Abstract
Patients with chronic kidney disease (CKD) have a high risk of hyperkalemia, which increases mortality and can lead to renin–angiotensin–aldosterone system inhibitor (RAASi) dose reduction or discontinuation. Patiromer, a nonabsorbed potassium binder, has been shown to normalize serum potassium in patients with CKD and hyperkalemia on RAASi. Here, patiromer's onset of action was determined in patients with CKD and hyperkalemia taking at least one RAASi. After a 3-day potassium- and sodium-restricted diet in an inpatient research unit, those with sustained hyperkalemia (serum potassium 5.5 – under 6.5 mEq/l) received patiromer 8.4 g/dose with morning and evening meals for a total of four doses. Serum potassium was assessed at baseline (0 h), 4 h postdose, then every 2–4 h to 48 h, at 58 h, and during outpatient follow-up. Mean baseline serum potassium was 5.93 mEq/l and was significantly reduced by 7 h after the first dose and at all subsequent times through 48 h. Significantly, mean serum potassium under 5.5 mEq/l was achieved within 20 h. At 48 h (14 h after last dose), there was a significant mean reduction of 0.75 mEq/l. Serum potassium did not increase before the next dose or for 24 h after the last dose. Patiromer was well tolerated, without serious adverse events and no withdrawals. The most common gastrointestinal adverse event was mild constipation in two patients. No hypokalemia (serum potassium under 3.5 mEq/l) was observed. Thus, patiromer induced an early and sustained reduction in serum potassium and was well tolerated in patients with CKD and sustained hyperkalemia on RAASis.
Keywords: chronic kidney disease, hyperkalemia, potassium binder, renin–angiotensin–aldosterone system inhibitor
Hyperkalemia may induce lethal cardiac arrhythmias.1 Patients with chronic kidney disease (CKD), especially when treated with inhibitors of the renin–angiotensin–aldosterone system (RAAS), have a high risk of hyperkalemia.1, 2, 3, 4, 5 Treatment of severe hyperkalemia involves pharmacologic stabilization of the cardiac membranes, moving potassium into cells, and finally removal of potassium from the body.6 With impaired renal function, removal of body potassium is limited to dialysis or augmentation of gastrointestinal potassium excretion.6
Sodium polystyrene sulfonate and calcium polystyrene sulfonate have been utilized to increase fecal potassium excretion; however, despite their long history of clinical use, there is little evidence from prospective clinical trials demonstrating their efficacy; moreover, they may cause serious, life-threatening gastrointestinal adverse effects.7, 8, 9, 10, 11, 12 The serious gastrointestinal adverse events, in combination with milder adverse reactions, including poor palatability, make these agents difficult for patients to tolerate, thereby limiting their utility in chronic care settings.4 As a consequence of the limitations to the use of medicines in chronically hyperkalemic individuals, clinicians frequently face the dilemma of reducing the dose of, or discontinuing, their patients' RAAS inhibitors (RAASis) in order to manage serum potassium elevations.3, 5 In doing so, the physician is often forced to choose between the established benefit of RAASis in patients with CKD and the avoidance of a potentially life-threatening hyperkalemic complication.
Patiromer for oral suspension is under investigation for the treatment of hyperkalemia. The active moiety, patiromer, is a nonabsorbed polymer that binds potassium in the gastrointestinal tract, leading to an increase in fecal potassium excretion and a decrease in serum potassium.13, 14 Patiromer consists of smooth, spherical beads ~100 μm in diameter that are free-flowing and do not swell appreciably when placed in liquids.13
Prior studies of patiromer have focused on its efficacy, safety, and tolerability in the treatment of hyperkalemic patients in studies that have ranged from 12 to 52 weeks in duration.13, 14, 15, 16 In those studies, the earliest time point at which the drug's effect on potassium was routinely measured was 48 h after the initial dose, by which time significant serum potassium reductions were seen. The impact of patiromer on serum potassium levels before 48 h was unknown. The purpose of this study was to characterize the onset-of-action of patiromer's potassium lowering activity in the interval between the initial dose and 48 h postdose. To accomplish this goal, hyperkalemic CKD patients taking at least one RAASi were admitted to an inpatient clinical research unit (CRU), entered a 3-day diet run-in, were treated with patiromer, and the patients' changes in serum potassium levels were documented at frequent intervals over the course of the next 3 days.
RESULTS
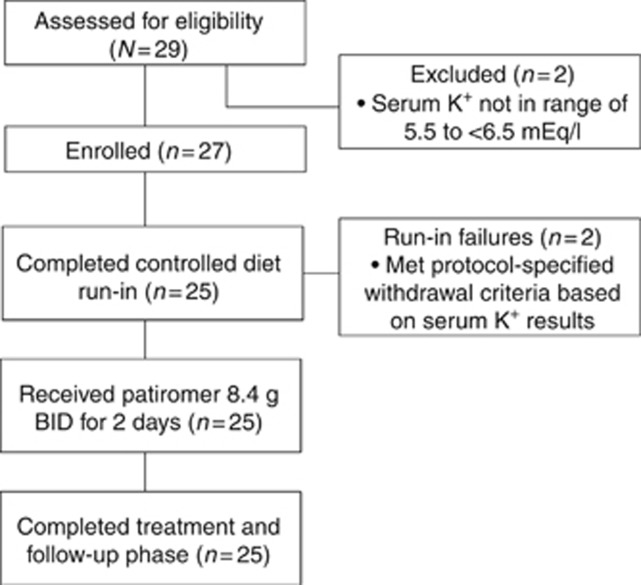
A total of 29 patients were screened (Figure 1). Twenty-seven patients met the eligibility criteria and were enrolled, entering the potassium- and sodium-restricted diet run-in phase. Two patients were withdrawn during run-in because they met protocol-specified withdrawal criteria (in both cases, serum potassium <5.5 mEq/l at the end of run-in). The remaining 25 patients had a sustained, elevated serum potassium during the run-in phase (Figure 2). These patients then entered the treatment phase, completed treatment with patiromer (4 doses over 2 days), and attended all follow-up visits. The study population was predominantly male, with a mean age of 58.7 years (Table 1). The majority had moderate-to-severe CKD as determined by the central laboratory. All patients were receiving at least one RAASi.
Figure 1.
Patient disposition. BID, twice daily; K+, potassium.
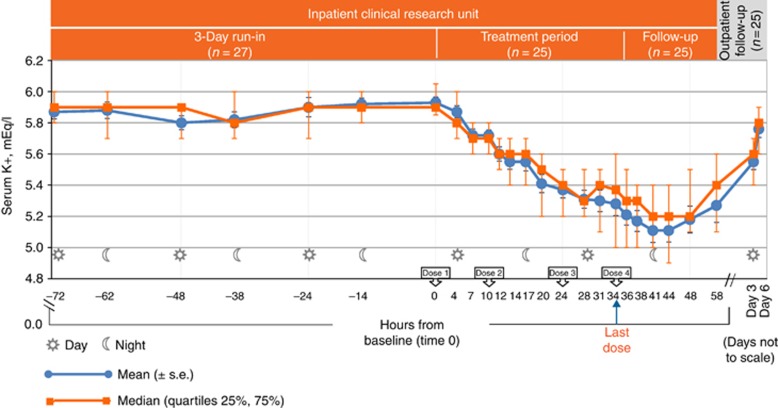
Figure 2.
Observed serum potassium (mEq/l) over time. Data presented as median (quartiles 25%, 75%), mean (s.e.). K+, potassium; s.e., standard error.
Table 1. Baseline demographics and clinical characteristics.
| Characteristic | Patiromer 16.8 g/day, N=25 |
|---|---|
| Age, years—mean (s.d.) | 58.7 (12.3) |
| Male—No. (%) | 15 (60) |
| Caucasian—No. (%) | 25 (100) |
| Body mass index, kg/m2—mean (s.d.) | 27.9 (3.9) |
| Type II diabetes mellitus—No. (%) | 15 (60) |
| Hypertension—No. (%) | 25 (100) |
| History of MI—No. (%) | 4 (16) |
| History of heart failure—No. (%) | 7 (28) |
| Duration of CKD, years—mean (s.d.) | 4.49 (4) |
| eGFR, ml/min per 1.73 m2—mean (s.d.) | 34.8 (20.7) |
| CKD stagea—No. (%) | |
| ⩾90 ml/min per 1.73 m2 | 1 (4) |
| 60–89 ml/min per 1.73 m2 | 2 (8) |
| 30–59 ml/min per 1.73 m2 | 9 (36) |
| 15–29 ml/min per 1.73 m2 | 11 (44) |
| <15 ml/min per 1.73 m2 | 2 (8) |
| Serum potassium,b mEq/l—mean (s.d.) | 5.93 (0.18) |
| Serum potassium >6.0 mEq/l—No. (%) | 8 (32) |
| On a RAASi—No. (%) | 25 (100) |
| Diuretic—No. (%) | 11 (44) |
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; RAASi, renin–angiotensin–aldosterone system inhibitor; s.d., standard deviation.
Stage 1 (normal): ⩾90 ml/min per 1.73 m2; Stage 2 (mild): 60–89 ml/min per 1.73 m2; Stage 3 (moderate): 30–59 ml/min per 1.73 m2; Stage 4 (severe): 15–29 ml/min per 1.73 m2; Stage 5 (end-stage): <15 ml/min per 1.73 m2.
Average of two central laboratory serum potassium values from samples collected at hour −1 and 0 on treatment day 1.
Efficacy
The study achieved its primary objective and found a significant reduction in serum potassium at 7 h after the first dose of patiromer (Figure 2). From a mean (s.e.) baseline serum potassium of 5.93 (0.04) mEq/l, a significant reduction in serum potassium of 0.21 (0.07) mEq/l (95% confidence interval (CI)=−0.35, −0.07, P=0.004) occurred 7 h after the first patiromer dose. The median (quartiles 25%, 75%) serum potassium was 5.90 (5.85, 6.05) at baseline, with a reduction in serum potassium of 0.20 (−0.3, −0.10) mEq/l at 7 h. Significant reductions occurred at all assessments from 7–48 h (P⩽0.004 at 7 h and 10h; P<0.001 for 12–48 h). Mean serum potassium decreased to 5.41 mEq/l by 20 h after the first patiromer dose (P<0.001), with a median serum potassium of 5.50 (5.20, 5.60) mEq/l. At 48 h (14 h after the last dose), a 0.75 (0.07) mEq/l mean reduction (95% CI=−0.89, −0.61, P<0.001), and a 0.65 (−0.95, −0.35) mEq/l median reduction from baseline were observed. Mean and median serum potassium did not increase before the next dose of patiromer.
Mean (s.e.) and median (25%, 75%) serum potassium at the time of the last patiromer dose (34 h) were 5.28 (0.06) and 5.37 (5.00, 5.60) mEq/l, respectively, and continued to decline, with significant (P<0.001) mean reductions from baseline observed from the last dose to the 38-, 41-, 44-, and 48-h time points. At 58 h (24 h after the last dose), the mean and median serum potassium were 5.27 (0.11) and 5.40 (5.10, 5.60), respectively, and the mean was not significantly different from the level at the time of the last patiromer dose (34 h). Five days after the first dose (~86 h after the last dose), mean (s.e.) serum potassium increased significantly from 34 h by 0.27 (0.05) mEq/l (95% CI=0.17, 0.36) to 5.55 (0.05) mEq/l, and the median serum potassium increased to 5.60 (5.40, 5.70) mEq/l. Eight days after the first dose (~158 h after the last dose), mean serum potassium increased significantly from 34 h by 0.48 (0.05) mEq/l (95% CI=0.38, 0.58) to 5.76 (0.05) mEq/l, and the median serum potassium increased to 5.80 (5.60, 5.90) mEq/l.
Seventeen patients had moderate hyperkalemia at baseline (mean (s.e.)=5.84 (0.03) mEq/l); eight patients had severe hyperkalemia (mean (s.e.)=6.14 (0.04) mEq/l). In a prespecified secondary analysis, mean changes in serum potassium over time in these two subgroups were consistent with those seen in the overall population. For patients with moderate and severe hyperkalemia, respectively, the mean (s.e.) change from baseline at 7 h was −0.17 (0.07) mEq/l (95% CI=−0.31, −0.03) and −0.29 (0.15) mEq/l (95% CI=−0.61, 0.04), respectively.
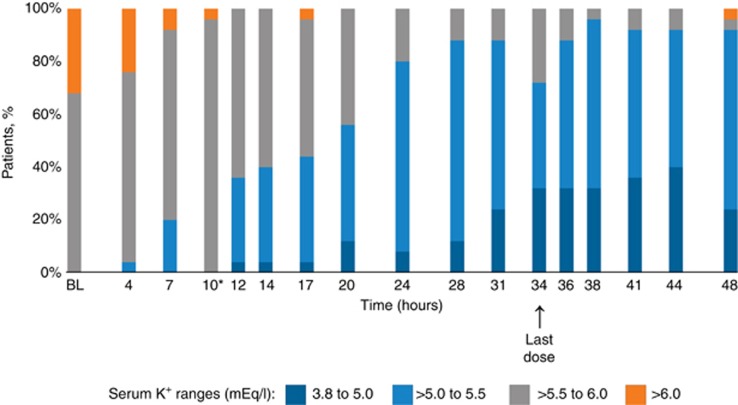
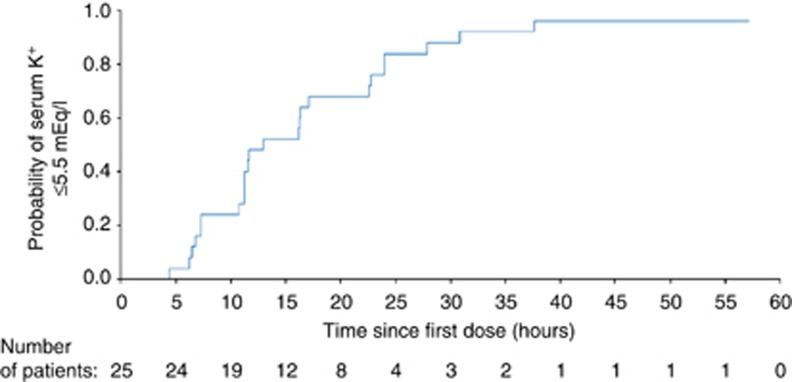
In a prespecified exploratory analysis, the proportion of patients with serum potassium values ⩽5.5 mEq/l was 20%, 36%, and 80% at 7-, 12-, and 24- h, respectively (Figure 3). In a post hoc analysis, the median time to achieve first serum potassium ⩽5.5 mEq/l was 12.7 h (95% CI=11.0, 22.6; Figure 4).
Figure 3.
Proportion of patients achieving serum potassium within the normal range (3.8–5.0 mEq/l). *Twelve (40%) patients had serum potassium values slightly above 5.5 mEq/l (i.e., 5.6 mEq/l) at 10 h, just before the second dose. BL, baseline; K+, potassium.
Figure 4.
Time to first serum potassium ⩽5.5 mEq/l. Patients whose change in serum potassium did not meet this threshold were censored at their last assessment time point. K+, potassium.
Safety
Patiromer was well tolerated over 2 days of treatment, with no serious or severe adverse events reported. Seven (28%) patients experienced eight adverse events during the active treatment phase through follow-up. The most common adverse events (occurring in two patients each) were mild constipation and mild hypotension (Table 2). No deaths occurred in the study, and no adverse events led to study drug withdrawal. No hypokalemic events (serum potassium <3.5 mEq/l) or hypomagnesemia (<1.4 mg/dl) were reported, and no clinically relevant alterations in other blood chemistry parameters were observed. Two patients (8%) experienced an increase from baseline in serum magnesium ⩾0.4 mg/dl, and no patient had a decrease of ⩾0.4 mg/dl.
Table 2. Adverse events.
| Adverse event category | Patiromer 16.8 g/day, N=25 |
|---|---|
| Patients with at least one AE, No. (%) | 7 (28) |
| Serious | 0 |
| Resulting in death | 0 |
| Leading to study drug discontinuation | 0 |
| Resulting in a change of dose | 0 |
| Related to patiromer | 3 (12) |
| Severe | 0 |
| Most common | 3 (12) |
| Constipation (mild) | 2 (8) |
| Hypotension (mild) | 2 (8) |
Abbreviation: AE, adverse event.
No patients had electrocardiogram (ECG) changes that were consistent with hypokalemia or hyperkalemia during active treatment through follow-up. There were no clinically relevant changes in vital signs by criteria prespecified in the protocol.
DISCUSSION
This study was designed to evaluate the onset-of-action of patiromer in representative patients with sustained hyperkalemia in the controlled environment of a CRU. We demonstrate that, after a single dose of patiromer, there was a significant reduction in mean serum potassium by 7 h in moderate-to-severe hyperkalemic CKD patients receiving at least one RAASi while consuming a 60 mEq/day potassium diet. Mean serum potassium continued to decrease and after a second dose of patiromer fell to <5.5 mEq/l by 20 h. By 24 h, more than 80% of patients had a serum potassium ⩽5.5 mEq/l. At 48 h, after four doses of patiromer, mean serum potassium had fallen by 0.75 mEq/l, and more than 90% of patients had serum potassium values ⩽5.5 mEq/l. Over the entire study, mean serum potassium did not increase before the next dose of patiromer. After the last dose of patiromer was administered at 34 h, mean serum potassium was 5.28 mEq/l. Mean serum potassium did not increase for the next 24 h and was 5.27 mEq/l at 58 h. However, mean serum potassium increased significantly at ~86 and 158 h after the last dose of patiromer. No patient developed hypokalemia, and patiromer was well tolerated with only mild adverse events.
The most common risk factors for hyperkalemia include the presence of CKD, diabetes mellitus, myocardial dysfunction, and the use of RAASis.17, 18 Thus, the study population was representative of patients who commonly present with hyperkalemia, as reflected by their baseline characteristics (i.e., 88% had estimated glomerular filtration rate <60 ml/min per 1.73 m2, 60% had diabetes mellitus, 28% had heart failure, all had hypertension, and all were on RAASis). Moreover, patients were on a potassium- and sodium-restricted diet during the inpatient phase of the study and were counseled to follow a low-potassium diet during the outpatient phase, similar to what is recommended for most patients who are at risk of hyperkalemia.19
In this study, eight adverse events were reported in seven patients. The most common side effects were mild constipation and mild hypotension, each occurring in two patients. There were no serious side effects and no side effects that led to a change in study drug dose or its discontinuation.
Patiromer has previously been reported to safely and effectively lower serum potassium and maintain normokalemia in several controlled trials,14, 15, 16 involving a total of 603 patients on active treatment. The need for chronic therapy with patiromer was demonstrated by the significant increase in serum potassium after discontinuation of patiromer in the AMETHYST-DN study.16 In the current study, a significant increase in mean serum potassium 4 and 7 days after the last dose of patiromer was also observed.
The strength of the efficacy findings derives from the study's rigorous design, including the inpatient treatment phase and enrollment of patients with persistent hyperkalemia while on a stable, potassium- and sodium-restricted diet. These design elements are optimal for determining the early onset effects of a drug on serum potassium. The 3-day run-in ensured the identification of patients with chronic or persistent hyperkalemia and also that patients with spurious (i.e., due to hemolysis), transient, and/or postprandial hyperkalemia were excluded.20 In addition, as serum potassium may vary secondary to diurnal variation21 and fasting/feeding cycles,22 serum potassium was recorded at prespecified intervals for 72 h, in the run-in period designed to confirm chronic hyperkalemia, reduce serum potassium variation, and establish a stable baseline serum potassium before treatment with patiromer.
Whether potassium would have decreased more rapidly or to a greater extent with a larger initial dose of patiromer or a different dosing regimen (e.g., 3 times daily dosing) cannot be determined from the data presented here. In the current study, the dose of patiromer was not titrated to the level of serum potassium. The total daily dose was lower than the mean 21.4 g daily that was used over the first 4 weeks in the phase 3 study in patients with moderate-to-severe hyperkalemia.14 Whether patiromer, as an adjunct to conventional therapy, will help lower potassium more rapidly in the emergency setting of acute life-threatening hyperkalemia remains to be evaluated.
Limitations of the study include the lack of a placebo control. All patients in this study had sustained, significantly elevated serum potassium levels (⩾5.5 mEq/l) for 3 days before the initiation of patiromer treatment. The significant reductions in serum potassium in this study are unlikely to have occurred without patiromer, given the stability of serum potassium values observed during the potassium- and sodium-restricted dietary run-in phase leading up to study qualification and enrollment, the use of a separate blood sample for assessment of baseline serum potassium, and the significant increase in serum potassium at ~86 and 158 h after the last dose of patiromer. The use of an active control such as sodium polystyrene sulfonate, the only drug specifically indicated for the treatment of hyperkalemia in the United States, was also considered but was felt to be inappropriate because of the lack of prospective, controlled trials demonstrating the efficacy of this agent, its potential to cause intestinal necrosis as detailed in the prescribing information,23 and its poor gastrointestinal tolerability making blinding difficult.8, 9, 10, 11, 12, 24, 25, 26, 27, 28, 29, 30, 31, 32
In conclusion, patiromer induced an early and a sustained, clinically significant reduction in serum potassium in moderately and severely hyperkalemic patients with CKD who were taking RAASis. After the first significant decrease in mean serum potassium at 7 h, mean serum potassium levels continued to decrease, never increasing before the next dose or for 24 h after the last dose of patiromer. Patiromer did not induce hypokalemia, and it was well tolerated.
MATERIALS AND METHODS
Design overview
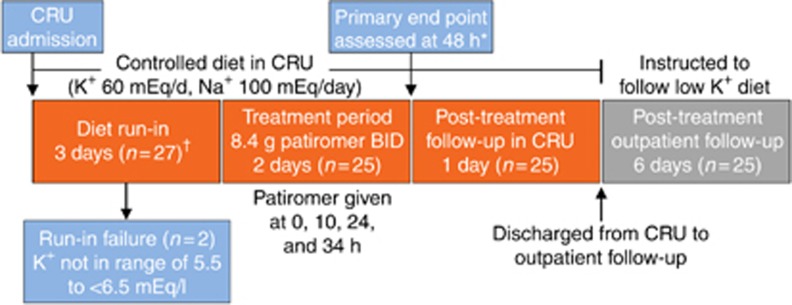
This phase 1, open-label, single-arm study was designed to evaluate the onset of effect of patiromer in patients with hyperkalemia and CKD who were receiving at least one RAASi. The study was conducted at CRUs and included an inpatient potassium- and sodium-restricted diet run-in and treatment phases and inpatient and outpatient follow-up phases (Figure 5).
Figure 5.
Study design. *A sequential testing procedure was used to determine the earliest time point at which the change from baseline in serum potassium was significant. Starting at 48 h, each time point was tested in order of reverse chronology (i.e., 48, 44, 41, 38 h, etc.), with the preceding time point assessed only if the change from baseline in serum potassium for the previously examined time point was statistically significantly. †Patients with serum potassium 5.5 to <6.5 mEq/l after the 3-day low-potassium, low-sodium diet run-in entered the treatment phase. BID, twice daily; CRU, clinical research unit; K+, potassium; Na+, sodium.
Study organization
The study was conducted at five sites in Europe. Relypsa, Inc. (Redwood City, CA, USA), sponsored the study; Worldwide Clinical Trials provided medical, safety, and site monitoring; and PAREXEL International provided overall study management, site monitoring, and data management. Statistical analyses were performed by PharmaStat, LLC (Newark, CA). ECGs were read at a core ECG laboratory, and an independent safety review board adjudicated all potassium-related ECG changes identified by the core laboratory. The protocol and informed consent forms were approved by the independent ethics committees for each participating site, and all patients provided written informed consent. The study was conducted in accordance with the International Conference for Harmonization Good Clinical Practice guidelines and local and national regulations.
Patient population
Patients aged 18–80 years with estimated glomerular filtration rate 15 to <90 ml/min per 1.73 m2 and serum potassium 5.5–6.2 mEq/l at screening were eligible to enter the run-in phase. Patients were required to be on a stable dose of at least one RAASi medication for 28 days before screening and, if taking antihypertensive medications (including diuretics and β-blockers), on a stable dose of those medications for at least 14 days before screening. Exclusion criteria included potassium-related ECG changes at screening, insulin-dependent or uncontrolled diabetes, severe swallowing disorder, moderate-to-severe gastroparesis, or a history of bariatric surgery, uncontrolled hypertension, significant (dialysis, transplant, acute injury, autoimmune) kidney disease, or major cardiovascular event within 2 months before screening. Use of potassium supplements, bicarbonate, or baking soda was not allowed in the 7 days before screening or during the study.
Procedures
Eligible patients were admitted to a CRU and began a 3-day controlled metabolic diet (potassium 60 mEq/day and sodium 100 mEq/day) run-in phase. The controlled diet continued throughout the 6-day inpatient stay (Figure 5). Serum potassium was assessed twice daily (i.e., every 10–14 h) during the run-in phase; a separate blood sample was drawn for the assessment of serum potassium at baseline. Assessments of serum potassium were performed by both the local and central laboratory. Local laboratory measurements were used for assessments of study inclusion criteria and for testing related to the clinical care of patients. Central laboratory measurements were used for assessments of baseline values and efficacy and safety; all samples were tested for evidence of hemolysis using a validated, semiquantitative method. Patients were withdrawn from the study at any time during the controlled diet run-in if serum potassium was ⩾6.5 mEq/l, increased by >0.5 mEq/l from screening, or decreased by >0.3 mEq/l within the final 15 h of the diet run-in phase.
At the end of the run-in, patients with serum potassium 5.5 to <6.5 mEq/l entered the treatment phase and began patiromer 8.4 g twice daily with meals for 2 days, for a total of four doses (i.e., at baseline (time 0) and 10, 24, and 34 h after the first dose). All patients received the same amount of patiromer regardless of their serum potassium. Serum potassium was assessed 1 h before treatment, at baseline just before treatment (time 0), and 4, 7, 10, 12, 14, 17, 20, 24, 28, 31, 34, 36, 38, 41, 44, 48, and 58 h after the first patiromer dose. Patients were discharged from the CRU at 58 h and were instructed to remain on a low-potassium diet (~50–75 mEq/day) during the post-discharge outpatient follow-up phase. Post-discharge follow-up assessments, including serum potassium, were performed on 5 and 8 days after the first dose (i.e., study days 9 and 12). Adverse events were collected throughout the inpatient phase and at each outpatient follow-up visit. Vital signs, 12-lead ECGs, and blood samples for serum chemistry and hematology were measured at various intervals throughout the study. High potassium values that, in the opinion of the investigator, warranted immediate treatment could be managed according to the local standard of care.
End points
The primary end point was the change in serum potassium from baseline during the 48 h after the first dose. The time of onset for patiromer was defined as the earliest time point at which the mean change in potassium from baseline was significant <0 mEq/l (P<0.05). The secondary end point was the change in serum potassium from baseline in prespecified subgroups defined by severity of hyperkalemia at baseline: moderate (serum potassium 5.5–6.0 mEq/l) and severe hyperkalemia (serum potassium >6.0–6.5 mEq/l).The proportion of patients achieving normokalemia (serum potassium 3.5–5.0 mEq/l) was a prespecified exploratory end point. An ad hoc exploratory analysis was conducted to evaluate the change in serum potassium from the last dose of patiromer and a post hoc analysis evaluated the time to first serum potassium ⩽5.5 mEq/l. Adverse events that occurred through the post-treatment follow-up phase are summarized descriptively.
Statistical analysis
The expected population mean (s.d.) change in serum potassium from baseline was −0.3 (0.473) mEq/l based on data from previous patiromer studies. Using a one group t-test, two-sided at a significance level of 0.05, it was estimated that a total of 22 patients were needed to provide 80% power to detect a mean change in serum potassium from baseline of −0.3 mEq/l.
All patients were included in the analyses who received at least one dose of patiromer and had baseline and at least one post-baseline serum potassium measurement. The least squares mean, standard error (s.e.), and two-sided 95% CIs for the change from baseline in serum potassium were calculated for each post-first dose time point through 48 h. The primary end point was evaluated using a mixed-effect model repeated measures analysis, with change from baseline in serum potassium as the dependent variable, time point as a fixed effect, and patient as a random effect. A sequential testing procedure was used to account for multiple comparisons of the primary end point,33 conducted from the latest to the earliest time points after the first patiromer dose (i.e., 48, 44, 41, 38, 36, 34, 31, 28, 24, 20, 17, 14, 12, 10, 7, and 4 h). Each subsequent time point was assessed only if all the changes in serum potassium from baseline were significantly <0 mEq/l (two-sided P<0.05) for all previously examined time points. For the secondary analysis, a longitudinal mixed-effect repeated measures model was used, similar to the primary analysis. The time to first reduction in serum potassium to ⩽5.5 mEq/l was assessed by the Kaplan–Meier method. Serum potassium values are presented as least squares mean (s.e.), mean (s.d.), and/or median (quartiles 25%, 75%), and change from baseline or change from last dose in serum potassium values are presented as least squares mean (s.e.), and/or median (quartiles 25%, 75%).
Acknowledgments
The authors had full access to the data. The first author wrote the first draft of the introduction and discussion. A medical writer (Wendy Gattis Stough, PharmD), funded by Relypsa, prepared the first draft of the methods and results sections under the supervision of the first author, and Jennifer Tyson (AlphaBioCom), funded by Relypsa, provided additional editorial support. All authors reviewed and revised the manuscript and made the decision to submit the manuscript for publication. This study was conducted by five principal investigators at one site in Bulgaria and four sites in Georgia. The results of the study were previously presented as a poster at the 2014 American Nephrology Society's Kidney Week. This study was funded by Relypsa, Inc. Clinical trial registration: EudraCT # 2013-000481-10.
Dr Bushinsky reports personal fees from Relypsa during the conduct of the study; he also reports personal fees outside of the submitted work from Amgen, Sanofi/Genzyme, Tricida, Fresenius Medical Care, and OPKO Health; he reports stock options in Relypsa, Amgen and Tricida. In addition, Dr Bushinsky receives grant support outside of the submitted work from the NIH and from the Renal Research Institute. Dr Williams reports personal fees from Relypsa; he also reports personal fees outside of the submitted work from Pfizer International, Merck, Tanebe, Novartis, and VitaPharm and grant support from Daiichi Sankyo, Otsuka, and NIH. Dr Pitt reports personal fees from Relypsa during the conduct of the study, and personal fees outside of the submitted work from Pfizer, Bayer, AstraZeneca, Tricida, scPharmaceuticals, DaVinci Therapeutics, Stealth Peptides, Aura Sense, Sarfez, Novartis, Johnson & Johnson, Oxygen Biotherapeutics, and Eli Lilly; he reports stock options in Relypsa, Tricida, scPharmaceuticals, DaVinci Therapeutics and Aura Sense. In addition, Dr Pitt has a patent EFS ID: 14916043, application number 61762661, /UM-33001/US-1PRO pending. Dr Weir reports personal fees from Relypsa and ZS Pharma, during the conduct of the study, and personal fees outside the submitted work from Akebia, Janssen, AstraZeneca, Amgen, MSD, Lexicon and Sandoz. Dr Freeman reports personal fees from Relypsa during the conduct of the study and outside the submitted work, and stock options for involvement in helping design the overall clinical trial program for patiromer. Dr Garza reports employment by Relypsa and stock options in Relypsa during the conduct of the study. Dr Stasiv reports employment by Relypsa and stock options in Relypsa during the conduct of the study. Ms Li reports consulting fees from Relypsa during the conduct of the study. Dr Berman reports employment by Relypsa and stock options in Relypsa, Pfizer and Merck during the conduct of the study. In addition, Dr Berman has a patent WO 2014/058905 pending. Dr Bakris reports personal fees from Relypsa during the conduct of the study and personal fees outside the submitted work from AbbVie, Takeda, Daiichi Sankyo, Boeringher-Ingelheim, Novartis, Bayer, and grants from Takeda, Boeringher-Ingelheim, and Medtronic.
References
- 1Einhorn LM, Zhan M, Hsu VD et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169: 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Juurlink DN, Mamdani MM, Lee DS et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004; 351: 543–551. [DOI] [PubMed] [Google Scholar]
- 3Albert NM, Yancy CW, Liang L et al. Use of aldosterone antagonists in heart failure. JAMA 2009; 302: 1658–1665. [DOI] [PubMed] [Google Scholar]
- 4Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004; 351: 585–592. [DOI] [PubMed] [Google Scholar]
- 5Yildirim T, Arici M, Piskinpasa S et al. Major barriers against renin-angiotensin-aldosterone system blocker use in chronic kidney disease stages 3-5 in clinical practice: a safety concern? Renal Fail 2012; 34: 1095–1099. [DOI] [PubMed] [Google Scholar]
- 6Elliott MJ, Ronksley PE, Clase CM et al. Management of patients with acute hyperkalemia. CMAJ 2010; 182: 1631–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Sterns RH, Rojas M, Bernstein P et al. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010; 21: 733–735. [DOI] [PubMed] [Google Scholar]
- 8Cheng ES, Stringer KM, Pegg SP. Colonic necrosis and perforation following oral sodium polystyrene sulfonate (Resonium A/Kayexalate in a burn patient. Burns 2002; 28: 189–190. [DOI] [PubMed] [Google Scholar]
- 9Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis 1992; 20: 159–161. [DOI] [PubMed] [Google Scholar]
- 10Harel Z, Harel S, Shah PS et al. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med 2013; 126: 264 e9–24. [DOI] [PubMed] [Google Scholar]
- 11McGowan CE, Saha S, Chu G et al. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J 2009; 102: 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Minford EJ, Hand T, Jones MC. Constipation and colonic perforation complicating calcium resonium therapy. Postgrad Med J 1992; 68: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Buysse JM, Huang IZ, Pitt B. PEARL-HF: prevention of hyperkalemia in patients with heart failure using a novel polymeric potassium binder, RLY5016. Future Cardiol 2012; 8: 17–28. [DOI] [PubMed] [Google Scholar]
- 14Weir MR, Bakris GL, Bushinsky DA et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372: 211–221. [DOI] [PubMed] [Google Scholar]
- 15Pitt B, Anker SD, Bushinsky DA et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J 2011; 32: 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Bakris GL, Pitt B, Weir MR et al.;AMETHYST-DN Investigators. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN Randomized Clinical Trial. JAMA 2015; 314(2): 151–161. [DOI] [PubMed] [Google Scholar]
- 17McCullough PA, Beaver TM, Bennett-Guerrero E et al. Acute and chronic cardiovascular effects of hyperkalemia: new insights into prevention and clinical management. Rev Cardiovasc Med 2014; 15: 11–23. [PubMed] [Google Scholar]
- 18Tamargo J, Caballero R, Delpon E. New drugs for the treatment of hyperkalemia in patients treated with renin-angiotensin-aldosterone system inhibitors — hype or hope? Discov Med 2014; 18: 249–254. [PubMed] [Google Scholar]
- 19National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 2007; 49: S12–154. [DOI] [PubMed] [Google Scholar]
- 20Gennari FJ, Segal AS. Hyperkalemia: an adaptive response in chronic renal insufficiency. Kidney Int 2002; 62: 1–9. [DOI] [PubMed] [Google Scholar]
- 21Schmidt ST, Ditting T, Deutsch B et al. Circadian rhythm and day to day variability of serum potassium concentration: a pilot study. J Nephrol 2015; 28(2): 165–172. [DOI] [PubMed] [Google Scholar]
- 22Allon M, Takeshian A, Shanklin N. Effect of insulin-plus-glucose infusion with or without epinephrine on fasting hyperkalemia. Kidney Int 1993; 43(1): 212–217. [DOI] [PubMed] [Google Scholar]
- 23Sanofi Aventis US LLC. Kayexalate Sodium Polystyrene Sulfonate, USP Cation-Exchange Resin Prescribing Information. Available at http://www.drugs.com/pro/kayexalate.html (accessed on 29 May 2015).
- 24Berlyne GM, Janabi K, Shaw AB. Dangers of resonium A in the treatment of hyperkalemia in renal failure. Lancet 1966; 1: 167–169. [DOI] [PubMed] [Google Scholar]
- 25Chaaban A, Abouchacra S, Gebran N et al. Potassium binders in hemodialysis patients: a friend or foe? Ren Fail 2013; 35: 185–188. [DOI] [PubMed] [Google Scholar]
- 26Chernin G, Gal-Oz A, Ben-Assa E et al. Secondary prevention of hyperkalemia with sodium polystyrene sulfonate in cardiac and kidney patients on renin-angiotensin-aldosterone system inhibition therapy. Clin Cardiol 2012; 35: 32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Fordjour KN, Walton T, Doran JJ. Management of hyperkalemia in hospitalized patients. Am J Med Sci 2014; 347: 93–100. [DOI] [PubMed] [Google Scholar]
- 28Gruy-Kapral C, Emmett M, Santa Ana CA et al. Effect of single dose resin-cathartic therapy on serum potassium concentration in patients with end-stage renal disease. J Am Soc Nephrol 1998; 9: 1924–1930. [DOI] [PubMed] [Google Scholar]
- 29Kessler C, Ng J, Valdez K et al. The use of sodium polystyrene sulfonate in the inpatient management of hyperkalemia. J Hosp Med 2011; 6: 136–140. [DOI] [PubMed] [Google Scholar]
- 30Sandal S, Karachiwala H, Noviasky J et al. To bind or to let loose: effectiveness of sodium polystyrene sulfonate in decreasing serum potassium. Int J Nephrol 2012; 2012: 940320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Scherr L, Ogden D, Mead A et al. Management of hyperkalemia with a cation-exchange resin. N Engl J Med 1961; 264: 115–119. [DOI] [PubMed] [Google Scholar]
- 32Mikrut M, Brockmiller-Sell H. Sodium polystyrene sulfonate dosing guidelines for the treatment of adult hyperkalemia. Hosp Pharm 2004; 39: 765–771. [Google Scholar]
- 33Dmitrienko A, Molenberghs G, Chuang-Stein C et alAnalysis of Clinical Trials Using SAS: A Practical Guide. SAS Institute, 2005. [Google Scholar]