Abstract
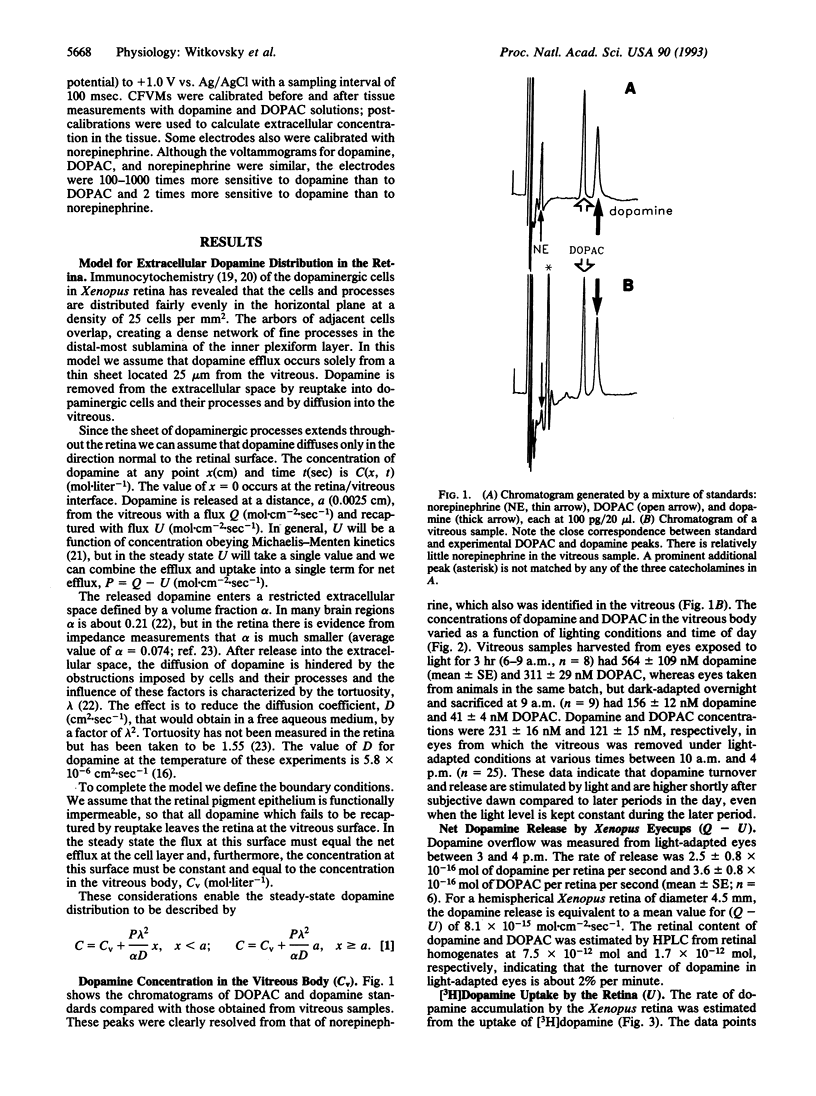
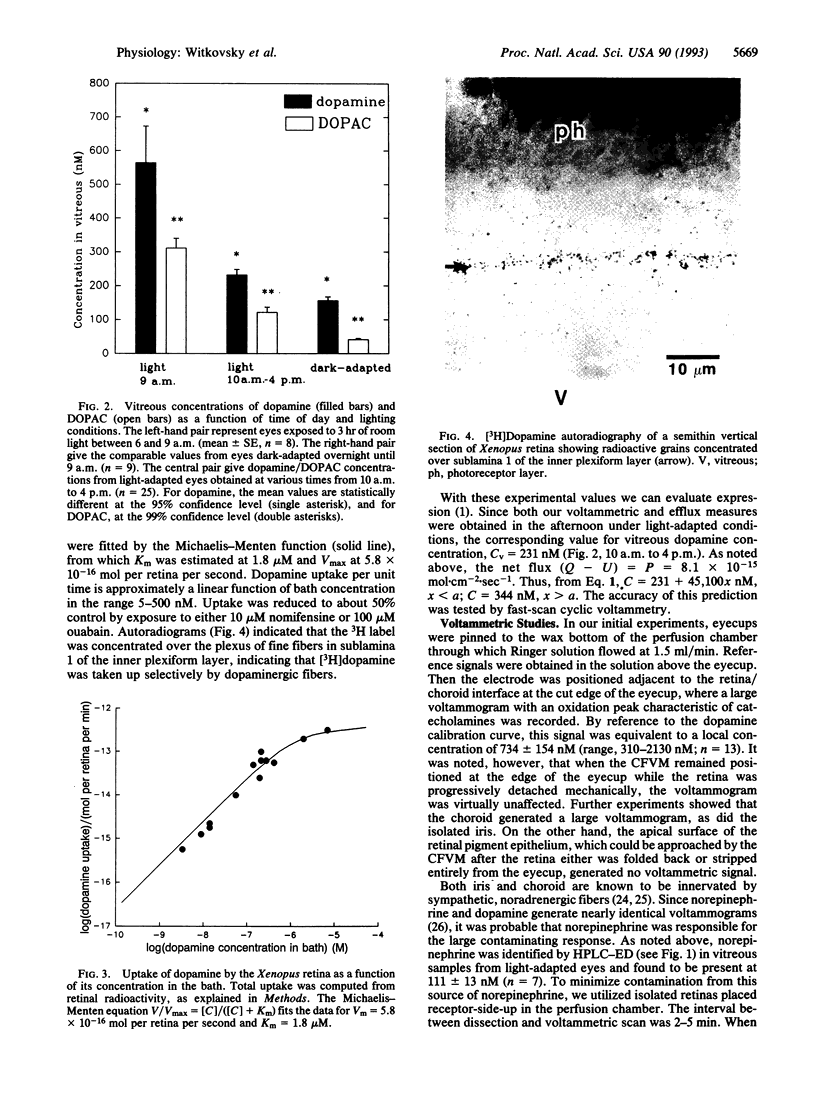
Dopamine reaches targets in the outer retina of the clawed frog (Xenopus laevis) by diffusion from a network of dopaminergic cells and processes located predominantly at the junction of inner nuclear and inner plexiform layers. We obtained values for the steady-state release, uptake, and extracellular concentration of dopamine in the retina by a combination of HPLC (with electrochemical detection), scintillation spectroscopy, and fast-scan cyclic voltammetry. Vitreal concentrations of dopamine varied from 564 +/- 109 nM in light-adapted eyes near the time of subjective dawn to 156 +/- 12 nM in dark-adapted eyes. The data are consistent with a simple model for steady-state dopamine diffusion from an appropriately sited thin-sheet source. This model was used to generate a profile of extracellular dopamine concentration as a function of retinal depth. The model predicted an increase in the dopamine concentration from the vitreous to the layer of dopaminergic cells, remaining constant from that layer to the distal tips of the photoreceptors. This prediction was borne out by comparing fast-scan voltammetric measures of dopamine at the distal tips of the receptors with the vitreal concentrations determined by HPLC using electrochemical detection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong-James M., Millar J. Carbon fibre microelectrodes. J Neurosci Methods. 1979 Oct;1(3):279–287. doi: 10.1016/0165-0270(79)90039-6. [DOI] [PubMed] [Google Scholar]
- Armstrong P. B., Bell A. L. Pupillary responses in the toad as related to innervation of the iris. Am J Physiol. 1968 Mar;214(3):566–573. doi: 10.1152/ajplegacy.1968.214.3.566. [DOI] [PubMed] [Google Scholar]
- Baur J. E., Kristensen E. W., May L. J., Wiedemann D. J., Wightman R. M. Fast-scan voltammetry of biogenic amines. Anal Chem. 1988 Jul 1;60(13):1268–1272. doi: 10.1021/ac00164a006. [DOI] [PubMed] [Google Scholar]
- Besharse J. C., Iuvone P. M. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature. 1983 Sep 8;305(5930):133–135. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- Boatright J. H., Hoel M. J., Iuvone P. M. Stimulation of endogenous dopamine release and metabolism in amphibian retina by light- and K+-evoked depolarization. Brain Res. 1989 Mar 13;482(1):164–168. doi: 10.1016/0006-8993(89)90555-6. [DOI] [PubMed] [Google Scholar]
- Cahill G. M., Besharse J. C. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991 Oct;11(10):2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M., Chan C. Y. Stimulus-induced extracellular pH transients in the in vitro turtle cerebellum. Neuroscience. 1988 Dec;27(3):941–948. doi: 10.1016/0306-4522(88)90197-2. [DOI] [PubMed] [Google Scholar]
- Dearry A., Burnside B. Dopaminergic regulation of cone retinomotor movement in isolated teleost retinas: I. Induction of cone contraction is mediated by D2 receptors. J Neurochem. 1986 Apr;46(4):1006–1021. doi: 10.1111/j.1471-4159.1986.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Dearry A., Edelman J. L., Miller S., Burnside B. Dopamine induces light-adaptive retinomotor movements in bullfrog cones via D2 receptors and in retinal pigment epithelium via D1 receptors. J Neurochem. 1990 Apr;54(4):1367–1378. doi: 10.1111/j.1471-4159.1990.tb01971.x. [DOI] [PubMed] [Google Scholar]
- Dubocovich M. L. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983 Dec 22;306(5945):782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Ehinger B. Neurotransmitter systems in the retina. Retina. 1982;2(4):305–321. [PubMed] [Google Scholar]
- HAEGGENDAL J., MALMFORS T. IDENTIFICATION AND CELLULAR LOCALIZATION OF THE CATECHOLAMINES IN THE RETINA AND THE CHOROID OF THE RABBIT. Acta Physiol Scand. 1965 May-Jun;64:58–66. doi: 10.1111/j.1748-1716.1965.tb04153.x. [DOI] [PubMed] [Google Scholar]
- Karwoski C. J., Frambach D. A., Proenza L. M. Laminar profile of resistivity in frog retina. J Neurophysiol. 1985 Dec;54(6):1607–1619. doi: 10.1152/jn.1985.54.6.1607. [DOI] [PubMed] [Google Scholar]
- Knapp A. G., Dowling J. E. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. 1987 Jan 29-Feb 4Nature. 325(6103):437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Leviel V., Gobert A., Guibert B. Direct observation of dopamine compartmentation in striatal nerve terminal by 'in vivo' measurement of the specific activity of released dopamine. Brain Res. 1989 Oct 16;499(2):205–213. doi: 10.1016/0006-8993(89)90768-3. [DOI] [PubMed] [Google Scholar]
- Louilot A., Le Moal M., Simon H. Differential reactivity of dopaminergic neurons in the nucleus accumbens in response to different behavioral situations. An in vivo voltammetric study in free moving rats. Brain Res. 1986 Nov 12;397(2):395–400. doi: 10.1016/0006-8993(86)90646-3. [DOI] [PubMed] [Google Scholar]
- Millar J., Stamford J. A., Kruk Z. L., Wightman R. M. Electrochemical, pharmacological and electrophysiological evidence of rapid dopamine release and removal in the rat caudate nucleus following electrical stimulation of the median forebrain bundle. Eur J Pharmacol. 1985 Mar 12;109(3):341–348. doi: 10.1016/0014-2999(85)90394-2. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Phillips J. M. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol. 1981 Dec;321:225–257. doi: 10.1113/jphysiol.1981.sp013981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M. E., Nicholson C. Measurement of nanomolar dopamine diffusion using low-noise perfluorinated ionomer coated carbon fiber microelectrodes and high-speed cyclic voltammetry. Anal Chem. 1989 Sep 1;61(17):1805–1810. doi: 10.1021/ac00192a005. [DOI] [PubMed] [Google Scholar]
- Schütte M., Witkovsky P. Dopaminergic interplexiform cells and centrifugal fibres in the Xenopus retina. J Neurocytol. 1991 Mar;20(3):195–207. doi: 10.1007/BF01186992. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Coyle J. T. Regional differences in H3-norepinephrine and H3-dopamine uptake into rat brain homogenates. J Pharmacol Exp Ther. 1969 Jan;165(1):78–86. [PubMed] [Google Scholar]
- Stone S., Witkovsky P. The actions of gamma-aminobutyric acid, glycine and their antagonists upon horizontal cells of the Xenopus retina. J Physiol. 1984 Aug;353:249–264. doi: 10.1113/jphysiol.1984.sp015334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P., Alones V., Piccolino M. Morphological changes induced in turtle retinal neurons by exposure to 6-hydroxydopamine and 5,6-dihydroxytryptamine. J Neurocytol. 1987 Feb;16(1):55–67. doi: 10.1007/BF02456697. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Schütte M. The organization of dopaminergic neurons in vertebrate retinas. Vis Neurosci. 1991 Jul-Aug;7(1-2):113–124. doi: 10.1017/s0952523800010981. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Shi X. P. Slow light and dark adaptation of horizontal cells in the Xenopus retina: a role for endogenous dopamine. Vis Neurosci. 1990 Oct;5(4):405–413. doi: 10.1017/s0952523800000493. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Stone S., Besharse J. C. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Res. 1988 May 24;449(1-2):332–336. doi: 10.1016/0006-8993(88)91048-7. [DOI] [PubMed] [Google Scholar]
- Wulle I., Kirsch M., Wagner H. J. Cyclic changes in dopamine and DOPAC content, and tyrosine hydroxylase activity in the retina of a cichlid fish. Brain Res. 1990 May 7;515(1-2):163–167. doi: 10.1016/0006-8993(90)90591-x. [DOI] [PubMed] [Google Scholar]
- Zhu B. S., Straznicky C. Morphology and retinal distribution of tyrosine hydroxylase-like immunoreactive amacrine cells in the retina of developing Xenopus laevis. Anat Embryol (Berl) 1991;184(1):33–45. doi: 10.1007/BF01744259. [DOI] [PubMed] [Google Scholar]




