Abstract
Purpose
Type II ovarian cancer (OC) and endometrial cancer (EC) are generally diagnosed at an advanced stage, translating into a poor survival rate. There is increasing evidence that Müllerian duct cancers may exfoliate cells. We have established an approach for lavage of the uterine cavity to detect shed cancer cells.
Patients and Methods
Lavage of the uterine cavity was used to obtain samples from 65 patients, including 30 with OC, five with EC, three with other malignancies, and 27 with benign lesions involving gynecologic organs. These samples, as well as corresponding tumor tissue, were examined for the presence of somatic mutations using massively parallel sequencing (next-generation sequencing) and, in a subset, singleplex analysis.
Results
The lavage technique could be applied successfully, and sufficient amounts of DNA were obtained in all patients. Mutations, mainly in TP53, were identified in 18 (60%) of 30 lavage samples of patients with OC using next-generation sequencing. Singleplex analysis of mutations previously determined in corresponding tumor tissue led to further identification of six patients. Taken together, in 24 (80%) of 30 patients with OC, specific mutations could be identified. This also included one patient with occult OC. All five analyzed lavage specimens from patients with EC harbored mutations. Eight (29.6%) of 27 patients with benign lesions tested positive for mutations, six (75%) as a result of mutations in the KRAS gene.
Conclusion
This study proved that tumor cells from ovarian neoplasms are shed and can be collected via lavage of the uterine cavity. Detection of OC and EC and even clinically occult OC was achieved, making it a potential tool of significant promise for early diagnosis.
INTRODUCTION
Ovarian cancer (OC) and endometrial cancer (EC) account for almost 40% of deaths from gynecologic cancers and share some similarities.1 Both develop from Müllerian epithelium and either show a more indolent growth pattern with a good prognosis (type I) or an aggressive phenotype with a poor prognosis (type II).2
Of all OCs, approximately 75% are type II, classified as high-grade serous cancers (HGSCs). HGSCs show frequent mutations in the TP53 gene (> 90%), early transperitoneal dissemination, and a poor prognosis with a 5-year survival rate of 10% to 30%.3 Over the last decade, the origin of OC has been reconsidered. The weight of current evidence suggests serous tubal intraepithelial carcinomas (STICs) to be precursor lesions of HGSC. There is growing evidence that the lag time from STIC to clinically overt HGSC is approximately 5 years.4 Recent studies demonstrated that intraluminal shedding of tumor cells from OCs and STICs occurred frequently.5
Of all ECs, approximately 10% are type II. Histologically, these tumors are classified as serous, clear cell, or poorly to undifferentiated endometrioid. Because of early transperitoneal dissemination and lymph node metastasis, prognosis of type II EC is poor, accounting for approximately 40% of EC mortality and with a 5-year survival rate of 50% to 60%.6
In addition, for type II EC, a lesion that seems to play an important role in carcinogenesis has been identified, called endometrial intraepithelial carcinoma. Similar to serous EC, endometrial intraepithelial carcinoma is associated with mutations and an abnormal accumulation of the TP53 protein.7
Late diagnosis is a large contributor to the poor prognosis of these cancers. Serum CA-125 measurement and transvaginal ultrasound are the most commonly used tests for diagnosis. Specificity and sensitivity of both are low, making them ineffective for screening and early or differential diagnosis.8,9 In the United States, 5% to 10% of women undergo surgery for a suspected ovarian neoplasm at least once during their life,10 and the majority of these procedures reveal only benign diseases.11–14 In part, this is a result of difficulties in discrimination before surgery and why only 30% to 50% of women with OC receive optimum treatment by a gynecologic oncologist.15,16 Studies have shown that surgical treatment in specialized centers results in a better survival, proving the benefit of a method able to discriminate between a benign or malignant lesion.17 Familial or inherited syndromes account for 10% to 15% of HGSCs.18 BRCA1 and BRCA2 mutation carriers have a 54% and 23% estimated lifetime risk of developing HGSC, respectively.19 Even in this high-risk group, screening with serum CA-125 measurement and transvaginal ultrasound was not able to improve clinical outcomes.8 At present, risk-reducing salpingo-oophorectomy (RRSO) is the only effective approach to reduce the risk of HGSC in these women.20,21 Thus, there is a clear and unmet clinical need for earlier detection of HGSC and type II EC.
A study published by Kinde et al22 made use of the so-called liquid Pap, which is routinely used for human papillomavirus detection, and showed that cells of gynecologic Müllerian duct cancers can be present in the uterine cervix. Massively parallel sequencing for tumor-specific mutations was performed on DNA from liquid Pap smear specimens. This technique was successfully applied to 24 (100%) of 24 patients with EC. In patients with OC, the sensitivity was less, with mutations identified in nine (41%) of 22 patients.
In this article, we describe a new method based on recent insights concerning the pathogenesis and characteristics of Müllerian tumors. The ovarian surface, fallopian tube, and uterine cavity form a communicating space. Peristaltic waves allow the fallopian tube to function as a conduit, possibly transporting exfoliated cells from HGSCs or STICs into the uterine cavity. We want to address whether cells from Müllerian duct cancers can be collected with a lavage of the uterine cavity. This lavage concept could improve both the earlier detection of type II Müllerian duct cancers and the differential diagnosis of adnexal masses.
PATIENTS AND METHODS
Patient Samples
Samples were collected in accordance with the institutional review boards of the Medical University of Vienna (EK#1148_2011, EK#1766_2013), the Catholic University Leuven (B322201214864/S54406), the Kliniken Essen-Mitte (LÄNR_2013456), and the Charité Medical University of Berlin (EA2/025/14). Informed consent was obtained from each participant. Patients with the following diagnoses were included (Table 1): OC (n = 30), EC (n = 5), benign gynecologic diseases (n = 27), and other malignancies with involvement of gynecologic organs (n = 7). To increase the number of patients with malignant diagnosis, three patients (patients 196, 197, and 198) were randomly selected from the prospective running Lavage of the Uterine Cavity for the Diagnosis of Ovarian and Tubal Carcinoma and Their Premalignant Changes (LUDOC) study (ClinicalTrials.gov identifier: NCT02387645). To prove that exfoliated cells may be present even in a clinically occult stage of disease, one patient (patient 205) from the prospective running Lavage of the Uterine Cavity for the Diagnosis of Serous Tubal Intraepithelial Carcinoma (LUSTIC) study (ClinicalTrials.gov identifier: NCT02039388) was included. In all 73 patients, lavage specimens were taken for subsequent mutation analysis. Eight patients were excluded from final analysis for one of the following reasons (Table 1): two or more malignant tumors were present, tubal ligation was performed previously, patients were suspected of harboring a germline mutation, or primary tumor tissue was available but did not harbor any of the tested mutations.
Table 1.
Characteristics of the Study Cohort and Index Mutation Assessed in Primary Tumor Tissues and Lavage Specimens by NGS and Singleplex Approaches
| Patient No. | Age (years) | Type of Disease | Histology | FIGO Stage | Grade | Mutations in Primary Tumor | Mutations in Lavage Specimen | Notes |
|---|---|---|---|---|---|---|---|---|
| 154 | 55 | OC | Endometrioid | IA | G1 | FGFR2 p.K659E, c.1975T>C | FGFR2 p.K659E, c.1975T>C (1) | |
| 155 | 45 | OC | Serous | IC | G1 | PIK3CA p.H1047R, c.3140A>G | PIK3CA p.H1047R, c.3140A>G (3) | |
| 152 | 50 | OC | Serous | IIC | G3 | TP53 p.R248Q, c.743G>A | TP53 p.R248Q, c.743G>A (1) | |
| 140 | 44 | OC | Serous | IIIA | G3 | TP53 p.H168R, c.503A>G | TP53 p.H168R, c.503A>G (3) | |
| 149 | 57 | OC | Serous | IIIA | G3 | TP53 p.V272E, c.815T>A | TP53 p.V272E, c.815T>A (3) | |
| 148 | 71 | OC | Serous | IIIB | G3 | TP53 p.V216M, c.646G>A | TP53 p.V216M, c.646G>A (1) | |
| 205 | 42 | OC | Serous | IIIB | G2 | TP53 p.V217fs, c.650delT | TP53 p.V217fs, c.650delT (1) | |
| 143 | 52 | OC | Serous | IIIC | G3 | TP53 p.C135Y, c.404G>A | TP53 p.L194R, c.581T>G (3) | |
| 158 | 49 | OC | Serous | IIIC | G3 | TP53 p.G245V, c.734G>T | TP53 p.G245V, c.734G>T (3) | |
| 130 | 72 | OC | Serous | IIIC | G2 | PPP2R1A p.W257C, c.771G>T | PPP2R1A p.W257C, c.771G>T (1) | |
| 132 | 64 | OC | Serous | IIIC | G2 | NA | TP53 p.M237I, c.711G>C (1) | |
| 139 | 70 | OC | Serous | IIIC | G3 | TP53 p.H193P, c.578A>C | TP53 p.H193P, c.578A>C (1) | |
| 144 | 43 | OC | Serous | IIIC | G3 | TP53 p.G245S, c.733G>A | KRAS p.G12C, c.34G>T (1) | |
| 160 | 35 | OC | Serous | IIIC | G3 | TP53 p.T170 (in-frame deletion), c.508_510delACG | TP53 p.T170 (in-frame deletion), c.508_510delACG (2) | |
| 168 | 58 | OC | Serous | IIIC | G3 | TP53 p.R273H, c.818G>A | TP53 p.R273H, c.818G>A (1) | |
| 197 | 73 | OC | Serous | IIIC | G3 | NA | KRAS p.G12A, c.35G>C (1) | |
| 137 | 61 | OC | Serous | IIIC | G3 | TP53 p.H179R, c.536A>G | TP53 p.H179R, c.536A>G (1) | |
| 194 | 49 | OC | Malignant mixed Müllerian tumor | IIIC | G3 | NA | NRAS p.Q61R, c.182A>G (1) | |
| 196 | 59 | OC | Malignant mixed Müllerian tumor | IIIA1(i) | G3 | NA | PIK3CA p.T1025N, c.3074C>A (1) | |
| 131 | 55 | OC | Endometrioid, partly serous | IIIC | G2 | KRAS p.G13D, c.38G>A | KRAS p.G13D, c.38G>A (1) | |
| 147 | 60 | OC | Endometrioid | IIIC | G3 | KRAS p.G12D, c.35G>A | KRAS p.G12D, c.35G>A (1) | |
| 204 | 61 | OC | Clear cell | IIIC | G3 | NA | TP53 p.A159I, c.475_476GC>AT (1) | |
| 198 | 59 | OC | Serous | IVB | G3 | NA | PIK3CA p.M1004V, c.3010A>G (1) | |
| 172 | 55 | OC | Serous | IV | G3 | NA | TP53 p.G262V, c.785G>T (1) | |
| 133 | 31 | OC LMP | Serous low malignant potential | NA | AKT1 p.E17K, c.49G>A (1) | |||
| 161 | 52 | OC meta | Signet ring carcinoma, spread in ovaries | TP53 p.R282W, c.844C>T | TP53 p.R282W, c.844C>T (1) | |||
| 164 | 72 | UC | Uterine carcinosarcoma | IIIC | G3 | NA | PTEN p.L193fs, c.578delT (1) | |
| 199 | 73 | EC | EC | IA | G2 | PTEN p.R130*, c.388C>T | PTEN p.R130*, c.388C>T (1) | |
| 200 | 70 | EC | EC | IA | G2 | PIK3CA p.H1047Y, c.3139C>T | PIK3CA p.H1047Y, c.3139C>T (1) | |
| 201 | 70 | EC | EC | IA | G2 | APC p.Q1469*, c.4405C>T | APC p.Q1469*, c.4405C>T (1) | |
| 202 | 63 | EC | EC | IA | G1 | NA | PTEN p.Y315*, c.945T>A (1) | |
| 203 | 55 | EC | EC | IA | G1 | NA | CTNNB1 p.S37F, c.110C>T (1) | |
| 165 | 61 | B | Fibroma | NA | FBXW7 p.R505S, c.1513C>A (1) | |||
| 175 | 50 | B | Ovarian cyst, teratoma | — | KRAS p.G12V, c.35G>T (1) | |||
| 177 | 31 | B | Secondary infertility | NA | KRAS p.G12C, c.34G>T (1) | |||
| 178 | 46 | B | Uterus myomatosus, ovarian cyst | — | KRAS p.G12C, c.34G>T (1) | |||
| 181 | 43 | B | Secondary infertility, polyp of the cervix mucosa | NA | KRAS p.G12V, c.35G>T (1) | |||
| 184 | 78 | B | Ovarian cyst | — | KRAS p.G12V, c.35G>T (1) | |||
| 190 | 58 | B | Teratoma | — | PIK3R1 p.R348*, c.1042C>T (1) | |||
| 193 | 54 | B | Inclusion cysts | — | KRAS p.G12D, c.35G>A (1) | |||
| 146* | 39 | BC | Breast cancer, uterine leiomyoma | NA | TP53 p.C242F, c.725G>T (1) | Germline mutation | ||
| 162* | 77 | B | Ovarian serous cystadenoma (previous leukemia, signet ring carcinoma, squamous cell carcinoma) | NA | TP53 p.N239D, c.715A>G (1) | Two or more malignant tumors | ||
| 129 | 82 | OC | Serous | IIIC | G2 | TP53 c.920-1G>A, g.7576927C>T (splice site) | — | |
| 150 | 51 | OC | Serous | IIIC | G3 | TP53 p.C176Y, c.527G>A | — | |
| 163 | 67 | OC | Serous | IIIC | G3 | TP53 p.C238R, c.712T>C | — | |
| 195 | 69 | OC | Serous | IIIC | G2 | NA | — | |
| 166 | 77 | OC | Serous | IV | G3 | NA | — | |
| 174 | 69 | OC | Serous | IV | G3 | TP53 p.R342*, c.1024C>T | — | |
| 145 | 52 | B | Cervix mucous polyp | — | — | |||
| 136 | 49 | B | Follicle cyst | NA | — | |||
| 138 | 47 | B | Uterus myomatosus | NA | — | |||
| 151 | 33 | B | Follicular cyst | NA | — | |||
| 156 | 45 | B | Serous cystadenoma | — | — | |||
| 157 | 58 | B | Thecofibroma | — | — | |||
| 159 | 49 | B | Cystic teratoma | — | — | |||
| 176 | 67 | B | Cystic teratoma of the ovary | — | — | |||
| 179 | 45 | B | Uterus myomatosus | — | — | |||
| 180 | 35 | B | Uterus myomatosus | — | — | |||
| 182 | 43 | B | CIN III, ovarian cyst, endometriosis | NA | — | |||
| 183 | 35 | B | Ovarian cyst, endometriosis | NA | — | |||
| 185 | 62 | B | Ovarian cyst, cystadenofibroma, | — | — | |||
| 186 | 36 | B | Teratoma of the ovary | — | — | |||
| 187 | 36 | B | Endometriosis | — | — | |||
| 188 | 72 | B | Thecofibroma | — | — | |||
| 189 | 45 | B | Ovarian cyst | — | — | |||
| 191 | 28 | B | Chronical salpingitis, oophoritis | — | — | |||
| 192 | 52 | B | Thecofibroma | — | — | |||
| 141* | 60 | OC | Low-grade endometrioid adenocarcinoma | IB | G1 | — | — | No mutation identified in primary tumor |
| 170* | 67 | OC | Serous carcinoma | IIIA | G1 | — | — | No mutation identified in primary tumor |
| 134* | 52 | OC | Serous | IIIC | G3 | — | — | No mutation identified in primary tumor |
| 142* | 58 | OC | Serous | IIIC | G2 | TP53 p.Q104*, c.310C>T | — | Tubal ligation |
| 153* | 62 | OC | Metastatic ovarian cancer from lobular mamma carcinoma | — | — | Two or more malignant tumors | ||
| 128* | 58 | BC, OC | Ductal breast cancer, mucinous adenocarcinoma ovary | IIIC | G2 | — | — | Two or more malignant tumors |
NOTE. Mutations detected by NGS, safe sequencing system (SafeSeqS), and digital droplet polymerase chain reaction (ddPCR).
Abbreviations: —, no mutation detected; B, benign disease; BC, breast cancer; CIN, cervical intraepithelial neoplasia; EC, endometrial cancer; FIGO, International Federation of Gynecology and Obstetrics; G, grade; LMP, low malignant potential; NA, not applicable; NGS, next-generation sequencing; OC, ovarian cancer; OC meta, cancer metastasized in ovaries; UC, uterine cancer;
Excluded from final analysis.
Corresponding tumor tissue was available for 21 of the included patients with OC (70%), three patients with EC (60%), 19 patients with benign diseases (70.4%), and the patient with a metastatic signet ring carcinoma. Uterine lavage was performed before surgery for suspected malignant adnexal pathology, immediately after induction of anesthesia. Lavage samples were centrifuged at 300 × g for 10 minutes at room temperature. DNA was isolated from the cell pellet (QIAamp MinElute Media Kit, No. 57414; Qiagen, Hilden, Germany), and concentration was determined using a Qubit fluorometer (ThermoFisher, Waltham, MA).
Lavage Technique
First, an antiseptic lotion was used for cleaning the cervix. Using a bullet forceps, the cervix was grasped at 12 o'clock. A three-way catheter (Coloplast AH5312; Coloplast, Minneapolis, MN) was inserted into the cervical canal, and the balloon was inflated with approximately 1 mL of saline to seal the cervical canal. If the cervical canal was too narrow to pass the catheter, it was dilated to 4 mm with Hegar dilators. Two 10-mL syringes, one of them containing 10 mL of saline, were connected to two of the catheter tubes. The patient was put into an anti-Trendelenburg position. By pushing on the plunger of the syringe containing saline, the uterine cavity and fallopian tubes were slowly perfused. Simultaneously, the plunger of the empty syringe was gently pulled out. Finally, the balloon was deflated, and the catheter was removed. This procedure was partially still under optimization during sample collection within this proof-of-concept study.
Massively Parallel Sequencing
Multiplex amplification and sequencing of 136 amplicons covering approximately 13,000 base pairs were applied to lavage samples, and the corresponding tumor specimens were22 modified as described earlier.23 Amplicons comprised gene regions of AKT1, APC, BRAF, CDKN2A, CTNNB1, EGFR, FBXW7, FGFR2, KRAS, NRAS, PIK3CA, PIK3R1, POLE, PPP2R1A, PTEN, and TP53 (Data Supplement). Confirmation of each detected mutation was carried out through independent singleplex amplification (safe sequencing system [SafeSeqS]) and sequencing using the same DNA sample, as described in Bettegowda et al.23
Digital Droplet Polymerase Chain Reaction
Lavage samples of six patients were analyzed by digital droplet polymerase chain reaction (ddPCR; QX100 Droplet Digital PCR System; Bio-Rad Laboratories, Hercules, CA). Mutations previously identified in tumor tissue were evaluated. For confirmation, DNA from tumor tissue was analyzed through ddPCR as well. Custom TaqMan SNP Genotyping Assays (No. 4331349; Life Technologies, Carlsbad, CA) were designed using Primer Express 3.0 software (ThermoFisher). A positive control and wild-type control were included in every polymerase chain reaction run. Ten to 20 ng of template DNA was used per amplification. All samples were analyzed at least in duplicates. To ensure specificity, all designed assays were also tested on samples obtained from three patients with benign diseases.
RESULTS
Identification of Tumor Cells in Lavage Samples
Sufficient amounts of DNA were obtained from each lavage sample (mean, 1.8 μg; range, 33 ng to 22.9 μg). Lavage samples were classified as positive when one or more mutations were detected in one sample.
Lavage specimens were analyzed by massively parallel sequencing. In patients with malignant diseases, a median mutant allele fraction of 13.9% (range, 1.5% to 44.6%) was detected (Data Supplement). All samples of patients with malignant diseases were analyzed in the manner described in Figure 1.
Fig 1.

Scheme of the procedure applied for mutation analysis of lavage samples from patients with malignant diseases. When a mutation was detected in the lavage specimen by next-generation sequencing (NGS), it was confirmed by safe sequencing system (SafeSeqS) and analysis of the corresponding tumor tissue. If no mutation was detected by NGS, singleplex methods (SafeSeqS for all 12 patients and digital droplet polymerase chain reaction for six patients) were used to detect a specific mutation previously determined in tumor tissue of 12 patients. (*) Excluded because of previous tubal ligation, germline mutation, or two or more malignant tumors present. MUT, mutation.
In case a mutation was detected in lavage specimens, the results were validated twice. First, the index mutation found in the lavage sample (mutation with highest mutant allele fraction) was assessed in an independent singleplex (SafeSeqS) experiment. In all malignant cases with previously identified mutations, the precise mutation was confirmed.
Second, if primary tumor tissue was available, mutation analysis was conducted in the primary sample as well. In all but two patients, the same mutation was identified. For patient 144, a KRAS G12C mutation (4.1%) was detected in the lavage fluid only, possibly because of heterogeneity or low tumor cell content of the tissue specimen. For patient 143, a mutation (TP53 p.L194R) was found that was present in a metastatic lesion but absent in the primary tumor.
When no mutation was found in the lavage DNA, we evaluated the primary tumors by multiplex sequencing, if available. Identified mutations could be verified in the lavage fluids by SafeSeqS (n = 1) or ddPCR (n = 5) with a sensitivity of 0.1% (Fig 2).
Fig 2.
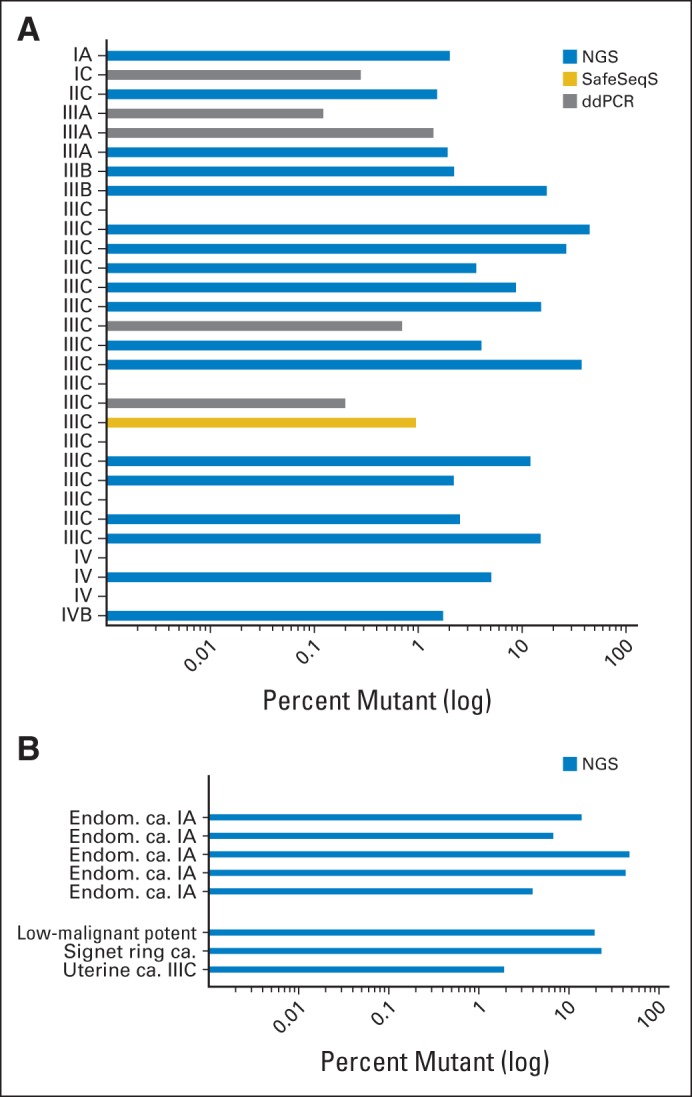
Mutation analysis of lavage specimens by next-generation sequencing (NGS) or singleplex approaches (safe sequencing system [SafeSeqS] and digital droplet polymerase chain reaction [ddPCR]) depicted as percentage of mutant alleles present. (A) Results obtained from analysis of patients with OC included in final analysis. All samples were analyzed by NGS, 12 initially negative samples were analyzed by SafeSeqS, and six of those were also analyzed by ddPCR. (B) Results obtained from analysis of patients with other malignancies, including low-malignant-potential ovarian tumor, uterine carcinosarcoma, and signet ring carcinoma metastasized to the ovaries, as well as five patients with endometrial carcinoma. Disease type and International Federation of Gynecology and Obstetrics stage of disease, if applicable, are listed on the y-axis. ca., carcinoma; Endom., endometrial; potent, potential.
Next-generation sequencing (NGS) identified at least one mutation in 18 (60%) of 30 patients with OC. Furthermore, a mutation was also identified in a lavage sample of a patient with a signet ring carcinoma that had spread to the ovaries, a uterine carcinoma, and a tumor with low malignant potential (Fig 2B). When no mutation was found in the lavage DNA (n = 15), we evaluated the available primary tumors by multiplex sequencing (n = 12). Identified mutations were verified in the lavage fluids by SafeSeqS (one of 12 tumors) or ddPCR (five of six tumors) with a sensitivity of 0.1% (Fig 2). Mutation types identified in tumor tissues or lavage samples by either method are listed in Table 1.
In total, 24 (80%) of 30 patients with OC were identified by either the NGS approach or singleplex analysis for the presence of a mutation previously identified in the primary tumor tissue. Applying NGS of the same panel of genes, lavage samples of five patients with stage IA EC were analyzed. In all five samples (100%), mutations were identified, with mutant allele frequencies ranging between 3.91% and 46.42% (Fig 2B).
Analysis of a Lavage Sample of a Woman With an Occult OC
A lavage sample was analyzed that was taken from a 41-year-old BRCA1 mutation carrier (patient 205) submitted for RRSO, who was recruited from the prospectively running LUSTIC trial. Although no signs of cancer were present (CA-125 and transvaginal ultrasound were inconspicuous) before surgery, laparoscopy and subsequent laparotomy revealed the presence of a few peritoneal nodules and a 15-mm peritoneal lesion on the right diaphragm as the only other site of metastatic spread. Final histopathology revealed a grade 2, pT3b, pN0, International Federation of Gynecology and Obstetrics stage IIIB serous OC. Both ovaries showed microscopic involvement, and STICs were found in both tubes. TP53 mutation analysis of the lavage sample was performed by NGS, an approach suitable for screening of a high-risk population. A frameshift mutation (TP53 p.V217fs, c.650delT) was detected and confirmed by ddPCR (16.8%) and SafeSeqS (17.3%) in DNA from the lavage and the corresponding tissue sample (Speiser et al, submitted for publication).
Analysis of Lavage Samples From Patients With Benign Gynecologic Diseases
Eight (29.6%) of 27 lavage samples from patients with benign gynecologic diseases were found to be mutated by NGS. In six of these patients, a KRAS mutation was detected (Table 1). No mutations were identified in 19 tested tissue samples of patients with benign diseases. Other genes affected only one or two patients (FBXW7, PIK3CA, PIK3R1, or PTEN), some of whom also harbored KRAS gene mutations. None of the lavage samples harbored TP53 mutations, the gene that was most commonly mutated in the patients with OC (Fig 3).
Fig 3.

Distribution of genes affected by mutations, leading to the identification of different sample types (index mutation). A mutation of the TP53 gene is the most important marker in identifying ovarian cancer (OC), whereas KRAS mutations can also be observed in patients with benign diseases. EC, endometrial cancer.
DISCUSSION
This pilot study demonstrates that cells shed from Müllerian duct cancers, including OC and EC, can be collected through a lavage of the uterine cavity, in which tumor-specific mutations can be detected through massively parallel sequencing. Sixty percent of patients with OC had mutations in their lavage samples detected with the NGS approach, a technology possibly suitable for routine application.
In patients with known mutation status of the primary tumor tissue but nondetectable mutation in the lavage by NGS, two methods with an even higher sensitivity were applied (SafeSeqS and ddPCR). Using these methods, mutation detection rates in patients with OC could be increased to 80%, demonstrating the general validity of this concept.
Because we could demonstrate that the lavage of a patient with occult OC was positive for cancer cells, shedding and transtubal transportation of cancer cells seems to take place early in OC propagation. Approximately one third of lavages from patients with benign disease tested positive with the panel of genes applied. In most cases, these were a result of mutations in the KRAS gene or other genes not commonly mutated in OC. All lavages of the five patients with EC tested positive for cancer cells using the NGS approach.
New insights into the biology of high-grade serous OC led to the hypothesis that cells of these tumors and possibly even their precursor lesions can be transported into the uterine cavity. Therefore, we established the lavage technique to collect and identify these cells. As opposed to Pap smears, as used in the study by Kinde et al,22 the lavage permits the evaluation of cells closer to the ovaries (ie, in the uterine cavity and the fallopian tubes). The evaluation of lavage fluids resulted in higher sensitivity than previously reported in Pap smear fluids (60% v 41%, respectively). We show here that, even in lavage samples, the fraction of mutant alleles can be below the detection limit of the NGS approach and that using individualized, singleplex methods (SafeSeqS or ddPCR) allows detection of such mutations. With the amount of DNA and depth of coverage used, the NGS approach could reliably detect mutations present in more than 1% of the total alleles examined. Only a multiplex assay such as the NGS method used here is applicable for screening of patients with OC as a result of the large number of different mutations in TP53 that can occur in OC. Therefore, advances in sequencing technologies should be able to increase detection of OC in lavage fluids in the future.
Both the lavage and liquid Pap smear suffer from the fact that few patients with early disease were analyzed because most OCs are not detected until they are advanced. The detection of early disease is one of the most important goals because of the expected greater impact on mortality along with higher chances of surgically curable disease. Still, it is encouraging that two of four stage I OCs were detected in the study by Kinde et al22 and two of two stage I OCs were detected in the current study. Even more promising for early diagnosis is our detection of a clinically occult OC that was missed by the current state-of-the-art diagnostic methods. As a result of transperitoneal spread, serous OC may not progress stage by stage, and earlier detection will not necessarily result in a stage shift. However, earlier detection of serous OC equates to less tumor burden at diagnosis, a substantial increase in patients who can undergo complete tumor resection, and less radical procedures necessary to achieve this result. Among women with an increased risk of HGSC who undergo RRSO, 10% to 15% are diagnosed with an STIC or an invasive neoplasm.11,24–31 Notably, more than 90% of STICs harbor TP53 mutations, like the more advanced lesions that evolve from them. Thus, the lavage technology has the potential to not only detect OC earlier, but also detect premalignant lesions. This is further supported by the progression time from STIC to an invasive carcinoma, opening a window for early detection or even prevention of HGSC. Cancers that the lavage procedure missed were International Federation of Gynecology and Obstetrics stage IIIC or IV. However, such a diagnostic method is not needed for advanced stages, and a successful detection of all of these advanced cancers was not expected as a result of physical reasons. A disadvantage of the invasive lavage method may be the theoretical risk of infection, although no such infections have been observed to date (P. Speiser, personal communication, September 2015).
Possible improvements for future follow-up studies comprise performing the lavage at specific time points during the menstrual cycle. Moreover, the lavage procedure is standardized now, and one Conformité Européene (CE)-certified catheter is used, in contrast to the proof-of-concept study, where these issues were still being optimized. Additional information will be obtained from comparing liquid Pap smears with lavage samples from the same patients. Both are expected to be exquisitely specific for neoplasia given the nature of the biomarker assessed (somatic mutations). However, it cannot be excluded that benign tumors, which are also neoplastic, contribute mutations to the analyzed fluids. To address this for the first time, we also analyzed samples of patients with benign diseases. In eight of 27 lavage samples, a mutation was detected. Some of these cases may be explained by the fact that low-grade serous carcinoma, in contrast to HGSC, shows a slow transition from benign to malignant stage and thereby accumulates mutations in KRAS, BRAF, or PPP2R1A and other genes.32 Whether these cases should be considered true-positive results is debatable; a similar debate has been waged for early cervical lesions in conventional Pap smears or small adenomas in colonoscopy. These results, especially the high frequency of KRAS mutations, suggest the exclusion of certain genes from mutation analysis for differential diagnosis. The clinically most relevant group is patients with HGSC, which in almost all patients can be detected via TP53 analysis. In patient 131, besides a predominant KRAS mutation, mutations in several other genes were detected, including TP53 (Data Supplement). Thus, the exclusion of KRAS will not alter the test result because of the present TP53 mutation. We were also able to reproduce the high frequency in EC detection, as published by Kinde et al.22 Although we analyzed lavage samples of only five patients with EC, the potential of the lavage technique is obvious.
In summary, this proof-of-concept study demonstrates the potentially high diagnostic power of the lavage approach for OC and EC detection, especially for early detection in high-risk populations. This is currently being investigated in a large study (ClinicalTrials.gov identifier: NCT02039388).
Footnotes
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Elisabeth Maritschnegg, Ignace Vergote, Luis A. Diaz Jr, Nickolas Papadopoulos, Kenneth W. Kinzler, Bert Vogelstein, Paul Speiser, Robert Zeillinger
Financial support: Luis A. Diaz Jr, Nickolas Papadopoulos, Kenneth W. Kinzler
Administrative support: Nina Pecha
Provision of study materials or patients: Nina Pecha, Ignace Vergote, Florian Heitz, Jalid Sehouli, Paul Speiser
Collection and assembly of data: Elisabeth Maritschnegg, Yuxuan Wang, Nina Pecha, Reinhard Horvat, Els Van Nieuwenhuysen, Ignace Vergote, Florian Heitz, Jalid Sehouli, Luis A. Diaz Jr, Nickolas Papadopoulos, Kenneth W. Kinzler, Bert Vogelstein, Paul Speiser
Data analysis and interpretation: Elisabeth Maritschnegg, Yuxuan Wang, Reinhard Horvat, Ignace Vergote, Florian Heitz, Jalid Sehouli, Isaac Kinde, Luis A. Diaz Jr, Nickolas Papadopoulos, Kenneth W. Kinzler, Bert Vogelstein, Paul Speiser, Robert Zeillinger
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Lavage of the Uterine Cavity for Molecular Detection of Müllerian Duct Carcinomas: A Proof-of-Concept Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Elisabeth Maritschnegg
No relationship to disclose
Yuxuan Wang
No relationship to disclose
Nina Pecha
No relationship to disclose
Reinhard Horvat
No relationship to disclose
Els Van Nieuwenhuysen
No relationship to disclose
Ignace Vergote
No relationship to disclose
Florian Heitz
Honoraria: Roche, AstraZeneca
Speakers' Bureau: Roche
Travel, Accommodations, Expenses: GlaxoSmithKline
Jalid Sehouli
No relationship to disclose
Isaac Kinde
Employment: PapGene
Leadership: PapGene
Stock or Other Ownership: PapGene
Patents, Royalties, Other Intellectual Property: Royalties through licensing managed by Johns Hopkins
Luis A. Diaz Jr
Employment: Personal Genome Diagnostics
Leadership: Personal Genome Diagnostics
Stock or Other Ownership: Personal Genome Diagnostics, PapGene
Consulting or Advisory Role: Personal Genome Diagnostics, PapGene
Patents, Royalties, Other Intellectual Property: Royalties from Johns Hopkins for licensed inventions
Nickolas Papadopoulos
Leadership: PapGene
Stock or Other Ownership: Personal Genome Diagnostics, PapGene
Consulting or Advisory Role: Personal Genome Diagnostics, Sysmex Inostics, PapGene
Patents, Royalties, Other Intellectual Property: Royalties from Johns Hopkins for licensed inventions
Travel, Accommodations, Expenses: Sysmex Inostics
Kenneth W. Kinzler
Leadership: PapGene
Stock or Other Ownership: Personal Genome Diagnostics, PapGene
Consulting or Advisory Role: Personal Genome Diagnostics, PapGene, Sysmex Inostics, Morphotek
Patents, Royalties, Other Intellectual Property: Royalties from Johns Hopkins for licensed inventions
Bert Vogelstein
Stock or Other Ownership: Personal Genome Diagnostics, PapGene
Consulting or Advisory Role: Personal Genome Diagnostics, PapGene, Sysmex Inostics, Morphotek
Patents, Royalties, Other Intellectual Property: Royalties from Johns Hopkins for licensed inventions
Paul Speiser
Patents, Royalties, Other Intellectual Property: Private patent
Travel, Accommodations, Expenses: Roche
Robert Zeillinger
Stock or Other Ownership: OncoLab Diagnostics GmbH, Dx4U GmbH
Research Funding: Bayer AG (Inst)
Patents, Royalties, Other Intellectual Property: OncoLab Diagnostics GmbH, Private patents, Dx4U GmbH
Travel, Accommodations, Expenses: Angle plc
REFERENCES
- 1.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Zorn KK, Bonome T, Gangi L, et al. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res. 2005;11:6422–6430. doi: 10.1158/1078-0432.CCR-05-0508. [DOI] [PubMed] [Google Scholar]
- 3.Stirling D, Evans DG, Pichert G, et al. Screening for familial ovarian cancer: Failure of current protocols to detect ovarian cancer at an early stage according to the International Federation of Gynecology and Obstetrics system. J Clin Oncol. 2005;23:5588–5596. doi: 10.1200/JCO.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 5.Kim A, Ueda Y, Naka T, et al. Therapeutic strategies in epithelial ovarian cancer. J Exp Clin Cancer Res. 2012;31:14. doi: 10.1186/1756-9966-31-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramus SJ, Gayther SA. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol Oncol. 2009;3:138–150. doi: 10.1016/j.molonc.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King MC, Marks JH, Mandell JB, et al. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 8.Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 9.Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 10.Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: Defining the target for early detection. PLoS Med. 2009;6:e1000114. doi: 10.1371/journal.pmed.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn E, Kurman RJ, Vang R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma: Evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226:421–426. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bijron JG, Seldenrijk CA, Zweemer RP, et al. Fallopian tube intraluminal tumor spread from noninvasive precursor lesions: A novel metastatic route in early pelvic carcinogenesis. Am J Surg Pathol. 2013;37:1123–1130. doi: 10.1097/PAS.0b013e318282da7f. [DOI] [PubMed] [Google Scholar]
- 13.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri: FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(suppl 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health Consensus Development Conference Statement. Ovarian cancer—Screening, treatment, and follow-up. Gynecol Oncol. 1994;55:S4–S14. doi: 10.1006/gyno.1994.1333. [DOI] [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Management of adnexal masses. Obstet Gynecol. 2007;110:201–214. doi: 10.1097/01.AOG.0000263913.92942.40. [DOI] [PubMed] [Google Scholar]
- 16.Drake J. Diagnosis and management of the adnexal mass. Am Fam Physician. 1998;57:2471–2476. 2479–2480. [PubMed] [Google Scholar]
- 17.Gallup DG, Talledo E. Management of the adnexal mass in the 1990s. South Med J. 1997;90:972–981. doi: 10.1097/00007611-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Hall TR, Randall TC. Adnexal masses in the premenopausal patient. Clin Obstet Gynecol. 2015;58:47–52. doi: 10.1097/GRF.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 19.Bast RC, Jr, Skates S, Lokshin A, et al. Differential diagnosis of a pelvic mass: Improved algorithms and novel biomarkers. Int J Gynecol Cancer. 2012;22(suppl 1):S5–S8. doi: 10.1097/IGC.0b013e318251c97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bristow RE, Smith A, Zhang Z, et al. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128:252–259. doi: 10.1016/j.ygyno.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 21.du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials—By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 22.Kinde I, Bettegowda C, Wang Y, et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013;5:167ra4. doi: 10.1126/scitranslmed.3004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colgan TJ, Murphy J, Cole DE, et al. Occult carcinoma in prophylactic oophorectomy specimens: Prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283–1289. doi: 10.1097/00000478-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Leeper K, Garcia R, Swisher E, et al. Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol Oncol. 2002;87:52–56. doi: 10.1006/gyno.2002.6779. [DOI] [PubMed] [Google Scholar]
- 26.Powell CB, Kenley E, Chen LM, et al. Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: Role of serial sectioning in the detection of occult malignancy. J Clin Oncol. 2005;23:127–132. doi: 10.1200/JCO.2005.04.109. [DOI] [PubMed] [Google Scholar]
- 27.Carcangiu ML, Peissel B, Pasini B, et al. Incidental carcinomas in prophylactic specimens in BRCA1 and BRCA2 germ-line mutation carriers, with emphasis on fallopian tube lesions: Report of 6 cases and review of the literature. Am J Surg Pathol. 2006;30:1222–1230. doi: 10.1097/01.pas.0000202161.80739.ac. [DOI] [PubMed] [Google Scholar]
- 28.Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58–64. doi: 10.1016/j.ygyno.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 29.Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 30.Hirst JE, Gard GB, McIllroy K, et al. High rates of occult fallopian tube cancer diagnosed at prophylactic bilateral salpingo-oophorectomy. Int J Gynecol Cancer. 2009;19:826–829. doi: 10.1111/IGC.0b013e3181a1b5dc. [DOI] [PubMed] [Google Scholar]
- 31.Reitsma W, de Bock GH, Oosterwijk JC, et al. Support of the “fallopian tube hypothesis” in a prospective series of risk-reducing salpingo-oophorectomy specimens. Eur J Cancer. 2013;49:132–141. doi: 10.1016/j.ejca.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Kessler M, Fotopoulou C, Meyer T. The molecular fingerprint of high grade serous ovarian cancer reflects its fallopian tube origin. Int J Mol Sci. 2013;14:6571–6596. doi: 10.3390/ijms14046571. [DOI] [PMC free article] [PubMed] [Google Scholar]


