Abstract
BACKGROUND
Patients with hippocampal epileptogenic foci may benefit from targeted intracranial monitoring of seizures and treatments such as hippocampal electrical stimulation, closed-loop stimulation, and stereotactic laser ablation. Each may benefit from a greater volume of hippocampal coverage with long-axis cannulation. Furthermore, an extraventricular trajectory avoids brain shift and reduces the risk of hemorrhage from ependymal breach. Unfortunately, detailed descriptions of the technical aspects of longitudinal cannulation of the hippocampus remain sparse.
OBJECTIVE
To develop a standard protocol for extraventricular longitudinal hippocampal cannulation.
METHODS
Images from 25 patients stereotactically implanted with 27 longitudinal hippocampal devices were retrospectively reviewed to determine the location of the burr hole or twist drill craniostomy. Simulated planning for bilateral occipital trajectories was then performed on a second cohort of 25 patients (50 trajectories) with mesial temporal sclerosis. An entry point derived from these 77 trajectories was subsequently validated on a third cohort of 25 patients (50 trajectories).
RESULTS
Extraventricular long-axis hippocampal implantation necessitates a lateral-to-medial and cephalad-to-caudal trajectory that skirts the inferomedial border of the temporal horn. Measurements from 64 trajectories suggested a consensus entry point that successfully facilitated 50 test trajectories as well as frame placement on 4 patients requiring long-axis hippocampal cannulation.
CONCLUSION
Although trajectories must be individually tailored for each patient, we recommend a starting entry point approximately 5.5 cm superior to the external occipital protuberance and 5.5 cm lateral to midline for extraventricular long-axis hippocampal cannulation in adult patients. Identification of this point is particularly important when positioning the stereotactic frame.
Keywords: Epilepsy, Hippocampus, Implanted stimulation electrodes, Lasers, Stereotaxic techniques
Advances in technology and our understanding of epileptogenic pathways have broadened the spectrum of interventions available for drug-resistant epilepsy. Hippocampal depth electrodes have been used during intracranial monitoring to improve the accuracy of seizure localization,1 while newer treatments such as electrical stimulation of the hippocampus,2 closed-loop electrical stimulation,3 and stereotactic laser ablation (SLA)4,5 have expanded the indications for hippocampal implantation. The hippocampus may be accessed orthogonally through a temporal burr hole or longitudinally through an occipital burr hole6 (R. E. Gross et al, unpublished data, 2013). The latter method places more contacts from a single electrode within the hippocampus, which increases the volume of recorded hippocampal tissue, programming flexibility, and possibly the effectiveness of stimulation.6–8 Despite these advantages, the techniques for safely placing longitudinal hippocampal electrodes have varied and detailed descriptions remain sparse.1,7,9,10 Furthermore, the proximity of the posterior post of the stereotactic frame to the occipital burr hole can hinder placement of the electrode or laser cannula. Previous knowledge of the intended entry point would help to guide frame placement and facilitate the procedure. Our objective, therefore, was to develop a standard protocol for extraventricular long-axis cannulation of the hippocampus.
METHODS
Review of Burr Hole Site in Patients Implanted With Hippocampal Electrodes
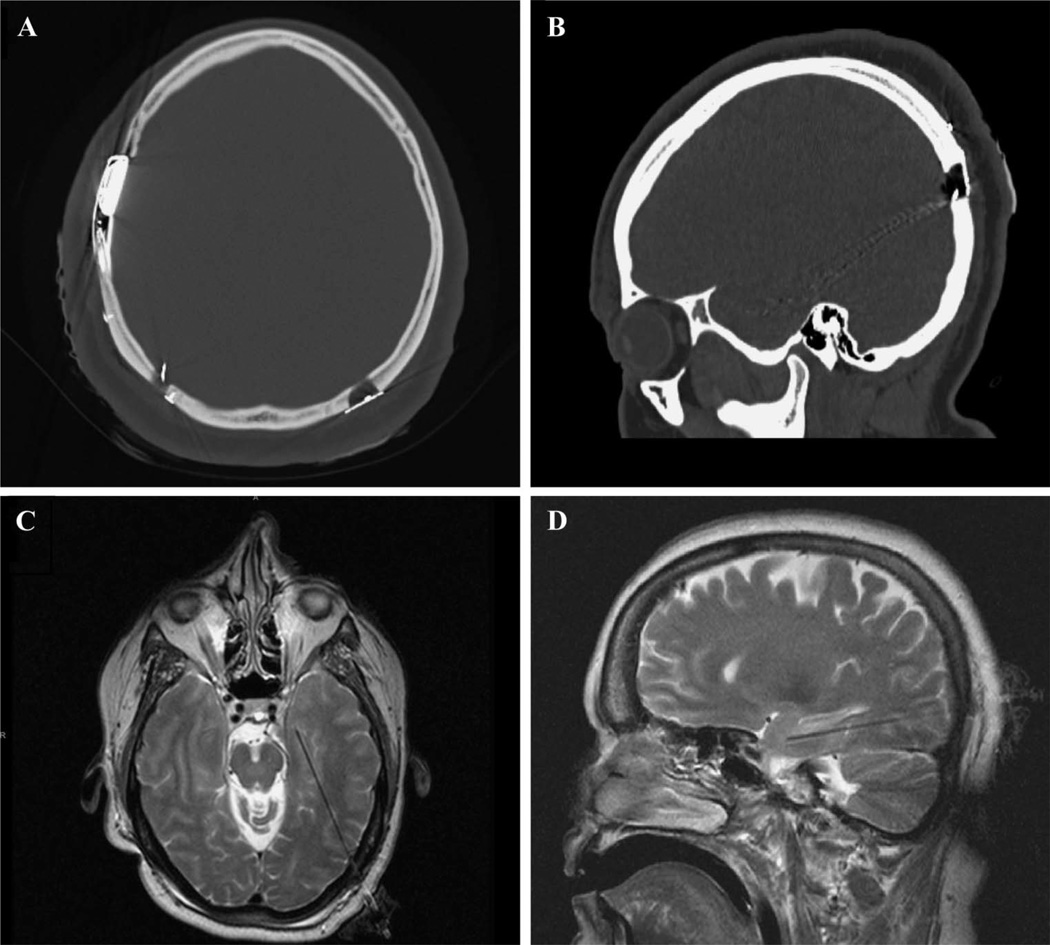
After approval by each institution’s Institutional Review Board, postoperative and intraoperative imaging was retrospectively reviewed to determine the site of burr hole placement in 25 patients implanted with 27 longitudinal hippocampal devices between September 2007 and March 2013 at Thomas Jefferson University and Emory University. This initial cohort consisted of 15 females and 10 males, with a mean age of 43 (s = 16; range, 11–66) years old. Five patients had 7 longitudinal hippocampal electrodes placed as part of a closed-loop stimulator for the RNS System Pivotal Clinical Investigation,3 and 20 patients underwent longitudinal hippocampal cannulation for magnetic resonance imaging (MRI)-guided SLA.5 For patients treated at Thomas Jefferson University, 1-mm-thick cut MRI sequences obtained preoperatively were fused with 1-mm-thick cut computed tomography (CT) sequences obtained after patients were placed in a Cosman-Roberts-Wells (CRW) frame (Integra Neurosciences, Plainsboro, New Jersey). For patients treated at Emory University, planning was performed directly on 1-mm-thick cut MRI sequences obtained with a MRI-compatible frame.11 Stereotactic coordinates generated from the planning process were transferred to the CRW frame for implantation of the electrode or laser along the long axis of the hippocampus. For patients implanted with hippocampal electrodes, routinely acquired postoperative high-resolution CT scans with 1-mm-thick cuts were analyzed; and for patients who underwent SLA, postablation high-resolution MRI scans with 1-mm-thick cuts were analyzed (Figure 1).
FIGURE 1.
Thin-cut postoperative CT (A and B) and intraoperative MRI scans (C and D) were used to retrospectively determine the entry point used for long-axis cannulation of the hippocampus. All images were loaded into OsiriX to standardize measurements.
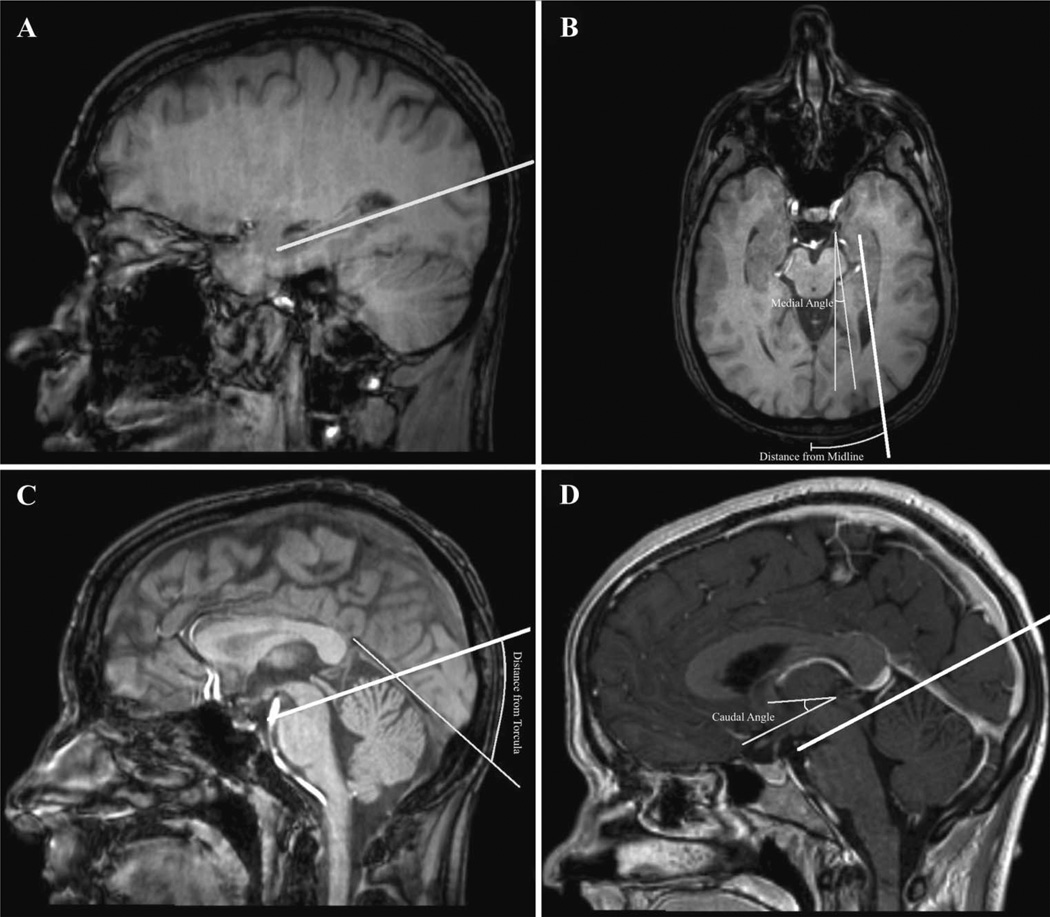
All images were loaded into the OsiriX imaging software package12 to generate 3-dimensional reconstructions and perform all measurements. The midpoint of each burr hole was measured relative to its distance above the torcula and its distance lateral to midline. Distances were measured along the skin surface to simulate preoperative determination of burr hole location. While typically palpable in the operating room, the point on the skin surface overlying the external occipital protuberance (EOP) cannot always be clearly defined on CT or MRI, particularly in patients with a large body habitus. In order to consistently extrapolate the internal landmark of the torcula onto the skin surface, a line along the cerebellar tentorium through the torcula was used. Trajectories were also measured: the cranial-caudal angle was measured relative to the anterior commissure-posterior commissure (AC-PC) line seen on sagittal imaging, and the lateral-medial angle was measured relative to midline (Figure 2B–D).
FIGURE 2.
Simulated trajectories through the long axis of the hippocampus (depicted as a thick white line) were planned using OsiriX (A). The distance from the midline over the surface of the skin and the medial angle relative to midline were measured from axial reconstructions (B). The distance from the torcula over the surface of the skin was measured from sagittal reconstructions. The exact point used on the skin surface was determined by extending a line along the cerebellar tentorium through the torcula (C). The caudal angle relative to the AC-PC line was measured from sagittal reconstructions at the midline (D). AC-PC, anterior commissure-posterior commissure.
Both horizontal and vertical measurement distributions were independently modeled in MatLab (MathWorks Inc, Natick, Massachusetts) with a logistic distribution with bin sizes determined by the Freedman-Diaconis rule.13 The means of each logistic distribution, which represent the highest probability distance for each measurement, were recorded along with the variance of the distribution. The means and standard deviations of trajectory angles and length of hippocampus cannulated were also recorded.
Trajectory for Longitudinal Hippocampal Cannulation
For all patients, trajectories were planned to cannulate a maximal length of the hippocampus, remain extraventricular, and avoid sulci and vasculature. The hippocampus is a curved structure that is concave both superiorly and medially with an axis generally parallel to the sylvian fissure.9 Although a trajectory along this same cranial-caudal angle would allow for maximal cannulation of this structure, such a path typically traverses the atrium of the lateral ventricle. We therefore applied a slightly shallower angle in order to remain extraventricular. Such trajectories were achieved by targeting the midportion of the amygdala or hippocampal head. Posterior to this target, the trajectory typically would run along the inferior border of the body of the hippocampus, below the tail of the hippocampus, and inferolateral to the atrium of the lateral ventricle before emerging at a gyral apex on the cortical surface (Figure 3). Minor adjustments were made at the discretion of the implanting surgeon, and final trajectories were created independently without consultation between surgeons.
FIGURE 3.
Probe’s eye view of trajectory along the long axis of the hippocampus generated from OsiriX. The trajectory begins in the middle of a gyrus (A) and remains inferior to the trigone and temporal horn of the lateral ventricle (B). It remains below the tail of the hippocampus at the level of the midbrain tectum (C) before entering the body of the hippocampus as it tracks along its inferior border (D). It ultimately terminates in the midportion of the hippocampal head (E).
Simulation of Cannulation on Database Images
To augment the retrospective analysis of 27 trajectories, simulations of longaxis hippocampal cannulations were performed on 50 consecutive patients with mesial temporal sclerosis from the Jefferson Comprehensive Epilepsy Center database. Patients were on average 42 (s = 13; range, 15–65) years old and included 30 females and 20 males. These patients were divided evenly into a planning group and a testing group by a random number generator.
Determining an Entry Point With Simulated Trajectory Plans
Simulated planning for bilateral long-axis hippocampal cannulation was performed on 25 patients assigned to the planning group (Figure 2A). A total of 50 trajectories were planned according to the previously delineated methods on 1-mm-thick cut MRI images with the OsiriX imaging software package.12 Measurements for the site of the planned entry point and the trajectory angles were recorded as described above (Figure 2B–D). The length of hippocampus that could be cannulated with the simulated trajectory was verified with a probe’s eye view and recorded.
Logistic distribution models were generated in MatLab for both the vertical and horizontal measurements from all 77 trajectories obtained from the combination of the retrospective cohort and the planning group. The means of each logistic distribution were rounded to the nearest half-centimeter and used as the recommended entry point.
Verification of the Recommended Entry Point
The recommended entry point was subsequently verified on 50 trajectories in 25 patients randomly assigned to the testing group. For each patient, the initial trajectory for bilateral long-axis hippocampal cannulation was based on this starting location. Necessary adjustments were made based on cortical, ventricular, and hippocampal anatomy in accordance with the previously describedmethods. The need for and degree of such adjustments were recorded along with the measurements for each trajectory. The suitability of the entry point was determined by its ability to allow for a trajectory that remained below the ventricle, did not traverse sulci or vasculature, and achieved longitudinal hippocampal cannulation.
The recommended entry point was subsequently used to assist with frame placement and trajectory planning in 4 patients undergoing MRIguided SLA of the hippocampus for the treatment of mesial temporal lobe epilepsy at Thomas Jefferson University.
RESULTS
Determining a Recommended Entry Point
Implantation along the long axis of the hippocampus necessitates a lateral-to-medial and slightly cephalad-to-caudad trajectory along the inferomedial border of the temporal horn. All 25 implanted patients tolerated the procedure without complications and there were no radiographic postoperative hemorrhages. The mean of the logistic distribution model indicated an entry point located 5.6 cm lateral to midline and 5.9 cm above the torcula (Table). From this entry point, electrode trajectory was 17.9 ± 7.3° caudal to the AC-PC line and directed 17.4 ± 5.9° medially (Figures 1 and 3).
TABLE.
Results of Logistic Regression Models for Distribution of Entry Point Locationsa
| Horizontal Distance From Midline, cm |
Vertical Distance From EOP, cm |
|||
|---|---|---|---|---|
| Mean | Variance | Mean | Variance | |
| Retrospective cohort | 5.55 | 1.37 | 5.90 | 3.99 |
| Planning group | 5.48 | 1.47 | 5.40 | 1.05 |
| Combined | 5.50 | 1.44 | 5.58 | 2.24 |
The logistic means were rounded to the closest half-centimeter to derive a recommended entry point 5.5 cm superior to the external occipital protuberance (EOP) and 5.5 cm lateral to midline.
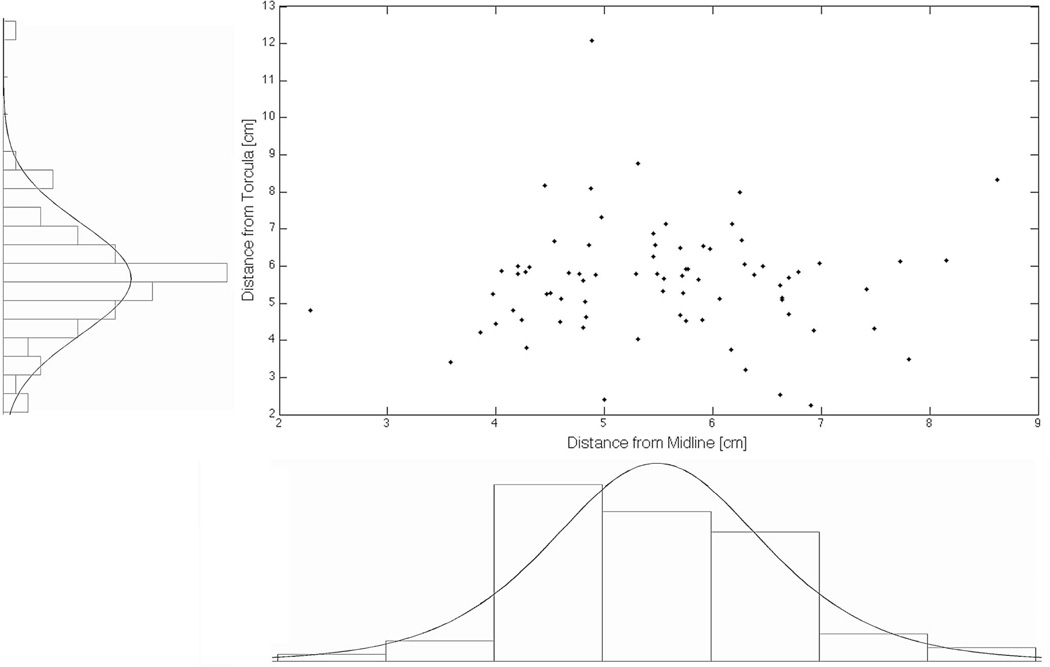
Incorporation of the 50 simulated trajectories yielded a logistic mean of 5.5 cm lateral to midline and 5.6 cm above the torcula (Figure 4; Table). As a matter of simplification and toward creating a general guideline for a recommended entry point that can easily be remembered, both measurements were rounded to the closest half-centimeter to yield a point 5.5 cm superior to the EOP and 5.5 cm lateral to midline. The trajectories were 20.0 ± 6.5° caudal to the AC-PC line, 18. ± 6 6.6° medial, and were able to cannulate 2.8 ± 0.5 cm of the hippocampus.
FIGURE 4.
Scatter distribution and histograms of measured distances for both the retrospective cohort and the planning group. Bin sizes were determined by the Freedman-Diaconis rule. Both the horizontal measurement from midline and the vertical measurement above the torcula were modeled with a logistic distribution to determine the optimal entry site for extraventricular long-axis cannulation of the hippocampus.
Verification of the Recommended Entry Point
When applied to 50 trajectories in the testing group, the recommended entry point of 5.5 cm superior to the torcula and 5.5 cm lateral to midline provided extraventricular long-axis hippocampal cannulation in 31 cases (62%) without need for further adjustment. In the remaining 19 trajectories (38%), minor adjustments had to be made (1.5 ± 0.7 cm in the cranial-caudal plane and 0.8 ± 0.6 cm in the lateral-medial plane) to meet our previously delineated criteria for successful cannulation. The logistic mean for the entry point in the testing group was 5.5 ± 0.2 cm lateral to midline and 5.2 ± 0.7 cm above the torcula with trajectories 17.8 ± 5.9° caudal to the AC-PC line, 17.2 ± 3.4° medial, and able to cannulate 2.4 ± 0.4 cm of the hippocampus. In addition, the subjective ease of planning was significantly improved with this recommended entry point.
DISCUSSION
The Role of Long-Axis Cannulation of the Hippocampus
Although some have questioned the benefit of the longitudinal occipital approach to the hippocampus over an orthogonal temporal approach for intracranial electroencephalographic (EEG) monitoring,14 (R. E. Gross et al, unpublished data, 2013), in a group of 41 patients undergoing intracranial EEG monitoring there was no difference in the efficacy of monitoring, complications, or outcomes between orthogonal and longitudinal hippocampal depth electrodes. This, therefore, remains a viable approach for hippocampal EEG monitoring, requiring fewer electrode insertions.
Essentially, in order to achieve the same volume of hippocampal coverage or ablation, multiple electrodes would be needed with the use of the orthogonal temporal approach in contrast to a single electrode with the longitudinal occipital approach. In a report by Parrent et al,15 in which orthogonal approaches to the amygdalohippocampal complex were used, patients generally had poor rates of seizure freedom. The benefit of a single trajectory is particularly relevant in patients requiring stimulation via implanted electrodes in whom only a single electrode can be implanted.3 Finally, in those undergoing SLA of the hippocampus, a longitudinal approach allows for the treatment of a larger volume of mesial structures through a series of ablations performed as the laser cannula is pulled back. These newer technologies highlight the growing need for standardization of the technique of long-axis cannulation of the hippocampus.
Comparison With Previously Described Techniques
The University of Bonn group described their method of longaxis cannulation, which utilizes a trajectory parallel to the sylvian fissure. In 141 patients, they used a more cephalad burr hole employing a transventricular trajectory to capture a greater length of the hippocampus. This approach was associated with an implantation morbidity of 5.7% and permanent neurological deficit in 0.7% of patients.9,16 More recently, Bahuleyan et al10 described a frameless stereotactic endoscope-assisted method of placing a transoccipital hippocampal depth electrode. In contrast to these methods, our method avoids the ventricular system, which may help to prevent brain shift and reduce the risk of hemorrhage from ependymal breach. Although a recent review of 65 patients at a single institution demonstrated that a transventricular approach for deep brain stimulation did not compromise safety or targeting accuracy,17 previous studies reported an increased risk of hemorrhage and associated neurological deficits with transventricular approaches.18,19 Given the limited surgical experience and lack of complications data for posterior extraventricular approaches, we can only speculate about its advantages at this time. Ultimately, careful avoidance of vessels along the anticipated trajectory on a probe’s eye view in stereotactic space remains paramount in reducing hemorrhagic complications.
In our simulations, efforts to maintain an extraventricular trajectory caused the morphology of the lateral ventricle (particularly the occipital horn and atrium) to be the most influential factor in the cranial-caudal location of the entry point. Although our lower trajectory maintains a path below the occipital and temporal horns, it unfortunately limits the length of hippocampus that can be cannulated. Because the hippocampal axis is roughly parallel to that of the sagittal view of the orbital floor, the temporal horn, and the sylvian fissure,9 a trajectory with a smaller caudal angle is less congruent with the longitudinal axis of the hippocampus. Although we would have liked to record the length of hippocampus cannulated in the retrospective cohort, such measurements could not be reliably acquired from the available postimplantation CTs and postablation MRIs. Nevertheless, for the 50 simulated trajectories in the planning group we were able to cover nearly 3 cm of hippocampus; and in the 50 additional trajectories in the testing group, we were able to cover nearly 2.5 cm of hippocampus. These lengths correspond to approximately 60% to 70% of the length of the hippocampus in healthy adults without hippocampal sclerosis.20
Anatomic Considerations
A nuance of our study protocol is the use of the torcula as a landmark. We used this structure rather than the EOP because all measurements were made on images in our database rather than on patients themselves. Although the EOP cannot always be clearly defined on CT or MRI, the torcula serves as an easily recognizable landmark. The point on the skin surface was extrapolated in a consistent manner and was typically positioned over the superior aspect of the torcula. As pointed out by Tubbs et al,21 however, the torcula does not necessarily correlate with the surface landmarks of the EOP or inion, but rather a point typically below the inion, at the point of insertion of the musculus semispinalis capitis. Therefore, in using the EOP as an intraoperative landmark, one must be aware of this nuance and potentially adjust the vertical distance, which may be slightly less than the 5.5 cm found in this study.
Our entry point is also reasonable in relationship to the well-described Frazier and Keen points for access to the ventricular system, yet utilizes a more inferior trajectory to avoid the temporal horn of the lateral ventricle. Historically, the Frazier point has been described as 6 to 7 cm superior to the inion and 3 to 4 cm lateral to midline and is commonly used for ventriculostomy placement when the patient is positioned prone.22,23 The Keen point has been described as 2.5 to 3 cm superior and 2.5 to 3 cm posterior to the pinna, provides access to the atrium, and was initially described as a method for drainage of cerebral abscesses extending from otitis media.22 It is therefore reasonable that our suggested entry point of 5.5 cm superior to the EOP with an inferior trajectory can remain below the ventricle en route to the long axis of the hippocampus.
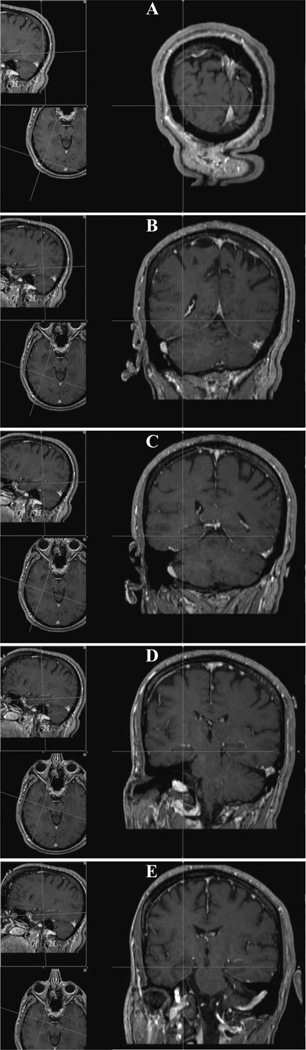
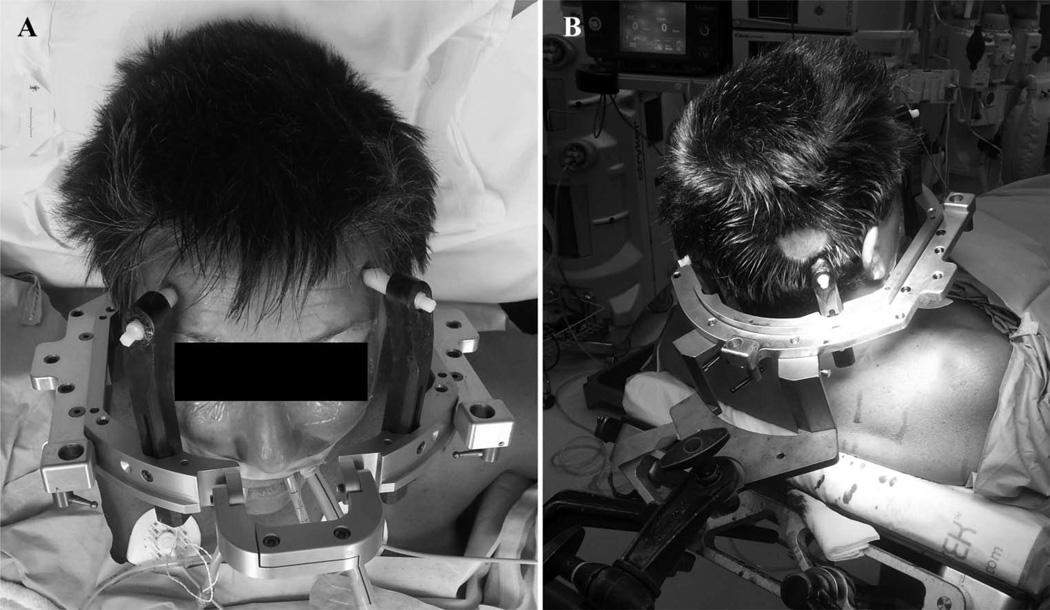
Facilitation of Stereotactic Frame Placement
A major advantage on understanding the entry point is it benefits in the facilitation of the proper placement of the stereotactic frame. Without a general idea of the entry point, we found that the inherent proximity of the posterior post of the CRW frame would obstruct access. In the last 4 patients undergoing MRI-guided SLA at our institution, a priori knowledge of this entry point led us to position the frame such that the pin for the posterior post was anterior and inferior to the recommended entry point—sufficiently distant from the surgical site. In fact, frequently, the frame must be rotated/shifted and placed off center to accommodate moving the posterior post away from the anticipated entry point (Figure 5). Without such compensatory moves, entry point access would be unnecessarily obstructed by the CRW frame itself. Although some may suggest that the use of a frameless registration system would provide similar benefits, we believe that the degree of accuracy required for hippocampal cannulation necessitates the use of a stereotactic frame, particularly in the setting of mesial temporal sclerosis.
FIGURE 5.
Two views of CRW frame placement adjusted for extraventricular long-axis cannulation of the hippocampus. For this right-sided procedure, the frame was rotated such that the front pins are both biased toward the patient’s left side (A) and the ipsilateral posterior pin is placed anteroinferior to the intended entry point (B). CRW, Cosman-Roberts-Wells.
Future Work
A major drawback of this study is that all measurements were taken retrospectively on CT or MRI scans, rather than on patients themselves. To further support this method, prospective evaluations should be performed in which hippocampal devices are placed via an occipital burr hole at our suggested entry point. Objective measures of appropriate placement and safety of this technique with complete complications data must be collected along with subjective measures of procedure facilitation. It is expected that the method described here will continue to aid in planning both frame placement and device trajectory. Such standardization not only streamlines procedures requiring longaxis cannulation of the hippocampus, but may also improve overall safety by increasing the uniformity of practice.24
CONCLUSION
Although the trajectories must be tailored for each patient, we recommend a burr hole approximately 5.5 cm superior to the EOP and 5.5 cm lateral to midline for extraventricular long-axis hippocampal cannulation in adult patients. Understanding this is particularly important when positioning the posterior posts on the stereotactic frame.
Acknowledgments
We would like to thank Andrew Hanson for his assistance in the image acquisition necessary for the execution of this study.
ABBREVIATIONS
- AC-PC
anterior commissure-posterior commissure
- CRW
Cosman-Roberts-Wells
- EEG
electroencephalographic
- EOP
external occipital protuberance
- SLA
stereotactic laser ablation
Footnotes
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Bekelis K, Desai A, Kotlyar A, et al. Occipitotemporal hippocampal depth electrodes in intracranial epilepsy monitoring: safety and utility. [Accessed April 23, 2013];J Neurosurg. 2013 118(2):345–352. doi: 10.3171/2012.9.JNS112221. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23082879. [DOI] [PubMed] [Google Scholar]
- 2.McLachlan RS, Pigott S, Tellez-Zenteno JF, Wiebe S, Parrent A. Bilateral hippocampal stimulation for intractable temporal lobe epilepsy: impact on seizures and memory. [Accessed March 5, 2013];Epilepsia. 2010 51(2):304–307. doi: 10.1111/j.1528-1167.2009.02332.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19817814. [DOI] [PubMed] [Google Scholar]
- 3.Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–1304. doi: 10.1212/WNL.0b013e3182302056. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21917777. [DOI] [PubMed] [Google Scholar]
- 4.Wu C, Sharan AD. Neurostimulation for the treatment of epilepsy: a review of current surgical interventions. [Accessed April 9, 2013];Neuromodulation. 2013 16(1):10–24. doi: 10.1111/j.1525-1403.2012.00501.x. discussion 24. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22947069. [DOI] [PubMed] [Google Scholar]
- 5.Sharan A, Wu C, Shetty A, et al. Preliminary Experience With Magnetic Resonance temperature Imaging (MRTI) and Stereotactic Laser Ablation (SLA) for Hippocampal Sclerosis (HS). 66th Annual Meeting of the American Epilepsy Society; San Diego, CA. 2012. [Google Scholar]
- 6.Spencer SS, Nguyen DK, Duckrow RB. Invasive EEG in presurgical evaluation of epilepsy. In: Shorvon SD, Perucca E, Engel J, editors. The Treatment of Epilepsy. 3rd ed. Oxford, United Kingdom: Wiley-Blackwell; 2009. pp. 767–798. [Google Scholar]
- 7.Shenai MB, Ross DA, Sagher O. The use of multiplanar trajectory planning in the stereotactic placement of depth electrodes. [Accessed April 7, 2013];Neurosurgery. 2007 60(4 suppl 2):272–276. doi: 10.1227/01.NEU.0000255390.92785.A4. discussion 276. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17415163. [DOI] [PubMed] [Google Scholar]
- 8.Olivier A. Diagnostic operative techniques in the treatment of epilepsy: depth electrodes. In: Schmidek HH, Sweet WH, editors. Operative Neurosurgical Techniques. 3rd ed. Philadelphia, PA: W.B. Saunders; 1995. pp. 1271–1285. [Google Scholar]
- 9.Van Roost D, Solymosi L, Schramm J, van Oosterwyck B, Elger CE. Depth electrode implantation in the length axis of the hippocampus for the presurgical evaluation of medial temporal lobe epilepsy: a computed tomography-based stereotactic insertion technique and its accuracy. [Accessed April 22, 2013];Neurosurgery. 1998 43(4):819–826. doi: 10.1097/00006123-199810000-00058. discussion 826–827. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9766309. [DOI] [PubMed] [Google Scholar]
- 10.Bahuleyan B, Omodon M, Robinson S, Cohen AR. Frameless stereotactic endoscope-assisted transoccipital hippocampal depth electrode placement: cadaveric demonstration of a new approach. [Accessed April 22, 2013];Childs Nerv Syst. 2011 27(8):1317–1320. doi: 10.1007/s00381-011-1489-7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21607640. [DOI] [PubMed] [Google Scholar]
- 11.Willie JT, Laxpati NG, Gowda A, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. doi: 10.1227/NEU.0000000000000343. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. [Accessed March 7, 2013];J Digit Imaging. 2004 17(3):205–216. doi: 10.1007/s10278-004-1014-6. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3046608&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman D, Diaconis P. On the histogram as a density estimator: L 2 theory. [Accessed November 7, 2013];Zeitschrift fur Wahrscheinlichkeitstheorie und Verwandte Gebiete. 1981 57(4):453–476. Available at: http://link.springer.com/10.1007/BF01025868. [Google Scholar]
- 14.Van Gompel JJ, Meyer FB, Marsh WR, Lee KH, Worrell GA. Stereotactic electroencephalography with temporal grid and mesial temporal depth electrode coverage: does technique of depth electrode placement affect outcome? [Accessed April 4, 2013];J Neurosurg. 2010 113(1):32–38. doi: 10.3171/2009.12.JNS091073. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3042277&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrent AG, Blume WT. Stereotactic amygdalohippocampotomy for the treatment of medial temporal lobe epilepsy. [Accessed December 20, 2013];Epilepsia. 1999 40(10):1408–1416. doi: 10.1111/j.1528-1157.1999.tb02013.x. Available at: http://doi.wiley.com/10.1111/j.1528-1157.1999.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 16.Fernández G, Hufnagel A, Van Roost D, et al. Safety of intrahippocampal depth electrodes for presurgical evaluation of patients with intractable epilepsy. [Accessed May 6, 2013];Epilepsia. 1997 38(8):922–929. doi: 10.1111/j.1528-1157.1997.tb01258.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9579894. [DOI] [PubMed] [Google Scholar]
- 17.Kramer DR, Halpern CH, Danish SF, Jaggi JL, Baltuch GH. The effect of intraventricular trajectory on brain shift in deep brain stimulation. [Accessed April 22, 2013];Stereotact Funct Neurosurg. 2012 90(1):20–24. doi: 10.1159/000332056. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22190056. [DOI] [PubMed] [Google Scholar]
- 18.Gologorsky Y, Ben-Haim S, Moshier EL, et al. Transgressing the ventricular wall during subthalamic deep brain stimulation surgery for Parkinson disease increases the risk of adverse neurological sequelae. [Accessed February 27, 2013];Neurosurgery. 2011 69(2):294–299. doi: 10.1227/NEU.0b013e318214abda. discussion 299–300. http://www.ncbi.nlm.nih.gov/pubmed/21389886. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Haim S, Asaad WF, Gale JT, Eskandar EN. Risk factors for hemorrhage during microelectrode-guided deep brain stimulation and the introduction of an improved microelectrode design. [Accessed April 8, 2013];Neurosurgery. 2009 64(4):754–762. doi: 10.1227/01.NEU.0000339173.77240.34. discussion 762–763. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19349834. [DOI] [PubMed] [Google Scholar]
- 20.Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42(9):1743–1750. doi: 10.1212/wnl.42.9.1743. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1513464. [DOI] [PubMed] [Google Scholar]
- 21.Tubbs RS, Salter G, Oakes WJ. Superficial surgical landmarks for the transverse sinus and torcular herophili. [Accessed April 22, 2013];J Neurosurg. 2000 93(2):279–281. doi: 10.3171/jns.2000.93.2.0279. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10930014. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg MS. Handbook of Neurosurgery. 7th ed. New York, NY: Thieme; 2010. [Google Scholar]
- 23.Connolley E, McKhann G, Choudhri T, Komotar R, Mocco J. Fundamentals of Operative Techniques in Neurosurgery. New York, NY: Thieme; 2010. [Google Scholar]
- 24.Babayan RK. Re: effect of a comprehensive surgical safety system on patient outcomes. J Urol. 2011;185(4):1329–1330. doi: 10.1016/j.juro.2011.01.004. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22115486. [DOI] [PubMed] [Google Scholar]