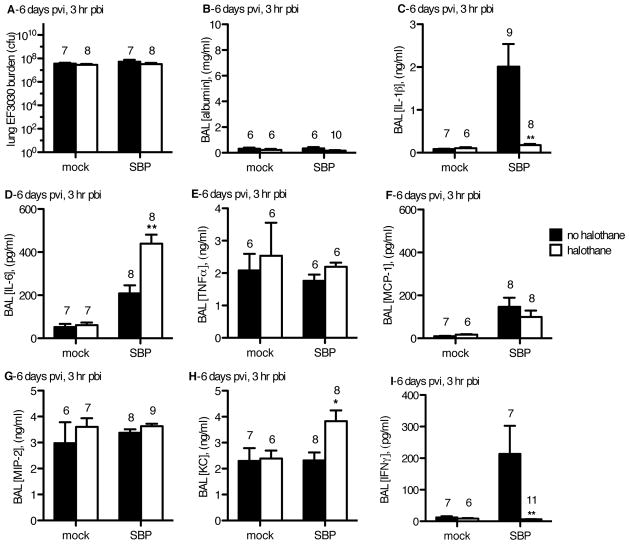
Figure 3. In a mouse model of secondary bacterial pneumonia (SBP), at 3 hr pbi, EF3030 burden and lung damage were equivalent, but halothane-induced alterations in cytokine expression were observed in flu-infected mice.
CD-1 mice were inoculated intranasally (i.n.) with 40 plaque-forming units A/PR/8/34 influenza virus (SBP) or mock-infected (mock). Mock-HAL and SBP-HAL mice (white bars) were exposed to 2% halothane for 2 hr just prior to infection (day 0) and again for 2 hr on day 4 post-viral infection (pvi). Mock-cont and SBP-cont mice (black bars) received ketamine sedation. On day 6 pvi, mice were inoculated with 2.5×106 cfu EF3030 and 3 hr later (3 hr post-bacterial infection; pbi) mice were sacrificed, BAL performed and lungs harvested. A) Non-clarified recovered BAL fluid and lung homogenates were titered for EF3030 to determine the total EF3030 lung burden. Cell-free BAL fluid was assessed for B) albumin, C) IL-1β, D) IL-6, E) TNFα, F) MCP-1, G) MIP-2, H) KC, and I) IFNγ. Sample sizes are displayed above groups. *p<0.05, **p<0.01 SBP-HAL compared to SBP-cont. Data expressed as mean +/− SEM.
Amongst SBP groups, halothane demonstrated the ability to minimize levels of the proximal inflammatory cytokine, IL-1β during SBP (Figure 3C). By contrast IL-6 was significantly elevated in the SBP-HAL group compared to the SBP-cont group (Figure 3D). However, TNF-α levels were unaffected by halothane in SBP-HAL mice (Figure 3E). This demonstrates that in the context of SBP post-flu, halothane exposure uncouples the immediate expression levels of these three proximal inflammatory cytokines that are thought to be co-regulated with one another by the p50 and p65 NF-κB heterodimer 23. Expression levels of these cytokines were unaffected by halothane in mock-flu infected mice (Figure 3C–3E). These results provide additional evidence that halothane exposure alters the host immune response to a secondary bacterial challenge following a prior flu infection, but not in mock-flu infected animals challenged only with pneumococci.
Next, we assessed levels of anti-viral cytokines (type I IFNs), as well as chemokines known to be critical for leukocyte recruitment to the lung environment. Flu infection with SBP (SBP-cont and SBP-HAL groups) resulted in elevated MCP-1 levels compared to the mock-flu infected mice challenged only with pneumococcus (mock-cont, mock-HAL). Halothane exposure had no effect on the production of this chemokine (Figure 3F). There was no significant difference in MIP-2 levels between the two mock-flu groups, or between the two SBP groups (Figure 3G). However, the neutrophilic chemokine KC was significantly elevated in the SBP-HAL mice compared to the SBP-cont group at this early 3 hour pbi time point despite the equivalent bacterial burden and lung injury (Figure 3H).
Type I and type II IFNs are essential elements that protect the host during virus infection, and both have been shown to inhibit anti-bacterial immunity in the lung post-flu through impaired PMN recruitment and alteration of macrophage phenotype 3–5. Given their potential to inhibit pulmonary anti-bacterial immunity, pulmonary expression levels of IFN-α (type I IFN) and IFN-γ (type II IFN) were investigated. On day 6 post-flu, IFN-α was low to undetectable in all groups of mice (data not shown), but the level of IFN-γ was elevated in the flu-infected SBP-cont mice compared to SBP-HAL. Halothane exposure reduced the IFN-γ levels to nearly undetectable levels (Figure 3I). From these findings we postulated that the alveolar macrophages’ anti-bacterial immune function may be compromised due to increased type II IFN levels and that halothane may confer protection by minimizing type II IFN production. Additionally, halothane may cause an increase in KC-mediated PMN recruitment to compensate for the altered macrophage phenotype, thus increasing anti-bacterial defense and decreasing pulmonary injury. To begin to test this hypothesis, we assessed levels of various immune cell infiltrates.
Halothane fosters rapid neutrophil recruitment post-influenza in response to a secondary pneumococcal challenge
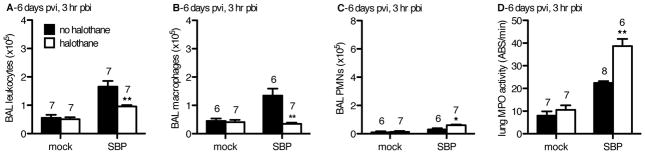
In SBP-cont mice, levels of total immune infiltrates were significantly enhanced as early as 3 hours pbi compared to all other groups (Figure 4A). Mock-flu infected control mice had 4.5±0.8×105 macrophages recovered from the BAL that is within the range of 2–5×105 airway macrophages expected to be isolated from a naïve mouse unchallenged with any pathogen from our lab’s past experience (Figure 4B and data not shown). Additionally, although PMN were the predominant leukocyte in all groups at 24 hours pbi (data not shown), mock-flu infected controls did not recruit significant numbers of PMN at 3 hours pbi, constituting less than 20% of total infiltrates (Figures 4A and 4C). These mock-flu infected mice also had the lowest bacterial burdens at 24 hours pbi, indicating that the macrophage population in these mice was more efficacious at controlling the bacterial challenge. By comparison, both SBP groups that had been challenged with flu 6 days prior had macrophage numbers equal or greater to those of mock-flu infected controls (Figure 4B). Despite having the highest macrophage numbers, the SBP-cont group was the least capable of mounting an anti-bacterial response. This finding supports our hypothesis that impairment of macrophage anti-bacterial function due to host immune responses to flu infection (i.e., enhanced type II IFN levels in the SBP-cont group (Figure 3I)) is mitigated by halothane. Also in agreement with our hypothesis, halothane enhanced KC levels in SBP-HAL mice (Figure 3H) that correlated with an increase in immediate airway PMN recruitment (Figure 4C). Levels of parenchymal PMN were also elevated in the SBP-HAL group (Figure 4D), as indicated by lung homogenate myeloperoxidase activity. The ratio and overall number of PMNs recruited relative to macrophage recruitment in SBP-cont compared to SBP-HAL mice was markedly different. Despite significantly less total immune infiltrates in the SBP-HAL group, these mice have significantly more total PMN than the SBP-cont mice (Figures 4A and 4C). It was found that 65% of all airway infiltrates are PMN in SBP-HAL mice, compared to only 27% in SBP-cont animals. Halothane reversed the PMN:macrophage recruitment ratio of 1:2 in SBP-cont mice to 2:1 in the SBP-HAL group. This finding indicates that the diminished expression of KC due to prior influenza infection is likely biologically significant, and results in less PMN recruitment in SBP-cont mice that may be necessary to compensate for the impaired anti-bacterial immune state in the lungs that we observe. The enhanced PMN recruitment mediated by halothane exposure may be required to compensate for this, resulting in the ~3-log less pneumococcal burden and less lung damage at 24 hours pbi.
Figure 4. In a mouse model of SBP, at 3 hr pbi, halothane exposure resulted in a decrease in the # of alveolar macrophages and an increase in the # of neutrophils (PMNs) in the pulmonary airspaces in flu-infected mice.
CD-1 mice were inoculated intranasally (i.n.) with 40 plaque-forming units A/PR/8/34 influenza virus (SBP) or mock-infected (mock). Mock-HAL and SBP-HAL mice (white bars) were exposed to 2% halothane for 2 hr just prior to infection (day 0) and again for 2 hr on day 4 post-viral infection (pvi). Mock-cont and SBP-cont mice (black bars) received ketamine sedation. On day 6 pvi, mice were inoculated with 2.5×106 cfu EF3030 and 3 hr later (3 hr post-bacterial infection; pbi) mice were sacrificed, BAL performed and lungs harvested. The cells recovered from the pulmonary airspaces by BAL were analyzed: A) total # of leukocytes, B) total # of macrophages, C) total # of PMNs. D) The harvested lungs were homogenized and myeloperoxidase (MPO) activity determined as a measure of PMN infiltration into the lung parachyma. Sample sizes are displayed above groups. *p<0.05, **p<0.01 SBP-HAL compared to SBP-cont. Data expressed as mean +/− SEM.
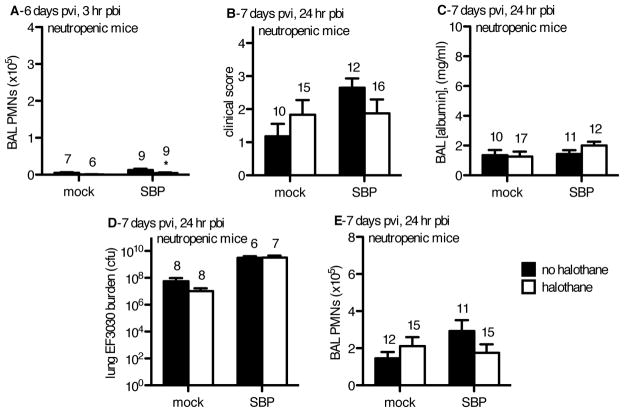
In order to test if early recruitment of PMN facilitated by halothane was central to this agent’s ability to minimize risk and severity of SBP, we predicted neutropenic mice challenged with SBP would no longer exhibit enhanced anti-bacterial immune function in the lungs following halothane exposure.
Neutropenic mice challenged with pneumococci demonstrate PMN recruitment is essential to halothane’s maintenance of anti-bacterial immunity post-influenza
To test if the early PMN recruitment facilitated by halothane exposure is central to this agent’s ability to benefit the host and minimize the risk and severity of SBP post-flu, we administered vinblastine on day 2 post-viral infection (pvi) to make the mice neutropenic by day 6 pvi, at the time of secondary pneumococcal challenge. Selective depletion of PMN by vinblastine, a chemotherapy agent, is accomplished due to the rapid generation time of these cells and their short lifespan (1–3 days), which is far shorter than other cells of the immune system 22. We predicted that halothane’s beneficial enhancement of the host’s anti-bacterial immune response post-influenza would not be exhibited in neutropenic mice. At the time of bacterial challenge (6 days pvi and 4 days post-vinblastine) circulating PMNs were reduced to approximately 1% of circulating leukocytes, as assessed by blood smear differential counts (data not shown). Also indicating successful PMN depletion by vinblastine (including the intravascular PMN margination pool), cell recovery from the BAL of the halothane SBP group at 3 hours pbi was 7.7% of the PMN recovery in the same group in non-neutropenic mice (4.7±1.4×103 PMNs (Fig 5A) compared to 6.1±0.5×104 PMNs (Figure 4C), p<0.05). This level of PMN recruitment was similar to that observed in the mock-flu infected groups (Figure 5A). Macrophage levels in the lungs at the time of pneumococcal challenge were not decreased due to our vinblastine regimen (data not shown). In neutropenic mice, halothane failed to minimize clinical symptoms (Figure 5B), lung injury (Figure 5C), and pneumococcal burden (Figure 5D) at 24 hours pbi in SBP mice. At day 7pvi/24 hours pbi (5 days post-vinblastine) there was an increase in PMNs recovered in the BAL in both flu-infected groups (Figure 5E). These findings indicate that early and immediate PMN recruitment mediated by halothane in the SBP-HAL group is essential for this agent’s ability to mitigate SBP.
Figure 5. In a mouse model of SBP, halothane exposure did not reduce lung injury or bacterial burden following vinblastine-induced neutropenia.
CD-1 mice were inoculated intranasally (i.n.) with 40 plaque-forming units A/PR/8/34 influenza virus (SBP) or mock-infected (mock). Mock-HAL and SBP-HAL mice (white bars) were exposed to 2% halothane for 2 hr just prior to infection (day 0) and again for 2 hr on day 4 post-viral infection (pvi). Mock-cont and SBP-cont mice (black bars) received ketamine sedation. On day 2 pvi, mice were administered 5 mg/kg vinblastine to induce neutropenia. On day 6 pvi, mice were inoculated with 2.5x106 cfu EF3030 and at 3 hr post-bacterial infection (pbi) or 24 hr pbi mice were sacrificed, BAL performed and lungs harvested. An indication of successful neutropenia, A) the # of neutrophils (PMNs) recovered by BAL on day 6 pvi and 3 hr pbi were markedly reduced in all injury groups. On day 7 pvi, 24 hr pbi, B) there was no difference in clinical score, or C) BAL [albumin] between all injury groups. D) Bacterial burden was higher in the influenza-infected mice and halothane exposure had no effect (cfu = colony forming units). E) There was no effect of influenza or halothane exposure on PMN recruitment into the lungs. Sample sizes are displayed above groups. *p<0.05, **p<0.01 SBP-HAL compared to SBP-cont. Data expressed as mean +/− SEM.
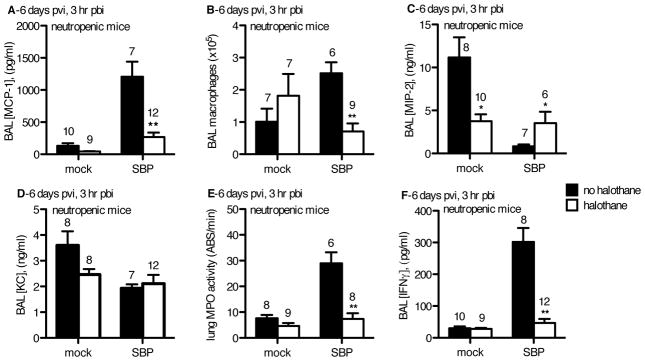
We assessed the immediate (3 hour pbi) inflammatory response in neutropenic animals on day 6 pvi. SBP-HAL mice had reduced levels of MCP-1 in the BAL (Figure 6A) with an associated decrease in numbers of macrophages recovered by BAL (Figure 6B) compared to SBP-cont mice. However, despite the early increase in macrophage recruitment, SBP-cont mice were not protected from SBP given the equivalent burdens in both SBP groups at 24 hours pbi (Figure 5D), potentially indicating that these macrophages were inefficient at limiting pneumococcal infection due to prior flu infection.
Figure 6.
CD-1 mice were inoculated intranasally (i.n.) with 40 plaque-forming units A/PR/8/34 influenza virus (SBP) or mock-infected (mock). Mock-HAL and SBP-HAL mice (white bars) were exposed to 2% halothane for 2 hr just prior to infection (day 0) and again for 2 hr on day 4 post-viral infection (pvi). Mock-cont and SBP-cont mice (black bars) received ketamine sedation. On day 2 pvi, mice were administered 5 mg/kg vinblastine to induce neutropenia. On day 6 pvi, mice were inoculated with 2.5×106 cfu EF3030 and at 3 hr post-bacterial infection (pbi) mice were sacrificed, BAL performed and lungs harvested. A) BAL [MCP-1], B) total # of macrophages recovered in the BAL, C) BAL [MIP-2], D) BAL [KC], E) lung homogenate myeloperoxidase (MPO) activity, and F) BAL IFNγ were determined. Sample sizes are displayed above groups. *p<0.05, **p<0.01 SBP-HAL compared to SBP-cont. Data expressed as mean +/− SEM.
The CXC neutrophilic chemokine MIP-2, but not KC, was elevated in SBP-HAL mice as compared to the SBP-cont group (Figure 6C and 6D). This is in contrast to our prior observations in PMN-replete mice of elevated KC levels (which was associated with rapid PMN recruitment), but no differences in MIP-2 levels in SBP-HAL mice compared to SBP-cont mice (Figures 3G and 3H). However, this elevation of MIP-2 was to no avail given PMN depletion, and SBP-HAL mice had even less airway and interstitial PMN than SBP-cont mice, and yet these two groups harbored equivalent E3030 burden at 3 hours and 24 hours pbi indicating that despite enhanced PMN levels in SBP-cont mice, this was insufficient for enhanced protection from pneumococcal infection post-flu (Fig 5E, 6C, 6D, 6E). Of note, IFN-γ levels in the lungs of mice in the SBP-HAL group were far less than that assessed in SBP-cont mice as we previously observed (Fig 6F). This indicated that halothane maintains its ability to minimize IFN-γ levels in PMN-depleted mice as it has done in PMN-replete mice.
In our PMN-depletion studies, halothane decreased MCP-1, macrophage recruitment, and IFN-γ in SBP-HAL compared to SBP-cont mice, and despite significantly more macrophages in the SBP-cont group, these mice harbored the same pneumococcal burden at 24 hours pbi as the SBP-HAL group. These findings imply that the anti-bacterial function of alveolar macrophages post-flu is superior in SBP-HAL mice compared to the SBP-cont group. To assess the ability of halothane to mitigate the dysfunction of alveolar macrophages post-flu, we challenged neutropenic mice post-flu with a small innoculum of EF3030 that does not require PMN recruitment for clearance, and assessed the ability of the lungs to clear the bacteria.
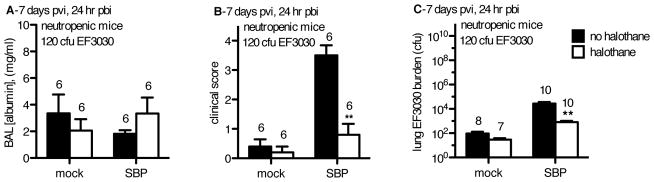
A minor insult of pneumococci in neutropenic mice is potentially infectious, indicating macrophage dysfunction post-flu that is mitigated by halothane
In both neutropenic and PMN-replete animals, IFN-γ levels were elevated in SBP-cont mice, but halothane exposure reduced those levels (Fig 3I, 6F). This cytokine has been shown to inhibit macrophage anti-bacterial function post-flu 4. To assess if halothane maintains macrophage anti-bacterial function, we employed our same model, but challenged neutropenic mice with only 120 cfu as opposed to the 2.5×106 cfu of EF3030 used previously. Although lung injury was not improved (Figure 7A), morbidity (Figure 7B) and pulmonary burden of EF3030 (Figure 7C) were reduced in neutropenic SBP-HAL mice compared to SBP-cont mice. While mock-flu controls, and SBP-HAL mice successfully managed to curtail pneumococcal outgrowth in the absence of PMN assistance, the SBP-cont mice failed to do so. The SBP-cont mice exhibited outgrowth of bacteria from the initial inoculum. This indicated that halothane not only fosters an initial and immediate recruitment of PMN post-flu upon secondary challenge, but also serves to maintain anti-bacterial competence of macrophages in the lung post-flu.
Figure 7.
CD-1 mice were inoculated intranasally (i.n.) with 40 plaque-forming units A/PR/8/34 influenza virus (SBP) or mock-infected (mock). Mock-HAL and SBP-HAL mice (white bars) were exposed to 2% halothane for 2 hr just prior to infection (day 0) and again for 2 hr on day 4 post-viral infection (pvi). Mock-cont and SBP-cont mice (black bars) received ketamine sedation. On day 2 pvi, mice were administered 5 mg/kg vinblastine to induce neutropenia. On day 6 pvi, mice were inoculated with 120 cfu EF3030 and at 24 hr post-bacterial infection (pbi) mice were sacrificed, BAL performed and lungs harvested. A) BAL [albumin], B) mouse clinical score, and C) total lung EF3030 burden were determined. Sample sizes are displayed above groups. *p<0.05, **p<0.01 SBP-HAL compared to SBP-cont. Data expressed as mean +/− SEM.
In the absence of type I IFN influence, halothane fails to protect the host from SBP
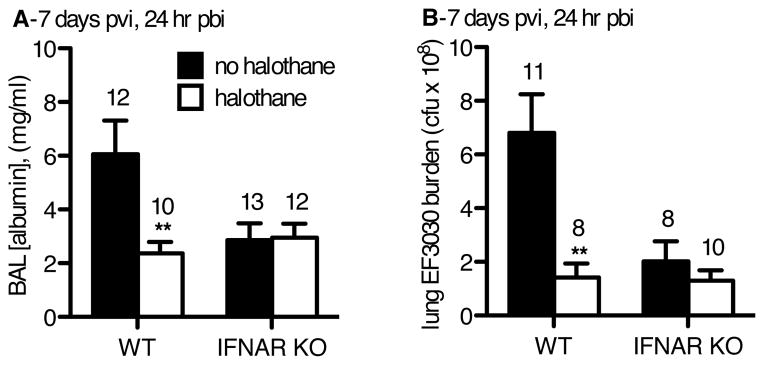
Our previous work has demonstrated that volatile anesthetics (i.e., halothane, isoflurane, sevoflurane) minimize the effects of type I IFNs on immune cell activation and clinical symptoms of patients afflicted with respiratory tract infections 2,15. Type I IFNs enhance the Th1 adaptive immune response following influenza infection, which enhances IFN-γ expression and inhibits both PMN recruitment and macrophage anti-bacterial capacity rendering the host highly susceptible to SBP. We hypothesized that halothane’s protection from SBP vulnerability would be dependent on type I IFN elaboration. To test this hypothesis we employed Ifnar1tm1Agt/Ifnar1tm1Agt mice devoid of the type I IFN receptor/interferon-α receptor (IFNAR), and assessed if these knockout mice had any benefit from halothane compared to wild-type controls.
Wild-type (C57BL/6) and IFNAR knockout mice were infected with flu and challenged with 2.5×106 cfu of EF3030 on day 6 pvi. At 24 hours pbi, mice were sacrificed and lung injury and EF3030 bacterial burden were assessed. Similar to the previous experiment in CD-1 mice (Figures 2C and 2D), halothane-exposed wild-type mice exhibited decreases in lung injury and bacterial burden compared to the wild-type control (not exposed to halothane) mice (Figures 8A and 8B). The levels of lung injury and bacterial burden in the IFNAR knockout mice, with or without halothane exposure, were similar to the levels attained in the wild-type mice that were exposed to halothane. Halothane did not produce any additional benefit in regards to both lung injury and bacterial burden in the IFNAR knockout mice than what was afforded by the loss of an intact type I IFN response. This result supports the hypothesis that halothane’s protection from SBP is conferred by halothane’s modulation of the type I IFN response to flu.
Figure 8.
C57BL/6 wild type (WT) and interferon-alpha receptor knock-out (IFNAR KO) mice were inoculated intranasally (i.n.) with 40 plaque-forming units A/PR/8/34 influenza virus day 0 post-viral infection (pvi), and exposed to 2.5 × 106 colony forming units (cfu) EF3030 (day 6 pvi) and sacrificed and harvested (24 hr pbi). Halothane exposed mice (white bars) were exposed to 2% halothane for 2 hr just prior to infection (day 0) and again for 2 hr on day 4 post-viral infection (pvi). Non-halothane exposed mice mice (black bars) received ketamine sedation. A) BAL [albumin], and B) total lung EF3030 burden were determined. Sample sizes are displayed above groups. *p<0.05, **p<0.01 WT or IFNAR KO halothane exposed mice compared to their non-halothane exposed control of their respective genetic background. Data expressed as mean +/− SEM.
DISCUSSION
Previously, we demonstrated that pediatric patients exposed to halothane for minor procedures that had viral upper respiratory tract infections had significantly fewer symptoms, and shorter duration of symptoms compared to patients that did not receive halothane 2. However, this study was not powered to address the risk of viral associated SBP, a major cause of morbidity and mortality worldwide. In the case of the 1918 “Spanish Flu”, SBP was the major cause of death, and S. pneumoniae was a major pathogen responsible for this 1,9,24. It is interesting that this commonly commensal bacterium of the nasopharynx can be so devastating. Yet it is evident that following influenza infection this bacterium possesses the capacity to wreak havoc on the lungs, compromising pulmonary function and in severe circumstances lead to death. Despite the advent of antibiotics, this pathogen is still a major cause of SBP post-flu.
Many studies have focused on the opportunistic nature and virulence factors of pathogens associated with SBP. These are worthwhile studies, but a common factor that underlies SBP regardless of the organism(s) responsible is the host immune status post-flu. In our studies, we demonstrate that immune-modulation by the halothane ameliorates symptoms, lung injury, and pulmonary burden of S. pneumoniae post-flu compared to controls. From our investigation, these benefits are due to halothane-mediated minimization of the effects of type I IFN. This maintains a host immune status in the pulmonary environment that is capable of combating SBP, which is typically impaired in patients post-flu.
Halothane did not enhance baseline anti-bacterial immune function, nor was the agent bactericidal as evidenced from our mock-flu infected controls (Fig 2D). Instead, halothane maintained baseline antibacterial immunity in previously flu-infected SBP mice, equivalent to that of mock-flu infected controls. This led us to our hypothesis that halothane alters aspects of the host anti-viral response that if left unchecked significantly impair anti-bacterial immunity and exacerbate the risk and severity of SBP.
We demonstrate that rapid PMN recruitment to the lungs is critical following SBP challenge, and halothane facilitates this, likely through enhanced production of neutrophilic chemokines such as KC, and MIP-2. When PMN are ablated, halothane no longer benefits the host and SBP severity is equivalent to that observed in controls. Mice challenged with SBP exhibited a significant increase in pulmonary IFN-γ levels, and this was minimized by halothane (Fig 3I). This cytokine has been shown to inhibit anti-bacterial functions of macrophages, rendering the host at increased risk of SBP 4. Minimization of this cytokine by halothane was associated with increased PMN recruitment, and decreased macrophage recruitment, and ultimately decreased bacterial infection and lung injury. We postulated that enhanced recruitment of PMN upon SBP challenge that we observed due to halothane was essential to compensate for impaired alveolar macrophage anti-bacterial function. Impaired macrophage anti-bacterial function post-flu was indeed observed in our PMN-depletion studies using a minor (120 cfu) EF3030 inoculum that does not require PMN recruitment, and halothane ameliorated this as well, whereas the SBP-cont group demonstrated bacterial burden greater than the inoculum (outgrowth). These findings indicate that halothane allows the host to combat bacterial infection post-flu through decreased IFN-γ expression, minimizing the impairment of macrophage anti-bacterial function, and by fostering compensatory PMN recruitment while diminishing newly-recruited, potentially damaging macrophages, to the pulmonary environment.
Others have shown the detrimental roles type I IFN can have post-flu that leave the host more susceptible to SBP. These effects of type I IFN include impaired expression of neutrophilic chemokines, alteration of bone-marrow monocyte precursors to a phenotype that preferentially recruits more inflammatory macrophages instead of PMN, and enhancement of numerous aspects of the adaptive Th1 response (of which IFN-γ is the quintessential cytokine) that results in increased pulmonary injury and diminished macrophage anti-bacterial activity 3–5,25–27. All of our biochemical and cytological studies indicate that halothane protects the host from these known responses to type I IFN post-flu, without altering type I IFN expression levels. These findings, coupled with our past work demonstrating that the effects of type I IFN on immune cells are significantly diminished by halothane led us to the hypothesis that modulation of the effects of type I IFN by this agent in the context of SBP is most likely responsible for the significant improvement in anti-bacterial immunity post-flu. We tested this through the use of mice devoid of the type I IFN receptor, and found that when the type I IFN signaling axis is absent, halothane no longer benefitted animals. Regardless of halothane exposure status, IFNAR knockout mice had low levels of lung injury and bacterial burden equivalent to that of WT SBP mice receiving halothane, and WT SBP mice that did not receive halothane had far more lung injury and pneumococcal infection. The lack of benefit from halothane in IFNAR KO mice can be explained by the fact that their genetic status provides protection from improperly delayed type I IFN expression post-flu and therefore halothane serves no additional benefit, unlike in WT controls 28,29. This confirmed that alteration of the effects of type I IFN by halothane was the major mechanism responsible for minimizing risk of SBP.
Type I IFN evolved to protect organisms from viral infections, and this is why all nucleated cells in the human body can express type I IFN. Clearly type I IFN did not evolve over eons to serve as an Achilles heel to the host post-flu. However, influenza perturbs the proper expression pattern of type I IFN (especially its timing), primarily through its virulence factor NS1, and this alteration is what we believe renders the host susceptible to catastrophic SBP following flu through both direct (inhibited PMN recruitment) and downstream actions (enhanced Th1 response; IFN-γ) 28,29. Instead of peak type I IFN expression occurring prior to peak viral titers, peak expression of type I IFN occurs at day 5 pvi in our model, well past peak viral titers that occur day 3 pvi. Altered type I IFN expression is found in patients suffering from flu as well 30. At this time, type I IFN cannot serve its appropriate role of minimizing viral titers, yet it can dramatically enhance the Th1 response, impair anti-bacterial immunity, and increase immune-mediated damage to host tissue. This explains what we dub the “interferon paradox”, wherein type I IFN may be both beneficial and detrimental to the host. Essentially, inappropriately timed type I IFN fails to minimize viral infection, but enhances Th1 immune response and impairs anti-bacterial immunity. Halothane ameliorates this.
Halothane and ketamine were employed in this study given our familiarity with these compounds in this model, and our well-established evidence that halothane possesses the ability to alter host immune responses which we sought to further elucidate. Although ketamine has potential side effects, its use was necessary to sedate the live animals to ensure accurate delivery of inoculum to the lower airways. Through years of use with this model, we have not detected any affects of ketamine on any of the immunological or clinical parameters assessed. We have also demonstrated that the commonly used clinical volatile anesthetics, isoflurane15 and sevofluorane (unpublished data in 2002 from Paul R Knight, M.D., Ph.D. indicating sevoflurane exposure reduced mortality in influenza-infected CD-1 mice), also possess the immunomodulatory abilities of halothane. It is possible that if these agents have the same efficacy in low concentrations, they might be employed prophylactically or therapeutically in patients with flu to mitigate the risk of SBP by modulating the errant type I IFN response. Additionally, compounds that possess the same immunological effects that lack anesthetic properties could be used clinically to enhance anti-bacterial immunity in the lungs post-flu. Halothane facilitates rapid PMN recruitment and minimizes the impairment of macrophage anti-bacterial immune function which is inhibited by host anti-viral factors such as type I IFN and IFN-γ, respectively. This immunomodulation greatly decreases risk and severity of SBP. Microbial resistance to antibiotics is always increasing, and is a major public health concern. Therefore these studies have important scientific and clinical implications. Our animal model aims to explain the clinical observations as published by Tait et. al. (2). In the absence of airway manipulation and bronchospasm, our findings in the mouse model support that on exposure to volatile anesthetics, animals have less predisposition to SBP. These findings should be taken into consideration when assessing patients with underlying viral infection that are planning to undergo a surgical procedure. Secondly, this work challenges the belief that neutrophilic influx is always highly detrimental to the lungs. To be sure, PMN cause significant collateral damage that compromise pulmonary function, as evidenced in chronic illnesses such as emphysema. However, during SBP post-flu, PMN recruitment to the lungs is essential. Finally, this work identifies a novel target that can be employed to combat flu and SBP; modulation of the type I IFN response. This approach, unlike vaccines, antimicrobials, or antivirals, is not pathogen-specific and therefore applicable to numerous diseases and has far less risk of organisms gaining resistance. Low concentration anesthetics, compounds with similar anti-type I IFN effects, and novel therapies such as nanoparticle-delivery of bioactive molecules targeting type I IFN are viable candidates to accomplish alterations in the host immune response 17,31.
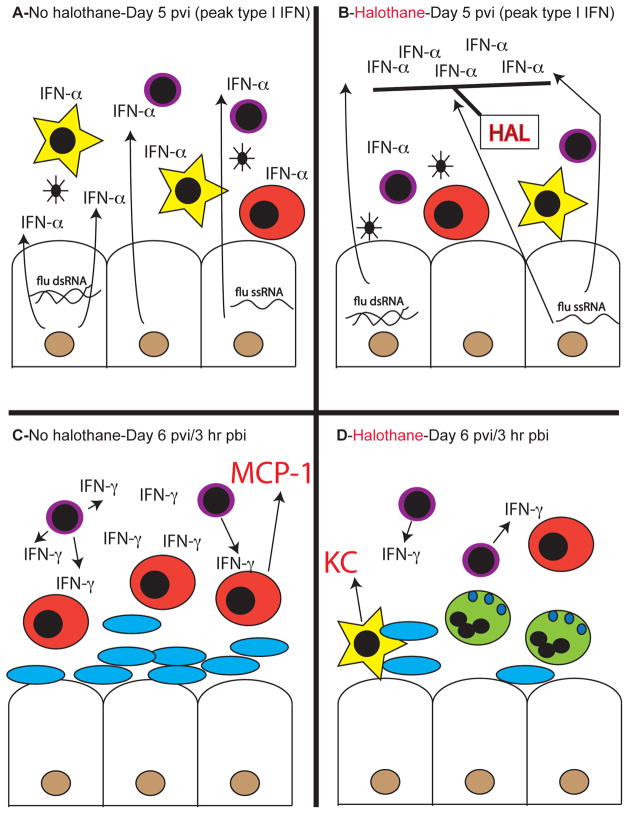
Figure 9. Proposed mechanism of halothane type I interferon (IFN) modulation during flu and SBP.
The influenza virulence factor, NS1, delays epithelial cell (white rhomboid, brown nucleus) type I IFN production in response to viral nucleic acids until day 5 post-viral infection (pvi). A) In the absence of halothane (HAL) exposure, the full effects of this delayed type I IFN (IFN-α) expression on both innate (phagocytic alveolar macrophages, yellow stars) and adaptive cells (lymphocytes, purple) is unopposed. B) Following halothane exposure, the effects of delayed type I IFN are effectively blocked, without altering type I IFN expression levels. This renders cells of the immune system less susceptible to phenotypic alteration due to this cytokine. C) At day 6 pvi, 3 hours post-bacterial infection (pbi), immune cells exhibit altered phenotypes as a result of type I IFN and lack of halothane exposure which would dampen these effects of type I IFN. This results in enhanced lymphocyte production of IFN-γ, decreased phagocytic activity of macrophages, and enhanced recruitment of inflammatory monocytes (red) via MCP-1 production. All of these factors result in increased bacterial infection (blue ovals) and pulmonary damage. D) Halothane mediated blockade of the effects of type I IFN results in reduced IFN-γ production, maintenance of phagocytic macrophage activity, decreased inflammatory monocyte recruitment, and preferential recruitment of neutrophils (green) via KC/MIP-2 expression following bacterial challenge post-flu. This results in bacterial clearance and decreased pulmonary damage.
Footnotes
Conflict of Interest Statement: The authors do not have any conflicts of interest to declare with respect to this manuscript.
Funding Disclosure:
This study was funded by the following grants from the National Institutes of Health (Bethesda, MD): HL048889 (to PRK, BAD), AI084410 (to PRK, BAD), and DC013554 (to APH). In addition, the work was supported with resources, and the use of facilities at the Veterans Administration Western New York Healthcare System, Buffalo, NY. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–82. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tait AR, Knight PR. The effects of general anesthesia on upper respiratory tract infections in children. Anesthesiology. 1987;67:930–5. doi: 10.1097/00000542-198712000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119:1910–20. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14:558–64. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 5.Seo SU, Kwon HJ, Ko HJ, Byun YH, Seong BL, Uematsu S, Akira S, Kweon MN. Type I interferon signaling regulates Ly6C(hi) monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathog. 2011;7:e1001304. doi: 10.1371/journal.ppat.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson S, Crotta S, McCabe TM, Wack A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nat Commun. 2014;5:3864. doi: 10.1038/ncomms4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 8.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 9.Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918–19 influenza pandemic. Emerg Infect Dis. 2008;14:1193–9. doi: 10.3201/eid1408.071313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salo M. Effects of anaesthesia and surgery on the immune response. Acta Anaesthesiol Scand. 1992;36:201–20. doi: 10.1111/j.1399-6576.1992.tb03452.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomson DA. Anesthesia and the immune system. J Burn Care Rehabil. 1987;8:483–7. [PubMed] [Google Scholar]
- 12.Tait AR, Davidson BA, Johnson KJ, Remick DG, Knight PR. Halothane inhibits the intraalveolar recruitment of neutrophils, lymphocytes, and macrophages in response to influenza virus infection in mice. Anesth Analg. 1993;76:1106–13. doi: 10.1213/00000539-199305000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Tait AR, Du Boulay PM, Knight PR. Alterations in the course of and histopathologic response to influenza virus infections produced by enflurane, halothane, and diethyl ether anesthesia in ferrets. Anesth Analg. 1988;67:671–6. [PubMed] [Google Scholar]
- 14.Penna AM, Johnson KJ, Camilleri J, Knight PR. Alterations in influenza A virus specific immune injury in mice anesthetized with halothane or ketamine. Intervirology. 1990;31:188–96. doi: 10.1159/000150153. [DOI] [PubMed] [Google Scholar]
- 15.Markovic SN, Knight PR, Murasko DM. Inhibition of interferon stimulation of natural killer cell activity in mice anesthetized with halothane or isoflurane. Anesthesiology. 1993;78:700–6. doi: 10.1097/00000542-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarthy KV, Bonoiu AC, Davis WG, Ranjan P, Ding H, Hu R, Bowzard JB, Bergey EJ, Katz JM, Knight PR, Sambhara S, Prasad PN. Gold nanorod delivery of an ssRNA immune activator inhibits pandemic H1N1 influenza viral replication. Proc Natl Acad Sci U S A. 2010;107:10172–7. doi: 10.1073/pnas.0914561107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks LR, Davidson BA, Knight PR, Hakansson AP. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. MBio. 2013;4:e00438–13. doi: 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonville CA, Bennett NJ, Koehnlein M, Haines DM, Ellis JA, DelVecchio AM, Rosenberg HF, Domachowske JB. Respiratory dysfunction and proinflammatory chemokines in the pneumonia virus of mice (PVM) model of viral bronchiolitis. Virology. 2006;349:87–95. doi: 10.1016/j.virol.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Raghavendran K, Davidson BA, Mullan BA, Hutson AD, Russo TA, Manderscheid PA, Woytash JA, Holm BA, Notter RH, Knight PR. Acid and particulate-induced aspiration lung injury in mice: importance of MCP-1. Am J Physiol Lung Cell Mol Physiol. 2005;289:L134–43. doi: 10.1152/ajplung.00390.2004. [DOI] [PubMed] [Google Scholar]
- 21.Davidson BA, Vethanayagam RR, Grimm MJ, Mullan BA, Raghavendran K, Blackwell TS, Freeman ML, Ayyasamy V, Singh KK, Sporn MB, Itagaki K, Hauser CJ, Knight PR, Segal BH. NADPH oxidase and Nrf2 regulate gastric aspiration-induced inflammation and acute lung injury. J Immunol. 2013;190:1714–24. doi: 10.4049/jimmunol.1202410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefort J, Motreff L, Vargaftig BB. Airway administration of Escherichia coli endotoxin to mice induces glucocorticosteroid-resistant bronchoconstriction and vasopermeation. Am J Respir Cell Mol Biol. 2001;24:345–51. doi: 10.1165/ajrcmb.24.3.4289. [DOI] [PubMed] [Google Scholar]
- 23.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tovey MG, Lallemand C, Thyphronitis G. Adjuvant activity of type I interferons. Biol Chem. 2008;389:541–5. doi: 10.1515/bc.2008.051. [DOI] [PubMed] [Google Scholar]
- 26.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 27.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayllon J, Garcia-Sastre A. The NS1 protein: a multitasking virulence factor. Curr Top Microbiol Immunol. 2015;386:73–107. doi: 10.1007/82_2014_400. [DOI] [PubMed] [Google Scholar]
- 29.Meunier I, von Messling V. NS1-mediated delay of type I interferon induction contributes to influenza A virulence in ferrets. J Gen Virol. 2011;92:1635–44. doi: 10.1099/vir.0.032193-0. [DOI] [PubMed] [Google Scholar]
- 30.Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, Rello J, Almansa R, Ramirez P, Martin-Loeches I, Varillas D, Gallegos MC, Seron C, Micheloud D, Gomez JM, Tenorio-Abreu A, Ramos MJ, Molina ML, Huidobro S, Sanchez E, Gordon M, Fernandez V, Del Castillo A, Marcos MA, Villanueva B, Lopez CJ, Rodriguez-Dominguez M, Galan JC, Canton R, Lietor A, Rojo S, Eiros JM, Hinojosa C, Gonzalez I, Torner N, Banner D, Leon A, Cuesta P, Rowe T, Kelvin DJ. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakravarthy KV, Davidson BA, Helinski JD, Ding H, Law WC, Yong KT, Prasad PN, Knight PR. Doxorubicin-conjugated quantum dots to target alveolar macrophages and inflammation. Nanomedicine. 2011;7:88–96. doi: 10.1016/j.nano.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]